Abstract
Phosphorus (P) is an indispensable element of living organisms and plays an irreplaceable role in the growth of crops. As a non-renewable element, the reserves of phosphorus rock, the primary source of phosphorus in nature, are facing the danger of exhaustion. As a phosphorus-rich solid waste, sewage sludge has gradually become a main renewable phosphorus resource. The combination of effective recycling of phosphorus and innocuous disposal of sewage sludge can not only alleviate the crisis of phosphate rock resources shortage but also reduce the environmental hazards of sewage sludge. This study reviewed the application of thermal treatment in sewage sludge disposal. Besides the advantages of reducing waste volume, decomposing organic pollutants, generating valuable byproducts, it can also significantly promote the recycling of phosphorus. Studies have shown that thermal treatment (incineration, pyrolysis, and hydrothermal) can enrich phosphorus in the products and transform the speciation of phosphorus to increase the bioavailability. The physical and chemical properties of different thermal treatment products and the speciation of phosphorus are different. The transformation and migration of phosphorus affect the efficiency of subsequent phosphorus recovery and reuse. At the same time, this study compared several general phosphorus recovery methods (wet extraction, thermochemical, and electrochemical methods), and further summarized the advantages and disadvantages of various methods and application conditions. This review summarizes recent advances in phosphorus recovery from sewage sludge, identifies challenges and knowledge gaps, and provides the foundation for future research aimed at achieving efficient, economic, and eco-friendly reclamation of phosphorus in sewage sludge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The importance of phosphorus
Phosphorus is a primary element in nature and an indispensable element in the process of life activities. Phosphorus is ubiquitous in all organisms and accounts for about 2–4% of the dry weight of most cells [1]. Phosphorus exists in the basic genetic structure of DNA and RNA and plays an irreplaceable role in the inheritance and reproduction of all living organisms. In addition, phosphorus is an essential component of ATP and ADP involved in energy production, transport, and storage activities in each biological process. Among humans, animals, fungi, and most bacteria, phosphorus participates in the process of respiration. Plants require phosphorus for photosynthesis, and phosphorus is closely related to plant metabolism.
Studies have shown that in plant tissues, phosphorus is concentrated in the most vigorously growing parts, and most of the phosphorus absorbed by plants is transported to and stored in fruits and grains. Therefore, high-yield crops require a large amount of phosphorus. Rational phosphorus application has a high impact on agriculture. Population estimates for the middle of this century exceed 9 billion, which means that food production will have to be increased by almost 30%. Currently, about 82% of the mined phosphorus is used in agriculture, while the production of animal feed uses 7%. The remaining 11% of the mined phosphorus is used in industry and medicine for the production of pharmaceuticals, oils, detergents, or even textiles [2,3,4]. Agricultural fertilizer manufacturing accounts for 90% of phosphorus resource use and traditional agricultural fertilization almost completely dominates the use of phosphorus resources [1]. As the world’s population continues to grow, human demand for food crops continues to rise sharply. The use of phosphate fertilizers and organophosphorus pesticides has played a significant role in the production of food crops. The supply of phosphorus resources is closely related to the sustainable development of agriculture. As the primary industry, agriculture provides the most fundamental guarantee for other sectors of the national economy and provides the primary industry to support the construction and development of the national economy. Therefore, the supply of sufficient phosphorus resources guarantees the security and adequate supply of global food resources, which is closely linked to the stable development of China and the rest of the world [5,6,7,8]. However, unlike nitrogen sources, phosphorus is found mainly in nature, such as phosphate rock and struvite, and phosphate ore of animal fossils. After artificial mining or natural erosion, it eventually falls with the water to the sedimentary layers of the deep sea. Only a small fraction of the phosphorus that sinks into the deep sea can return to land through shallow sea fish or seabirds. Therefore, most of the phosphorus in the biosphere is unidirectional. Phosphorus has become a valuable resource that cannot be created.
The situation of phosphorus resource
As mentioned above, phosphorus in nature mainly exists as ore. There are about 120 naturally occurring, widely distributed types of phosphorus-containing minerals. However, it is possible to only exploit a few types of phosphorus-containing minerals due to their quality and quantity. In industrial applications, the main phosphorus-containing mineral for extracting phosphorus is apatite, followed by svanbergite, struvite, and bluestone. Apatite contains about 95% of the natural phosphorus.
According to the US Geological Survey, in 2016, the economic reserves of the world’s phosphate rock were about 17 billion tons, and the basic reserves were nearly 50 billion tons [9]. Distribution of phosphate rocks occurs in more than 60 countries and regions such as Africa, North America, Asia, Middle East, and South America, and more than 80% of them are concentrated in Morocco, the United States, South Africa, Jordan, and China. Based on economic reserves and basic reserves, Morocco ranks first, China ranks second, and the United States ranks third [10]. Figure 1 shows the phosphate rock reserves of China and some other main countries.
The annual output of world phosphate rock has remained above 100 million tons, and it shows a growing trend. Two-thirds of the world’s phosphate rock comes from the United States, China, and Morocco. Although the reserves of phosphate rock in the world are abundant, the natural phosphate rock is non-renewable, and the annual mining volume will increase year by year as the demand for phosphate rock increases, making natural phosphate rock reserves increasingly exhausted. Experts predict the annual consumption and available time of world phosphate rock in the coming decades [11]. If the estimated annual consumption of phosphate rock is predicted at an annual growth rate of 3%, the annual consumption of phosphate rock will reach 170 million tons by 2050 and the global phosphate rock resources will be completely depleted by 2065. With an estimated 2% annual growth in the consumption of phosphate rock, annual consumption will reach 100 million tons by 2050, with 80% of phosphate rock reserves consumed by 2070. Even if the annual growth rate of phosphate ore is extremely weak, the annual consumption of phosphate rock will reach 50 million tons by 2070, and the global phosphate resources will only contain 40% of current reserves. Recently, with the development of industrial and agricultural production, the demand for and consumption of phosphate ore have been increasing. From the end of the 1990s to the present, the actual annual consumption growth rate of phosphate rock is about 2.5%. Therefore, according to the proven phosphorus reserves and mining rates in the world, global phosphate rock resources can only last for about 100 years.
China is one of the major producing phosphorus countries in the world. The proven reserves of phosphate ore have been about 6.6 billion tons and the basic reserves are about 13 billion tons. The reserves of phosphate ore in China have surpassed that of the United States, ranking second only to Morocco. Although the total amount of phosphate ore in China is among the highest in the world, the quality of phosphate ore is not high. The average grade of phosphate ore is only 16.9%. Among the proven reserves of phosphate ore, high-grade ore in which the content of P2O5 exceeds 30% of the total mass accounts for only 8%, and the rest is medium-grade ore in which the content of P2O5 is 12–30%, and low-grade ore in which the content of P2O5 is less than 12%. The content of colloidal phosphate ore at the medium and low grade is relatively high. The mineral particles are fine and densely embedded and contain more harmful impurities. Therefore, there are a series of problems in the mining process, such as high mining difficulty, high depletion rate, high loss rate, and low recovery rate. According to the current annual consumption and consumption growth rate of China’s phosphorus resources, phosphorus-rich mines can still supply ore for 15–20 years. The Ministry of Land and Resources has listed phosphate rock as one of the mineral resources that cannot meet the requirements of national economic development after 2010. The long-term supply situation of phosphate rock at home and abroad is not optimistic, and will gradually show a trend of decline in ore quality and rise in processing costs.
Despite the declining reserves of phosphate rock, the demand for phosphorus resources in China and even in the world has not decreased due to population and food problems. Phosphorus resources are limited and increasingly scarce, which can no longer meet the development needs of China and the world. Therefore, it is critical to look for an alternative and renewable resource of P. Phosphorus is recovered from phosphorus-rich residues such as manure, meat, bone meal, and agricultural waste, even sewage sludge can be a part of the solution. In the process of municipal sewage treatment, sewage treatment plants widely use enhanced biological phosphorus removal (EBPR), which makes activated sludge rich in a large number of phosphorus accumulating organisms (PAOs) and denitrifying phosphorus accumulating bacteria (DPAOs). These two microorganisms accumulate much more phosphorus than their physiological need by their special metabolism. The excess phosphorus accumulates in the form of polyphosphates in the cells forming a high-phosphorus sludge, which will be discharged at the end of the aerobic section, thereby achieving the effect of efficient phosphorus removal from the sewage. This process produces a large amount of phosphorus-rich by-products, sewage sludge. 25% of the total phosphorus in sewage is enriched in sludge through this process. If the EBPR process treats the raw sewage, more than 90% of the phosphorus load in the sewage transfers to the sludge. Moreover, the phosphorus in the sludge mainly exists in the form of inorganic phosphorus (IP), and the content of organic phosphorus (OP) is relatively low, which reduces the difficulty of phosphorus recovery from the sludge. Therefore, this is an effective way to recover phosphorus from sludge (Fig. 2).
