Abstract
Sheep-associated malignant catarrhal fever (SA-MCF), the form of MCF that occurs in Brazil, is a severe, frequently fatal, infectious disease caused by ovine gammaherpesvirus-2 (OvHV-2), in which sheep are the asymptomatic hosts and cattle and other cloven-hoofed animals are the accidental hosts. This review provides a critical analysis of the historical, epidemiological aspects and the estimated economic impacts associated with SA-MCF in Brazil. Moreover, the clinical manifestations and pathological lesions associated with SA-MCF in cattle are reviewed and discussed and the phylogenetic distribution of OvHV-2 in Brazil is presented. OvHV-2 is the only MCF virus identified in animals from Brazil. It is recommended that a histopathologic diagnosis of SA-MCF be based on all aspects of vascular disease in the affected animal and not only lymphocytic/necrotizing vasculitis and/or fibrinoid change. Conformation of the intralesional participation of OvHV-2 in these alterations can be achieved by immunohistochemistry and/or in situ hybridization assays. Additionally, it is proposed that OvHV-2 should be considered as a possible infectious disease agent associated with the development of bovine respiratory disease in cattle. Furthermore, the possible role of the small intestine in the dissemination of OvHV-2 is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Historical aspects of malignant catarrhal fever in Brazil
It is rather likely that the first occurrence of malignant catarrhal fever (MCF) in domestic cattle in association with sheep occurred in Europe by the end of 1700 [1]. By 1850, MCF was recognized in Africa and the relationship of the wildebeest in the transmission of MCF was already suspected [2]. However, the first experimental study with MCF was probably done in 1923 in South Africa by Mettam, where MCF is known as snotziekte [3]. In this featured article, Mettam [3] described that the histopathological findings “reveals the presence of hyperplasia of the lymphoid tissues, and infiltrations of lymphocytes in the parenchyma of the organs, especially in situations closely related to blood vessels”; these are the hallmarks for the histologic diagnosis of MCF that is now known as disseminated vasculitis with fibrinoid change.
The documented chronology of events relative to the characterization of MCF in Brazil based on published manuscripts is given in Table 1. The first mention of MCF in Brazil probably dated back to 1924, during which several outbreaks of cattle mortality that occurred in various cities within the State of Paraíba, Northeastern Brazil, were investigated [4]. However, the detailed report described the clinical manifestations and gross findings of 7.9% (3/38) cows [4] that could have been simultaneously affected by several infectious disease entities, probably including MCF, but there is no description of the histopathologic findings. These clinical syndromes were referred to as “hallow” (“oca”), “horn disease” (“mal de chifres”), or gangrenous coryza (“coriza gangrenosa”) and were associated with progressive emaciation, corneal ulcerations, ulcerative gingivitis, bronchopneumonia, enlarged lymph nodes, fibrinous and/or necrotizing tracheitis and laryngitis, and purulent sinusitis [4]. Nevertheless, an extensive study done within the North and Northeastern regions of Brazil in 1959 revealed that the horn affections could have been confused with nutritional deficiencies of cattle, purulent empyema of the frontal sinuses, and/or chronic sinusitis [5]. Moreover, it seemed that at that time, “hallow” and “horn disease” as well as other similar terms, were unintentionally used to indicate or were confused with gangrenous coryza [6].
The first description of the gross and histopathologic findings associated with MCF in Brazil was done by Döbereiner and Tokarnia in 1956 who investigated cattle mortality in Rio Grande do Norte [6]. At that time, MCF was known as gangrenous coryza in Brazil [6] and was referred to as bovine malignant catarrh in Europe and North America [7,8,9], and snotziekte in Africa [3, 10]. Although Döbereiner and Tokarnia tried unsuccessfully to reproduce the disease in rabbits, they concluded that their histopathologic diagnosis was consistent with similar reports worldwide [6]. In fact, the histopathologic lesions described in the cases reported by Döbereiner and Tokarnia [6] were similar to the ongoing studies worldwide [3, 9, 11], where experimental infection was confirmed in cattle [2], the passive role of sheep in the development of MCF was discussed [12], and the utilization of rabbits as a model to study this disease was proposed [7, 13]. Consequently, it can be argued that Döbereiner and Tokarnia were the first to effectively describe the occurrence of MCF in Brazil, since the vascular lesions associated with MCF continue to be fundamental for the histopathologic diagnosis of this disease, being consistent with those described by Mettam in 1924 [3]. Alternatively, Torres in 1924 reported the clinical aspects of several diseases [4], and described gross manifestations including corneal edema and nasal secretions, which are frequently observed in cattle infected with MCF [14, 15]; but Torres did not provide any associated histologic record of alterations associated with the clinical diseases. It must be highlighted that some of those alterations observed by Torres in 1924 could have been attributed to those seen in cattle with MCF but are easily confused with the clinical manifestations of other diseases such as blue tongue (BT) [14], bovine viral diarrhea (BVD) [14, 16], infectious bovine rhinotracheitis (IBR) [16,17,18], foot-and-mouth disease (FMD) [14, 16], and other vesicular infectious diseases that affect the oral and respiratory tracts of cattle. Moreover, the clinical manifestations of MCF are variable and a diagnosis cannot be made exclusively on gross findings [19].
There was an apparent 13-year absence of scientific data relative to the occurrence of MCF in Brazil between the cases described by Döbereiner and Tokarnia and the outbreak reported in São Paulo in 1972 [20]. In 1983, Barros and colleagues [17] investigated and described another outbreak of MCF in cattle that occurred in 1973 at the city of Julio de Castilhos, approximately 60 km from Santa Maria, Rio Grande do Sul. These authors clearly differentiated the histopathologic findings associated with MCF from other similar infectious diseases [17], and probably marked the turning point for the histopathologic characterization of MCF in Brazil by presenting detailed histologic findings observed in several organs. MCF was then described in outbreaks affecting cattle in the States of São Paulo in 1983 [21] and Paraná in 1985 [22]. It must be highlighted, that up to this point, all confirmations of cases of MCF in cattle from Brazil was done exclusively using the classic histopathologic vascular alterations that are characteristic of this disease.
During 1959–1960, experimental studies confirmed that Alcelaphine herpesvirus, now renamed Alcelaphine gammaherpesvirus 1 (AlHV-1), was the etiologic agent associated with MCF in Africa, after the successful identification of this virus from blue wildebeest (Connochaetes taurinus) in Kenya [11]. The first molecular diagnostic method for the characterization of MCF was done to amplify AlHV-1 DNA from blue wildebeest in 1990 [23]. In 1992, ovine gammaherpesvirus 2 (formerly ovine herpesvirus, OvHV-2) DNA was identified in normal sheep and clinically affected Balinese cattle (Bos javamcus) and water buffalo (Bubalus bubalis) from Indonesia [24]. These studies demonstrated that there are at least two principal syndromes with similar clinical sings and pathologic alterations associated with MCF in distinct geographical locations: wildebeest-associated MCF (WA-MCF) in Africa and sheep-associated MCF (SA-MCF) worldwide. In 1993, Baxter and others developed a PCR assay that successfully amplified OvHV-2 DNA from several ruminant species with clinical manifestations of MCF [25]. Seven years later, a quantitative PCR (qPCR) technique was developed to detect the number of OvHV-2 DNA copies in sheep [26]. In 2002, Flach and colleagues designed PCR assays that are able to differentiate between OvHV-2 and AlHV-1 [27]. In addition, Cunha and others in 2009, introduced a multiplex PCR assay to differentiate between five viral agents associated with MCF [28]. These molecular assays are considered as fundamental for the efficient identification of MCF in a wide range of mammalian species [29].
Although molecular methods to amplify viral nucleic acids associated with MCF were established and used worldwide since 1990, the first molecular detection of OvHV-2 DNA in Brazil occurred more than a decade later in 2002 [30]. This turning point occurred when Driemeier and colleagues revised the findings of an MCF outbreak that occurred in 1994, during which brown Brocket deer (Mazama gouazoubira) maintained in a zoological park in Cuiabá, Mato Grosso, were clinically ill and died with histopathologic findings consistent with this disease [30]. Thereafter, PCR assays were used to identify OvHV-2 DNA in outbreaks of MCF from the States of Rio Grande do Sul [31, 32], Minas Gerais [33,34,35], Mato Grosso [36, 37], Rio Grande do Norte [38], Paraná [39, 40], Pernambuco [41], and Rio de Janeiro [42]. Consequently, the utilization of molecular testing associated with typical histopathologic findings is now frequently used to efficiently diagnose MCF in Brazil, except for the descriptions in the States of São Paulo-Mato Grosso do Sul [43], Paraíba [44], Espírito Santo [45], Rio de Janeiro [46], and Bahia [47], in which diagnosis was based exclusively on histopathologic findings. An in situ hybridization (ISH) assay was designed to effectively identify OvHV-2 nucleic acids in tissues with lesions typical of MCF [48]. The probes used in that diagnostic method were specific in detecting only OvHV-2 and did not cross-react with other members of the MCF complex of organisms, including AlHV-1, AlHV-2, caprine herpesvirus 2 (CpHV-2) and 3 (CpHV-3) and ibex-MCF virus [48]; this diagnostic combination confirms an active infection due to the intralesional detection of the MCF viruses. To this end, Headley and others [49] have standardized an immunohistochemical (IHC) assay designed to identify OvHV-2 antigens from formalin-fixed paraffin-embedded (FFPE) tissues sections; these in situ diagnostic methods are excellent tools for retrospective studies using archival material.
In 2001, Garmatz and colleagues successfully transmitted MCF after an outbreak of the disease in cattle, by using blood derived from a cow with clinical manifestations of MCF that was transferred to five asymptomatic calves [31]. Three calves that were experimentally infected and developed clinical disease and pathological findings consistent with those described in MCF, with the amplification of OvHV-2 DNA [31]. These results represent the first confirmed transmission of MCF in animals from Brazil, and are similar to the early experimental studies done in North America [50, 51] and Africa [2, 7, 12].
Macêdo and collaborators [44] did a retrospective study of the cases of SA-MCF diagnosed in cattle from Paraíba, Northeastern Brazil, and demonstrated that only 3.2% (6/190) of the submitted cases were infected by OvHV-2. However, the diagnosis of SA-MCF in this study was based exclusively on the histopathologic findings recorded without any molecular analysis.
Costa and others [35] identified typical histopathologic lesions of SA-MCF in Murrah buffalos and amplified OvHV-2 DNA from multiple tissues of four buffalos that died of the head and eye form of MCF; these findings represent the only documentation of MCF in buffalos from Brazil. The clinical findings and lesions observed during that outbreak were similar to those described in buffalos worldwide with SA-MCF [52,53,54]. Additional details of the outbreak of SA-MCF in buffalos from Brazil are provided below.
In 2013, Headley and others [40] demonstrated transplacental transmission of OvHV-2 from a cow to her offspring due to the combination of characteristic histopathologic findings in several tissues with the concomitant amplification of OvHV-2 DNA from both animals. Although vertical transmission is not frequently observed in SA-MCF, and is not the most frequent form of dissemination of OvHV-2 to susceptible hosts [19, 29, 55], these findings demonstrate the possibility of OvHV-2 transplacental infection to be associated with SA-MCF. Additionally, congenital transmission of WA-MCF was experimentally demonstrated in cattle [56]. However, further epidemiologic data and studies are required to associate OvHV-2 as a possible reproductive disease pathogen of cattle.
Eloi and colleagues [57], evaluated the occurrence of OvHV-2 DNA in sheep (n = 188), compared the efficiency of detection of viral DNA in blood as against nasal secretions from eight sheep farms, and amplified OvHV-2 DNA from 41.5% (78/188) of the nasal samples. However, the results of the blood samples are somewhat obscure, while qPCR and statistical analyses were not used to compare the efficiency of these results. Nevertheless, these authors indicated that the efficiency of molecular detection of OvHV-2 DNA was twofold higher in nasal secretions relative to blood. Additionally, sheep from two of the farms evaluated had direct contact with cattle but without clinical manifestations associated with MCF [57]. Notwithstanding the above, similar investigations done by Li and colleagues [58, 59] demonstrated, by using qPCR assays, that up to a 100,000-fold increase per copy of OvHV-2 DNA can be found in nasal samples relative to blood in sheep during shedding episodes and confirmed that nasal secretions are the principal means of transmission of MCF by sheep .
