Abstract
Two interrelated problems exist: the non-renewability of phosphate rock as a resource and the excess phosphate in the water system lead to eutrophication. Removal and recovery of phosphorus (P) from waste streams at wastewater treatment plants (WWTPs) is one of the promising solutions. This paper reviews strategies for P recovery from waste streams in WWTPs are reviewed, and the main P recovery processes were broken down into three parts: enrichment, extraction, and crystallization. On this basis, the present P recovery technology was summarized and compared. The choice of P recovery technology depends on the process of sewage treatment and sludge treatment. Most P recovery processes can meet the financial requirements since the recent surge in phosphate rock prices. The safety requirements of P recovery products add a high cost to toxic substance removal, so it is necessary to control the discharge of toxic substances such as heavy metals and persistent organic pollutants from the source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is an essential element for life’s survival and P fertilizer has made a significant breakthrough in agriculture. Ensuring the long-term availability and accessibility of P sources is critical to the future of humanity. P occurs in nature mainly in the form of apatite and is released from rock into the soil, recycled by plants and animals, and may also be lost due to soil leaching and erosion into streams and rivers (Azam et al. 2019). Mining and agriculture have accelerated this loss, and large amounts of phosphate fertilizers are used for agriculture and livestock, entering natural water bodies through rainfall, the food chain, and eventually the sea. When P reaches the ocean and reacts with other seawater chemicals, it becomes insoluble. This insoluble P sinks to the seabed and is lost. Excessive P in natural water bodies can lead to the explosive growth of algae, which affects the ecological function of water bodies, known as eutrophication.
Currently, the world’s reserves of natural P ore are available for approximately 50–100 years (Cordell et al. 2009). Recycling lost P resources will partly alleviate the P crisis. An average of 21 million tons of phosphate rock is mined yearly and goes into human activities as fertilizer and other industrial products. About 15% of this P goes into wastewater treatment plants (WWTPs) (Venkiteshwaran et al. 2018). Compared with dispersed livestock manure and non-point agricultural sources, municipal wastewater has a complete collection network and centralized treatment facilities, which makes it easier to recover phosphorus from it and is the most promising of all the available sources of recovery of P in the waste sector (Rahman et al. 2019). P recovery from WWTPs’ waste streams can alleviate the P crisis and effectively reduce the emission P concentration and the contribution of WWTPs’ discharge to eutrophication.
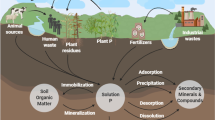
Many technologies have been developed to recover P from WWTPs waste streams (Cordell et al. 2011), including some full-scale technologies (Diaz-Elsayed et al. 2019). A schematic overview of P recovery processes is shown in Fig. 1. Although there are many technologies, most are based on the same principles and follow the basic steps of P recovery from wastewater, including enrichment, extraction, and recovery as bioavailable products. We review the last decade of research aimed at P recovery based on WWTPs. The classification was carried out from recovery location, enrichment, extraction methods, recovery products, scale, etc. The network diagram is shown in Fig. 2. Currently, the primary P enrichment method is the excessive uptake of P by phosphorus-accumulating organisms (PAOs). Of course, there are other physical, chemical, and biological P enrichment methods, such as membrane separation processes (Li et al. 2021b), adsorption (Bacelo et al. 2020), and microalgae (Roy 2017). The P recovery processes can be divided into three ways according to the recovery location: direct recovery from sewage, recovery from sewage sludge (SS), and recovery from sewage sludge ash (SSA) (Cieslik and Konieczka 2017; Donatello and Cheeseman 2013; Zhang et al. 2022). Different P crystallization products can be obtained by adding various metal salts, including struvite generated by magnesium salt, apatite induced by calcium salt, and vivianite caused by iron salt. Most P recovery is ultimately achieved in the form of Mg, Ca, or Fe crystallization products, although some P recovery is conducted in P-rich biochar.

Adapted from Jupp et al. (2021)
Schematic overview of P recovery processes.
In this paper, the P forms in different waste streams of WWTPs are introduced, and the enrichment, extraction, and recovery technologies into bioavailable products are reviewed. The principle, progress, and limitations of the technologies are introduced, and the possible obstacles to P recovery were evaluated.