Apart from sewage sludge, many organic waste materials contain significant amounts of phosphorus. Table 1 shows the phosphorus concentrations of different typical wastes. Sewage sludge contains the second greatest amounts of phosphorus [12]. The only organic waste containing more phosphorus is bone meal, but on a global scale, it is produced in much smaller quantities than sludge. Therefore, sludge is considered a very promising source of phosphorus [13].
Sewage sludge treatment
According to statistics, the sludge produced by only developed countries in the world exceeds 30 million tons per year, and this number is still on the rise [14, 15]. There is a large amount of sludge produced globally. In addition to its rich organic matter and nutrient elements such as nitrogen, phosphorus, and potassium, it also contains various pathogens and heavy metal elements. If not disposed properly, it will not only waste resources but also emit odor, spread germs, and pollute water sources, causing severe pollution to the human living environment, which will endanger human health. Nowadays, the main ways of disposing of sewage sludge can be classified into three categories: landfill,agricultural use, and incineration [14]. Resource utilization is the main idea for sludge treatment in developed countries. For example, Japan technically uses sludge for building materials after incineration. In developed countries of Europe, land use is the primary disposal method for sludge, and in the United States, 60% of sludge is treated to form bio-solids for use as farmland fertilizer.
Sludge contains an elevated amount of organic matter, which generates a landfill gas rich in CH4, contributing even more to the greenhouse effect than CO2. Moreover, the cost of the land needed for a landfill is increasing because of its decreasing availability. Besides, the disposal of a large amount of sewage sludge to landfills would inevitably induce some geo-environmental problems, such as compression, differential settlement, local instability, and slope instability of the landfill [16]. Slope stability refers to the stability of slope rock and soil under certain slope height and slope angle conditions.
The second disposal method of sewage sludge is agricultural use because of its abundant organic matter and large amount of nitrogen, phosphorus, and potassium, which can be used as fertilizer for crops. However, the direct use in agriculture is controversial because sewage sludge as a sink for pollutants in wastewater treatment plants has the possibility of contaminating the environment with heavy metals and organic pollutants, such as pharmaceuticals, parasites, and pathogens, which are a focus of discussion and limit the direct application of sewage sludge in agriculture [15].
Incineration, the final disposal method, is an exothermic oxidation process of biosolids resulting in flue gasses comprising of CO2, H2O, ash, and a certain amount of heat. Incineration reduces the volume of sludge by 90% with the simultaneous destruction of pathogens. The residual ash and bottom slag from incineration (about 30 wt% of the original sludge) can be disposed in landfills or utilized for building material production [17, 18].
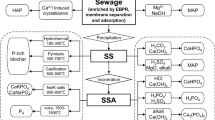
In addition to the three main sludge disposal methods mentioned above, some other sludge treatment technologies are also under study, such as gasification, pyrolysis, and hydrothermal. Figure 3 systematically demonstrates the source and use of phosphorus and the treatment technologies by which sewage sludge is eventually disposed of after phosphorus has been used and enriched by humans.
Gasification is the thermal process during which the carbonaceous content of MSS converts to a combustible gas and ash in the presence of a reactive atmosphere (generally air or steam). Gasification mainly transforms organic materials to combustible gas or syngas, using between 20 and 40% of the oxygen required for total combustion [19].
Pyrolysis is a thermal decomposition process under low-oxygen or anoxic conditions at relatively high temperature (300–1000 °C), which provides three basic products such as pyrolysis gas, bio-oil, and solid product namely char [19,20,21].
Hydrothermal is a wet thermochemical process in a closed system that operates under certain pressure and relatively lower temperature compared to incineration or pyrolysis. Moreover, hydrothermal does not require a specific atmosphere. In addition, hydrothermal reactions are exothermic processes [22,23,24]. All these features make the hydrothermal process more energy efficient.
Figure 4 summarizes the common process for recovering phosphorus from sludge and its thermal-treated products.
Effect of different thermal treatments on the utilization of phosphorus resource in sewage sludge
Sewage sludge has been used for direct agriculture application as fertilizer for decades. Due to high contents of heavy metals and organic pollutants [25,26,27], concerns about the direct agriculture application of sewage sludge are on the rise. Legal regulations restricting the use of sewage sludge as fertilizers are increasingly strict all over the world, especially those defining the maximum allowable concentrations of heavy metals in sewage sludge introduced into the soil [28,29,30]. For this reason, technologies for sewage sludge treatment and indirect recovery of phosphorus from it are becoming increasingly arresting. Thermal treatment of sewage sludge can reduce waste volume, decompose organic pollutants, reuse the energy contained in the sludge, and generate valuable byproducts [31, 32]; therefore, it is regarded as the best way to dispose sewage sludge. Presently, the thermal treatment of sewage mainly includes incineration, pyrolysis, and hydrothermal. During the thermal treatment of sewage sludge, P was enriched in ash/char, which has several consequences [33, 34]. Thermal processing modifies the chemical and physical structure of the feedstock while affecting P speciation. Different thermal processes have a different impact on the migration and transformation of phosphorus, resulting in different phosphorus speciation. For any P recycling/reclamation methods, one fundamental influencing factor is the P speciation in the sewage sludge and treatment products, because speciation of an element largely determines its mobility and bioavailability. This review summarizes the speciation and migration characteristics of P in sludge during the thermal treatment processes of incineration, pyrolysis, and hydrothermal.
Incineration
The incineration process of sewage sludge completely neutralizes the vast majority of potentially hazardous organic compounds, as well as all parasites and pathogenic microorganisms [35, 36]. Ashes from sewage sludge incineration contain a much higher content of P, mainly due to a significant reduction (70–90%) in the volume of the incinerated material [35, 37]. The content of phosphorus in ash is 5–11% (maximum concentration is up to 20%) [37,38,39]. Some authors claim that the concentration of phosphorus in ash is from 70 to 134 g/kg [40]. The fact that ash is a dry and free-flowing powder greatly simplifies processing operations for subsequent phosphate extraction when compared to either phosphate rock or liquid and diluted sewage sludge.
The composition and relative abundance of metals (particularly metals with strong affinities for P) affect the P speciation in both sewage sludge and treated products. The main elements of ash fraction are: Ca, Si, Al, Fe, P, and O. Most of them occur as oxides forming such compounds as CaO, SiO2, Al2O3 or Fe2O3. Incineration allows complex management of all solid residues formed during the P recovery processes [41]. Wang et al. [42] found the formation of calcium phosphate (Ca3(PO4)2), calcium pyrophosphate (Ca2P2O7), and hydroxyapatite (Ca5(PO4)3(OH)) at high temperatures during incineration. Incineration of sewage sludge at 750–950 °C also showed the formation of apatite from Al and Fe phosphates and amorphous Ca phosphate phases [43]. Some evidence has suggested the partial substitution of Ca2+ in whitlockite in ash for Mg2+, Fe3+ or Al3+ [37, 44,45,46,47]. Phosphorus often occurs as Fe4(P4O12)3, Al(PO3)3, mainly because AlCl3, Al2(SO4)3, FeCl3, Fe2(SO4)3 compounds are wildly used as phosphorus precipitating agents [36,37,38]. However, a high concentration of Al compounds may cause a greater threat to the environment than a high concentration of Fe compounds.
Incineration of sewage sludge is often carried out at a temperature of about 850 °C. At this temperature, phosphorus takes a form of volatile oxides, which then condense upon cooling to a temperature of 40–600 °C to form P4O10, becoming a component of ash retained by the filters. According to the results of [33], P is a typical lithophilic element with approximately 80–90% of P remaining in the bottom ash after incineration. The rest is transferred into fly ash, due to mechanical carryover. However, during the process of sewage sludge incineration, a large amount of fly ash is produced [45], which reduces the amount of phosphorus in the bottom ash. Thygesen et al. [48] reported that incineration temperatures should be kept below 700 °C to avoid the formation of insoluble hydroxyapatite in the ash. Some authors have also claimed that ash obtained during sewage sludge incineration above 700 °C is unsuitable for use as fertilizers, mainly due to the bio-unavailable form of phosphorus compounds occurring in the mentioned materials [35, 36, 38, 49, 50]. Moreover, incineration of sewage sludge in 1250 °C results in a lower concentration of P in ash fraction and a higher concentration of P in dust fraction [51].
Various innovative incineration process parameters are proposed to increase the available and soluble phosphorus in ash (e.g., additives and temperature). Zhao et al. [52] claimed that cotton stalk (CS) was added into sewage sludge to improve the bioavailability of phosphorus in the fly ash during incineration. The addition of CS was advantageous for the conversion of non-apatite inorganic phosphorus (NAIP) to apatite phosphorus (AP). The Ca and Mg compounds in CS can provide reactive chemical sites for phosphorus to form Ca18Mg2H2(PO4)14 and Ca2P2O7. Ren et al. [53] also found that sewage sludge co-combustion with wheat straw leads to the formation of alkali-rich phosphate silicate that restrains the reaction between the alkali and quartz. Reactions between the P-rich additive and alkali metal lead to the formation of K–Ca–P compounds. Beck and Unterberger [54] reported that phosphorus preferred to combine with calcium and was enriched in the fly ash during co-combustion of sewage sludge with coal. Han et al. [55] showed that CaO additives could increase the phosphorous and heavy metals during incineration of sewage sludge, while with HCl additives, the amounts of phosphorous and heavy metals decreased in the bottom ash. At the same time, the removal efficiency of heavy metals in sewage sludge or ash exhibited an increasing tendency with the addition of chloride, especially in the cases of Cu, Zn, and Pb. MgCl2 is more effective than KCl in improving the removal efficiency of heavy metals in sewage sludge [56].