Martins and colleagues [60] did a retrospective study using a combination of histopathology and qPCR to review a collection of 290 brain samples of cattle derived from several states of Brazil, between 2012 and 2014. These authors demonstrated that 5.9% (17/290) of the samples evaluated contained OvHV DNA by qPCR, while histopathologic lesions were identified in 47.1% (8/17) of the samples that contained OvHV-2 DNA. However, there is no consistent information relative to the anatomic locations of the brain evaluated in these cases. Although these authors [60] correctly argued that molecular testing is more sensitive than histopathologic findings to confirm the presence of OvHV-2, they erroneously suggested that MCF may be underdiagnosed in Brazil “since there are no characteristic macro- or microscopic lesions, and some lesions can be observed only upon post-mortem examination.” The histopathologic lesions associated with MCF are characteristic [19, 61, 62], fundamental for the diagnosis of this disease, have been used since 1923 [3], and are recognized by the World Organization for Animal Health (OIE) as diagnostic [16, 19]. Another negative note in this article was the overstatement made by these authors indicating that “Malignant catarrhal fever diagnosis in Brazil is usually made by clinical signs, necropsy, and histology” and cited only a few of the articles published in Brazil relative to MCF to corroborate their statement, but excluded the manuscripts that used molecular testing to identify OvHV-2 DNA in cattle. It is interested to note that the paper by Martins and colleagues [60] was published in 2017, while the utilization of molecular testing to identify OvHV-2 in Brazil dated back to 1994. Additionally, the discrepancy between molecular and histological data in that study [60] may be directly related to the type of samples examined. Brain does not seem to be very reliable for the diagnosis MCF, since usually very low amount of virus can be detected in the brain as compared to other organs.
Oliveira and colleagues [42] identified OvHV-2 DNA in an outbreak of MCF in 2017, during which neurological manifestations were observed in a group of Sambar deer (Rusa unicolor), maintained in a park within the state of Rio de Janeiro. Interestingly, one of these deer was gravid and borne a 20 cm long fetus [42], which was not evaluated neither for possible typical histopathologic lesions nor the presence of OvHV-2 DNA. Nonetheless, this is the second confirmation of MCF in a wild ruminant from Brazil.
In 2019, Pinheiro de Oliveira and others [63] developed a digital droplet PCR (ddPCR) methodology to identify OvHV-2 DNA in cattle and sheep, and have indicated that this strategy has elevated sensitivity and specificity for the identification of viral particles from secretions and tissues of animals. OvHV-2 DNA was detected in blood from asymptomatic sheep, tissues of a MCF affected cow, as well as nasal and ocular swabs and milk from a sheep that had recently lambed [63]. However, milk does not seem to play a role in the transmission of SA-MCF, as demonstrated in experimental studies [64, 65].
A significant breakthrough to understand the pathogenesis of OvHV-2 induced SA-MCF was achieved by Pesavento and collaborators [48] and Headley and colleagues [49] by using in situ diagnostic methods. Headley and colleagues used the monoclonal antibody (MAb-15A) in IHC assays to identify intralesional antigens of OvHV-2 in cattle with neurological manifestations [49], but without the head and eye form (HEF) of MCF and that contained OvHV-2 DNA in brain tissues by PCR [66]. These authors have demonstrated that the MAb-15A, which binds to a common epitope in MCF viruses, and previously used exclusively in serological assays to identify OvHV-2 [67,68,69], can efficiently detect OvHV-2 antigens within the cytoplasm of epithelial, some endothelial cells, and leukocytes [49]. This IHC assay will be a landmark in the diagnosis of MCF since retrospective studies can be easily achieved using FFPE archival blocks. Another interesting finding from the IHC study [49] was widespread angiopathy in cattle that died with acute manifestations of neurological disease but without the head and eye form of MCF. These findings contrast previous descriptions of arterial proliferative lesions in MCF in which the affected animals had chronic manifestations of MCF [70,71,72]; consequently, angiopathy in MCF may occur in animals with or without the classical head and eye form and during acute manifestations of neurologic disease.
Etiology of malignant catarrhal fever
Malignant catarrhal fever is a frequently lethal, lymphotropic and vasotropic [7, 50], viral-induced infectious disease, that is caused by several agents of the malignant catarrhal fever virus (MCFV) complex group, subfamily Gammaherpesvirinae [55, 73], genus Macavirus [74], and is associated with the proliferation of dysregulated lymphocytes [75, 76]. These viral disease pathogens infect several members of the orders Artiodactyla (cloven hoofed animals), including those from the families Bovidae, Giraffidae, Cervidae, and Suidae [55, 73]. Infections are also described in Equidae from the order Perissodactyla, odd-hoofed mammals [33]. With the recent reorganization, the Macavirus genus now contains nine members [74], and some of these species have been incriminated in the development of MCF in specific mammalian hosts [73]. However, not all recognized MCFVs are currently included in the Macavirus genus listed by ICTV. An updated list of MCF species with their associated mammalian hosts that are pathogenic and the known geographical distribution is given in Table 2; additional wildlife species that are susceptible to members of the MCF complex can be consulted [1]. Within these pathogenic species, OvHV-2, AlHV-1, and CpHV-2 are more frequently studied, and are considered of moderate economic importance due to mortality in susceptible mammalian species [55]. Moreover, phylogenetic analyses have revealed new species of the MCFV complex that are distinct from previously described members of the MCFV including, Ibex-MCF [77], CpHV-3 [78], and recently a new species subtype of OvHV-3 [79].
Two syndromes of epidemiological importance are accepted for MCF: wildebeest-associated MCF (WA-MCF) and sheep-associated MCF, (SA-MCF) [19, 55, 73]. The wildebeest is the asymptomatic carrier of WA-MCF, which is induced by AlHV-1 and occurs predominantly in ruminants from Africa and in wildlife worldwide [19, 61]. Alternatively, SA-MCF occurs worldwide, is caused by OvHV-2, with sporadic outbreaks in cattle, bison and deer, while sheep are subclinically infected [19, 55, 61]. Although MCFV carrier animals are usually asymptomatic, OvHV-2 nucleic acids were detected by ISH associated with PCR amplification of OvHV-2 DNA and histopathologic evidence of disseminated vasculitis in sheep with clinical disease [80], and viral load in affected sheep being 100–1000 times more elevated relative to asymptomatic sheep [81]. Therefore, sheep may also demonstrate histopathologic manifestations associated with MCF in specific cases. In Brazil, SA-MCF is the only syndrome, thus far, identified in cattle and other ruminants infected by OvHV-2; clinically infected animals are epidemiologically considered as dead-end hosts and do not transmit the virus to other susceptible animals [82]. Consequently, sheep are the natural host, while cattle are the accidental hosts of OvHV-2.
Epidemiology of sheep-associated malignant catarrhal fever in Brazil
Sheep-associated malignant catarrhal fever in cattle
As indicated previously, SA-MCF in Brazil was initially diagnosed in cattle from Rio Grande do North in 1956 [6]. Since that early description, SA-MCF was described in all geographical regions of Brazil, but only one outbreak occurred within the Northern region of the country. The few outbreaks of SA-MCF in Northern Brazil may represent the corresponding reduced cattle populations in that geographical region. All SA-MCF outbreaks in Brazil were sporadic, predominantly affecting beef cattle [17, 20, 22, 31, 36,37,38, 40, 44, 60], with few cases in dairy cows [6, 21, 43, 46, 83]. However, all cattle regardless of breed, age, sex, and age are equally susceptible to SA-MCF [82].
Most outbreaks of SA-MCF affected the major cattle producing states of Brazil, with the largest number of outbreaks and associated cattle mortality occurring in Rio Grande do Sul and São Paulo (Fig. 1). The notable difference was with the states of Mato Grosso do Sul (MS) and Minas Gerais (MG); these are major regions of cattle production in Brazil [84, 85], but with few published outbreaks of SA-MCF. Although the exact reason for this difference is unknow, the sporadic occurrence of MCF [55], associated with the number of similar infectious diseases that must be included in the differential diagnosis [16, 19] may have resulted in the underdiagnosis of cases of SA-MCF and could have contributed to the reduced number of cases in these areas of elevated cattle production. Additionally, the reduced number of cases of SA-MCF in these states may be associated with the absence of contact between asymptomatic sheep and susceptible cattle due to technification in cattle production and the reduced number of sheep populations in these states, considering that the States of MS and MG contributed towards 2.3% and 1%, respectively, of the number of sheep from Brazil in 2018 [86]. Underreporting of MCF seems common worldwide, since MCF is not on the OIE official list of diseases that must be communicated by member countries as mandated by the World Trade Organization [87]; consequently, there is no organized and enforced reporting system for this disease [73]. Underreporting can also be attributed to the development of chronic manifestations of MCF in cattle that have survived the acute infections and remained persistently infected with OvHV-2 [70, 72]. Consequently, the reported morbidity, mortality, and lethality rates associated with MCF may be more elevated than the documented cases.
A graphical overview of the geographical distribution of the relationship between the reported number of susceptible cattle to OvHV-2 and the morbidity rate in Brazil based on published articles is provided at Fig. 1, revealing that morbidity was more elevated in the State of Rio Grande do Norte, followed by Paraná, Rio Grande do Sul, and Rio de Janeiro. Consequently, SA-MCF-2 was identified in four of the five geographical regions of Brazil, except the Northern region, demonstrating that this disease is endemic in this country. When all outbreaks of SA-MCF in cattle from Brazil were considered based on published articles, an average morbidity of 5.6% (818/14,521) was observed since the first description of MCF, while the average lethality was estimated at 99.3% (818/824), since most cattle with clinical manifestations of SA-MCF died (Table 3). This overall estimated SA-MCF associated mortality in Brazil is similar to that identified in cattle with neurological disease from the States of Paraná, 2.1%; 5/236 [66], Rio Grande do Sul, 3.3%; 10/305 [88] and the semiarid states (6.3%; 7/111) of Northeastern Brazil [89]. Alternatively, a review of the diagnosis of neurological diseases of cattle from the State of Mato Grosso do Sul revealed that MCF contributed to only 0.4% (2/534) of cattle mortality [90]; however, almost half (46.3%; 247/534) of the cases of cattle with neurologic disease from this study remained undiagnosed. The incidence of WA-MCF was estimated as 5.6–6.2% in cattle from high risk regions of the Ngorongoro District, Tanzania [91], and varies between 3 and 7% in southern Kenya [92]. Consequently, the estimated 5.6% cattle morbidity associated with SA-MCF in Brazil is a reasonable rate that can be used nationally.
In most outbreaks of MCF, there is a description of the concomitant rearing of sheep and cattle on the same farm or pastures. Nevertheless, the association of sheep was unknown in several outbreaks of SA-MCF in cattle from Rio Grande do Sul [32], and in swine from Minas Gerais [34]. Interestingly, bison (Bison bison) reared less than 1 km distant from sheep were considered at higher risk to develop MCF [93], compared to animals separated by greater distances, as there seems to be a direct relationship between the distance of sheep herds and flocks of bison in the development of SA-MCF bison mortality [94]. Consequently, direct comingling with sheep may not be absolutely necessary for the development of MCF in susceptible animal populations, since aerosol transmission associated with local climatic conditions, such as wind direction and speed, temperature, and moisture, and possible mechanical vectors may participate in the dissemination of OvHV-2 [94].
In two outbreaks, goats and not sheep were reared simultaneously with cattle [39] and horses [33] that developed SA-MCF. Although goats are the natural hosts of CpHV-2, which forms part of the viral infectious disease agents within the MCF group [55, 73], the possibility of simultaneous infections due to CpHV-2 and OvHV-2 in these two outbreaks from Brazil [33, 39] or the detection of CpHV-2 in the asymptomatic goats was not explored. Goats have been infected by OvHV-2 [95, 96] and are associated with the transmission of MCF to several wild ruminant species [55], but the role of goats in the dissemination of MCF to cattle is currently unknown.
Sheep-associated malignant catarrhal fever in other animals
Outbreaks of SA-MCF occurred in other production animals from Brazil, including buffalos [35], horse [33], and pig [34]. Brazil has a considerably population (1.3 million heads) of buffalos distributed throughout most states [84], but only one outbreak of SA-MCF was located in buffalos [35]; a similar trend of MCF occurs in outbreaks of buffaloes worldwide [55]. The outbreak from Brazil occurred in Minas Gerais where Murrah buffalos (Bubalus bubalis) were raised simultaneously with cattle and sheep, during which buffalo morbidity was reduced (3.4%; 5/145) but with elevated lethality (100%; 5/5). The clinical manifestations and pathological findings observed in the affected buffalos [35] were similar to that previously described in cattle [55, 61, 73] and buffalos from diverse geographical locations with MCF due to OvHV-2 [52,53,54, 97] and in Africa associated with AlHV-1 [97]. Moreover, one cow that was at the farm died with clinical and pathological manifestations consistent with those induced by OvHV-2, where asymptomatic and affected buffalos that contained OvHV-2 DNA comingled with infected, asymptomatic sheep [35]; these authors then confirmed the association of OvHV-2 with the development of the clinical and pathological findings observed in this herd of buffalos. Nevertheless, this is one of the few studies from Brazil that have identified OvHV-2 DNA in sheep during outbreaks of SA-MCF.
The report of OvHV-2 in a foal from Minas Gerais [33] represents the unique demonstration of SA-MCF in a member of the Equidae family of domestic animals, since as of the time of writing, similar cases have not been published. In that study, a foal died after acute onset of neurological manifestations; histopathology revealed lymphocytic nephritis and granulomatous hepatitis with fibrinoid change at both organs, while molecular testing amplified OvHV-2 DNA from the affected arteries by using laser capture microdissection array. The remarkable event in that case, was the absence of adequate of histopathologic evidence of brain disease to support the neurological manifestations observed clinically; the authors described non-specific findings in the brain, such as congestion and hemorrhage [33]. Additionally, OvHV-2 DNA was amplified from goats and adult horses, including the dam of the foal, that comingled at the farm where the foal was maintained, while molecular testing to eliminate other possible infectious causes of neurological diseases in horses resulted in negative results [33]. Although this case clearly demonstrated the possibility of SA-MCF to be a potential infectious disease for members of the Equidae family, additional reports are required to assess the importance, if any, of this disease to horses.