P in sewage, sewage sludge, and sewage sludge ash
P in sewage
P exists in many forms in sewage and changes with the sewage treatment processes. The chemical form of P affects its removal and recovery. P can be divided into particulate and dissolved states according to physical properties. According to the chemical structure, P can be divided into inorganic P and organic P. And according to bioavailability, P can be divided into reactive P (RP) and non-reactive P (NRP). In practice, separating inorganic P and organic P in a particulate state is difficult. Therefore, P in sewage is generally divided into particulate phosphorus (pP), dissolved inorganic phosphorus (DIP), and dissolved organic phosphorus (DOP).
P that is physically transported as a particle by adsorption onto hydroxides and clay particles is called pP. In nature, the P transported in physical form accounts for about 95% of the total P, while in sewage, the proportion of pP is low, accounting for about 16 ± 11% of the TP (Venkiteshwaran et al. 2018). In the general process of sewage treatment, pP can be quickly and thoroughly removed from the sewage stream in the primary treatment (Dueñas et al. 2003) and transferred into the sludge.
DIP mainly exists in water in orthophosphate or polyphosphate, which is also the main P form in sewage. In an aqueous solution, orthophosphates may exist in PO43−, HPO42−, H2PO4−, and H3PO4, and each part’s relative proportion (i.e., distribution coefficient) varies with pH. Polyphosphates in sewage come from various sources, such as detergents, scale inhibitors, and food additives. In sewage, polyphosphates are hydrolyzed to orthophosphates, a process accelerated by organisms and enzymes.
DOP in sewage refers to P combined with organic matter. It mainly comes from the decomposition of organisms and the use of pesticides, flame retardants, and surfactants. Components of DOP derived from organic decomposition include phosphoprotein, nucleoprotein, phospholipid, and carbohydrate phosphate (ester). Organophosphorus can be divided into biodegradable parts and non-biodegradable parts. Most biodegradable organophosphorus contain a P–O–C bond, common in natural organophosphorus compounds, while synthetic compounds containing a P–C bond, such as phosphonates, are difficult to biodegrade. Due to the extensive use of phosphonates, their transformation and fate in the sewage treatment process and water environment cannot be ignored.
P in sewage sludge
The characteristics of SS are highly dependent on the technology and location of the WWTP (Wollmann and Möller 2018). Decisions made within a WWTP about physical, biological, or chemical separation techniques for partitioning solid from liquid have downstream effects on SS characteristics (Ma and Rosen 2021). Yu et al. (2021) reviewed the species, fractions, and characterization of P in SS from the perspective of P recovery and compared waste-activated sludge (WAS) and chemically enhanced primary sludge (CEPS). The P form distribution is shown in Fig. 3. In WAS, polyphosphate is the main form of P, accounting for about 30–80%, while other inorganic P and organic P account for 10–30%, respectively. In iron-based CEPS, 83 ~ 96% of the TP is IP, of which 68 ~ 73% is chemically precipitated P. 45 ~ 59% of the chemically precipitated metal oxides and metal hydroxide adsorb P, and 20 ~ 52% is Fe–P in the form of FePO4.
P in sewage sludge ash
The characteristics of SSA depend on the source SS. In addition, different incineration technologies have unique physical mechanisms and incineration temperatures that will change SSA’s physical and chemical properties and may impact the quality of the SSA products produced.
Phosphate does not volatilize during the drying or incineration of sludge. Instead, phosphate is concentrated in the SSA as whitlockite type, tri-calcium phosphates (Ca3(PO4)2) (Donatello and Cheeseman 2013). Sometimes Ca2+ is partially replaced by Mg2+, Fe3+, or Al3+ (Adam et al. 2009; Donatello 2009). Based on previous research data, the proportion of P in SSA ranged from 1.0 to 14.4%, with an average ratio of 7.6% (n = 101). The primary forms of P are dolomite (Ca9Al(PO4)7), aluminum phosphate (AlPO4), and iron phosphate (FePO4). Nanzer et al. (2014) conducted a series of analyses, including X-ray powder diffraction, solid-state 31P direct-polarization magic-angle spinning nuclear magnetic resonance, and X-ray absorption near-edge structure, to determine the direct P speciation in four SSAs. Their Analysis found that overall speciation depended on the calcium-P mole ratio (Ca/P). Specifically, if Ca/P > 2, P is mainly bound to the apatite-like structure. In contrast, P is bound to aluminum phosphate, limestone, and apatite-like structures.