Pyrolysis
Among various treatment techniques for sewage sludge, pyrolysis has been recognized as an effective alternative to primarily reduce the volume, destroy pathogens, and convert the sludge into high-valued carbonaceous materials (biochar) for multiple applications like soil amendment, carbon sequestration, and contaminants mitigation [57,58,59,60]. P remains mostly in the solid phase, and its partitioning in liquid and gas phases is limited. The relative fractions of P in solid, liquid, and gas phases are related to P speciation and treatment conditions (primarily temperature). Pyrolysis (or carbonization, for dry thermochemical processes) occurs at low heating temperatures with slow heating rate, where the mass predominantly remains in the solid products. For example, when pyrolyzed at 250–600 °C, P recovery in the solid phase is nearly 100% for sewage sludge [23, 61, 62]. Liquefaction and hydrothermal liquefaction are the processes occurring at medium temperature range, with a fast heating rate and short residence time, where liquid products account for a large fraction of the mass [63]. Gasification and hydrothermal gasification occur at higher temperatures [64].
The composition of sewage sludge depends strongly on the sewage source, wastewater treatment techniques, and subsequent handling processes. The organic matter in sewage sludge is primarily microbial biomass; thus, the main organic components in sludge are organic compounds constituting cellular structures, such as polysaccharides, proteins, lipids, and DNA. The amounts of organic P in raw sewage sludge are more sensitive to pyrolysis so that they are more likely to convert into the gas phase. Among them, organophosphates in sewage sludge can undergo dramatic transformations during pyrolysis, such as dehydration, decarboxylation, and polymerization [65]. These reactions can lead to the decomposition of organophosphates and formation of orthophosphates, pyrophosphates, and/or organophosphates with more condensed functional groups. Polyphosphates in sewage sludge were found to degrade into shorter chain lengths (including pyrophosphate) during slow pyrolysis, with the chain length decreasing with increasing pyrolysis temperature. In general, pyrolysis of sewage sludge can convert organic P into inorganic P, decrease the organophosphates and polyphosphate, and increase the total P content by 2–3-fold.
Regarding the transformation of P in sewage sludge during pyrolysis, several consistent trends have been observed on the effect of treatment temperature. Pyrophosphate forms during pyrolysis and is most abundant at medium temperature ranges (300–600 °C) [66, 67]. Almost all P exists as orthophosphate in chars produced at high temperatures (mostly > 700 °C) [67,68,69]. Higher heating temperatures promote P migration into the gaseous phase. For example, P can be volatilized during gasification [70, 71], and the main gaseous P species was PO2+ during gasification (900–1400 °C), whereas hydrogen phosphate and two organophosphates were also identified in the volatiles of pyrolyzed Tris (2-butoxyethyl) phosphate [70, 72].
Pyrolysis affects both quantity and speciation of P in the biochar, and studies show that the mobility and bioavailability of P are closely related to its speciation [23, 73,74,75,76]. Decreases of soluble and Olsen P (sodium bicarbonate extractable) was observed during the pyrolysis of sewage sludge [62, 77]. In these cases, the fractions of HCl extractable P increased, consistent with the decrease of organophosphates and the increase of crystalline Ca phosphates. Such immobilization effect generally increases with increasing pyrolysis temperature. Meng et al. [68], Nakakubo et al. [49], and Xu et al. [67] also reported that the increase of pyrolysis temperature would lead NAIP to transform into AP. The content of calcium phosphate and orthophosphate in the sludge sample increased with the increase of temperature. As for the parameters of heating rate and soaking duration, they have minimal effects on P transformation. For example, no obvious differences were observed for the liquefaction (500 °C) and flash carbonization of sludge [69]. Pyrolysis under different inert atmospheres has little influence. For sewage sludge pyrolyzed under N2 and CO2, similar P speciation and relative abundance were observed in chars [75].
Kleemann et al. [78] compare the physical and chemical characteristics of incinerated sewage sludge ash (ISSA) and pyrolyzed sewage sludge char (PSSC) to recover phosphorus. Both PSSC and ISSA contain whitlockite with PSSC containing more whitlockite than ISSA. Heavy metals are less solution from PSSC because they are more strongly incorporated in the particles. Moreover, the application of biochar to soil can increase soil C sequestration, reduce atmospheric CO2 concentrations, the bioavailability of some heavy metals, improve physicochemical soil quality, and alter the content and availability of nutrients [79,80,81].
Hydrothermal
In general, hydrothermal techniques can be divided into two main categories: thermal treatments (operating under inert atmosphere and dry conditions) and hydrothermal treatments operating in a closed pressurized system and under wet conditions. Each category can be further divided into carbonization, liquefaction, and gasification treatments, based on the operating temperature and phase partitioning of the products [65, 82]. The application of hydrothermal techniques for biowaste managements can accommodate a broad range of wastes with different properties (dry vs. wet, uniform vs. heterogeneous) [83, 84]. They are highly tunable techniques that can convert solid biowastes into products with broad applications (for example, chars, fuel, and organic products) [85, 86], thus achieving effective resource recovery.
Hydrothermal treatment of sewage sludge has certain advantages. Sewage sludge has high water contents, with a significant fraction present in the form of intracellular or bound water that cannot be efficiently pre-dried for thermal treatments. The treatment can be used for converting high moisture content of sewage sludge at mild reaction temperature into valuable self-separating products, such as biochar with aqueous products and gasses [87]. In these cases, hydrothermal treatments can be used to avoid drying processes [84, 88]. Hydrothermal treatments generally operate in closed systems and at lower temperatures, compared to the higher operating temperatures required by other thermal treatments and are also exothermic processes [89]. Similar to thermal treatments, hydrothermal treatments can also serve as sanitation methods to degrade pathogens and organic contaminants. Studies have shown that organic contaminants in sewage sludge can be degraded after HTC treatment, with more than 90% of common pharmaceuticals degraded following HTC treatment at 210 °C [90, 91].
Hydrothermal treatment can be proposed to release phosphate from sewage sludge. P recovery in HTC-produced hydrochars from sludge is commonly above 80% [92,93,94]. Moreover, metal-complexed and mineral-associated P species in the sewage sludge can also be transformed into more stable species under hydrothermal conditions. Huang and Tang [77] claimed that hydrothermal treatment could achieve the transformation of all kinds of P species like phosphonate, organic phosphates, and polyphosphate mainly into inorganic orthophosphate. In general, hydrothermal treatments impose more extensive alterations to P speciation than thermal treatments. Orthophosphates dissociated from organophosphates are available for forming phosphate precipitates or adsorption to minerals. Unstable inorganic P species may also undergo dissolution and reorganization/recrystallization. These two processes will lead to the formation of more stable and insoluble species. Sequential extraction of sewage sludge hydrochars showed significant decreases of soluble and Olsen P fractions, and increases of both NaOH and HCl fractions [77, 95]. Thus, P speciation in the treatment products is primarily governed by the presence of P-binding metals (which determines the complexation and mineralogy state of phosphate species) and the hydrothermal conditions (which determines the stability of different P species). For example, HTC treatment of sewage sludge at 225 °C induced the increasing association of phosphate with Fe (as Fe oxide adsorbed species) and Ca (as Ca phosphate minerals), because both cations are abundant in sludge and have high affinities for P to form precipitates and/or surface complexes [77]. The transformation of Ca phosphate phases is, thus, an important reaction during hydrothermal processes. AP can be a primary form of P species in hydrochars produced from sewage sludge containing abundant Ca. The majority of heavy metals can be immobilized into a solid phase during the hydrothermal reaction [96]. Moreover, hydrothermal treatment is considered as an appropriate pretreatment method of sludge for magnesium ammonium phosphate (MgNH4PO3·6H2O) crystallization. Figure 5 summarizes the transformation characteristics of phosphorus in the sewage sludge during thermal treatments.
Phosphorus recovery from sewage sludge and thermal products
Direct extraction and reclamation of P from sewage sludge and its by-products are generally not practical or efficient due to the physicochemical properties. Today, there are numerous (> 30) kinds of technologies of P recovery from sewage sludge and new ones are continually being developed [12, 13, 97,98,99,100], varying on type of matrix (e.g., wastewater, sewage sludge, and its ashes/chars) and the type of process (e.g., precipitation, wet chemical extraction, and thermal treatment). The methods for P recovery from sewage sludge generally involve a step that solubilized P via precipitation in the form of struvite, monoammonium phosphate, or other phosphate phases, adsorption, or ion exchange [101, 102]. For P recovery, the speciation of P in sewage sludge and the by-products controls its extractability and speciation in the liquid extracts [12, 98, 103], and ultimately its recovery efficiency. The optimal scenario is to achieve maximum P recovery with the least operational steps and low costs. The section below introduces the main methods of P recovery and recycling from sewage sludge and its by-products.