There is only one report of SA-MCF in pigs from Brazil; this isolated description may reflect the rarity of porcine MCF worldwide [98] as compared to cattle. The outbreak in Brazil occurred between 2004 and 2008, at two commercial pig farms that had no reported contact with sheep, during which 0.6% (28/4370) of the sows and gilts developed ataxia, convulsions, tremors, and aggressive behavior, while fever followed by abortion was observed in pregnant gilts and sows [34]. Histopathology revealed nonsuppurative meningoencephalitis with fibrinoid change in several parts of the brain and molecular testing revealed OvHV-2 DNA from the brainstem [34]; however, histopathologic evaluation was not reported for non-neurologic tissues and it is not clear which viral disease agents, other than rabies and porcine lymphotropic herpesviruses, were eliminated as possible causes of the neurologic manifestations, while the cause of the aborted fetus were not evaluated. This differential investigation would have been important to exclude the possibility of simultaneous infections at this outbreak, since additional infectious diseases, including porcine teschovirus, Aujeszky’s disease, classical swine fever, African swine fever, and porcine circovirus are included in the differential diagnosis of porcine MCF [98].
The first identification of OvHV-2 in pigs was published in 1998 and described two outbreaks of SA-MCF from Norway, during which respiratory difficulties and the inability to stand were observed clinically, with widespread fibrinoid change in multiple tissues, and the amplification of OvHV-2 DNA from the affected pigs [99]. Since the first report in Norway in 1998 [99], MCF was diagnosed in pigs from the USA [100,101,102], Switzerland [103], Finland [104], Norway [105], the UK [106], and Vietnam [107]. It is worthy to mention that most of the cases of SA-MCF in swine reported lesions affecting multiple systems [99,100,101, 108], while the outbreak from Brazil described lesions restricted to the brain [34]. Studies have demonstrated that domestic pigs are highly susceptible to infection by OvHV-2 [108], develop multiple organ disease [48, 99, 101, 104, 106], and are excellent models to investigate the pathogenesis of MCF [108]. Nevertheless, porcine MCF may not be of significant threat to the commercial pig industry [98].
Thus far, outbreaks of SA-MCF in non-domestic animals in Brazil occurred in the Brocket [30] and Sambar [42] deer maintained within the states of Mato Grosso and Rio de Janeiro, respectively. SA-MCF seems to be more severe and fatal in deer relative to cattle [82]. The first outbreak occurred in deer maintained in a zoological park at the city of Cuiabá, Mato Grosso where morbidity was 60% (6/10) and all affected deer died within 3 days since the onset of acute clinical manifestations associated with MCF [30]. The other outbreak affected deer maintained at a conservationist estate within the city of Casimiro de Abreu, Rio de Janeiro; morbidity was more elevated (82.6%; 19/23), with 100% lethality, but the reported clinical manifestations were predominantly of acute neurological disease, with death occurring with 2 days after the onset of initial signs [42]. In both outbreaks, the affected deer had contact with sheep, developed typical histopathologic lesions in multiple tissues, and OvHV-2 DNA was amplified by PCR [30, 42]. The findings of these outbreaks are similar to those described in other countries where deer developed manifestations of MCF associated with OvHV-2 [109,110,111,112,113], CpHV-2 [109, 113, 114], and/or an AlHV-2-like virus [115]. Consequently, it seems that deer are highly susceptible to infections by members of MCF complex of organisms. However, the clinical manifestations of MCF in exotic ruminants may not be that characteristic as observed in cattle, and may be manifested only by conjunctivitis, photophobia, unilateral corneal edema, fever, depression, lymphadenopathy, occasionally diarrhea, mild serous nasal discharge, and sudden death [1].
Phylogenetic analysis of OvHV-2 in cattle from Brazil
Most studies [31, 33,34,35,36, 38,39,40, 60] that have used molecular assays to identify OvHV-2 in Brazil have used the classic identification method with the Baxter primers [25] based on the tegument protein gene. However, not all studies that have performed molecular identification of OvHV-2 in animals from Brazil have deposited the obtained nucleotide sequences in public databases. Consequently, when public databases were consulted, 12 nucleotides sequences suitable for phylogenetic analysis were obtained. Sequences were obtained from four federative states of Brazil, which represent the principal cattle producing regions (South, Southeastern, Midwest, and Northeastern) of Brazil; the animal host of the sequences were derived predominantly from cattle, with few sequences from the horse, sheep, pig, and goat. Authors and/or related scientists are encouraged to deposit nucleotide sequences in public databases so that detailed epidemiological surveys and phylogenetic analyses of OvHV-2 in Brazil can be achieved.
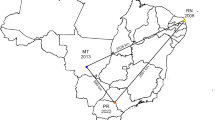
The phylogenetic analysis revealed two distinct groups (Fig. 2), with all sequences derived from cows from the cattle producing regions of Brazil grouped with the reference strain of OvHV-2 (DQ198083.1) obtained in the US [116]; this group also contained sequences derived from horses, sheep, and goats from the state of Minas Gerais. The other group consisted of nucleotides that clustered around the UK reference strain (NC_007646.1; [117]) and contained non-cow sequences from Brazil. Additionally, sequences from Brazil clustered with sequences derived from South Africa, Germany, Turkey, and Italy.
Phylogenetic relationship of OvHV-2 identified in cattle from Brazil based on the tegument protein gene. The sequences derived from Brazil are highlighted (●); the evolutionary history was inferred by using the maximum likelihood method based on the Jukes-Cantor model. The geographical origins and the animal hosts of the sequences used are provided. MG, Minas Gerais; MT, Mato Grosso; PR, Paraná; RN, Rio Grande do Norte
Additionally, all sequences derived from Brazil demonstrated 99% and 100% nucleotide identity, indicating that the type of OvHV-2 circulating in the major cattle producing regions of Brazil is remarkably similar, if not identical. Moreover, the sequences from Brazil demonstrated the same percentage of nucleotide identity with sequences derived South Africa, Germany, Turkey, and Italy. Similar findings were obtained in a study done to evaluate the distribution of OvHV-2 in Mongolian livestock [118], which demonstrated remarkably high (99–100%) nucleotide identity with sequences derived from several countries, including Brazil.
Monitoring of malignant catarrhal fever
The MCF complex of organisms is considered as a high consequence livestock pathogen [119] and SA-MCF is a reportable disease that in the USA [120]. In Brazil, SA-MCF is also listed as a disease that requires automatic notification when diagnosed in any species of domestic animals [121]. However, MCF is not on the list of the notifiable disease published by the OIE in 2019 [87], and even in India where the disease is considered as an emerging infection [122], as well as in Africa where WA-MCF is endemic, with elevated cattle fatality (95–100%) resulting in severe economic losses to farmers in several countries within the African continent [123]. The absence of worldwide regulation as to the notification of MCF may have contributed towards the apparent reduced global prevalence of this disease, probably due to under notification [73]. Nonetheless, initial retrospective studies suggest that OvHV-2 may be associated with the development of bovine respiratory disease (BRD) in cattle from Brazil, acting individually or in association with other disease pathogens of BRD (Oliveira et al. manuscript in preparation). Therefore, the real prevalence of OvHV-2 may be underdiagnosed worldwide (see discussion below).
Estimated direct economic impacts of sheep-associated malignant catarrhal fever on cattle production in Brazil
There is wide variation of the economic impacts associated with losses due to MCF [73]. Losses have been associated with death, slaughter, the emergency sale of affected animals, and reduced price for MCF affected cattle in Ngorongoro district, Tanzania [91], while the emergency sale of diseased animals was reported as impacting the economy in Southern Kenya [92]. However, data relative to the actual financial loss of cattle due to MCF is unknown. Additionally, there is no available data of the possible impacts of MCF on the cattle industry in Brazil. Consequently, knowledge of the economic impacts of SA-MCF is fundamental for the implementation of governmental policies related to this disease.
The estimated direct economic losses due to cattle mortality associated with SA-MFC in Brazil are summarized in Table 4, considering that the average annual cattle morbidity due to SA-MCF is 5.6% (185/14,521), with lethality of 99.3% (Table 3). The average live body weight of cows that died due to SA-MCF probably varied between 200 to 300 kg, which would represent a hypothetical annual value of $264 (USD) and $396 per each category of cows. Since the operational therapeutic cost associated with feedlot cattle reared in Brazil is 26.62 USD/animal [124], the estimated annual cost of therapy due to SA-MCF infections may vary between $8.59 and $12.89 per category of cows. It must be underscored that there is no effective therapy for MCF [16, 73]. However, due to the similarity of lesions seen in other infectious diseases of cattle, most affected cattle will probably be subjected to some therapeutic approach before MCF is eventually diagnosed. When the average costs associated with morbidity and mortality related to SA-MCF were estimated, morbidity would have resulted in an economic loss that varied between $215,592 and $323,928, with mortality costs accounting for $214,368 to $321,552, considering each category of cow.
If these data are projected nationally and considering an annual mortality rate, due to SA-MCF, of 5.6%, an estimated 217 M heads of cattle from the national herd [84, 85] can possibly be affected, resulting in a projected economic loss of $3.2 to 4.8 billion per category of cattle. However, these estimates represent less than 0.0001% of the gross domestic product (GDP) of Brazil in 2017 [125], and less than 0.004% of the GDP [126] derived from livestock production within the same year; livestock farming contributed to 31% of the GDP generated by agribusiness in 2017 [126]. Although these are estimated costs, the results demonstrate the reduced impact of SA-MCF on the local economy. Consequently, these estimates revealed that the possible direct economic losses due to SA-MCF related morbidity and mortality of cattle on the livestock industry of Brazil are inconsequential when the economy of the country is considered and may explain the absence of a systematic obligatory reporting system for this disease [73], not only in Brazil, but worldwide. Alternatively, these numbers are probably underestimated, considering the larger number of unreported cases [73], so the economic impact on individual business may be extremely significant, although when considered at a country level the impact seems irrelevant. Additionally, the economic scenario may be different when highly susceptible mammalian species to MCFV, such as bison and some cervids, are considered, since higher morbidity rates are usually observed in these species [1, 73].
Clinical forms of sheep-associated malignant catarrhal fever in cattle from Brazil
Several clinical forms have been associated with SA-MCF in cattle, including the head and eye form [55, 61, 127,128,129], acute [51, 71] and chronic manifestations [70, 72, 127, 128], as well as hematuria [127]. The head and eye form (HEF) of SA-MCF is generally of sudden onset [128] and is the most frequently occurring clinical manifestation in spontaneous outbreaks of MCF affecting cattle [55, 127]. This form is characterized by persistent, elevated fever, 40.5–42.2 °C [127,128,129], lymphadenopathy, ocular lesions, depression [127], severe dyspnea [128] and profuse mucopurulent nasal and oral discharges [127, 128]. The HEF manifestation of MCF is the most frequent form of SA-MCF in cattle from Brazil where the associated clinical manifestations (Fig. 3a, b) were commonly observed. Table 5 presents the frequency of the principal clinical findings described in outbreaks of SA-MCF in cattle from Brazil and reveals that corneal opacity, fever, nasal and ocular discharges (Fig. 3c), and profuse salivations were the clinical manifestations frequently observed in affected cattle. Other frequent clinical findings included oral ulcerations, blindness, apathy, nasal ulcerations, and respiratory distress. Less frequently identified clinical manifestations included interdigital ulcerations, ulcerative glossitis, gingival ulcerations, depression, and tenesmus. Nevertheless, corneal opacity, the most frequently described clinical manifestation observed in SA-MCF from Brazil, is always present in some form [127, 128, 130, 131], and occurs due to inflammatory reactions and accumulations of exudative cellular deposits at the corneal endothelium [127]. Furthermore, a retrospective study revealed that most cows that survived (90%; 9/10) and succumbed (80%; 12/15) to MCF had corneal edema [131]. It must be highlighted that oral, gingival, and interdigital ulcerations in cattle caused by OvHV-2 can be easily confused with similar clinical manifestations observed in several infectious diseases including FMD, BVD, IBR, and BT [14, 16].