Enrichment methods
Due to the lack of specific P recovery policies and the demand for P recovery products, the main driving force of P enrichment in wastewater is to remove P from wastewater to meet the increasingly strict P discharge standards. Therefore, from the perspective of feasibility, the full-scale P recovery process is established based on mature P removal processes (EBPR and chemical precipitation P removal). However, some emerging laboratory and pilot-scale processes such as sorption, membrane separation, and microalgae-based processes have shown good P removal and enrichment effects, but there are still some obstacles to overcome before full-scale application.
Enhanced biological phosphorus removal
At present, the mainstream P removal method is Enhanced biological P removal (EBPR). The EBPR process relies on a specific group of bacteria, called polyphosphate accumulating organisms (PAOs), to accumulate phosphate from wastewater more than its growth requirements under alternating anaerobic and aerobic/anoxic conditions to complete the separation and enrichment of P from wastewater. The P enrichment mechanism is shown in Fig. 4. In this way, 90% of the P in sewage can be removed, the P content of the resulting sludge is 5–7%, and the net P removal of the whole system is achieved by discharging the excess sludge. Through a complete aerobic (anoxic) and anaerobic process, EBPR can obtain a higher concentration of P in the anaerobic supernatant, thus completing the enrichment of P in the liquid phase, which provides a pre-stage for a series of mainstream P-recovery strategies (Zhang et al. 2022).

Modified from Yuan et al. (2012)
Schematic diagrams of the PAO metabolism.
Chemical precipitation
Chemical precipitation is the earliest method to remove P from sewage. In response to eutrophication, chemical precipitation has been widely used in Switzerland since the 1950s to remove P from wastewater (Morse et al. 1998). At present this simple method is widely used in most countries of the world. Chemical precipitation is essentially a physicochemical process involving the addition of a bivalent or trivalent metal salt to sewage resulting in the precipitation of insoluble metallic phosphate. The most suitable metals are iron and aluminum, added in the form of chloride or sulfate.
Although chemical precipitation is simple and does not require additional processes, chemically precipitated P is difficult to separate from sludge. And phosphates, in the form of iron phosphate or aluminum phosphate, are difficult to use in agriculture (iron phosphate is insoluble in acidic conditions and aluminum phosphate is toxic to plant roots). Therefore, many alternative P recovery technologies have been developed. However, the latest study found that vivianite would form in iron-based P removal sludge under anaerobic conditions (Wilfert et al. 2016), which brings a new way for P recovery, which will be introduced in the “Vivianite” section.
Adsorption
Adsorption is generally considered an effective and attractive treatment process because of its ease of operation, simplicity of design, ability to remove P at very low concentrations, and minimal waste production (Loganathan et al. 2014). There are five main mechanisms of phosphate adsorption: (a) ion exchange (outer-sphere surface complexation), (b) ligand exchange (inner-sphere surface complexation), (c) hydrogen bonding, (d) surface precipitation, and (e) diffusion into the interior structure of the sorbent (Loganathan et al. 2014). The predominant type of mechanism operating in an adsorption process depends on the physical and chemical characteristics of the adsorbents and the environmental/operational conditions. Adsorbents can be divided into four categories: inorganic adsorbents, organic adsorbents, industrial by-products, and biological waste. Applications of adsorption on P recovery can be divided into two ways: adsorbed by the renewable adsorbents then desorption to form P-rich solutions, or by the low-cost adsorbents then direct land application.
Among the adsorbents, metal oxides and hydroxides generally have a high phosphate adsorption capacity. Thermal treatment and acid treatment can enhance the adsorption capacity of some adsorbents, such as layered double hydroxides (LDHs) (Cheng et al. 2010), Fe, Al oxides and hydroxides, and red mud (Bhatnagar et al. 2011). Surface modification of adsorbents by grafting metal or organic groups will also improve the adsorption capacity of adsorbents (Bacelo et al. 2020). However, the modification of adsorbents increases the costs. These materials with high adsorption properties are promising in the case of very low P emission concentration requirements.
An adsorbent suitable for P recovery should not only have a high phosphate adsorption capacity and cost-effectiveness but also be easy to desorb phosphate and be efficiently regenerated and used for a long time. Desorption of phosphate is the leaching of adsorbed phosphate with acid, base, or salt. Phosphate in desorption solutions can be recovered in the form of calcium phosphate compounds by adding CaCl2 or Ca(OH)2 and used as fertilizers or feedstock for fertilizers.