Wet chemical
Chemical washing is the most pervasive method for P extraction from sewage sludge ash (SSA) due to the simple process and low expenditure [104]. Several research articles have been published on the recovery of phosphate from SSA using the leaching process. Phosphorus can be recovered from SSA by leaching with acidic, alkaline solutions, and, sequentially, with acidic and alkaline solutions [15, 37, 46, 47, 105,106,107,108,109,110]. Both acidic and alkaline washing processes have the benefit of potentially lower energy consumption and the production of phosphoric acid and phosphate products that could be tailored to products, such as struvites, hydroxyapatites, or calcium phosphates with potentially higher market prices.
The extractants substantially comprise of inorganic acids (H2SO4, HCl, and HNO3), organic acids (citric, oxalic acids), alkali (NaOH, CaO), inorganic chemicals (e.g., ferric chloride), and a chelating agent [111]. Fang et al. [107] showed that organic acids leach more trace elements, particularly Cu, Zn, Pb, and As. Normally, inorganic acids can accomplish a higher P extraction efficiency (> 90%) [37, 105, 108, 111, 112], where H2SO4 has been widely used from an economic point of view [37]. Also, it can easily remove unwanted Ca2+ from mixtures by the controlled precipitation of gypsum (CaSO4·2H2O). The acid concentration and liquid to solid (L/S) ratio are critical factors that are affecting the leaching efficiency. Pettersson et al. [36] claimed that sulphuric acid was the most efficient for P recovery from SSA and achieved 94% of total extraction under the optimal conditions, which were a 2 h reaction with 0.2 mol/L H2SO4 at a liquid to solid ratio of 20. Donatello et al. [105] used a sulfuric acid washing procedure to produce a technical-grade phosphoric acid. The optimized conditions were the minimum stoichiometric acid requirement, a reaction time of 120 min, and a liquid to solid ratio of 20. These conditions obtained average recoveries of 72–91% of total phosphorus. Franz [108] showed that 66–99% of total phosphate could be dissolved by washing ISSA with 14% H2SO4 for 10 min at L/S ratio of 2. Stark et al. [113] reported the extraction of up to 87% of total phosphate in ISSA with 1 mol/L HCl at L/S ratio of 50 for 2 h.
Taking into account that in most SSAs, the P component is mainly present in the form of calcium phosphates (Ca–P), aluminum phosphates (Al–P), and iron phosphates (Fe–P), the chemical demand for such a ‘complete acidic dissolution of P’ can be estimated as follows [114]:
During the leaching process, major elements (predominantly Ca, Al, Fe) and heavy metals are co-dissolved with P [104, 115]. P recovery from the heavy metal-containing leachate is the pivotal issue. So, the leaching process followed further steps to separate P from the leached impurities like heavy metals by means of sequential precipitation [41], sulfide precipitation [108], liquid–liquid extraction (PASCH-process) [114], solvent extraction, ion exchange columns [105, 108], as well as through the chemical precipitation of P as Ca–phosphates.
In the case of sequential precipitation, in a first step, acid is added, dissolving P and metals, and separating the remaining solids from the P-containing acidic leachate. The separation of dissolved P from heavy metals is achieved by raising the pH value in the acidic leachate to induce the precipitation of Al–P while most heavy metals remain in solution [41]. Precipitating heavy metals with sulfide can further decrease the heavy metal content of the Al–P product.
The SESAL-Phos process (sequential elution of sewage sludge ash for aluminum and phosphorus recovery) (Fig. 6) presented by [46] is an interesting alternative to the process used by [41], as this ultimately produces a solid Ca–phosphate precipitate and soluble AlCl3 solution. A pH of 3 is maintained with HCl, under which conditions Ca–P compounds dissolve and Al–P compounds simultaneously precipitate. Al–phosphates and acid insoluble SSA residues are retained on the filter, while soluble heavy metals and Ca2+ pass to the filtrate. The solid fraction is then treated with NaOH at pH 13, where the Al–phosphate is dissolved and separated from the insoluble components of residues. Finally, the Al–phosphate filtrate is treated with CaCl2 to precipitate P as Ca–phosphate.
Chemical precipitation has been extensively used to recover phosphate in the liquid phase, and calcium or magnesium is the pervasive precipitator, which can react with phosphate to form hydroxyapatite or struvite correspondingly [42, 116]. Compared with the struvite, hydroxyapatite requires additional treatments before its utilization, while struvite can be used directly as a fertilizer, which exhibits comparable agronomic performance to commercial fertilizers [116,117,118,119,120]. Furthermore, struvite is considered poorly soluble, which means that even applying a vast amount of such fertilizer to the ground, the possibility of polluting the environment with a high load of phosphorus and enhancing the eutrophication process is low [121, 122]. Another advantage of such a technology is the spontaneous struvite precipitation phenomenon. In some specific conditions, especially if a sewage sludge treatment plant runs the process of biogas recovery, struvite may cause clogging of the pipes. This problem occurs in most of such facilities when the Mg2+, NH4+, and PO43+ concentrations are high and the pH is in the range 7.0 < pH < 10.7. However, the solution pH always is over 8.0 to facilitate the formation of struvite. During the adjustment of the solution pH, the co-dissolved metal ions can inhibit/contaminate P reclamation process. Al and Fe ions have strong affinities to P under pH 4.0 [112, 123]. Another major element Ca, along with other heavy metals such as Zn and Cu, can co-precipitate with P at elevated pH values and contaminate the struvite [124, 125]. The separation of metals from P in the leachate is the prerequisite for struvite crystallization. As Fig. 7 shows, Wang et al. [42] and Xu et al. [126] utilized a cation exchange resin (CER) to purify the leachate by HCl leaching of the ISSA followed by performing a struvite crystallization experiment. Wang et al. [42] transform P in the ISSA to amorphous iron phosphate (Fe–P) and aluminum phosphate (Al–P) by acid washing followed by alkali precipitation, re-dissolve Fe–P and Al–P via acid washing, and adsorption of Fe and Al ions by a CER, and struvite crystallization. Some authors claim that struvite may be recovered up to 97% if the Mg concentration in sewage sludge is sufficiently high. The best P:Mg ratio is 1:1.05, but on a technical scale, it should generally be maintained at a level of 1:1.3. Generally, sewage sludge does not contain sufficient amounts of Mg, resulting in only 72% efficiency of the recovery process. In such cases, MgCl2, or another Mg source (wood ashes, magnesite, magnesia, bittern, seawater, or by-products of MgO production) is often added during the thermal treatment or the extraction process [3, 121, 127].
An alternative to the acidic dissolution of P followed by a separation of dissolved P from the dissolved metals is a direct alkaline dissolution of P. Direct extraction using NaOH is possible, but the efficiency of such a P recovery process reaches only 40% [37, 49]. In this case, only the amphoteric Al–P compounds dissolve while most (heavy) metals remain in the SSA. The dissolved P can be precipitated from the alkaline solution (pH > 13) as Ca–P with a very low impurity level, via the addition CaCl2. The amount of Al–P directly leachable via alkaline treatment depends on both the Al content and the Ca content of the SSA. In the case of SSA with very low Ca contents, direct alkaline elution can dissolve a significant amount of P. The wastewater treatment plant of Gifu, Japan, recovers 75% of Ptotal in a full-scale wet chemical P-recovery plant by direct alkaline elution from Ca-poor and Al-rich SSA. The mean value of the P/Ca ratio calculated from the composition of six Japanese SSAs published by [128] is 2.00 (± 0.23). In Germany, the Ca content of raw SSA is generally high due to the geological conditions (hard drinking water). Consequently, merely 0–35% of the Ptotal can be recovered by direct alkaline elution, even with the addition of Al for chemical P removal. However, for one German Al-rich SSA [112] showed that the amount of P that could be dissolved and recovered by alkaline leaching increased from 25 to 67% with the application of an acidic pre-treatment step that removes Ca.
Thermochemical
One of the alternatives for P recovery from SSA is via thermal methods. As with acid leaching, one issue is how to separate the valuable P from problematic heavy metals. To enhance the P bioavailability and remove heavy metals of SSC/A, several thermochemical treatment has been investigated with promising outcomes [44, 129,130,131,132,133]. Subjects of these investigations were the influence of different parameters of thermal processing (temperature, oxidative or reductive conductions, and additives) on the P compounds and P bioavailability of the products.
Regarding temperature, with an increase in the ISSA treatment temperature, the bio-available P content increased. Treatment at 800 °C resulted in similar available P levels to a commercial fertilizer. The thermal removal of heavy metals at high temperatures (above 1400 °C) [134] also allows the separation of the primary metals like Fe, which at the same time increases the bioavailability of P in the ash. These process intend to increase the P bioavailability via the formation of soluble P-bearing mineral phases, specifically, which include the conversion of whitlockite (Ca3(PO4)2) to chlorapatite (Ca5(PO4)3Cl1−x(OH)x) via an intermediary chlorspodiosite (Ca2PO4Cl) species, with the formation of new Mg–phosphates (farringtonite Mg3(PO4)2) or Mg–Ca–phosphates.