Clinical presentations and pathologic manifestations of sheep-associated malignant catarrhal fever in cattle from Brazil. The cow is depressed with corneal edema and nasal discharge (a); observe severe conjunctivitis (b) and profuse nasal discharge (c). There are ulcerative lesions at the gingiva (d, e), hard palate (f), tongue (g), and esophagus (h) of cows with SA-MCF. c, d, f Reproduced with the kind permission of Headley et al. (2012). Molecular confirmation of ovine herpesvirus 2-induced malignant catarrhal fever lesions in cattle from Rio Grande do Norte, Brazil. Pesqui Vet Bras. 32:1213–1218
Corneal edema in SA-MCF normally occurs 2–5 days post-infection, begins initially at the limbus and then moves centripetally within the cornea [127, 128], and occurs with hyperemia of the conjunctiva and episclera [128]. This centripetal dissemination of corneal edema in SA-MCF distinguishes these lesions from the edema observed in contagious keratoconjunctivitis [127], while the histopathologic features are fundamental to differentiate this lesion from those observed in contagious keratoconjunctivitis, BVD, IBR, and thrombotic meningoencephalitis (TME) [130]. BVD and IBR are endemic diseases of cattle throughout continental Brazil, while the real incidence of TME induced by Histophilus somni is unknown and probably underdiagnosed in cattle herds from Brazil [132, 133]. Furthermore, concomitant uveitis, scleritis, conjunctivitis, and retinitis may occur with corneal edema in cattle with MCF [127]. Additionally, corneal edema may serve as a prognostic factor for the progression of MCF in affected cattle, since it was demonstrated that the clinical manifestations of corneal edema were improved or unchanged in cattle that survived MCF, and there was progressive deterioration of corneal edema in cattle that died due to MCF [131].
The important neuropathologic manifestation frequently observed in the HEF of MCF included profound depression [127] and motor incoordination [128]. Depression in MCF may be associated with severe widespread vasculitis of the brain [127], which can also occur in other neurological diseases of cattle. Motor incoordination was frequently diagnosed in cattle with MCF from Brazil, while depression was not a common finding (Table 5). The reduced frequency of depression associated with SA-MCF in Brazil may be because not all cattle with this clinical manifestation progress to fatality with consequent autopsy to determine the cause of death or may be related to the lack of detectable neurologic symptoms in clinically affected animals. Additionally, underdiagnosis and/or misdiagnosis of depression in MCF can be associated with the neurologic manifestations that can be easily confused with other infectious disease of cattle such as IBR, FMD, BVD, and TME, resulting in reduced frequency. Although encephalitic listeriosis due to Listeria monocytogenes is not a common disease of cattle in Brazil [66, 134], this disease can produce lesions in other parts of the brain other than the brainstem [135], and as such can be confused, when the classical listeria-related neurological lesions are absent, with the neurological manifestations of SA-MCF.
The acute manifestations of MCF normally last for 2–3 days, with elevated fever, dyspnea and diarrhea without the typical clinical findings observed in the HEF of MCF [82]; several cases of SA-MCF in cattle from Brazil were classified as acute manifestations of MCF with corresponding histopathologic findings in multiple tissues. The salient histopathologic findings include systemic ulcerations of epithelial surfaces, ophthalmitis, widespread lymphoid vasculitis with lymphocytic accumulations in the kidney, liver, and meninges [51, 71].
Cases of chronic MCF were described in cattle that demonstrated clinical and pathologic alterations of OvHV-2 after survival of an initial infection for at least 3 months [70] or 3 years [72]; a similar disease was also described in bison [71]. Two reports of chronic SA-MCF were described in cattle from Brazil: in the first, one animal developed SA-MCF after 3 months of evolution with progressive emaciation, bilateral corneal opacity, and profuse salivation [43], and in the other case, typical clinical manifestations were observed during 40 days, the animal recuperated after maintenance therapy, became progressively emaciated, and was humanely put-down [44]. The clinical manifestations observed in chronic MCF are associated with the HEF of SA-MCF and included corneal ulceration, panophthalmitis that progressed to corneal perforation and iridial prolapse [72], nasal discharge, blepharospasm, and epiphora [70]. Both cows from Brazil with chronic manifestations of SA-MCF demonstrated the HEF of MCF, but progressive emaciation as observed in the cows from Brazil [43, 44] was not reported in the cases from the USA [70, 72]. By histopathology, the chronic manifestations of MCF were characterized by widespread vascular lesions that resulted in arteriopathy in which lymphocytic arteritis, fibrinoid change, and atherosclerosis were the predominant vascular lesions [44, 50, 70,71,72]. In addition, corneal edema [70,71,72] and ulceration, uveitis, keratitis [71, 72], portal lymphocytic hepatitis [71], degeneration of corneal endothelium [70], lymphocytic interstitial nephritis, and nonsuppurative meningoencephalitis [70, 71] were described in cases of chronic SA-MCF. Intriguingly, proliferative vascular lesions with the simultaneous intralesional detection of OvHV-2 DNA by IHC and PCR, similar to those described in chronic SA-MCF were observed in cattle from Southern Brazil that demonstrated acute manifestations of neurological disease but without the HEF of SA-MCF [49].
Gross lesions observed in sheep-associated malignant catarrhal fever in cattle from Brazil
The frequency of the principal gross findings observed in outbreaks of cattle diagnosed with SA-MCF from Brazil are given in Table 6 and consisted of lesions typical of the HEF affecting principally the lymphoid tissue, oral cavity, urinary system, respiratory tract, and gastrointestinal organs. Generalized lymphadenopathy (n = 41), chronic interstitial nephritis (n = 39), and corneal opacity (n = 30) were the most frequently observed gross alterations in cattle with SA-MCF (Table 6); other lesions that were very frequently identified included ulcerative abomasitis (n = 19), glossitis (n = 18; Fig. 3d), stomatitis (n = 15; Fig. 3e,f), and esophagitis (n = 15; Fig. 3g). Frequent gross lesions associated with SA-MCF included conjunctivitis (n = 12), erosive esophagitis (n = 11), interstitial pneumonia (n = 8), and ulcerative tracheitis (n = 6). Less frequently gross alterations included pulmonary emphysema (n = 5), interdigital ulcerations (n = 4), suppurative bronchopneumonia (n = 4), ulcerative palatitis (n = 4; Fig. 3h), fetal deaths (n = 2), and enlarged Payer patches (n = 1). The importance and differential diagnoses associated with the gross manifestations observed in cattle with SA-MCF from Brazil were presented within the clinical findings section (see above).
However, special attention must be given to the report of fetal deaths in cattle infected with OvHV-2 from Brazil. In one report [40], a cow with clinical and pathological manifestations of SA-MCF was carrying a 4-month-old fetus when succumbed to OvHV-2. Moreover, histopathology of the fetus revealed lymphoplasmacytic myocarditis; the brain of the fetus and multiple tissues of the cow contained OvHV-2 DNA, while other common abortive agents were not identified [40]. This case demonstrated transplacental transmission of OvHV-2, and it can be inferred that fetal death could have been associated with infection due to OvHV-2; transplacental transmission of OvHV-2 was suspected in an asymptomatic calf born to a cow that was persistently infected and recovered from episodes of SA-MCF [72]. Alternatively, transplacental dissemination of AlHV-1 was experimentally demonstrated in calves [56]. However, the actual impact of transplacental transmission of OvHV-2 on the epidemiology of MCF is probably low. Nevertheless, a retrospective study (manuscript in preparation) identified MCFV/OvHV-2 antigens in the lungs of bovine fetuses (13.1%; 3/23) with interstitial pneumonia.
Histopathologic findings observed in sheep-associated malignant catarrhal fever in cattle from Brazil
The principal histopathologic findings observed per outbreak of SA-MCF in cattle from Brazil are summarized in Fig. 4, while the frequency observed per animal from these outbreaks is provided in Table 7. The most frequently diagnosed histopathologic findings in animals derived from outbreaks of SA-MCF from Brazil (Table 7) were disseminated vasculitis (n = 45), nonsuppurative meningoencephalitis (n = 25), lymphocytic interstitial nephritis (n = 22), and portal lymphoplasmacytic hepatitis (n = 16). Frequent histopathologic findings described were lymphoplasmacytic myocarditis (n = 9), lymphocytic uveitis (n = 8), lymphocytic vasculitis of the carotid rete mirabile, CRM (n = 6), interstitial pneumonia (n = 5), and thrombosis (n = 5). Infrequent histopathologic findings described in SA-MCF in outbreaks of cattle from Brazil lymphoplasmacytic rhinitis (n = 4), lymphoplasmacytic cystitis (n = 3), lymphocytic uveitis (n=3), and atrophic lymphoplasmacytic enteritis (n = 1).
Lymphocytic interstitial nephritis with vasculitis (Fig. 5a, b) seems to be the one of most frequently occurring histopathologic finding associated with infections induced by OvHV-2, and as such, the kidney is one of the organs of choice to be collected for evaluation during outbreaks of SA-MCF [19]. However, marked vasculitis nor interstitial nephritis is not always present in the kidneys of cattle with SA-MCF. In these cases, angiopathy of renal vessels (Fig. 5c, d) with concomitant vascular lesions in additional tissues may be helpful to establish a histopathologic diagnosis of MCF. The extent of arterial proliferative lesions in MCF may vary from mild to severe [50, 70, 72], and is characterized by severe proliferation at the tunica media with endothelial hypertrophy that may terminate in partial [49] or total [70] occlusion of the affected vascular lumen. Additionally, intralesional antigens of OvHV-2 (Fig. 5e, f) can be easily identified within the endothelium of renal capillaries and epithelial cells of renal tubules by IHC [49], thereby confirming the histopathologic diagnosis. An interesting feature observed in the kidneys of cattle with SA-MCF was ballooning degeneration (Fig. 5g, h) of the uroepithelium at the renal pelvis with intralesional detection of OvHV-2 antigens by IHC [49]. Ballooning degeneration is the initial histologic manifestation of viral entry into epithelial tissues. However, it would be interesting to investigate the intralesional presence of OvHV-2 in the transitional epithelium of the urinary bladder and kidneys in cattle by IHC and/or ISH to determine the role of the urinary system in disease pathogenesis.
Histopathologic and immunohistochemical findings observed in the kidneys of cattle infected by OvHV-2. Histopathologic demonstration of lymphocytic nephritis (a) with vasculitis and perivasculitis (b) and angiopathy (c, d) in the kidneys of cattle. Observe positive intracytoplasmic immunoreactivity to antigens of OvHV-2 within the epithelial cells of renal tubules in sections of the kidney with lymphocytic interstitial nephritis (e) and angiopathy (arrow, f). There is ballooning degeneration of the uroepithelium of the renal pelvis (g); a closer view showing the severely degenerated epithelial cells (h). Hematoxylin and eosin stain (a–d, g, h); immunoperoxidase counterstained with hematoxylin (e, f). Bar, a–c 100 μm; d–g 50 μm; h 20 μm
Nonsuppurative meningoencephalitis with vasculitis and/or perivasculitis (Fig. 6a, b) in cattle with MCF must be differentiated from other neurological disease of this species. In Brazil, the principal differential histopathologic diagnosis will be nonsuppurative meningoencephalitis associated with BoHV-5, due to the extensive perivascular cuffings and neuronal necrosis [136, 137] that are common to both infectious diseases. However, severe cerebral necrosis and intranuclear inclusion bodies (IB) in astrocytes and neurons are described within areas of malacia and/or nonsuppurative inflammation in herpetic meningoencephalitis induced bybovine alphaherpesvirus-5 (BoHV-5) [137, 138]. Alternatively, malacia and IB are not typical neurohistopathological findings of MCF, while extraneural IB have not been described in cattle with SA-MCF, since animals that succumb to MCF do not present IB in affected tissues [55, 129]. This is noteworthy, since most gammaherpesviruses are known to produce intranuclear IB [139] due to the entry of nucleocapsids into the nucleus with subsequent intranuclear release of viral DNA [140]. Furthermore, IB were not observed in experimentally infected rabbits [13], and in situ detection methods did not identify intralesional antigens of OvHV-2 within neurological tissues of cattle with SA-MCF by IHC [49] and ISH [48] assays. Notwithstanding the above, intranuclear IB were reported in neurons but the identity of these were not confirmed [82], while IB were identified in experimentally infected rabbits [82] and sheep [55]. Consequently, although the neurological manifestations associated with infections due to OvHV-2 and BoHV-5 may be similar, there are histopathologic features that can be used to differentiate between these two neurological diseases of cattle.
Histopathologic and immunohistochemical findings associated with OvHV-2 in the brain, liver, and intestine of cattle with SA-MCF. There is nonsuppurative meningoencephalitis with vasculitis (a, b) and portal lymphocytic hepatitis (c) with intracytoplasmic accumulations of OvHV-2 antigens within degenerated bile duct epithelial cells and the endothelia of a capillary vessel (arrow) at the portal region of the liver (d). Observe atrophic enteritis (e) associated with intralesional intracytoplasmic OvHV-2 antigens within cryptal epithelial cells (f). Hematoxylin and eosin stain (a–c, e); immunoperoxidase counterstained with hematoxylin (d, e). Bar, a, e 100 μm; b, f 50 μm; c 200 μm; d 20 μm
Lymphocytic or lymphoplasmacytic hepatitis (Fig. 6c) in SA-MCF is frequently observed at the portal traits of the liver in infected cattle [17, 38, 43, 48, 49] without any significant lesion to hepatocytes. In these cases, intracytoplasmic OvHV-2 antigens are demonstrable within degenerated epithelial cells of the bile ducts (Fig. 6d) and not within hepatocytes. Similarly, ISH detected OvHV-2 within leucocytes at the portal regions of the kidney in cattle [48]. This specific pattern of hepatitis in cattle may suggest an extra-hepatic origin of the inflammatory infiltrate, probably enteric via the portal system. Moreover, rabbits [76, 141,142,143,144] and bison [145, 146] experimentally infected with OvHV-2 demonstrated predominately portal lymphocytic hepatitis [76, 142,143,144, 147], with multifocal hepatocellular necrosis being identified in rabbits [76, 141,142,143,144], buffalos [53], and sheep [81]. Although rabbits and bison are more sensitive to infections by OvHV-2 relative to cattle [142, 143, 145, 148], and rabbits are considered as the ideal model to investigate the pathogenesis of SA-MCF [142, 144], meningoencephalitis with vasculitis at the rete carotid that is frequently diagnosed in cattle, seems not to occur in rabbits. The absence of necrotizing hepatitis in cattle relative to other animals and the infrequent occurrence of meningoencephalitis in rabbits may represent species characteristics relative to infection by OvHV-2.