Although many industrial by-products and organic wastes have low absorbability, they are attractive because of their low cost (mainly transport costs). The low absorbability can be overcome with the large usage of these materials. These materials are recommended for use in areas where they are locally available to reduce transport costs. Low-cost adsorbents with low toxic substance concentrations can be used directly after phosphate adsorption.
Membrane separation processes
The membrane separation process is considered a promising P enrichment method because it is selective for pollutants and can be targeted to separate specific toxic substances (Gerardo et al. 2015). Compared to the other P removal and enrichment processes mentioned earlier, the membrane separation process is more stable in operation, more selective for P, and produces less waste (Azam et al. 2019; Rittmann et al. 2011). According to the driving force, the membrane separation processes can be divided into pressure-driven, osmotic pressure-driven, and electric-driven. In the P recovery process, membrane separation is first applied in the separation of P recovery products, such as the use of microfiltration and ultrafiltration for the separation of P recovery products generated by chemical precipitation (Disha et al. 2012; Gerardo et al. 2013). Membrane separation processes including nanofiltration, forward osmosis, membrane distillation, and electrodialysis are also used to separate the liquid phase and sludge phase after P extraction from sludge. Relevant studies are shown in Table 1.
Nanofiltration, reverse osmosis and other membrane separation processes with high phosphate retention rates have also been extensively studied (Li et al. 2021b). However, due to the serious membrane fouling, these pressure-driven membrane separation processes cannot adapt to the complexity of wastewater. Emerging technologies such as forward osmosis and electrodialysis have lower membrane fouling tendencies and are expected to help solve this problem. In particular, the forward osmosis process can be used in the MBR process, which has better pre-preparation. Xie et al. (2014) coupled forward osmosis and membrane distillation processes for P recovery from sludge. OMBR P recovery system using forward osmosis combined with microfiltration also shows its potential (Qiu and Ting 2014; Qiu et al. 2016a, b). Meanwhile, nanofiltration, electrodialysis, and other membrane separation processes can complete the separation of heavy metals and organic pollutants and improve the safety of P recovery products (Li et al. 2021b). However, the regeneration of the draw solution in the forward osmosis process is a problem to be solved. For electrodialysis, high energy consumption and long separation time are the main factors that limit its large-scale application.
Extraction methods
After enrichment by the processes mentioned above, P-rich liquid phases (anaerobic supernatant, anaerobic sludge digestion, P-rich desorption solutions, and P-rich solutions formed by membrane separation, etc.) or solid phases (waste activated sludge, chemically precipitated sludge, P-rich adsorbents, etc.) are usually included. Some P-rich solutions and solids can be used directly as fertilizer, but most P-rich SS and SSA need to be extracted for recovery.
Extracted from sewage sludge
As mentioned earlier, in existing wastewater treatment plants, approximately 90% of P from influent water enters the sludge. Most of the P in the sludge is stored in adenosine triphosphate by phospho-accumulating bacteria through the EBPR process, and some of the P is precipitated into the sludge by aluminum salts or iron salts in the process of chemical P removal. The concentration of total P in sludge depends on the inlet water quality and the method used in the sewage treatment plant. Sludge contains many pathogenic bacteria, parasite eggs, heavy metals, and some toxic and harmful organic substances that are difficult to degrade, which limits its direct land use (Jupp et al. 2021). As a result, in Germany, the Netherlands, Switzerland, and other countries, the agricultural use of sludge has been banned by legislation, so P needs to be extracted from sludge and its leachate. For EBPR sludge, the supernatant with a high concentration of P can be obtained by anaerobic digestion, and then P can be recovered by crystallization. In addition, ways to recover P in the form of vivianite by adding iron salts to sludge are also being established. Other methods of extracting P directly from sludge are mainly divided into wet-chemical and thermochemical treatments.
Wet-chemicaltreatment
Wet-chemical processes use acidification or alkalization to treat sludge and enhance P release. The extractants used for P extraction in SS are shown in Table 2. P release can be improved by pretreatment methods such as ultrasound and microwave but at an additional cost. Treating sludge with inorganic acid will release heavy metals synchronously, so measures should be taken to prevent heavy metals from entering the recycled products during P recovery. For example, the polarity of the nanofiltration membrane is used to exclude cations through phosphoric acid ions, and the phosphoric acid and heavy metals are separated by electrodialysis (Guedes et al. 2014).
