Abstract
Malignant catarrhal fever (MCF) presents a sporadic yet significant threat to livestock and wildlife. A comprehensive investigation in Karnataka, India into the prevalence and transmission patterns of sheep-associated MCF (SA-MCF) was conducted. A total of 507 sheep peripheral blood leukocyte samples from 13 districts along with 27 cows and 10 buffalo samples from various regions in Karnataka were tested for SA-MCF infection i.e. Ovine gammaherpesvirus 2 (OvHV-2) using heminested PCR. Furthermore, serum samples collected from 73 cows and 15 buffalo suspected of MCF were tested using a commercially available ELISA kit. Additionally, histopathological examinations of affected tissues and phylogenetic analysis of viral tegument protein sequences were conducted. Our findings indicated a 20.11%, 33.33% and 20% positivity for OvHV-2 in sheep, cows and buffalo respectively by PCR. Statistical analysis revealed a significant association between the age of sheep and the detection of OvHV-2. Seven cows and one buffalo serum samples tested positive for ELISA. Clinical findings in bovids were consistent with typical MCF signs, and histopathological results revealed multi-organ involvement characterised by necrotising vasculitis and lymphoid hyperplasia. The nucleotide pairwise identity matrix revealed 99.5% identity between the sequences obtained in the study with sequences from other states. The phylogenetic analysis of partial tegument protein sequences from bovid and sheep samples suggested a close genetic relationship between the local OvHV-2 strains and those from various global regions. Crucially, this study underscores the widespread presence of SA-MCF in Karnataka, with significant implications for both livestock management and wildlife conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant Catarrhal Fever (MCF) is a perplexing disease that impacts a variety of animals, including cattle, water buffalo, bison, deer, pigs, and horses (Plowright et al. 1960; Oğuzoğlu et al. 2020; Madrigal-Valencia et al. 2023). The causative agents of MCF belong to the Macavirus genus (Gammaherpesvirinae subfamily, Herpesviridae family), which currently comprises ten known viruses (O’Toole and Li 2014; Bianchessi et al. 2022). The most prevalent of these are Alcelaphine gammaherpesvirus 1 (AIHV-1) and Ovine gammaherpesvirus 2 (OvHV-2). In India, domestic and wild animals are more frequently affected by OvHV-2 (Kumar et al. 2014, 2021). Sheep, acting as carriers, do not show any clinical signs of disease (Li et al. 2014) and can transmit MCF to other species through aerosols and direct contact (Kim et al. 2003). Typical clinical signs in affected hosts include anorexia, nasal and ocular discharge, fever, bilateral corneal opacity, mucosal ulceration, and neurological signs in advanced stages (Foyle et al. 2009; Headley et al. 2020b). The World Organization of Animal Health recommends heminested PCR targeting the tegument protein coded by the ORF75 region for OvHV-2 detection (Baxter et al. 1993), a method previously employed in various parts of India (Sood et al. 2014).
Though there are multiple reports of MCF in cattle in India, no study with detailed molecular epidemiology in sheep and seroprevalence in bovids using ELISA is available. Sheep are reared in nearby forest areas without any restrictions and no data is available regarding the prevalence of OvHV-2 in them. Considering these limitations, the current study was carried out to identify the carrier status of OvHV-2 in sheep.
Materials and methods
Sample collection
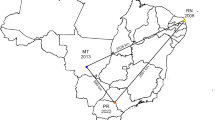
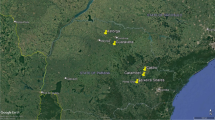
Karnataka, situated in southern India, encompasses 31 districts across latitudes 11°N to 18°N and longitudes 74°E to 78°E, with a sheep population of approximately 11.05 million. In this study, we adopted a random sampling method in which districts with high sheep populations and those with sheep in forest fringe areas were selected. We collected peripheral blood leukocytes from sheep in 13 strategically chosen districts (n = 507) along with details of their age (up to one year old/one-two-year/> two years) and breed (Bannur/Ballari/Deccani/Hassan/Kenguri/Nellur/Yalaga). Five districts - Chikkamagaluru, Shivamogga, Davanagere, Mysuru, and Bengaluru Urban - were specifically selected for their practice of daily forest grazing. The sheep in the remaining eight districts were predominantly owned by nomadic and unorganized farmers (rear sheep, cow and buffalo in a single enclosure). We collaborated with local veterinarians across Karnataka to monitor and report clinical signs indicative of MCF and to provide information regarding migration of sheep. Additionally, we collected swabs (n = 6), post-mortem samples (n = 3), and blood (n = 37) from 27 cows and 10 buffalo exhibiting suspected MCF signs across various parts of the state (Fig. 1a, b and c). When interpreting the results, several samples taken from a single animal were treated as a single sample. These samples were promptly stored at 4 °C in vaccine carriers and transported to ICAR-NIVEDI, Bengaluru, for further analysis.