Although the participation of the intestines in the pathogenesis of OVHV-2 is not well elucidated [49], there are descriptions of intestinal lesions in SA-MCF affecting cattle [8, 17, 49, 51, 72, 148, 149], bison [71, 145, 146], buffalos [52,53,54, 97], and sheep [81]. To this end, elevated loads of OvHV-2 were demonstrated within intestinal segments of sheep with OvHV-2-induced systemic vasculitis [80], as well as in sheep naturally infected by OvHV-2 [150] and in sheep with a SA-MCF like syndrome [81]. Furthermore, OvHV-2 proteins were detected within intestinal epithelia and the intestinal M cells of rabbits experimental infected with OvHV-2 [151], in cattle and buffalo with corresponding histopathologic evidence of disease [149], while OvHV-2 antigens were identified within the intestines of cattle (Fig. 6e, f) with or without enteric disease [49]. Although two of these animals were concomitantly infected by BVDV, there was comparatively more intralesional OvHV-2 antigens relative to those of BVDV [49]. Additionally, in several cases of SA-MCF the adjacent mesenteric lymph nodes were either infected by OvHV-2 [49, 51, 147, 149], or contained elevated viral loads of OvHV-2 [48, 150]. Moreover, the small intestine and the mesenteric lymph nodes received a comparatively more elevated score, based on viral load, relative to the lungs [150], with similar results occurring in FFPE tissue sections [152]. However, the current pathogenesis theory for the dissemination of OvHV-2 indicates that primary lytic replication occurs in the lungs [142, 143, 145, 153], between 1 and 2 weeks postinfection [145, 153], after which viral dissemination occurs via infected lymphocytes. In addition, there was positive correlation between viral load and lesion score in affected tissues [145], with infected lymphocytes being the key participants in determining lytic or non-lytic infections in infected animals [75, 154]. It must be underscored that lytic infection does not occur in other tissues before the initial reaction in the lungs [143, 145, 153], confirming the lungs as the initial site of replication for OvHV-2 in both the carrier and clinically susceptible hosts. Even though initial pulmonary replication with lytic infection is characteristic of gammaherpesvirus [140, 155], the participation of the intestine via the mesenteric lymph nodes in the dissemination and/or maintenance of OvHV-2 warrants additional investigations. Consequently, one wonders if the gut-lung axis phenomenon [156] is associated with the development of SA-MCF.
However, this possible viral maintenance must be differentiated from latency associated with OvHV-2, since gammaherpesviruses maintain latency in persistently infected lymphocytes without any lytic replication [154, 155]. In fact, it is now known that latency in infections due to herpesviruses is associated with viral encoded microRNAs (miRNAs) that are fundamental for the regulation of gene expression via post-transcriptional mechanisms [155]. Moreover, latency induced specifically by OvHV-2 seems to be controlled by miRNAs located at the open reading frame (ORF) 73 of the viral genome [75]. Additionally, experimental studies have identified ORF73 in association with ORF25 (lytic expression) and 50 (viral activation) transcripts in the lungs, lymph nodes, spleen, liver, and urinary bladder of rabbits [144]. Consequently, the identification of microRNAs are fundamental to understand the pathogenesis of MCF; thus far, 35 OvHV-2 microRNAs were discovered [157], and it would be interesting to see the role of these in the development of MCF.
Vascular alterations are fundamental to obtain a consistent histopathologic diagnosis of MCF, and have ranged from fibrinoid change (previous referred to as fibrinoid degeneration/necrosis), lymphocytic and/or necrotizing vasculitis, angiopathy, and arteriopathy [3, 12, 49, 50, 55, 70]. However, these vascular lesions are more consistent, exacerbated, and dramatic when observed at the eye and CRM, and are probably the results of a direct viral-mediated inflammatory and/or degenerative reaction [48]. Consistent histopathologic ocular lesions frequently observed in SA-MCF include corneal edema, lymphocytic vasculitis, uveitis (Fig. 7a, b), and keratitis with vasculitis of small vessels of the retina [51, 72, 130]; thrombosis (Fig. 7c) can also be observed. The CRM is the tissue of choice to confirm a histopathologic diagnosis of SA-MCF in cattle [55], but is not frequently collected at post-mortem evaluations due to its anatomic location at the base of the brain. The most frequently diagnosed histologic lesions at the CRM are necrotizing lymphocytic vasculitis (Fig. 7d, e) and fibrinoid change; in some of these lesions, there is severe proliferation of the intima and media of affected arteries resulting in total or partial occlusion of the vascular lumen (Fig. 7f). Proliferative vascular lesions at the CRM (Fig. 7g, h), due to marked proliferation of the intima and media of affected vessels with or without an related inflammatory or necrotic exudate, was described in cattle with chronic SA-MCF with the HEF of the disease [70, 72] and in cattle with acute neurological manifestations but without the HEF form of SA-MCF [49]. Therefore, when considering a histopathologic diagnosis of vascular lesions at the CRM, all spectrum of arterial lesions must be considered, since these lesions are progressive relative to the degree of degeneration and/or necrosis and the related inflammatory exudate [50].
Histopathologic lesions observed at the eye and carotid rete mirabile (CRM) of cattle infected with OvHV-2. There is lymphocytic uveitis (a) and vasculitis (b) with thrombosis (c) of the eye. Observe lymphocytic vasculitis (d, e) of arteries of the CRM. There is proliferation of the tunica media resulting in partial occlusion (star) of the affected vessel (f), and angiopathy (g, h) due to marked proliferation of the vascular endothelium. Hematoxylin and eosin stain; Bar, a, b, e, f 200 μm; c, d 500 μm; g 50 μm; h 100 μm
It is worthy to mention that proliferative vascular lesions in SA-MCF is not restricted to the CRM but can be observed in any organ of the affected animal, principally the lungs (Fig. 8a, b), spleen, (Fig. 8c, d), lymph nodes (Fig. 8e), and kidneys (Fig. 8f). Although the sequential events of OvHV-2 induced vasculitis are not fully known, it seems logical that the progression of vascular lesions in SA-MCF initiates with ballooning degeneration of the vascular endothelium (Fig. 8g, h) due to the direct effects of OvHV-2 [48], there is then progressive step-wise damage from the intima to the adventitia [50], considering that endothelial damage is the first step towards the obliteration of vascular lumens [70]. Persistent injury to the damaged endothelium will then induce the liberation of inflammatory mediators such as the platelet-derived growth factor (PDGF) [70]. One of the functions of PDGF is the stimulation of smooth muscle cells (SMCs) [158] within the damaged vessel. The subsequent migration of SMC from the media to the intima [158] is fundamental for the development of angiopathy, which probably occurs before vasculitis, that is associated with an influx of T lymphocytes [76, 80, 147], while fibrinoid change and/or vascular occlusion are the likely end results of the damaged vascular wall. Nevertheless, experimental studies are required to determine the correct sequence of vascular events in SA-MCF. However, if the sequence of these vascular events is correct, the observed histopathologic findings will vary with diseases progression. Consequently, one specific histologic vascular alteration should not be used to arrive at a histopathologic diagnosis of SA-MCF, and in situ confirmation of the participation of OvHV-2 in the development of these lesions can be achieved by IHC [49] and ISH [48] assays.
Histopathologic demonstration of angiopathy induced by OvHV-2 in nonneurologic tissues of cattle infected by OvHV-2. Observe proliferating vascular lesions at the lungs (a, b), spleen (c, d), lymph node (e), and kidney (f). There is ballooning degeneration (arrows) of vascular endothelial cells of an artery at the spleen (g) and lung (h), with the intravascular accumulation of a lipid-like (star) material within the artery of the spleen. Hematoxylin and eosin stain. Bar, a–c 100 μm; d, g, h 20 μm; e, f 50 μm
The case for OvHV-2 as a viral disease pathogen associated with the development of bovine respiratory disease
Although there is convincing evidence that OvHV-2 initially replicates within the epithelial cells of the lungs [145, 153, 159], the participation of OvHV-2 towards the development of BRD in feedlot cattle was never formally proposed. In most cases of SA-MCF, the associated pulmonary disease is frequently classified as interstitial pneumonia [6, 22, 38, 49, 55, 142, 153, 159]; there are also descriptions of histiocytic bronchointerstitial pneumonia [160] and granulomatous pneumonia [142] associated with infections due to OvHV-2. Interstitial pneumonia (IP) of infectious origin in domestic animals occurs either due to the aerogenous or hematogenous dissemination of infectious disease pathogens, with lesions to either the endothelial, basement membrane or epithelial components of the pulmonary interstitium [161, 162].
Although IP in domestic animals are predominantly induced by viral agents; pneumotropic viral disease pathogens induce lesions to the alveolar epithelium (type I and II pneumocytes), while endotheliotropic viruses affect the vascular endothelium [161], so the origin of IP is based on the histologic structure of the pulmonary parenchyma primarily affected. The alveolar parenchyma of domestic animals is lined by epithelial cells referred to as pneumocytes, with most epithelial cells being predominantly type I pneumocytes, with less than 7% being of type II pneumocytes, which are located at the junction of two adjacent alveolar septa [162]. Moreover, experimental studies have demonstrated that infection by OvHV-2 in sheep results in lytic replication predominantly within type II pneumocytes [159]. The reduced amount of type II alveolar cells would probably be responsible for the scattered and discrete accumulation of OvHV-2 within epithelial cells of infected pulmonary tissues [159]. Alternatively, the scattered lytic replication observed [159] may represent a transient infection frequently observed in acute interstitial pneumonia [161]. However, the specific pattern of pneumonia associated with OvHV-2 was never fully investigated. This is of fundamental importance to effectively characterize the pulmonary lesions of OvHV-2 in cattle, considering that pneumonia of domestic animals can be classified in four broad groups: namely, bronchopneumonia (suppurative and fibrinous), interstitial, embolic, and granulomatous pneumonia [161].
A retrospective review of pulmonary tissues (n = 144) from beef and dairy cattle submitted to our laboratory for etiologic diagnosis revealed that IP was the most frequent (71.5%; 103/144) pattern of pulmonary disease diagnosed using IHC assays to detect several pathogens associated with BRD (manuscript in preparation). Additionally, MCFV/OvHV-2 antigens were associated with IP in 45.6% (47/103) of the cases evaluated. Moreover, singular infections due to OvHV-2 represented 19.1% (9/47) of all pulmonary patterns of IP identified, with mixed infectious, particularly with BVDV, contributing towards 80.9% (38/47) of these. OvHV-2 antigens were identified within several epithelial cells of the lungs, including endothelial cells (Fig. 9a), type II pneumocytes (Fig. 9b), bronchial and bronchiolar epithelium (Fig. 9c), chondrocytes of hyaline cartilage (Fig. 9d), bronchial mixed glands (Fig. 9e), and leukocytes within the alveoli of the lungs of cattle with IP in field cases of SA-MCF. Additionally, OvHV-2 antigens were identified within epithelial cells in field cases of cattle with suppurative bronchopneumonia (Fig. 9f) and cuffing pneumonia (Fig. 9g); the latter frequently associated with pneumonia induced by Mycoplasma bovis. More surprisingly, OvHV-2 antigens were identified in the bronchiolar epithelium of a “normal-looking” pulmonary section of a cow (Fig. 9h). Therefore, it may seem that OvHV-2 pneumonia may be more frequent than previously diagnosed in cattle, since the associated pulmonary disease may not have a characteristic pattern or OvHV-2 may occur simultaneously with other infectious pathogens of BRD. Collectively, these factors may contribute to the underdiagnosis of SA-MCF worldwide [73], considering that BRD is a major economic problem of beef cattle in the Americas, Australia, the UK, and some European countries.