Thermochemical treatment
Thermochemical treatment is a series of sludge treatment methods utilizing high temperature, mainly including the hydrothermal method (Shi et al. 2019; Yu et al. 2017), pyrolysis (Tang et al. 2018), and gasification (Acelas et al. 2014). The main difference between these methods lies in the temperature and oxygen content used. Thermochemical treatment can simultaneously remove pathogens and organic matter in sludge, but inorganic metal and non-metal pollutants are still retained. Therefore, heavy metal pollutants in P recovery products obtained after pyrolysis and sludge gasification are concentrated, limiting its application prospects. It is more suitable for treating phospho-rich wastes such as kitchen waste and livestock manure with less heavy metal pollutants.
Physical approaches enhance the extraction of P from sludge
In addition to the two types of chemical treatment, physical approaches can also be used to enhance the release of P from SS, including microwave, ultrasound, and ozonation, but additional costs are required. Examples of enhanced P extraction using physical approaches are shown in Table 3.
Extracted from sewage sludge ash
Incineration of SS can reduce solid volume by more than 90%. Phosphate is thermally stable and will not volatilize during sludge drying or incineration at 800–900℃ but is concentrated in SSA in the form of calcium phosphate (Mg2 + , Fe3 + , or Al3 + may partially replace Ca2+). The P content of SSA produced is between 9 and 13.1%. In addition, the incineration process will destroy organic matter and pathogens that interfere with P recovery. Therefore, P recovery from SSA has excellent advantages. Its primary disadvantage is that the construction of standard sludge incineration facilities requires a significant investment, which has high requirements for the scale and centralization of sludge treatment, so it may not be suitable for decentralized and small sewage treatment facilities. Since most heavy metals do not volatilize during sludge incineration, the content of heavy metals in ash is higher. Meanwhile, due to the high content of Fe–P and Al–P in SSA, it is restricted from being directly used as fertilizer. Therefore, it is necessary to extract P from SSA to remove heavy metals and recover P in a bioavailable form.
Wet-chemical treatment
Like the wet-chemical treatment of SS, the wet-chemical treatment of SSA generally leaches P by organic or inorganic acids, and studies on SSA with high aluminum and P content are also carried out by alternating acid–base leaching (Petzet et al. 2011). The extractants used for P extraction in SSA are shown in Table 4. The synchronous release of heavy metals usually accompanies the process of extracting P in SSA, and P and heavy metals can be separated by electrodialysis (Guedes et al. 2014) and ion exchange (Xu et al. 2012) and adsorption (Li et al. 2021a). Other studies (Fang et al. 2018b; Gorazda et al. 2012) reduced the release of heavy metals by changing the type and concentration of acid, but the P release efficiency also decreased. Besides, the P-rich solution extracted by wet-chemical treatment will produce waste acid after P recovery, which needs further treatment and disposal. Therefore, the P leaching process with low environmental impact can also be considered. Söldner et al. (2019) used deep eutectic solvents (DESs) based on natural products as extraction media for P from incinerated sewage sludge ash. Semerci et al. (2019) carried out batch bioleaching experiments with Sulfur oxidizing bacteria (SOB) to optimize the process in terms of P dissolution. Generally, the P-rich solution extracted by the wet-chemical method needs to recover P in the form of MAP or Ca–P by adjusting pH to alkaline. There have also been studies on the use of acid-resistant adsorbents to adsorb P in leaching solutions (Li et al. 2021a).
Thermochemical treatment
The focus of thermal-chemical treatment is to remove heavy metals from SSA and improve the bioavailability of P. At high temperatures of 900 ~ 2000℃, the chlorine donor can react with the heavy metal oxides to produce chlorides, which are easily gasified or liquefied, thus separating from the P remaining in the solid phase. This is because the chlorides of most heavy metals have lower melting and boiling points. The melting and boiling points of chlorides and oxides of primary heavy metals are shown in Table 5.