Serum samples were also collected from 73 cows and 15 buffalo in apparently healthy animals in areas of suspected cases/hotspots of the disease. Samples subjected to PCR and ELISA were from different animals.
DNA extraction and PCR
Genomic DNA was extracted from sheep and bovine samples using the QIAamp® DNA Mini and Blood Mini kit (Qiagen, Cat. No. 51306, Germany). Each sample underwent heminested PCR following the protocols of Baxter et al. (1993) and Sood et al. (2014) for the detection of partial tegument gene coded by ORF75. Post-PCR, DNA was eluted using the QIAquick® Gel Extraction Kit (Qiagen, Cat. No. 28704, Germany) and subjected to bidirectional Sanger’s dideoxy sequencing. The sequences were edited using Gene Tool 1.0 software and submitted to NCBI.
Histopathology
Postmortem samples (Spleen, Liver and Intestine) from a PCR-positive cow collected in 10 per cent neutral buffered formalin underwent histopathological examination using Hematoxylin and Eosin (H&E) staining (Feldman and Wolfe 2014). The samples followed a standardized procedure for processing (Gurina and Simms 2023). Three heminested PCR-positive samples from a single PCR-positive cow were subjected to histopathology.
Enzyme-linked immunosorbent assay
A commercially available Bovine Malignant Catarrhal Fever Antibody (Anti-BMCF) indirect ELISA Kit (Abbexa, Cat. No. abx055892, UK) was utilized for qualitative analysis of 73 cow and 15 buffalo serum samples. The test was carried out based on the protocol provided by manufacturers. OD was measured spectrophotometrically at 450 nm in a microplate reader. The cutoff value was calculated to be 0.23 based on OD values of controls.
Phylogenetic analysis
A partial tegument gene sequence of 422 bp, obtained from the first stage of heminested PCR in this study, along with sequences from NCBI, were used. The Kimura 2-parameter model and the Maximum Likelihood approach were used to derive the evolutionary history (Kimura 1980). By employing the Neighbor-Join and BioNJ algorithms on a matrix of pairwise distances calculated using the Maximum Composite Likelihood (MCL) technique, the initial tree or trees for the heuristic search were automatically generated with a bootstrap value of 1000. The analysis involved 48 nucleotide sequences, with a total of 7414 positions in the final dataset. Evolutionary analyses were conducted in MEGA11 (Molecular Evolutionary Genetics Analysis) software (Tamura et al. 2021).
Statistical analysis
The chi-square test was used to calculate whether there was a significant association between the age/breed of sheep and the likelihood of detecting OvHV-2 in sheep using R software (http://www.rstudio.com/) by PCR with function chisq—test () and ‘stats’ package.
Results
Detection of OvHV-2
A total of 37 bovine samples (cows-27, buffalo-10) were collected from unorganised farms and 11 (cows-9, buffalo-2) of the samples reacted in the heminested PCR and this represents 29.72% of the samples.
Out of the 507 sheep peripheral blood leukocyte samples collected from 13 districts in Karnataka, Ramanagara district, having fewer sheep blood samples, was excluded from the positivity discussion. Among the 12 analysed districts, 42.5% samples from Ballari district reacted in the assay, followed by Shivamogga (30%), Tumakuru (29.4%), Mysuru (23.6%), Vijayapura (22.4%), Chikkamagaluru (16%), Bengaluru Urban (18.4%), Raichur (14.8%), Yadgiri (13.6%), Bagalakote (12.5%), Davanagere (10%), and Chitradurga (7%).
Overall, 102 out of the 507 sheep samples reacted in heminested PCR for OvHV-2. This equals a rate of 20.11% of samples reacting.
Histopathology
Histopathological examination of MCF-affected cow suggested that tissues were actively participating in the process. A severe lymphocytic periarteritis with thickening of the vessel wall and accumulation of lymphocytes was observed in the spleen (Fig. 2d) sample. Some regions exhibited proliferating vascular lesions with mild to moderate endothelial cell hyperplasia, indicative of lymphoid hyperplasia with lymphocytic vasculitis.