Immunohistochemical evidence of the participation of OvHV-2 in the development of bovine respiratory disease. There is positive intracytoplasmic immunoreactivity to OvHV-2 antigens in the vascular endothelial cells (a, arrows), in type II pneumocytes (black arrow), and leucocytes (open arrows) in the alveolar space (b). Observe positive immunolabeling of OvHV-2 antigens in the bronchiolar epithelium (c), within chondrocytes (arrows) of the hyaline cartilage of the bronchus (d), and within epithelial cells of bronchial mixed glands (e). There is positive immunoreactivity to epithelial cells of the bronchus that is filled with neutrophilic exudate (star) in a cow with purulent bronchopneumonia (f) and identification of OvHV-2 antigens in the epithelia in case of cuffing pneumonia (g). Observe immunolabeling of OvHV-2 antigens at the bronchiolar epithelium in a normally looking section of the lungs (h). Immunoperoxidase counterstained with hematoxylin. Bar, a, b 20 μm; c, g 100 μm; d–f 50 μm; h 200 μm
The current role of OvHV-2 in the pathogenesis of BRD in cattle is unknown. Consequently, studies must be done to understand the contribution of OvHV-2 towards the development of BRD and provide answers to basic questions. Is OvHV-2 an innocent bystander or a primary infectious disease pathogen of BRD? Do lytic replication in type II pneumocytes results in cell death via apoptosis, oncosis, pyroptosis, or simply necrosis? What are the histopathologic and/or ultrastructural manifestations of viral replication in type II pneumocytes? What mechanisms are responsible for the development of OvHV-2 associated IP? Does the virus act as a pneumotropic or endotheliotropic agent? Is there any evidence for the gut-lung axis in the pathogenesis of OvHV-2 pneumonia? What are the effects of concomitant infections relative to the occurrence of OvHV-2 induced pneumonia in cattle? Are there specific histologic features associated with OvHV-2 that can facilitate a histopathologic diagnosis of pulmonary disease, since IHC and/or ISH assays may not be present in all diagnostic laboratories worldwide? Adequate responses to these initial doubts will provide substantial answers to the possible role of OvHV-2 in the development of BRD and can be achieved by using a combination of IHC and/or ISH assays in association with molecular evaluations.
Conclusions
Sheep-associated malignant catarrhal fever is endemic in all geographical regions of Brazil, produces reduced morbidity with elevated mortality and lethality in affected cattle herds, but has no significant impact on the economy of the country. Sheep are the asymptomatic hosts of OvHV-2 while cattle and other cloven-hooved animals are the accidental hosts that present a wide spectrum of clinical manifestations. Moreover, OvHV-2 is the only MCFV identified in animals from this geographical region. Phylogenetic analysis suggests that the type of OvHV-2 circulating in Brazil is similar within all major cattle producing regions of the country. An adequate histopathologic diagnosis of SA-MCF in cattle requires the collection of specific tissues at post-mortem, particularly representative samples from the mesenteric lymph node, small intestine, lung, eye, carotid rete mirabile, kidney, and urinary bladder. Attention must be paid to the wide variation of vascular alterations that are characteristic of SA-MCF in multiple tissues and the participation of OvHV-2 in these lesions can be achieved by the intralesional identification via available IHC and IHS assays. With modern diagnostic pathology, the combined use of PCR assays associated with the salient histopathologic findings and in situ identification of OvHV-2 is recommended for an efficient confirmation of MCF in Brazil and must be encouraged so that data can be accumulated for molecular epidemiological studies. There is accumulating evidence to demonstrate that OvHV-2 may contribute to the development of BRD in cattle. Confirmation of this will significantly increase the worldwide prevalence of MCF, considering the known economic impacts related with BRD in feedlot cattle. Future studies investigating the role of the intestine in the pathogenesis of SA-MCF will be necessary to understand the pathogenesis of OvHV-2 in susceptible hosts. The utilization of in situ diagnostic methods will result in the identification of more cases of SA-MCF in cattle and other susceptible species via retrospective studies using archival FFPE blocks.
References
Heuschele WP, Reid HW (2001) Malignant catarrhal fever. In: Williams S, Barker IK (eds) Infectious diseases of wild mammals. Iowa, Iowa State University Press, Ames, pp 157–164
Piercy SE (1952) Studies in bovine malignant catarrh: I. Experimental infection in cattle. Br Vet J 108(2):35–47
Mettam RWM (1923). Snotsiekte in cattle. Ninth and Tenth Reports of the Director Veterinary Education and Research Union of South Africa 395–432 pp.
Torres S (1924) Oca, mal do chifre, ou coriza gangrenosa dos bovinos. Bol Soc Bras Med Vet 1(4):144–159
Tokarnia CH, Döbereiner J, Canela CFC (1959) Estudo sobre o “mal dos chifres” em gado do Nordeste e Norte do Brasil. Arq Inst Biol Animal 2:39–64
Döbereiner J, Tokarnia CH (1959) Ocorrência da coriza gangrenosa dos bovinos no município de Serra Negra do Norte, Rio Grande do Norte. Arq Inst Biol Animal 2:65–82
Daubney R, Hudson JR (1936) Transmission experiments with bovine malignant catarrh. J Comp Pathol Ther 49:63–89
Murray RB, Blood DC (1961) An outbreak of bovine malignant catarrh in a dairy herd I: I. Clinical and pathologic observations. Can Vet J 2(8):277–281
Danskin D (1955) Elementary bodies in bovine malignant catarrh. Nature. 176(4480):518
de Kock G, Neitz WO (1950) Sheep as a reservoir host of snotsiekte (or bovine malignant catarrhal fever) of cattle in South Africa. S Afr J Sci 46(6):176–180
Plowright W, Ferris RD, Scott GR (1960) Blue wildebeest and the ætiological agent of bovine malignant catarrhal fever. Nature. 188(4757):1167–1169
Piercy SE (1954) Studies in bovine malignant catarrh: V. The role of sheep in the transmission of the disease. Br Vet J 110(12):508–516
Piercy SE (1955) Studies in bovine malignant catarrh: VI Adaptation to rabbits. Br Vet J 111(11):484–491
Bexiga R, Guyot H, Saegerman C, Mauroy A, Rollin F, Thiry E, Philbey AW, Logue DN, Mellor DJ, Barrett DC, Ellis K (2007) Clinical differentiation of malignant catarrhal fever, mucosal disease and bluetongue. Vet Rec 161(25):858–859
Kalunda M, Dardiri AH, Lee KM (1981) Malignant catarrhal fever. I. Response of American cattle to malignant catarrhal virus isolated in Kenya. Can J Comp Med 45(1):70–76
OIE. (2013).Malignnat catarrhal fever. Technical disease cards. Retrieved 25/02/2019, from http://www.oie.int/animal-health-in-the-world/technical-disease-cards/. Accessed 25 Feb 2019
Barros SS, Santos MN, Barros CSL (1983) Surto de febre catarral maligna em bovinos no Rio Grande do Sul. Pesqui Vet Bras 3(3):81–86
Munday JS, French AF, Smith A, Wang J, Squires RA (2008) Probable malignant catarrhal fever presented as transient generalised crusting dermatitis in a cow. N Z Vet J 56(2):89–93
Russell G (2018). Malignant catarrhal fever. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018. OIE-World Organisation Anim Health 1-12
Corrêa WM, Gottschalk AF, Corrêa CNM, Zezza NL (1972) Febre catarral maligna em bovinos no estado de São Paulo. Biol 38:67-72
Marques LC, Alessi A, Bechara BH et al (1986) Surtos de febre catarral maligna em bovinos no estado de São Paulo. Arq Bras Med Vet Zoot 38(5):719–729
Baptista FQ, Guidi PC (1988) Febre catarral maligna no estado do Paraná. Hora Vet 45:33–37
Hsu D, Shih LM, Castro AE, Zee YC (1990) A diagnostic method to detect alcelaphine herpesvirus-1 of malignant catarrhal fever using the polymerase chain reaction. Arch Virol 114(3–4):259–263
Wiyono A, Baxter SI, Saepulloh M et al (1994) PCR detection of ovine herpesvirus-2 DNA in Indonesian ruminants-normal sheep and clinical cases of malignant catarrhal fever. Vet Microbiol 42(1):45–52
Baxter SI, Pow I, Bridgen A, Reid HW (1993) PCR detection of the sheep-associated agent of malignant catarrhal fever. Arch Virol 132(1–2):145–159
Hussy D, Stauber N, Leutenegger CM, Rieder S, Ackermann M (2001) Quantitative fluorogenic PCR assay for measuring ovine herpesvirus 2 replication in sheep. Clin Diagn Lab Immunol 8(1):123–128
Flach EJ, Reid H, Pow I, Klemt A (2002) Gamma herpesvirus carrier status of captive artiodactyls. Res Vet Sci 73(1):93–99
Cunha CW, Otto L, Taus NS, Knowles DP, Li H (2009) Development of a multiplex real-time PCR for detection and differentiation of malignant catarrhal fever viruses in clinical samples. J Clin Microbiol 47(8):2586–2589
Li H, Cunha CW, Taus NS (2011) Malignant catarrhal fever: understanding molecular diagnostics in context of epidemiology. Int J Mol Sci 12(10):6881–6893
Driemeier D, Brito MF, Traverso SD, Cattani C, Cruz CE (2002) Outbreak of malignant catarrhal fever in brown brocket deer (Mazama gouazoubira) in Brazil. Vet Rec 151(9):271–272
Garmatz SL, Irigoyen LF, Rech RR, Brown CC, Zhang J, Barros CSL (2004) Febre catarral maligna em bovinos no Rio Grande do Sul: transmissão experimental Para bovinos e caracterização do agente etiológico. Pesqui Vet Bras 24(2):93–103
Rech RR, Schild AL, Driemeier D, Garmatz SL, Oliveira FN, Riet-Correa F, Barros CSL (2005) Malignant catarrhal fever in cattle in Rio Grande do Sul, Brazil: epidemiology, clinical signs and pathology. Pesqui Vet Bras 25:97–105
Costa EA, Bomfim MR, Fonseca FG et al (2009) Ovine herpesvirus 2 infection in Foal, Brazil. Emerg Infect Dis 15(5):844–845
Costa ÉA, Viott AM, Machado GS et al (2010) Transmission of ovine herpesvirus 2 from asymptomatic boars to sows. Emerg Infect Dis 16(12):2011–2012
Costa EA, Bastianetto E, Vasconcelos AC, Bomfim MRQ, Fonseca FG, Gomes AD, Leite RC, Resende M (2009) An outbreak of malignant catarrhal fever in Murrah buffaloes in Minas Gerais. Brazil Pesq Vet Bras 29(5):395–400
Mendonça FS, Dória RGS, Schein FB, Freitas SH, Nakazato L, Boabaid FM, Paula DAJ, Dutra V, Colodel EM (2008) Febre catarral maligna em bovinos no Estado de Mato Grosso. Pesqui Vet Bras 28(3):155–160
Luvizotto M, Ferrari H, Cardoso T (2010) Malignant catarrhal fever-like lesions associated with ovine herpesvirus-2 infection in young calves (Bos indicus): a case report. J Venom Anim Toxins incl Trop Dis 16:178–185
Headley SA, Sousa IKF, Minervino AHH, Barros IO, Barrêto Júnior RA, Alfieri AF, Ortolani EL, Alfieri AA (2012) Molecular confirmation of ovine herpesvirus 2-induced malignant catarrhal fever lesions in cattle from Rio Grande do Norte, Brazil. Pesqui Vet Bras 32(12):1213–1218
Headley SA, Lisbôa JAN, Fritzen JTT et al (2013) Ovine herpesvirus type 2-induced malignant catarrhal fever in a heifer. Semin: Cenc-Agrar 34(6,S2):3903–3908
Headley SA, Pimentel LA, Oliveira VH et al (2015) Transplacental transmission of ovine herpesvirus 2 in cattle with sheep-associated malignant catarrhal fever. J Comp Pathol 153(4):206–211
Silva Filho GB, Silva Chaves HA, Aires LDA et al (2017) Febre catarral maligna em bovinos no Agreste de Pernambuco. Med Vet UFPRE 11(3):192–196
Oliveira MC, Pereira GO, Daoualibi Y, Dutra V, Brito MF, Caldas SA, Balthazar DA, Ubiali DG (2018) An outbreak of malignant catarrhal fever in Sambar deer (Rusa unicolor). Pesqui Vet Bras 38:1675–1680
Lemos RAA, Rech RR, Guimarães EB, Kadri A, Dutra IS (2005) Febre catarral maligna em bovinos do Mato Grosso do Sul e de São Paulo. Cienc Rural 35(4):932–934