When the chlorine donor is MgCl2 or CaCl2, the conversion of Al–P and Fe–P to Mg–P or Ca–P in SSA can be promoted, and the bioavailability of SSA can be improved (Li et al. 2015; Nowak et al. 2012). This process can be carried out simultaneously with sludge incineration (Jeon and Kim 2018). In addition, adding Na/K salt during thermochemical treatment (Herzel et al. 2016; Stemann et al. 2015) or co-incineration sludge with biomass (Zhao et al. 2018) can also improve the bioavailability of SSA. After being treated by these methods, most of the P in SSA exists in Ca-Mg-P or Ca-Mg-P with Na and K, and the heavy metal content is low, which can be directly used as a slow-release fertilizer.
Crystallization methods
Except for P-rich biochar and treated SSA used directly as fertilizer, most P recovery configurations eventually precipitate P as phosphate by crystallization, both from the liquid phase and sludge. The primary product forms are struvite, hydroxyapatite, and vivianite.
Struvite
Struvite, chemically composed of magnesium ammonium phosphate (MAP, MgNH4PO4·6H2O), first came to the attention of the wastewater treatment industry for scaling in the pipes of WWTPs. Generally, MAP crystals are generated by adding MgCl2 and NaOH to the P-rich solution. The reaction process is shown in Eq. 1, where n is 0, 1, 2.
Therefore, the controlled recovery of struvite from sewage can help save maintenance costs for pipes and machinery while completing the recovery of ammonium from sewage. Its disadvantages are that the precipitation of MAP requires a higher pH value, and because of the large solubility product of MAP (Table 6), it has a higher requirement for P concentration, and the P recovery efficiency is relatively low. Struvite requires a higher concentration of ammonium in the solution, and the product is susceptible to Ca2+ and other impurities. Struvite can be used as a slow-release fertilizer.
Hydroxyapatite (calcium phosphates)
Calcium ions can form a variety of possible precipitates with phosphate groups in solution, the most stable form being hydroxyapatite (HAP, Ca5(PO4)3OH). In the process of P recovery with calcium salts, precursors such as amorphous calcium phosphate (ACP) are usually formed and then gradually transformed into HAP, which is the most thermodynamically stable. The chemical formula and solubility product of the main calcium phosphate are shown in Table 5. After aging, the main hydroxyapatite products can be used as slow-release fertilizers and as raw materials in the fertilizer industry. At present, some studies (Guo and Li 2020; Magrí et al. 2021) have shown that mineral cores composed of HAP will form in the interior of anammox granular sludge, which also provides a new idea of the P recovery process, but it is not within the scope of this paper.
Vivianite
Wilfert et al. (2016) found that vivianite (Fe3(PO4)2·8H2O) accounted for 10 to 30% of the total P in biological P removal sludge and 40 to 50% in iron-based P removal sludge in WWTPs, which provided a new insight for P recovery. Vivianite is mainly formed by reduced Fe3+ induced by dissimilatory metal-reducing bacteria (DMRB) under reductive conditions (O’Loughlin et al. 2013; Rothe et al. 2016). Compared with MAP and HAP, the recovery of P in vivianite does not rely on EBPR processes and can be used in WWTPs with an iron-based P removal. Meanwhile, vivianite can exist in a wide range of pH, and the most suitable pH for precipitation is 6–8, which can fully adapt to the fluctuation of sewage pH. Vivianite formed in sludge can be separated by magnetic separation. Prot et al. (2019) used a lab-scale Jones magnetic separator to treat chemical P removal sludge and realized a concentration factor for P and iron of 2 to 3. The separation product contained 52–62% of vivianite. Wijdeveld et al. (2022) used a pilot-scale magnetic separator (capacity of 1.0 m3/h) to recover vivianite from the digestive sewage sludge and recovered more than 80% vivianite in three cycles. In the concentrated P products produced, the content of vivianite was up to 800 mg/g, and the range of P was 98 mg/g. However, vivianite is incompatible with the mainstream fertilizer industry, and further research is needed to market it. In addition, due to the potent inhibition of the vivianite formation by Al3+, this method is not recommended for treating aluminum-based P removal sludge.