Normal tissues (a, b, c) and tissues affected with MCF (d, e, f) a. SpleenH&E(10X), b. Liver (10X), c. Intestine (10X), d. Spleen showing lymphoid hyperplasia(10x), e. Liver showing lymphocytic vasculitis (10x), f. Intestine showing lymphocytic enteritis (10x). Arrows indicate a low number/absence of lymphocytes in normal tissues and an increased number of lymphocytes in affected tissues
A moderate hepatocyte swelling, with fatty degeneration in multifocal areas, was observed in the liver sample (Fig. 2e). Lymphocyte accumulation around portal blood vessels was noted. These features suggested lymphocytic vasculitis.
In the glandular epithelium of the intestine (Fig. 2f), mild to severe degenerative changes along with lymphocytic infiltration in the submucosal area were observed. Loss of villus architecture due to severe inflammation and necrosis of epithelium and accumulation of lymphoplasmacytic cells in the lamina propria and crypts were observed. The lamina propria was infiltrated by large lymphocytes and plasma cells widely separating the crypts, suggestive of lymphocytic enteritis.
Enzyme-linked immunosorbent assay
The results of the ELISA were interpreted as per the kit manufacturer’s instructions. A total of 8 serum samples (cows-7, buffalo-1) reacted in ELISA indicating the presence of specific antibodies to MCF.
Phylogenetic analysis
A total of 113 positive results were obtained from the sheep, cow and buffalo samples. Of these, 23 (one buffalo; six cows and 16 sheep) were selected (on a regional basis) for bidirectional Sanger’s dideoxy sequencing.
The phylogenetic tree was constructed using 23 sequences from this study and 25 sequences available in GenBank including Brazil, Egypt, South Africa, Italy, the United Kingdom Turkey, Iraq, Mongolia and Pakistan (Fig. 3). Phylogenetic analysis of the viral tegument protein, coded by ORF 75, showed that local sequences clustered with those from various regions, including India, Pakistan, Egypt, Turkey, Mongolia, and Iraq.
Statistical analysis
Sample results of PCR assay in sheep were separated based on breed and age. Samples from the Hassan breed of sheep were observed to have more OvHV-2 positivity (27%) compared to the other six breeds in this study. Samples from sheep aged up to one year showed the highest OvHV-2 positivity (22.88%) compared to older sheep.
Statistical analysis using the chi-square test demonstrated a significant association between age and detection of OvHV-2 (p-value: 0.0406), whereas no significant association was found between breeds and detection of OvHV-2 (p-value > 0.05).
The evolutionary history was inferred by using the Maximum Likelihood method and Kimura 2-parameter model. The tree with the highest log likelihood (-11887.35) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. The sequences marked in red colour were obtained in this study
Discussion
Sheep-associated MCF is considered sporadic with varying incidence rates, influenced by carrier prevalence and local management practices (Li et al. 2014; Sood et al. 2014). This study was carried out to identify the carrier status of MCF in sheep, transmission patterns and seroprevalence in apparently healthy bovids.
In this study, 11 out of 37 suspected bovine cases reacted positively in PCR assay for OvHV-2. Notably, in areas where sheep and bovids were co-housed, both species tested positive, indicating a shared source of infection and similar transmission patterns to previous studies (Kumar et al. 2014, 2021). In contrast, in areas where sheep and bovids were not co-housed, they reacted positively in PCR potentially due to unrestricted livestock movement. The antibodies against MCF were identified by ELISA in apparently healthy animals. Both PCR and ELISA revealed their significance in screening MCF whenever new animals are introduced to domestic animal farms or wildlife enclosures. In this study, we observed characteristic lymphoid hyperplasia in lymphoid organs (such as the spleen) and lymphocyte accumulation in non-lymphoid organs (including the liver and intestine) in a PCR-positive MCF case which was in concordance with previous reports (Headley et al. 2020a) and highlighted the specificity of PCR.
Our findings revealed the presence of OvHV-2 by PCR in sheep of different regions. In samples from the Ballari district, a high positivity (42.5%) was observed among nomadic flocks. Further investigation with local veterinarians revealed seasonal migration of sheep to Shivamogga district, wherein 30% of samples reacted indicating they were latently infected. This migratory pattern is a key factor in disease transmission. Raichur, Yadagiri, Vijayapura and Bagalakote belong to the semi-arid region of the northern part of Karnataka, wherein, the nomadic sheep farmers move towards the west coast tropical semi-evergreen forest areas of the southern part of Karnataka during the summer season in search of fodder similar to Ballari district, resulting in transmission of disease throughout the state.