Chlorine donor is the most widely used additive in thermochemical treatment processes. Figure 8 shows the process of the thermochemical treatment method using chloride as an additive. Thermochemical treatment with 5–15% of KCl or MgCl2 and heating at 900–1000 °C resulted in high percentage removals of Pb, Cd, Cu, and Zn [133]. MgCl2 was used under oxidative conditions at different temperatures (450–1000 °C) to eliminate heavy metals and to increase the P bioavailability due to the formation of farringtonite (Mg3(PO4)2) and stanfieldite (Ca4Mg5(PO4)6) [44, 135]. Vogel et al. [136] used HCl to remove heavy metals from sewage sludge during thermochemical treatment. Adding MgCO3 increased the bioavailability of P. Using a gaseous chlorine donor has the advantage of not needing to be mixed with ash before treatment. Fraissler et al. [131] used CaCl2 as a Cl donor and found that Cd and Pb were readily volatile, while Cu and Zn were semi-volatile and Cr and Ni were not very volatile. They also concluded that the material bed temperature of 1000 °C leads to better removal results. The result of [44] showed that Hg could be removed by thermochemical treatment to meet the limits of fertilizer ordinances. Treatment with 15% MgCl2 at 1000 °C for 60 min was shown to remove over 90% of Cu and Zn by volatilization as CuCl2 or ZnCl2.
Furthermore, the use of sodium salts under reductive conditions reduces heavy metals and increases the P bioavailability of the product. SSA blended with Na2CO3 or Na2SO4 using dried sewage sludge as a reducing agent was carried out at 1000 °C. Thus, the Ca3(PO4)2 or whitlockite component of raw sewage sludge ash, which is not readily plant available, was converted to CaNaPO4 (buchwaldite), which has a much higher P bioavailability than chlorapatite or whitlockite [15, 137, 138]. Sludge can adequately be treated at high temperatures under reductive conditions with sodium additives to form highly bioavailable calcium–sodium–phosphate. Highly heavy-metal contaminated sludge can be thermochemically treated at high temperatures to achieve the legal requirements for fertilizers [139].
Under the above conditions, metal or its chlorides are volatilized, and P is converted to a more bio-available form so that products can be used directly as a fertilizer. However, thermochemical treatment processes have often been associated with high specific energy demands. There are concerns over the operating costs of thermochemical treatment and with equipment lifetime due to the highly corrosive conditions generated.
Electrokinetic (electrodialytic)
Electrokinetically based technologies can be effective and viable options for P recovery from sewage sludge. The electrokinetic (EK) process is based on the application of a low-level current (direct or alternate; DC or AC) density and low potential gradient [140], between suitably located electrodes. The contaminants are moved out of the matrix towards one of the electrode compartments by three main transport processes: electromigration, electroosmosis, and electrophoresis [141].
The electrodialytic (ED) process is based on the combination of the electrokinetic movement of ions and electrodialysis. The general principle is very similar to EK, but instead of using passive membranes, it uses ion exchange membranes (anion exchange membrane and cation exchange membrane). The ion exchange membranes prevent the spare of inefficient use of the current in transporting ions from one electrode compartment through the matrix into the second electrode compartment. Furthermore, the ion exchange membranes make the conditions in the central compartment less dependent on the choice of electrolyte solution than the use of passive membranes. Figure 9 shows the schematic diagram of ED and EK processes in a three-cell.
Many reported electrodialytic remediation (EDR) experiments for stirred suspensions consist of a three (or more) compartments experimental setup [142,143,144]. The ash suspension in a three-compartment setup can be acidified through water splitting at the anion membrane, proton leakage from the anode compartment, or the ion exchange from the cathode compartment [145]. Recently, the new development in ED is the two-compartment (2C) electrodialytic setup, in which the anode is placed directly in the compartment where the contaminated matrix is suspended and stirred simultaneously. In this setup, the anode compartment contains SSA suspended in water. The major objective with this setup is a simultaneous extraction of P and removal of heavy metals from the liquid with P. With the application of electrical DC to the electrodes, the SSA suspension will gradually be acidified due to electrolysis at the anode (H2O − 2e− → 2H+ + 1/2O2(g)). During the acidification, heavy metals and P are extracted from the ash. The heavy metals are transported by electromigration over the cation exchange membrane (CEM) and concentrated in the cathode compartment. The extracted P remains in the filtrate of the ash suspension. With this, simultaneous extraction and separation are obtained.
Dissolution of phosphorus and heavy metals is faster in the 2C experiments than for the 3C experiments where their dissolution depends on acidification through water splitting and migration of H+ to the suspension compartment or addition of mineral acid. Protons are concurrently produced from water electrolysis at a node, which increases the solubilization of the different elements present in the SSA [146, 147]. Acidic leaching, combined with cation migration into the cathode compartment achieved a high recovery of P (> 80%) for SSA. In addition, P was effectively separated from Al, Ca, Fe, Mg, and most heavy metals. This is due to the alkaline pH of the ash suspension in one of the steps of ED treatment, where P bound to Fe and Al was extracted, and most metals were insoluble.
Guedes et al. [148] recovered P from sewage sludge through electrodialytic treatment. They assessed simultaneous P recovery and removal of emerging organic contaminants (caffeine, bisphenol A, 17α-ethinylestradiol, and oxybenzone) from sewage sludge (SS). 70% of P was recovered in the anolyte, and between 79 and 96% of organic compounds were degraded. Still, the obtained anolyte enriched in P was not completely free of organic contaminants.
Summary
In general, P recovery from sewage sludge can be achieved via two different strategies: reclamation of P as pure P compounds, or direct application of the P-rich products as P fertilizers. The comparison of different methods for recovering phosphorus from sewage sludge and its thermal products is shown in Table 2. There is no doubt that the recovery of P from sewage sludge is more environmental friendly than the direct use of sludge as a fertilizer. Studies demonstrate the potentials of thermal treatments in improving P recovery from sewage sludge. They can facilitate more effective P recovery via waste volume reduction and P speciation modulation. They can also accommodate sewage sludge with high contaminant contents that are technically or environmentally unfavorable for P recovery via conventional practices.
Technologies based on phosphorus recovery from sewage sludge and thermal products, such as struvite and hydroxyapatite recovery, gained popularity more than 10 years ago. However, the migration and transformation of P in thermal treatment processes and the specific influence of the speciation of P in the thermally treated products on subsequent leaching, extraction or direct utilization are still problems to be solved urgently. In addition, based on these conversion mechanisms and the influence of P morphology on the product’s availability, the comprehensive evaluation of phosphorus resource utilization in sludge and the selection of the most appropriate treatment method according to the specific situation will be the future research trend.
Specifically, previous studies have demonstrated the potentials of thermal treatments and chemical recovery methods in facilitating P reclamation and recycling, and the critical role of P speciation in these processes. Further studies are needed to technically improve the application of these techniques for P reclamation and recovery. Regarding the application for P reclamation, the thermal treatment and P recovery processes need to be integrated and optimized to recover P at a low cost efficiently. For example, whether precipitation or adsorption is a better P reclamation option for the extracts from different thermal conditions needs to be tested. For the Fe-rich sludge and Al-rich sludge obtained by using different flocculants, what kind of extraction and precipitation methods should be adopted for each? The thermal treatment and reclamation methods need to be optimized to minimize metal contents and/or bioavailability in the reclaimed P products. Moreover, the effects of thermal treatment, recovery methods, and their interactions on the morphology of heavy metals in the products need further comparison and analysis.
Regarding agriculture application of purified P-rich products from thermal-treated sewage sludge as a P recycling strategy, further research efforts are needed to gather relevant information to evaluate the environmental and economic benefits and provide guidance for regulations and usages. A systematic comparison of products from different treatment conditions and their correlations to P speciation is needed. Particularly, technologies based on P recovery from ashes are gaining popularity for socioeconomic reasons, whereas very few focused on products from the treatment of pyrolysis, hydrothermal, and co-combustion/incineration with biomass or other additions. Also, P speciation in the sewage sludge and its thermal products was rarely characterized and related to the product performance. This information is necessary for providing feedbacks for wastewater and thermal treatment processes to select the optimal treatment technique and condition. For example, the incineration in fluidized bed furnaces method and separate collection of ashes seems to be the most environmental friendly methods. However, it is essential to select the most suitable method of P recovery for every single facility.
To evaluate the overall benefits of agriculture application of the treated products, measurements on the recycling of P and crop productivity should also be conducted and compared to those of conventional fertilization strategies. The effects of P speciation and impurities in the products on P absorption of crops need to be specifically analyzed. For another, there is a need for the development of standardized test procedures to generate quantitative parameters that will allow the comparison among different studies. For example, in the existing literature, different leaching protocols (e.g., different leaching solutions and soil mixing ratios) were used for the assessment of P mobility and bioavailability.