Macêdo JTSA, Riet-Correa F, Simões SVD, Dantas AFM, Nobre VMT (2007) Febre catarral maligna em bovinos na Paraíba. Pesqui Vet Bras 27:277–281
Carmo PMS, Oliveira KD, Barioni G, Oliveira-Filho JC, Souza TD (2011) Malignant catarrhal fever in a calf in Espírito Santo State, Brazil: report of the first case. Braz J Vet Pathol 4(1):44–46
Galvão A, Galvão CF, Caldas SA et al (2016) Febre catarral maligna em bovino no estado do Rio de Janeiro – Relato de caso. Rev Bras Med Vet 38(Supl.1):108–114
Peixoto TC, Cunha VAF, Silva DN, Farias SS, Madureira KM (2015) Febre catarral maligna em bovino no estado da Bahia – relato de caso. Encicl Biosf 11(21):1092-1101
Pesavento PA, Cunha CW, Li H, Jackson K, O'Toole D (2019) In situ hybridization for localization of ovine herpesvirus 2, the agent of sheep-associated malignant catarrhal fever, in formalin-fixed tissues. Vet Pathol 56:78–86
Headley SA, Oliveira TES, Li H, Lisbôa JAN, Queiroz GR, Fritzen JTT, Flores EF, Alfieri AA, Cunha CW (2020) Immunohistochemical detection of intralesional antigens of ovine gamaherpesvirus-2 in cattle with sheep-associated malignant catarrhal fever. J Comp Pathol 174:86–98
Liggitt HD, DeMartini JC (1980) The pathomorphology of malignant catarrhal fever: I. generalized lymphoid vasculitis. Vet Pathol 17(1):58–72
Liggitt HD, DeMartini JC, McChesney AE, Pierson RE, Storz J (1978) Experimental transmission of malignant catarrhal fever in cattle: gross and histopathologic changes. Am J Vet Res 39(8):1249–1257
Hill FI, Arthur DG, Thompson J (1993) Malignant catarrhal fever in a swamp buffalo (Bubalus bubalis) calf in New Zealand. N Z Vet J 41(1):35–38
Hoffmann D, Soeripto S, Sobironingsih S, Campbell RS, Clarke BC (1984) The clinico-pathology of a malignant catarrhal fever syndrome in the Indonesian swamp buffalo (Bubalus bubalis). Aust Vet J 61(4):108–112
Teankam K, Tantilertcharoen R, Boonserm T, Suadsong S, Banlunara W (2006) Malignant catarrhal fever in swamp buffaloes (Bubalus bubalis): a retrospective pathological study of outbreaks in Thailand. Thai J Vet Med 36:19–30
O'Toole D, Li H (2014) The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet Pathol 51(2):437–452
Plowright W, Kalunda M, Jessett DM, Herniman KAJ (1972) Congenital infection of cattle with the herpesvirus causing malignant catarrhal fever. Res Vet Sci 13(1):37–45
Eloi RSA, Marçola TG, Paludo GR, Araújo RR, Colodel EM, Lima EMM, Castro MB (2017) Taxa de infecção pelo Herpesvírus ovino tipo 2 (OvHV-2)em rebanhos de ovinos no Distrito Federal. Pesqui Vet Bras 37:657–661
Li H, Hua Y, Snowder G, Crawford TB (2001) Levels of ovine herpesvirus 2 DNA in nasal secretions and blood of sheep: implications for transmission. Vet Microbiol 79(4):301–310
Li H, Taus NS, Lewis GS, Kim O, Traul DL, Crawford TB (2004) Shedding of ovine herpesvirus 2 in sheep nasal secretions: the predominant mode for transmission. J Clin Microbiol 42(12):5558–5564
Martins MSN, Castro AMMG, Lima MS et al (2017) Malignant catarrhal fever in Brazilian cattle presenting with neurological syndrome. Braz J Microbiol 48:366–372
Russell GC, Stewart JP, Haig DM (2009) Malignant catarrhal fever: a review. Vet J 179(3):324–335
Liggitt HD, DeMartini JC (1980) The pathomorphology of malignant catarrhal fever: II multisystemic epithelial lesions. Vet Pathol 17(1):78–83
Pinheiro de Oliveira TF, Laguardia-Nascimento M, Xavier FG, do Amaral Pinto C, Ferreira LR, de Castro Campos de Souza I, Hammerschmitt ME, Bianchi RM, Wronski JG, Etges RN, Rigon GM, Camargos MF, Júnior AVR, Fonseca Junior AA (2019) Quantification of ovine herpesvirus 2 by digital PCR in an outbreak of malignant catarrhal fever. Arch Virol 164(12):3045–3050
Li H, Snowder G, Crawford TB (1999) Production of malignant catarrhal fever virus-free sheep. Vet Microbiol 65(2):167–172
Li H, Snowder G, O'Toole D, Crawford TB (1998) Transmission of ovine herpesvirus 2 in lambs. J Clin Microbiol 36(1):223–226
Queiroz GR, de Oliveira RA, Flaiban KKMC et al (2018) Diagnóstico diferencial das doenças neurológicas dos bovinos no estado do Paraná. Pesqui Vet Bras 38:1264–1277
Li H, Shen DT, O'Toole D, Knowles DP, Gorham JR, Crawford TB (1995) Investigation of sheep-associated malignant catarrhal fever virus infection in ruminants by PCR and competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol 33(8):2048–2053
Müller-Doblies UU, Li H, Hauser B, Adler H, Ackermann M (1998) Field validation of laboratory tests for clinical diagnosis of sheep-associated malignant catarrhal fever. J Clin Microbiol 36(10):2970–2972
Li H, McGuire TC, Muller-Doblies UU, Crawford TB (2001) A simpler, more sensitive competitive inhibition enzyme-linked immunosorbent assay for detection of antibody to malignant catarrhal fever viruses. J Vet Diagn Investig 13(4):361–364
O'Toole D, Li H, Roberts S, Rovnak J, DeMartini J, Cavender J, Williams B, Crawford T (1995) Chronic generalized obliterative arteriopathy in cattle: a sequel to sheep-associated malignant catarrhal fever. J Vet Diagn Investig 7(1):108–121
Schultheiss PC, Collins JK, Austgen LE, DeMartini JC (1998) Malignant catarrhal fever in bison, acute and chronic cases. J Vet Diagn Investig 10(3):255–262
O'Toole D, Li H, Miller D, Williams WR, Crawford TB (1997) Chronic and recovered cases of sheep-associated malignant catarrhal fever in cattle. Vet Rec 140(20):519–524
Li H, Cunha CW, Taus NS, Knowles DP (2014) Malignant catarrhal fever: inching toward understanding. Annul Rev Anim Biosci 2:209–233
ICTV. (2019). International committee on taxonomy of viruses. Virus taxonomy: 2018 release - order herpesvirales. Retrieved from https://talk.ictvonline.org/taxonomy/. Accessed 13 Feb 2019
Riaz A, Dry I, Levy CS, Hopkins J, Grey F, Shaw DJ, Dalziel RG (2014) Ovine herpesvirus-2-encoded microRNAs target virus genes involved in virus latency. J Gen Virol 95(Pt 2):472–480
Buxton D, Reid HW, Finlayson J, Pow I (1984) Pathogenesis of ‘sheep-associated’ malignant catarrhal fever in rabbits. Res Vet Sci 36(2):205–211
Li H, Gailbreath K, Bender LC, West K, Keller J, Crawford TB (2003) Evidence of three new members of malignant catarrhal fever virus group in muskox (Ovibos moschatus), Nubian ibex (Capra nubiana), and gemsbok (Oryx gazella). J Wildl Dis 39(4):875–880
Li H, Cunha CW, Abbitt B, deMaar TW, Lenz SD, Hayes JR, Taus NS (2013) Goats are a potential reservoir for the herpesvirus (MCFV-WTD), causing malignant catarrhal fever in deer. J Zoo Wildl Med 44(2):484–486
Cunha CW, Slater OM, Macbeth B, Duignan PJ, Warren A, Highland MA, Li H (2019) Domestic sheep and bighorn sheep carry distinct gammaherpesviruses belonging to the genus Macavirus. Virus Res 272:197729
Pesavento PA, Dange RB, Ferreras MC, Dasjerdi A, Pérez V, LaRoca A, Silván JB, Diab S, Jackson K, Phillips IL, Li H, Cunha CW, Wessels M (2019) Systemic necrotizing vasculitis in sheep is associated with ovine herpesvirus 2. Vet Pathol 56:87–92
Phillips IL, Cunha CW, Galbraith D, Highland MA, Bildfell RJ, Li H (2018) High copy number of ovine gammaherpesvirus 2 DNA associated with malignant catarrhal fever-like syndrome in a lamb. J Vet Diagn Investig 30(4):623–627
Constable P, Hinchcliff KW, Done S, Gruenberg W (2017) Malignant catarrhal fever (ed). Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs, and goats. Elsevier, St. Louis, Missouri, pp 2076–2080
Furlan FH, Amorim TM, Justo RV et al (2012) Febre catarral maligna em bovinos no norte de Mato Grosso. Brasil Acta Sci Vet 40(2):1043
MAPA (2017) Ministério da agricultura, pecuária e abastecimento. Dados de rebanho bovino e bubalino no Brasil – 2017. Retrieved from http://www.agricultura.gov.br. Accessed 26 Mar 2019
IBGE (2017) Instituto brasileiro de geografia e estatistica. Efetivo dos rebanhos, por tipo de rebanho. Retrieved from https://sidra.ibge.gov.br/. Accessed 26 Mar 2019
IBGE (2018). Efetivo dos rebanhos, por rebanho ovino, Instituto Brasileiro de Geografia e Estatística
OIE (2019) World organization for animal health. Official disease status. Retrieved from http://www.oie.int/en/animal-health-in-the-world/official-disease-status/. Accessed 31 Mar 2019
Sanches AWD, Langohr IM, Stigger AL, Barros CSL (2000) Doenças do sistema nervoso central em bovinos no Sul do Brasil. Pesqui Vet Bras 20:113–118
Galiza GJN, Silva MLCR, Dantas AFM, Simões SVD, Riet-Correa F (2010) Doenças do sistema nervoso de bovinos no semiárido nordestino. Pesqui Vet Bras 30:267–276
Ribas NLKS, Carvalho RI, Santos AC et al (2013) Doenças do sistema nervoso de bovinos no Mato Grosso do Sul: 1082 casos. Pesqui Vet Bras 33:1183–1194
Cleaveland S, Kusiluka L, Kuwai JO, Bell C, Rudovick K (2004). Assessing the impact of malignant catarrhal fever in Ngorongoro District, Tanzania. Animal Health Programme, Department for International Development, Tanzania
Bedelian C, Nkedianye D, Herrero M (2007) Maasai perception of the impact and incidence of malignant catarrhal fever (MCF) in southern Kenya. Prev Vet Med 78(3):296–316
Epp T, Waldner C, Woodbury M (2016) An observational study of mortality on bison farms in Saskatchewan with special emphasis on malignant catarrhal fever. Can Vet J 57(1):37–45
Li H, Karney G, O'Toole D, Crawford TB (2008) Long distance spread of malignant catarrhal fever virus from feedlot lambs to ranch bison. Can Vet J 49(2):183–185
Jacobsen B, Thies K, von Altrock A, Förster C, König M, Baumgärtner W (2007) Malignant catarrhal fever-like lesions associated with ovine herpesvirus-2 infection in three goats. Vet Microbiol 124(3):353–357
Foster AP, Twomey DF, Monie OR, Kiupel M, Hoffmann I, Willoughby K (2010) Diagnostic exercise: generalized alopecia and mural folliculitis in a goat. Vet Pathol 47(4):760–763
Pfitzer S, Last R, Espie I, van Vuuren M (2015) Malignant catarrhal fever: an emerging disease in the African buffalo (Syncerus caffer). Transbound Emerg Dis 62(3):288–294
Mettenleiter TC, Ehlers B, Müller T, Yoon KJ, Teifke JP (2019) Ovine herpesvirus 2 causing porcine malignant catarrhal fever. In: Zimmerman JJ, Karriker LA, Ramirez A et al (eds) Diseases of swine, Hoboken, NJ. USA, Wiley-Blackwell, pp 569–571
Løken T, Aleksandersen M, Reid H, Pow I (1998) Malignant catarrhal fever caused by ovine herpesvirus-2 in pigs in Norway. Vet Rec 143(17):464–467
Albini S, Zimmermann W, Neff F, Ehlers B, Hani H, Li H, Hussy D, Engels M, Ackermann M (2003) Identification and quantification of ovine gammaherpesvirus 2 DNA in fresh and stored tissues of pigs with symptoms of porcine malignant catarrhal fever. J Clin Microbiol 41(2):900–904
Alcaraz A, Warren A, Jackson C, Gold J, McCoy M, Cheong SH, Kimball S, Sells S, Taus NS, Divers T, Li H (2009) Naturally occurring sheep-associated malignant catarrhal fever in north American pigs. J Vet Diagn Investig 21(2):250–253
Gauger PC, Patterson AR, Kim WI et al (2010) An outbreak of porcine malignant catarrhal fever in a farrow-to-finish swine farm in the United States. J Swine Health Prod 18(5):244–248
Albini S, Zimmermann W, Neff F, Ehlers B, Häni H, Li H, Hüssy D, Casura C, Engels M, Ackermann M (2003) Porcines Bösartiges Katarrhalfieber: Diagnostische Befunde und erstmaliger Nachweis des Erregers bei erkrankten Schweinen in der Schweiz. Schweiz Arch Tierheilkd 145(2):61–68
Syrjala P, Saarinen H, Laine T, Kokkonen T, Veijalainen P (2006) Malignant catarrhal fever in pigs and a genetic comparison of porcine and ruminant virus isolates in Finland. Vet Rec 159(13):406–409