Karnataka’s rich forest cover, home to diverse wildlife, presents a unique challenge in managing SA-MCF. The detection of the disease in sheep from forest fringe areas was done for the first time, with an average positivity rate of 19.12%, underscores the risk to local fauna. OvHV-2 in various herbivores highlights the potential for broader ecological impacts, including disruptions to the food chain.
Chitradurga district samples were observed to have only 7% positivity in this study in sheep which is the least compared to other districts. It may be because the population of nomadic farmers in this study were moving around in a very small restricted area with limited to no contact with other herds within the district. Tumakuru district samples with 29.4% positivity and its proximity to Andhra Pradesh and Tamil Nadu may result in the transmission of disease between the states during the movement of sheep which is discussed in phylogenetic analysis.
Statistical analysis showed a significant association between the age of sheep and OvHV-2 positivity. Further, it was revealed that sheep aged up to one year are more likely to transmit the disease and these findings were similar to previous studies (Li et al. 2001). In sheep, aged more than two years, any difficulties in identifying the latent virus by heminested PCR should be further studied. The phylogenetic tree suggested that the other sequences that clustered along with the Indian sequences are probably from animals with a similar heritage (Kamalakkannan et al. 2021). The pairwise identity matrix of 48 sequences revealed that the sequences from Karnataka and Andhra Pradesh state, showed homogeneity of 99.5 to 100%. The sequences obtained from Egypt were closely related to Indian sequences (99 to 100%) whereas the sequences from South Africa showed homogeneity of 64 to 70%. The sequences from other countries were found to be showing heterogeneity of 10% with the sequences obtained in this study.
Given the incidence of OvHV-2 in sheep of forest fringe regions, measures have to be taken to restrict sheep movement into forest areas to prevent potential spillover of MCF to susceptible wildlife. The migration of sheep facilitates the introduction of the disease into new areas and alters disease transmission patterns, posing a continuous threat. The study highlights the critical role of mixed farming in the propagation of MCF in Karnataka and use of ELISA in identifying seroprevalence. The particular issue requires targeted awareness programs to educate farmers about the risk of transmission of disease and subsequent preventive measures.
Data availability
No datasets were generated or analysed during the current study.
References
Baxter SI, Pow I, Bridgen A, Reid HW (1993) PCR detection of the sheep-associated agent of malignant catarrhal fever. Arch Virol 145–159. https://doi.org/10.1007/BF01309849
Bianchessi L, Rocchi MS, Maley M, Piccinini R, Turin L (2022) Molecular Tools to identify and characterize malignant Catarrhal Fever viruses (MCFV) of ruminants and Captive Artiodactyla. Viruses 14:2697. https://doi.org/10.3390/v14122697
Feldman AT, Wolfe D (2014) Tissue processing and hematoxylin and eosin staining. Methods Mol Biol 1180:31–43. https://doi.org/10.1007/978-1-4939-1050-2_3
Foyle KL, Fuller HE, Higgins RJ, Russell GC, Willoughby K, Rosie WG, Stidworthy MF, Foster AP (2009) Malignant catarrhal fever in sika deer (Cervus nippon) in the UK. Vet Rec 165:445–447. https://doi.org/10.1136/vr.165.15.445
Gurina TS, Simms L (2023) Histology, Staining. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL)
Headley SA, Oliveira TES, Li H, Lisbôa JAN, Queiroz GR, Fritzen JTT, Flores EF, Alfieri AA, Cunha CW (2020a) Immunohistochemical Detection of Intralesional Antigens of Ovine Gammaherpesvirus-2 in cattle with Sheep-associated Malignant Catarrhal Fever. J Comp Pathol 174:86–98. https://doi.org/10.1016/j.jcpa.2019.11.002
Headley SA, de Oliveira TES, Cunha CW (2020b) A review of the epidemiological, clinical, and pathological aspects of malignant catarrhal fever in Brazil. Braz J Microbiol 51:1405–1432. https://doi.org/10.1007/s42770-020-00273-6