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42768-021-00078-9
29 January 2020
A Correction to this paper has been published: https://doi.org/10.1007/s42768-019-00028-6
References
Desmidt E, Ghyselbrecht K, Zhang Y, et al. Global phosphorus scarcity and full-scale P-recovery techniques: a review. Crit Rev Environ Sci Technol. 2015;45(4):336–84.
Cordell D, Drangert JO, White S. The story of phosphorus: global food security and food for thought. Global Environ Change. 2009;19(2):305.
De-Bashan LE. Bashan, recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004;38:4222–46.
SøRensen BL, Dall OL, Habib K. Environmental and resource implications of phosphorus recovery from waste activated sludge. Waste Manag. 2015;45:391–9.
Liu Y, Villalba G, Ayres RU, et al. Global phosphorus flows and environmental impacts from a consumption perspective. J Ind Ecol. 2008;12(2):229–47.
Chowdhury RB, Moore GA, Weatherley AJ, et al. A review of recent substance flow analyses of phosphorus to identify priority management areas at different geographical scales. Resour Conserv Recycl. 2014;83:213–28.
Koppelaar RHEM, Weikard HP. Assessing phosphate rock depletion and phosphorus recycling options. Glob Environ Change. 2013;23(6):1454–66.
Suh S, Yee S. Phosphorus use-efficiency of agriculture and food system in the US. Chemosphere. 2011;84(6):806–13.
Dawson CJ, Hilton J. Fertiliser availability in a resource-limited world: production and recycling of nitrogen and phosphorus. Food Policy. 2012;36(1):S14–22.
Mineral Commodity Summaries 2019. In: Tolcin AC, editor. Mineral Commodity Summaries, Reston, VA; 2019. https://doi.org/10.3133/70202434.
Shepherd JG, Kleemann R, Bahri-Esfahani J, et al. The future of phosphorus in our hands. Nutr Cycl Agroecosyst. 2016;104(3):281–7.
Cordell D, Rosemarin A, Schrder JJ, et al. Towards global phosphorus security: a systems framework for phosphorus recovery and reuse options. Chemosphere. 2011;84(6):747–58.
Cieślik B, Konieczka P. A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J Clean Prod. 2017;142:1728–40.
Fonts I, Gea G, Azuara M, et al. Sewage sludge pyrolysis for liquid production: a review. Renew Sustain Energy Rev. 2012;16(5):2781–805.
Herzel H, Krüger O, Hermann L, et al. Sewage sludge ash—a promising secondary phosphorus source for fertilizer production. Sci Total Environ. 2016;542(Pt B):1136–43.
Lo IMC, Zhou WW, Lee KM. Geotechnical characterization of dewatered sewage sludge for landfill. Revue Canadienne De Géotechnique. 2002;39(5):1139–49.
Raheem A, Sikarwar VS, He J, et al. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: a review. Chem Eng J. 2017;337:616–41.
Wu MH, Lin CL, Huang WC, et al. Characteristics of pervious concrete using incineration bottom ash in place of sandstone graded material. Constr Build Mater. 2016;111:618–24.
Samolada MC, Zabaniotou AA. Comparative assessment of municipal sewage sludge incineration, gasification and pyrolysis for a sustainable sludge-to-energy management in Greece. Waste Manag. 2014;34(2):411–20.
Frišták V, Pipíška M, Soja G. Pyrolysis treatment of sewage sludge: a promising way to produce phosphorus fertilizer. J Clean Prod. 2018;172:1772–8.
Lehmann J, Joseph S. Biochar for environmental management science. Technol Implement. 2015;25:15801–11. https://doi.org/10.4324/9780203762264.
Huang R, Fang C, Lu X, et al. Transformation of phosphorus during (Hydro)thermal treatments of solid biowastes: reaction mechanisms and implications for P reclamation and recycling. Environ Sci Technol. 2017;51(18):10284–98.
Huang R, Tang Y. Speciation dynamics of phosphorus during (hydro)thermal treatments of sewage sludge. Environ Sci Technol. 2015;49:14466–74.
Xue X, Chen D, Song X, et al. Hydrothermal and pyrolysis treatment for sewage sludge: choice from product and from energy benefit. Energy Proc. 2015;66:301–4.
Carbonell G, Pro J, Gómez N, et al. Sewage sludge applied to agricultural soil: ecotoxicological effects on representative soil organisms. Ecotoxicol Environ Saf. 2009;72(4):1319.
Laturnus F, von Arnold K, Grøn C. Organic contaminants from sewage sludge applied to agricultural soils. False alarm regarding possible problems for food safety? Environ Sci Pollut Res. 2007;14:53–60.
Harrison EZ, Oakes SR, Hysell M, et al. Organic chemicals in sewage sludges. Sci Total Environ. 2006;367(2–3):481–97.
Suciu NA, Lamastra L, Trevisan M. PAHs content of sewage sludge in Europe and its use as soil fertilizer. Waste Manag. 2015;41:119–27.
Tarayre C, Clercq LD, Charlier R, et al. New perspectives for the design of sustainable bioprocesses for phosphorus recovery from waste. Biores Technol. 2016;206:264–74.
Zhou K, Barjenbruch M, Kabbe C, et al. Phosphorus recovery from municipal and fertilizer wastewater: China’s potential and perspective. J Environ Sci. 2016;52:10.
Hartman M, Svoboda K, Pohořely M, et al. Combustion of dried sewage sludge in a fluidized-bed reactor. Ind Eng Chem Res. 2005;44(10):3432–41.
Hossain MK, Strezov V, Chan KY, et al. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J Environ Manage. 2011;92(1):223–8.
Belevi H, Langmeier M. Factors determining the element behavior in municipal solid waste incinerators. 2. Laboratory experiments. Environ Sci Technol. 2000;34(12):2507–12.
Wang T, Camps-Arbestain M, Hedley M, et al. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil. 2012;357(1–2):173–87.
Guedes P, Couto N, Ottosen LM, et al. Phosphorus recovery from sewage sludge ash through an electrodialytic process. Waste Manag. 2014;34(5):886–92.
Pettersson A, Amand LE, Steenari BM. Leaching of ashes from co-combustion of sewage sludge and wood—Part I: recovery of phosphorus. Biomass Bioenerg. 2008;32(3):224–35.
Biswas BK, Harada H, Ohto K, et al. Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. J Environ Sci. 2009;21(12):1753–60.
Ottosen LM, Kirkelund GM. Jensen, Extracting phosphorous from incinerated sewage sludge ash rich in iron or aluminum. Chemophere. 2013;91:963–9.
Weigand H, Bertau M, Hübner W, et al. RecoPhos: full-scale fertilizer production from sewage sludge ash. Waste Manag. 2013;33(3):540–4.
Couto N, Guedes P, Ferreira AR, et al. Electrodialytic process of nanofiltration concentrates—phosphorus recovery and microcystins removal. Electrochim Acta. 2015;181:200–7.
Takahashi M, Kato S, Shima H, et al. Technology for recovering phosphorus from incinerated wastewater treatment sludge. Chemosphere. 2001;44(1):23–9.
Wang Q, Li J-S, Tang P, et al. Sustainable reclamation of phosphorus from incinerated sewage sludge ash as value-added struvite by chemical extraction, purification and crystallization. J Clean Prod. 2018;181:717–25.
Li R, Zhang Z, Li Y, et al. Transformation of apatite phosphorus and non-apatite inorganic phosphorus during incineration of sewage sludge. Chemosphere. 2015;141:57–61.
Adam C, Peplinski B, Michaelis M, et al. Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag. 2009;29(3):1122–8.
Donatello S. Characteristics of incinerated sewage sludge ashes: potential for pozzolanic material in construction products. Imp Coll Lond. 2009. https://doi.org/10.13140/RG.2.2.33926.63040.
Petzet S, Peplinski B, Cornel P. On wet chemical phosphorus recovery from sewage sludge ash by acidic or alkaline leaching and an optimized combination of both. Water Res. 2012;46(12):3769–80.
Wzorek Z, Jodko M, Gorazda K, et al. Extraction of phosphorus compounds from ashes from thermal processing of sewage sludge. J Loss Prev Process Ind. 2006;19(1):39–50.
Thygesen AM, Wernberg O, Skou E, et al. Effect of incineration temperature on phosphorus availability in bio-ash from manure. Environ Technol. 2011;32(6):633–8.
Nakakubo T, Tokai A, Ohno K. Comparative assessment of technological systems for recycling sludge and food waste aimed at greenhouse gas emissions reduction and phosphorus recovery. J Clean Prod. 2012;32:157–72.
Yuan Z, Pratt S, Batstone DJ. Phosphorus recovery from wastewater through microbial processes. Curr Opin Biotechnol. 2012;23(6):878–83.
Zhang L, Ninomiya Y. Transformation of phosphorus during combustion of coal and sewage sludge and its contributions to PM10. Proc Combust Inst. 2007;31(2):2847–54.
Zhao Y, Ren Q, Na Y. Promotion of cotton stalk on bioavailability of phosphorus in municipal sewage sludge incineration ash. Fuel. 2018;214:351–5.