Comprehensive discussion
Technology selection based on upstream processes
Overall, different sets of the P recovery process are decided by the objective of sewage treatment and wastewater treatment process. Although the concept of a water resource recovery factory (WRRF) has been put forward and conceptualized, the WRRF demonstration plant has been established all over the world, the target of the sewage treatment plant design is still on pollutant removal, and this situation will continue for decades. First, it is challenging to transform the sewage treatment plant process and train technical personnel. Second, further economic development and environmental protection level have different requirements for the function of WWTPs. Therefore, the research on P recovery technology based on the widely used sewage P removal process is currently mainstream. Although a wide range of technologies exists, the technology choices for a given WWTP will be limited by local policies and regulations, the characteristics of the effluent accepted, the sewage treatment processes (especially P removal processes) used in sludge treatment facilities, methods, etc. For example, in the typical case of WWTPs using the EBPR process, P recovery from the waterline or untreated P-rich sludge can only be considered because agricultural use of P-rich sludge is prohibited, and sludge disposal is mainly landfill or mixed with industrial sludge and incinerated for use as construction material. For WWTPs that use Fe salt to remove P, recovering vivianite from sludge is almost the only way to recover P, while for WWTPs that use Al salt to remove P, it is not easy to find a location for P recovery. Therefore, to achieve comprehensive P recovery from sewage, it is necessary to change the goal of sewage treatment fundamentally, consider P recovery, and do overall planning in the design of WWTPs and sludge treatment and disposal facilities. And that needs to be driven by policy.
Economic and environmental impact
The main reason that restricts the development of P recovery and the application of most emerging P recovery technologies is not economical enough. The cost of P recovery from WWTPs includes the price of P enrichment and separation. Due to the strict restrictions on wastewater P discharge in most areas, the cost of P enrichment is included in the P removal processes. Therefore, only the cost of P separation must be considered when P recovery is established based on biological P removal or chemical P removal processes currently widely used in WWTPs. But at present, the cost of P recovery is still higher than the value of its products, and even in most parts of the world, barriers to the entry of P recovery products into the market have not been cleared. The value chains are hard to build.
The cost of the P recovery process can be divided into construction cost, operation cost, and product storage and transport cost. The main cost of direct P recovery from sewage is in the chemicals (calcium salts, magnesium salts, and NaOH) added. In contrast, the recovery of P from SS or SSA requires additional cost of P extraction, such as acid or alkali required by wet-chemical processes and high temperature and chlorine donors required by thermochemical processes. However, even after EBPR enrichment, the concentration of P in the liquid phase is still low, with low P recovery efficiency and high treatment flux, resulting in higher facility construction and operation costs. The efficiency of P recovery from SSA is very high, and the transportation cost is the lowest. For the recovery of P from SS and SSA, the highest cost lies in the construction cost of pre-processing. The cost of P recovery from SS and SSA will be significantly reduced for the area with centralized sludge treatment and incineration facilities. Cost savings can also be achieved by using waste heat, renewable energy sources, and concentrated seawater as precipitators.
In addition to cost, the economics of P recovery processes also depend on the value and bioavailability of the recovered products. Magnesium ammonium phosphate can be directly used as a slow-release fertilizer, and calcium phosphate can be well-compatible with the mainstream fertilizer production industry. The use of bipolar membrane electrodialysis can be used to recover P in the form of phosphoric acid. Of course, some pretreatment or post-treatment to ensure the purity and safety of the product. At the same time, there are emerging technologies to recover P in the form of more valuable P compounds and simultaneous recovery of organic matter and heavy metals. These valuable by-products will further promote P recovery in the market. With the further improvement of the cost of P ore mining and the quality of P recovery products, the price gap between phosphate rock (PR) and P recovery products will be narrowed. It is estimated that when the price of PR increases to 100 $US/t, it is expected to achieve true self-sufficiency in wastewater P recovery. By the time the paper was written, the price had risen to $US/t (for Moroccan PR) as the international situation changed, so if a market for P recovery products could be created, most of the current P recovery processes would be profitable.
Toxic substances and safety
The safety of the P recovery process and products mainly lies in the migration of heavy metals, persistent organic pollutants, endocrine-disrupting chemicals, and other emerging contaminants from sewage and sludge to P recovery products. For the process of P recovery from the liquid phase, because the heavy metals, antibiotics, and other emerging contaminants in sewage are concentrated in the sludge, the concentration of toxic substances in the liquid phase is low. However, it may also be enriched through the adsorption and co-precipitation of seed and crystallization products. Since SS accumulates toxic substances in sewage, it is necessary to consider the transformation and fate of almost all inorganic and organic pollutants when recovering P from SS. Incineration removes virtually all organic impurities and concentrates inorganic contaminants such as heavy metals in SSA. However, due to the relatively single types of toxic substances, it is easier to develop targeted technologies for separation. The evaluation and realization of the safety of P recovery products need additional costs, even more than the cost of P recovery itself. Therefore, it is of great significance to limit and divert the discharge of toxic substances from the stage of sewage generation and discharge into the sewer line.