Kamalakkannan R, Kumar S, Bhavana K, Prabhu VR, Machado CB, Singha HS, Sureshgopi D, Vijay V, Nagarajan M (2021) Evidence for independent domestication of sheep mtDNA lineage A in India and introduction of lineage B through arabian sea route. Sci Rep 11:19733. https://doi.org/10.1038/s41598-021-97761-y
Kim O, Li H, Crawford TB (2003) Demonstration of sheep-associated malignant catarrhal fever virions in sheep nasal secretions. Virus Res 98:117–122. https://doi.org/10.1016/j.virusres.2003.09.002
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Kumar VN, Sreedevi B, Karthik A, Vijaya Lakshmi S, Geetha Reddy A, Sreenivasulu D (2014) Detection of OvHV-2 from an outbreak of sheep associated malignant catarrhal fever from crossbred cattle of Southern India. Vet Res Commun 38:323–328. https://doi.org/10.1007/s11259-014-9612-3
Kumar N, Sood R, Pateriya AK, Venkatesakumar E, Ramprabhu R, Dixit R, Bhatia S, Singh VP (2021) First molecular evidence and genetic characterization of Ovine Herpesvirus 2 in multiple animal species in India. Front Vet Sci 8:610178. https://doi.org/10.3389/fvets.2021.610178
Li H, Hua Y, Snowder G, Crawford TB (2001) Levels of ovine herpesvirus 2 DNA in nasal secretions and blood of sheep: implications for transmission. Vet Microbiol 79:301–310. https://doi.org/10.1016/s0378-1135(00)00367-9
Li H, Cunha CW, Taus NS, Knowles DP (2014) Malignant catarrhal fever: inching toward understanding. Annu Rev Anim Biosci 2:209–233. https://doi.org/10.1146/annurev-animal-022513-114156
Madrigal-Valencia TL, Saavedra-Montañez M, Pérez-Torres A, Hernández J, Segalés J, Hernández YD, Candanosa-Aranda IE, Pérez-Guiot A, Ramírez-Mendoza H (2023) First identification and characterization of ovine gammaherpesvirus type 2 in horses and artiodactyla from an outbreak of malignant catarrhal fever in Mexico. PLoS ONE 18:e0290309. https://doi.org/10.1371/journal.pone.0290309
O’Toole D, Li H (2014) The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet Pathol 51:437–452. https://doi.org/10.1177/0300985813520435
Oğuzoğlu TÇ, Salar S, Adıgüzel E, Demirden C, Ülgenalp O (2020) Detection and characterisation of sheep-associated malignant catarrhal fever infection from ruminants by using tegument and gB gene sequences of OvHV-2. Onderstepoort J Vet Res 87:e1–e4. https://doi.org/10.4102/ojvr.v87i1.1886
Plowright W, Ferris RD, Scott GR (1960) Blue wildebeest and the aetiological agent of bovine malignant catarrhal fever. Nature 188:1167–1169. https://doi.org/10.1038/1881167a0
Sood R, Khandia R, Bhatia S, Hemadri D, Kumar M, Patil SS, Pateriya AK, Siddiqui A, Kumar MS, Venkatesha MD, Kulkarni DD (2014) Detection and molecular characterization of naturally transmitted sheep-associated malignant catarrhal fever in cattle in India. Trop Anim Health Prod 46:1037–1043. https://doi.org/10.1007/s11250-014-0611-8
Tamura K, Stecher G, Kumar S (2021) MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Acknowledgements
The authors acknowledge the Director, ICAR-NIVEDI, Bengaluru and Dean, Veterinary College (KVAFSU), Bengaluru for their constant support and guidance during the study. Farmers of respective villages in Karnataka are thanked for their unwavering cooperation during our visit to the fields.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
The current work is part of a doctoral thesis in philosophy of science written by student KAS, who completed all thesis requirements and research practically and, in a lab, while being supervised by professors and scientists who also served as co-authors on the manuscript and who helped conceptualise and design the current study to meet its objective. The research was conceptualised with the help of DR and BRG. The samples were collected with the help of SI, BMC and RS. BPS, KPS and SR helped analyse data and contributed new methods or models. SSP was instrumental in conceptualization, analysis, providing lab facility and editing the paper. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shyamsundar, K.A., Rathnamma, D., Gulati, B.R. et al. Transmission patterns of malignant catarrhal fever in sheep and cattle in Karnataka, India. Vet Res Commun (2024). https://doi.org/10.1007/s11259-024-10486-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11259-024-10486-x