Ren Q, Li L. Co-combustion of agricultural straw with municipal sewage sludge in a fluidized bed: role of phosphorus in potassium behavior. Energy Fuels. 2015;29(7):4321–7.
Beck J, Unterberger S. The behaviour of phosphorus in the flue gas during the combustion of high-phosphate fuels. Fuel. 2006;85(10):1541–9.
Han J, Kanchanapiya P, Sakano T, et al. The behaviour of phosphorus and heavy metals in sewage sludge ashes. Int J Environ Pollut. 2009;37(4):357.
Saleh Bairq ZA, Li R, Li Y, et al. New advancement perspectives of chloride additives on enhanced heavy metals removal and phosphorus fixation during thermal processing of sewage sludge. J Clean Prod. 2018;188:185–94.
Feng H, Zheng M, Dong H, et al. Three-dimensional honeycomb-like hierarchically structured carbon for high-performance supercapacitors derived from high-ash-content sewage sludge. J Mater Chem A. 2015;3:15225–34.
Liu XQ, Ding HS, Wang YY, et al. Pyrolytic temperature dependent and ash catalyzed formation of sludge char with ultra-high adsorption to 1-Naphthol. Environ Sci Technol. 2016;50:536.
Yuan S-J, Dai X-H. Heteroatom-doped porous carbon derived from “all-in-one” precursor sewage sludge for electrochemical energy storage. RSC Adv. 2015;5:45827–35.
Zhang J, Lü F, Zhang H, et al. Multiscale visualization of the structural and characteristic changes of sewage sludge biochar oriented towards potential agronomic and environmental implication. Sci Rep. 2015;5:9406.
Azuara M, Kersten SRA, Kootstra AMJ. Recycling phosphorus by fast pyrolysis of pig manure: concentration and extraction of phosphorus combined with formation of value-added pyrolysis products. Biomass Bioenerg. 2013;49:171–80.
Bridle TR, Pritchard D. Energy and nutrient recovery from sewage sludge via pyrolysis. Water Sci Technol. 2004;50(9):169–75.
Toor SS, Rosendahl L, Rudolf A. Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy. 2011;36(5):2328–42.
Kruse A. Hydrothermal biomass gasification. J Supercrit Fluids. 2009;47(3):391–9.
Libra JA, Ro KS, Kammann C, et al. Kern, Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels. 2011;2:71–106.
Uchimiya M, Hiradate S. Pyrolysis temperature-dependent changes in dissolved phosphorus speciation of plant and manure biochars. J Agric Food Chem. 2014;62(8):1802–9.
Xu G, Zhang Y, Shao H, et al. Pyrolysis temperature affects phosphorus transformation in biochar: chemical fractionation and 31P NMR analysis. Sci Total Environ. 2016;569–570:65–72.
Meng X, Huang Q, Gao H, et al. Improved utilization of phosphorous from sewage sludge (as fertilizer) after treatment by low-temperature combustion. Waste Manag. 2018;80:349–58.
Uchimiya M, Hiradate S, Antal MJ. Dissolved phosphorus speciation of flash carbonization, slow pyrolysis, and fast pyrolysis biochars. ACS Sustain Chem Eng. 2015;3(7):1642–9.
Bläsing M, Zini M, Müller MJE. Influence of feedstock on the release of potassium, sodium, chlorine, sulfur, and phosphorus species during gasification of wood and biomass shells. Energy Fuels. 2013;2013(27):1439–45.
Bourgel C, Véron E, Poirier J, et al. Behavior of phosphorus and other inorganics during the gasification of sewage sludge. Energy Fuels. 2011;25(12):5707–17.
Qian TT, Li DC, Jiang H. Thermochemical Behavior of Tris(2-Butoxyethyl) Phosphate (TBEP) during Co-pyrolysis with Biomass. Environ Sci Technol. 2014;48(18):10734–42.
Liu J, Yang J, Cade-Menun BJ, et al. Complementary phosphorus speciation in agricultural soils by sequential fractionation, solution P nuclear magnetic resonance, and phosphorus K-edge X-ray absorption near-edge structure spectroscopy. J Environ Qual. 2013;42:1763–70.
Nanzer S, Oberson A, Huthwelker T, et al. The molecular environment of phosphorus in sewage sludge ash: implications for bioavailability. J Environ Qual. 2014;43(3):1050.
Qian TT, Jiang H. Migration of phosphorus in sewage sludge during different thermal treatment processes. ACS Sustain Chem Eng. 2014;2(6):1411–9.
Wisawapipat W, Charoensri K, Runglerttrakoolchai J. Solid-phase speciation and solubility of phosphorus in an acid sulfate paddy soil during soil reduction and reoxidation as affected by oil palm ash and biochar. J Agric Food Chem. 2017;65(4):704–10.
Huang R, Tang Y. Evolution of phosphorus complexation and mineralogy during (hydro)thermal treatments of activated and anaerobically digested sludge: Insights from sequential extraction and P K-edge XANES. Water Res. 2016;100:439–47.
Kleemann R, Chenoweth J, Clift R, et al. Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolysed sewage sludge char (PSSC). Waste Manag. 2017;60:201–10.
Adnan A, Karin HA, Gerhard S, et al. Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp. lycopersici. Front Plant Sci. 2015;6:529.
Frišták V, Soja G. Effect of wood-based biochar and sewage sludge amendments for soil phosphorus availability. Nova Biotechmologica et Chimica. 2015;14(1):104–15. https://doi.org/10.1515/nbec-2015-0020.
Karer J, Wawra A, Zehetner F, et al. Effects of biochars and compost mixtures and inorganic additives on immobilisation of heavy metals in contaminated soils. Water Air Soil Pollut. 2015;226(10):342.
Bridgwater AV. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenerg. 2012;38:68–94.
Funke A, Ziegler F. Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin. 2010;4(2):160–77.
Zumbühl K. Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: a feasibility study on a laboratory scale. Fuels. 2013;27:454–60.
Maria-Magdalena T, Markus AJCSR. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem Soc Rev. 2009;39:103–16.
Titirici MM, White RJ, Falco C, et al. Black perspectives for a green future: hydrothermal carbons for environment protection and energy storage. Energy Environ Sci. 2012;5(5):6796.
Valdez PJ, Nelson MC, Wang HY, et al. Hydrothermal liquefaction of Nannochloropsis sp.: systematic study of process variables and analysis of the product fractions. Biomass Bioenerg. 2012;46:317–31.
Pavlovic I, Knez Z, Skerget, Mojca A. Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: a review of fundamentals, mechanisms and state of research. J Agric Food Chem 2013;61(34):8003–8025.
Funke A, Ziegler F. Heat of reaction measurements for hydrothermal carbonization of biomass. Bioresour Technol. 2011;102(16):7595–8.
Vom Eyser C, Palmu K, Otterpohl R, et al. Determination of pharmaceuticals in sewage sludge and biochar from hydrothermal carbonization using different quantification approaches and matrix effect studies. Anal Bioanal Chem. 2015;407(3):821–30.
Vom Eyser C, Palmu K, Schmidt TC, et al. Pharmaceutical load in sewage sludge and biochar produced by hydrothermal carbonization. Sci Total Environ. 2015;537:180–6.
Heilmann SM, Jader LR, Harned LA, et al. Hydrothermal carbonization of microalgae II. Fatty acid, char, and algal nutrient products. Appl Energy. 2011;88(10):3286–90.
Heilmann SM, Jader LR, Sadowsky MJ, et al. Hydrothermal carbonization of distiller’s grains. Biomass Bioenergy. 2011;35(7):2526–33.
Heilmann SM, Molde JS, Timler JG, et al. Phosphorus reclamation through hydrothermal carbonization of animal manures. Environ Sci Technol. 2014;48:10323–9.
Zhu W, Xu ZR, Li L, et al. The behavior of phosphorus in sub- and super-critical water gasification of sewage sludge. Chem Eng J. 2011;171(1):190–6.
Shi W, Liu C, Ding D, et al. Immobilization of heavy metals in sewage sludge by using subcritical water technology. Biores Technol. 2013;137:18–24.
Bright DA, Healey N. Contaminant risks from biosolids land application—contemporary organic contaminant levels in digested sewage sludge from five treatment plants in Greater Vancouver, British Columbia. Environ Pollut. 2003;126(1):39–49.
Cordell D, Rosemarin A, Schrder JJ, et al. Towards global phosphorus security: a systems framework for phosphorus recovery and reuse options. Chemosphere. 2011;84(6):747–58.
Rajasulochana P, Preethy V. Comparison on efficiency of various techniques in treatment of waste and sewage water—a comprehensive review. Resour Effic Technol. 2016;2(4):175–84.
Sartorius C, von Horn J, Tettenborn F. Tettenborn, Phosphorus recovery from wastewater–expert survey on present use and future potential. Water Environ Res. 2012;84:313–22.
Egle L, Rechberger H, Zessner M. Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour Conserv Recycl. 2015;105:S0921344915300938.
Rittmann BE, Brooke M, Paul W, et al. Capturing the lost phosphorus. Chemosphere. 2011;84(6):846–53.