Loken T, Bosman AM, van Vuuren M (2009) Infection with ovine herpesvirus 2 in Norwegian herds with a history of previous outbreaks of malignant catarrhal fever. J Vet Diagn Investig 21(2):257–261
Wessels M, Harwood D, Maley M, Willoughby K, Balfour C (2011) Malignant catarrhal fever in kune kune pigs in the UK. Vet Rec 169:156
Lapp S, Forster C, Kummrow M, Wohlsein P, Haist V (2015) Malignant catarrhal fever in a Vietnamese pot-bellied pig. A potential threat to pigs in mixed-species exhibits? Tierarztl Prax Ausg G Grosstiere Nutztiere 43(3):165-168
Li H, Brooking A, Cunha CW, Highland MA, O’Toole D, Knowles DP, Taus NS (2012) Experimental induction of malignant catarrhal fever in pigs with ovine herpesvirus 2 by intranasal nebulization. Vet Microbiol 159(3):485–489
Foyle KL, Fuller HE, Higgins RJ, Russell GC, Willoughby K, Rosie WG, Stidworthy MF, Foster AP (2009) Malignant catarrhal fever in sika deer (Cervus nippon) in the UK. Vet Rec 165(15):445–447
Burrells C, Reid HW (1991) Phenotypic analysis of lymphoblastoid cell lines derived from cattle and deer affected with “sheep-associated” malignant catarrhal fever. Vet Immunol Immunopathol 29(1–2):151–161
Palmer MV, Thacker TC, Madison RJ, Koster LG, Swenson SL, Li H (2013) Active and latent ovine herpesvirus-2 (OvHV-2) infection in a herd of captive white-tailed deer (Odocoileus virginianus). J Comp Pathol 149(2):162–166
Frontoso R, Autorino GL, Friedrich KG, Li H, Eleni C, Cocumelli C, di Cerbo P, Manna G, Scicluna MT (2016) An acute multispecies episode of sheep-associated malignant catarrhal fever in captive wild animals in an Italian zoo. Transbound Emerg Dis 63(6):621–627
Vikoren T, Li H, Lillehaug A et al (2006) Malignant catarrhal fever in free-ranging cervids associated with OvHV-2 and CpHV-2 DNA. J Wildl Dis 42(4):797–807
Zhu H, Huang Q, Hu X, Chu W, Zhang J, Jiang L, Yu X, Zhang X, Cheng S (2018) Caprine herpesvirus 2-associated malignant catarrhal fever of captive sika deer (Cervus nippon) in an intensive management system. BMC Vet Res 14(1):38
Klieforth R, Maalouf G, Stalis I, Terio K, Janssen D, Schrenzel M (2002) Malignant catarrhal fever-like disease in barbary red deer (Cervus elaphus barbarus) naturally infected with a virus resembling alcelaphine herpesvirus 2. J Clin Microbiol 40(9):3381–3390
Taus NS, Herndon DR, Traul DL, Stewart JP, Ackermann M, Li H, Knowles DP, Lewis GS, Brayton KA (2007) Comparison of ovine herpesvirus 2 genomes isolated from domestic sheep (Ovis aries) and a clinically affected cow (Bos bovis). J Gen Virol 88(Pt 1):40–45
Hart J, Ackermann M, Jayawardane G, Russell G, Haig DM, Reid H, Stewart JP (2007) Complete sequence and analysis of the ovine herpesvirus 2 genome. J Gen Virol 88(Pt 1):28–39
Ochirkhuu N, Konnai S, Odbileg R, Murata S, Ohashi K (2017) Molecular epidemiological survey and genetic characterization of ovine gammaherpesvirus-2 in Mongolian livestock. J Vet Med Sci 79(12):2040–2042
ISU (2019). High consequence livestock pathogens and toxins. Center for Food Security and Public Health, College of Veterinary Medicine, Iowa State University, Ames, Iowa, USA: 1
USDA-APHIS (2019). U.S. National List of Reportable Animal Diseases (NLRAD) Framework. Animal and Plant Health Inspection Service, U
MAPA (2013). Ministério da Agricultura, Pecuária e Abastecimento. Instrução normativa No 50, de 24 de setembro de 2013. 50. Brasília, DF
Sood R, Hemadri D, Bhatia S (2013) Sheep associated malignant catarrhal fever: an emerging disease of bovids in India. Indian J Virol 24(3):321–331
Wambua L, Wambua PN, Ramogo AM, Mijele D, Otiende MY (2016) Wildebeest-associated malignant catarrhal fever: perspectives for integrated control of a lymphoproliferative disease of cattle in sub- Saharan Africa. Arch Virol 161(1):1–10
Baptista AL, Rezende AL, Fonseca PA et al (2017) Bovine respiratory disease complex associated mortality and morbidity rates in feedlot cattle from southeastern Brazil. J Infect Dev Ctries 11:791–799
Wikipedia (2018) Economy of Brazil. Retrieved from https://en.wikipedia.org/wiki/Economy_of_Brazil. Accessed 30 Mar 2019
ABIEC (2018) Brazilian livestock profile. Brazilian Beef Exporters Association, São Paulo
Van Metre D, Tennant BC, Whitlock RH (2008) Malignant catarrhal fever. In: Divers TJ, Peck SF (eds) Diseases of dairy cattle. Saunders Elsevier, St Louis, pp 276–279
Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2006) Malignant catarrhal fever. In: Radostits OM, Gay CC, Hinchcliff KW, Constable PD (eds) Veterinary medicine, A textbook of the diseases of cattle, horses, sheep, pigs and goats. Saunders/Elsevier, St. Louis MO, USA, pp 1245–1248
Rossiter PB (1985) Immunology and immunopathology of malignant catarrhal fever. Prog Vet Microbiol Immunol 1:121–144
Whiteley HE, Young S, Liggitt HD, DeMartini JC (1985) Ocular lesions of bovine malignant catarrhal fever. Vet Pathol 22(3):219–225
Zemljič T, Pot SA, Haessig M, Spiess BM (2012) Clinical ocular findings in cows with malignant catarrhal fever: ocular disease progression and outcome in 25 cases (2007-2010). Vet Ophthalmol 15(1):46–52
Headley SA, Bracarense APFRL, Oliveira VHS, Queiroz GR, Okano W, Alfieri AF, Flaiban KKMC, Lisbôa JAN, Alfieri AA (2015) Histophilus somni-induced thrombotic meningoencephalitis in cattle from northern Paraná,Brazil. Pesqui Vet Bras 35(2):329–336
Headley SA, Oliveira VH, Figueira GF et al (2013) Histophilus somni-induced infections in cattle from southern Brazil. Trop Anim Health Prod 45(7):1579–1588
Headley SA, Fritzen JT, Queiroz GR et al (2014) Molecular characterization of encephalitic bovine listeriosis from southern Brazil. Trop Anim Health Prod 46(1):19–25
Oevermann A, Di Palma S, Doherr MG et al (2010) Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol 20(2):378–390
Del Medico Zajac MP, Ladelfa MF, Kotsias F et al (2010) Biology of bovine herpesvirus 5. Vet J 184(2):138–145
Rissi DR, Oliveira FN, Rech RR, Pierezan F, Lemos RAA, Barros CSL (2006) Epidemiologia, sinais clínicos e distribuição das lesões encefálicas em bovinos afetados por meningoencefalite por herpesvírus bovino-5. Pesqui Vet Bras 26:123–132
Salvador SC, Lemos RAA, Riet-Correa F, Roehe PM, Osório ALAR (1998) Meningoencefalite em bovinos causada por herpesvírus bovino-5 no Mato Grosso do Sul e São Paulo. Pesqui Vet Bras 18:76–83
MacLachlan NJ, Dubovi EJ (2017). Herpesvirales. In: MacLachlan, N.J. and E.J, D. (ed). Fenner’s veterinary virology. San Diego, California, Elsevier: 190-216
Ackermann M (2006) Pathogenesis of gammaherpesvirus infections. Vet Microbiol 113(3–4):211–222
Gailbreath KL, Taus NS, Cunha CW, Knowles DP, Li H (2008) Experimental infection of rabbits with ovine herpesvirus 2 from sheep nasal secretions. Vet Microbiol 132(1–2):65–73
Cunha CW, O’Toole D, Taus NS, et al. (2019) A rabbit model for sheep-associated malignant catarrhal fever research: from virus infection to pathogenesis studies and vaccine development. Curr Clin Micro Rpt 6(148)
Cunha CW, O'Toole D, Taus NS, Knowles DP, Li H (2013) Are rabbits a suitable model to study sheep-associated malignant catarrhal fever in susceptible hosts? Vet Microbiol 163(3–4):358–363
Li H, Cunha CW, Gailbreath KL, O’Toole D, White SN, Vanderplasschen A, Dewals B, Knowles DP, Taus NS (2011) Characterization of ovine herpesvirus 2-induced malignant catarrhal fever in rabbits. Vet Microbiol 150(3–4):270–277
Cunha CW, Gailbreath KL, O’Toole D, Knowles DP, Schneider DA, White SN, Taus NS, Davies CJ, Davis WC, Li H (2012) Ovine herpesvirus 2 infection in American bison: virus and host dynamics in the development of sheep-associated malignant catarrhal fever. Vet Microbiol 159(3):307–319
Liggitt HD, McChesney AE, DeMartini JC (1980) Experimental transmission of bovine malignant catarrhal fever to a bison (Bison bison). J Wildl Dis 16(2):299–304
Anderson IE, Buxton D, Campbell I, Russell G, Davis WC, Hamilton MJ, Haig DM (2007) Immunohistochemical study of experimental malignant catarrhal fever in rabbits. J Comp Pathol 136(2–3):156–166
Taus NS, Oaks JL, Gailbreath K, Traul DL, O’Toole D, Li H (2006) Experimental aerosol infection of cattle (Bos taurus) with ovine herpesvirus 2 using nasal secretions from infected sheep. Vet Microbiol 116(1–3):29–36
Tham KM (1997) Molecular and clinicopathological diagnosis of malignant catarrhal fever in cattle, deer and buffalo in New Zealand. Vet Rec 141(12):303–306
Hüssy D, Janett F, Albini S et al (2002) Analysis of the pathogenetic basis for shedding and transmission of ovine gamma herpesvirus 2. J Clin Microbiol 40(12):4700–4704
Meier-Trummer CS, Tobler K, Hilbe M, Stewart JP, Hart J, Campbell I, Haig DM, Glauser DL, Ehrensperger F, Ackermann M (2009) Ovine herpesvirus 2 structural proteins in epithelial cells and M-cells of the appendix in rabbits with malignant catarrhal fever. Vet Microbiol 137(3–4):235–242
Crawford TB, Li H, O'Toole D (1999) Diagnosis of malignant catarrhal fever by PCR using formalin-fixed, paraffin-embedded tissues. J Vet Diagn Investig 11(2):111–116
Li H, Cunha CW, Davies CJ, Gailbreath KL, Knowles DP, Oaks JL, Taus NS (2008) Ovine herpesvirus 2 replicates initially in the lung of experimentally infected sheep. J Gen Virol 89(Pt 7):1699–1708
Levy CS, Hopkins J, Russell GC, Dalziel RG (2012) Novel virus-encoded microRNA molecules expressed by ovine herpesvirus 2-immortalized bovine T-cells. J Gen Virol 93(Pt 1):150–154
Grey F (2015) Role of microRNAs in herpesvirus latency and persistence. J Gen Virol 96(Pt 4):739–751
He Y, Wen Q, Yao F, Xu D, Huang Y, Wang J (2017) Gut-lung axis: the microbial contributions and clinical implications. Crit Rev Microbiol 43(1):81–95
Nightingale K, Levy CS, Hopkins J, Grey F, Esper S, Dalziel RG (2014) Expression of ovine herpesvirus −2 encoded microRNAs in an immortalised bovine - cell line. PLoS One 9(5):e97765
Andrae J, Gallini R, Betsholtz C (2008) Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22(10):1276–1312
Taus NS, Schneider DA, Oaks JL, Yan H, Gailbreath KL, Knowles DP, Li H (2010) Sheep (Ovis aries) airway epithelial cells support ovine herpesvirus 2 lytic replication in vivo. Vet Microbiol 145(1):47–53
Li H, O'Toole D, Kim O, Oaks JL, Crawford TB (2005) Malignant catarrhal fever-like disease in sheep after intranasal inoculation with ovine herpesvirus-2. J Vet Diagn Investig 17(2):171–175
López A, Martinson SA (2017) Respiratory system, mediastinum, and pleurae. In: Zachary JF (ed) Pathologic basis of veterinary disease. St. Louis, Missouri, Elsevier, pp 471–560
Caswell JL, Williams KJ (2016) Respiratory system. In: Maxie MG (ed) Jubb, Kennedy, and Palmer’s pathology of domestic animals, vol 2. Saunders/Elsevier, Philadelphia, pp 465–591
Acknowledgments
SA Headley and TES Oliveira are recipients of the National Counsel of Scientific and Technological Development (CNPq; Brazil) fellowships. The authors are grateful to Dr. Elis Lorenzetti for initial assistance with the phylogenetic analysis and Bruno Silva Dias for assistance with the graphical demonstration of data generated with the QGIS software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Fernando R. Spilki.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Headley, S.A., de Oliveira, T.E.S. & Cunha, C.W. A review of the epidemiological, clinical, and pathological aspects of malignant catarrhal fever in Brazil. Braz J Microbiol 51, 1405–1432 (2020). https://doi.org/10.1007/s42770-020-00273-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00273-6