Hunger S, Sims JT, Sparks DL. How accurate is the assessment of phosphorus pools in poultry litter by sequential extraction? J Environ Qual. 2005;34(1):382.
Donatello S, Cheeseman CR. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): a review. Waste Manag. 2013;33(11):2328–40.
Donatello S, Tong D, Cheeseman C. Production of technical grade phosphoric acid from incinerator sewage sludge ash (ISSA). Waste Manag. 2010;30(8):1634–42.
Fang L, Li JS, Donatello S, et al. Recovery of phosphorus from incinerated sewage sludge ash by combined two-step extraction and selective precipitation. Chem Eng J. 2018;348:74–83.
Fang L, Li JS, Guo MZ, et al. Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere. 2018;193:278–87.
Franz M. Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 2007;28(10):1809–18.
Li JS, Chen Z, Wang QM, et al. Change in re-use value of incinerated sewage sludge ash due to chemical extraction of phosphorus. Waste Manag. 2018;74:72.
Stasta P, Boran J, Bebar L, et al. Thermal processing of sewage sludge. Appl Therm Eng. 2006;26(13):1420–6.
Li Y, Cui R, Yang T, et al. Distribution characteristics of heavy metals in different size fly ash from a sewage sludge circulating fluidized bed incinerator. Energy Fuels. 2017;31(2):2044–51.
Petzet S, Peplinski B, Bodkhe SY, et al. Recovery of phosphorus and aluminium from sewage sludge ash by a new wet chemical elution process (SESAL-Phos-recovery process). Water Sci Technol. 2011;64(3):693.
Stark K, Plaza E, Hultman B. Phosphorus release from ash, dried sludge and sludge residue from supercritical water oxidation by acid or base. Chemosphere. 2006;62(5):832.
Levlin E, Hultman B. Phosphorus recovery from sewage sludge-Ideas for further studies to improve leaching. Stockholm, Sweden: Department of Land and Water Resources Engineering; 2004. p. 61–77.
Li M, Tang Y, Lu XY, et al. Phosphorus speciation in sewage sludge and the sludge-derived biochar by a combination of experimental methods and theoretical simulation. Water Res. 2018;140:90.
Ye Y, Ngo HH, Guo W, et al. Insight into chemical phosphate recovery from municipal wastewater. Sci Total Environ. 2017;576:159–71.
Antonini S, Arias MA, Eichert T, et al. Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere. 2012;89(10):1202–10.
César P, Rafael S, Cristina C, et al. Greenhouse evaluation of struvite and sludges from municipal wastewater treatment works as phosphorus sources for plants. J Agric Food Chem. 2007;55(20):8206–12.
Ryu HD, Lim CS, Kang MK, Lee S. Evaluation of struvite obtained from semiconductor wastewater as a fertilizer in cultivating Chinese cabbage. J Hazard Mater. 2012;221:248–55.
Yu Y, Lei Z, Yuan T, et al. Simultaneous phosphorus and nitrogen recovery from anaerobically digested sludge using a hybrid system coupling hydrothermal pretreatment with MAP precipitation. Biores Technol. 2017;243:634.
Kataki S, West H, Clarke M, et al. Phosphorus recovery as struvite: recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour Conserv Recyc. 2016;107:142–56.
Shu L, Schneider P, Jegatheesan V, et al. An economic evaluation of phosphorus recovery as struvite from digester supernatant. Biores Technol. 2006;97(17):2211–6.
Yan H, Shih K. Effects of calcium and ferric ions on struvite precipitation: a new assessment based on quantitative X-ray diffraction analysis. Water Res. 2016;95:310–8.
Corre KSL, Valsami-Jones E, Hobbs P, et al. Impact of calcium on struvite crystal size, shape and purity. J Cryst Growth. 2005;283(3–4):514–22.
Muryanto S, Bayuseno AP. Influence of Cu2+ and Zn2+ as additives on crystallization kinetics and morphology of struvite. Powder Technol. 2014;253:602–7.
Xu H, He P, Gu W, et al. Recovery of phosphorus as struvite from sewage sludge ash. J Environ Sci. 2012;24:1533–8.
Pastor L, Marti N, Bouzas A, et al. Sewage sludge management for phosphorus recovery as struvite in EBPR wastewater treatment plants. Biores Technol. 2008;99(11):4817–24.
Ohbuchi A, Sakamoto J, Kitano M, et al. X-ray fluorescence analysis of sludge ash from sewage disposal plant. X-Ray Spectrom. 2008;37(5):544–50.
Adam C, Kley G. Simon, thermal treatment of municipal sewage sludge aiming at marketable P-fertilisers. Mater Trans. 2007;48(12):3056–61.
Vogel C, Adam C. Heavy metal removal from sewage sludge ash by thermochemical treatment with gaseous hydrochloric acid. Environ Sci Technol. 2011;45(17):7445–50.
Fraissler G, JöLler M, Mattenberger H, et al. Thermodynamic equilibrium calculations concerning the removal of heavy metals from sewage sludge ash by chlorination. Chem Eng Process. 2009;48(1):152–64.
Havukainen J, Mai TN, Hermann L, et al. Linnanen, Potential of phosphorus recovery from sewage sludge and manure ash by thermochemical treatment. Waste Manag. 2016;49:221–9.
Mattenberger H, Fraissler G, Brunner T, Herk P, Hermann L, Obernberger I. Sewage sludge ash to phosphorus fertiliser: variables influencing heavy metal removal during thermochemical treatment. Waste Manag. 2008;28:2709–22.
Kleemann R, Morse S. Sustainable phosphorus management—a global transdisciplinary roadmap In: Scholz RW, Roy A, Brand FS, et al. ISBN: 978-94-007-7249-6. Ecol Econ. 2015;114(3):245–246.
Adam C, Michaelis M, Kley G, et al. Reaction sequences in the thermochemical treatment of sewage sludge ashes revealed by X-ray powder diffraction—a contribution to the European project SUSAN. Zeitschrift Für Kristallographie Supplements. 2009;2009(30):459–64.
Vogel C, Adam C, Peplinski B, et al. Chemical reactions during the preparation of P and NPK fertilizers from thermochemically treated sewage sludge ashes. Soil Sci Plant Nutr. 2010;56(4):627–35.
Jan S, Burkhard P, Christian AJWM. Thermochemical treatment of sewage sludge ash with sodium salt additives for phosphorus fertilizer production—analysis of underlying chemical reactions. Waste Manag. 2015;45:385–90.
Vogel C, Krüger O, Adam C. Thermochemical treatment of sewage sludge ash with sodium additives under reducing conditions analyzed by thermogravimetry. J Therm Anal Calorim. 2016;123(2):1045–51.
Steckenmesser D, Vogel C, Adam C, et al. Effect of various types of thermochemical processing of sewage sludges on phosphorus speciation, solubility, and fertilization performance. Waste Manag. 2017;62:194–203.
Acar YB, Alshawabkeh AN. Principles of electrokinetic remediation. Environ Sci Technol. 1993;27:2638–47.
Viader RP, Jensen PE, Ottosen LM, et al. Sequential electrodialytic recovery of phosphorus from low-temperature gasification ashes of chemically precipitated sewage sludge. Waste Manag. 2016;60:211–8.
Jakobsen MR, Fritt-Rasmussen J, Nielsen S, et al. Electrodialytic removal of cadmium from wastewater sludge. J Hazard Mater. 2004;106(2–3):127–32.
Ottosen LM, Jensen PE, Kirkelund GM. Electrodialytic separation of phosphorus and heavy metals from two types of sewage sludge ash. Sep Sci Technol. 2014;49(12):1910–20.
Ottosen LM, Pedersen AJ, Hansen HK, et al. Screening the possibility for removing cadmium and other heavy metals from wastewater sludge and bio-ashes by an electrodialytic method. Electrochim Acta. 2007;52(10):3420–6.
Nystroem GM, Ottosen LM, Villumsen A. Acidification of harbor sediment and removal of heavy metals induced by water splitting in electrodialytic remediation. Sep Sci Technol. 2005;40(11):2245–64.
Ebbers B, Ottosen LM, Jensen PE. Comparison of two different electrodialytic cells for separation of phosphorus and heavy metals from sewage sludge ash. Chemosphere. 2015;125:122–9.
Viader RP, Jensen PE, Ottosen LM, et al. Electrodialytic extraction of phosphorus from ash of low-temperature gasification of sewage sludge. Electrochim Acta. 2015;181:100–8.
Guedes P, Mateus EP, Almeida J, et al. Electrodialytic treatment of sewage sludge: current intensity influence on phosphorus recovery and organic contaminants removal. Chem Eng J. 2016;306:1058–66.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51621005) and Key research and development plan of the Yunnan Science and Technology Department (2018IB026). The authors declare no competing financial interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, X., Huang, Q., Xu, J. et al. A review of phosphorus recovery from different thermal treatment products of sewage sludge. Waste Dispos. Sustain. Energy 1, 99–115 (2019). https://doi.org/10.1007/s42768-019-00007-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-019-00007-x