Conclusion
The P entering the WWTPs can be transformed and enriched to form a P-rich liquid phase and sludge phase. P-rich SS and SSA can be treated by wet-chemical or thermochemical methods and then recovered in the form of HAP, struvite, and vivianite by crystallization through the liquid and sludge. P-adsorbed biochar and treated SSA can also be used directly as fertilizer. The choice of P recovery technology depends on upstream processes and infrastructure design and construction. With the significant increase in PR price, most P recovery processes can now meet economic requirements if a P recovery market is established. The main limiting factor is that the safety requirement of P recovery products will lead to a considerable cost for pollutant removal. Therefore, it is necessary to control the discharge of toxic substances such as heavy metals and persistent organic pollutants from the source.
Data availability
All data generated or analyzed during this study are included in this published article. More detailed data is available from the corresponding author upon reasonable request.
References
Acelas NY, Lopez DP, Brilman DWF, Kersten SRA, Kootstra AMJ (2014) Supercritical water gasification of sewage sludge: gas production and phosphorus recovery. Biores Technol 174:167–175
Adam C, Peplinski B, Michaelis M, Kley G, Simon FG (2009) Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manage 29:1122–1128
Ansari AJ, Hai FI, Price WE, Nghiem LD (2016) Phosphorus recovery from digested sludge centrate using seawater-driven forward osmosis. Sep Purif Technol 163:1–7
Atienza-Martínez M, Gea G, Arauzo J, Kersten SRA, Kootstra AMJ (2014) Phosphorus recovery from sewage sludge char ash. Biomass Bioenergy 65:42–50
Azam HM, Alam ST, Hasan M, Yameogo DDS, Kannan AD, Rahman A, Kwon MJ (2019) Phosphorous in the environment: characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ Sci Pollut R 26:20183–20207
Bacelo H, Pintor AMA, Santos SCR, Boaventura RAR, Botelho CMS (2020) Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem Eng J 381:122566
Bhatnagar A, Vilar VJP, Botelho CMS, Boaventura RAR (2011) A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ Technol 32:231–249
Bi W, Li Y, Hu Y (2014) Recovery of phosphorus and nitrogen from alkaline hydrolysis supernatant of excess sludge by magnesium ammonium phosphate. Bioresource Technol 166:1–8
Biswas BK, Inoue K, Harada H, Ohto K, Kawakita H (2009) Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. J Environ Sci-China 21:1753–1760
Boniardi G, Turolla A, Fiameni L, Gelmi E, Malpei F, Bontempi E, Canziani R (2021) Assessment of a simple and replicable procedure for selective phosphorus recovery from sewage sludge ashes by wet chemical extraction and precipitation. Chemosphere 285:131476
Chang X, Zeng W, Li N, Li S, Peng Y (2019) Phosphorus recovery from freeze-microwave pretreated sludge supernatant by phosphate sedimentation. Environ Sci Pollut Res Int 26:12859–12866
Chen Y, Lin H, Yan W, Huang J, Wang G, Shen N (2019) Alkaline fermentation promotes organics and phosphorus recovery from polyaluminum chloride-enhanced primary sedimentation sludge. Bioresource Technol 294:122160
Cheng X, Huang X, Wang X, Sun D (2010) Influence of calcination on the adsorptive removal of phosphate by Zn–Al layered double hydroxides from excess sludge liquor. J Hazard Mater 177:516–523
Cieslik B, Konieczka P (2017) A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J Clean Prod 142:1728–1740
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: Global food security and food for thought. Glob Environ Chang 19:292–305
Cordell D, Rosemarin A, Schroder JJ, Smit AL (2011) Towards global phosphorus security: a systems framework for phosphorus recovery and reuse options. Chemosphere 84:747–758
Diaz-Elsayed N, Rezaei N, Guo TJ, Mohebbi S, Zhang Q (2019) Wastewater-based resource recovery technologies across scale: A review. Resour Conserv Recy 145:94–112
Disha VJ, Aravindakumar CT, Aravind UK (2012) Phosphate Recovery by High Flux Low Pressure Multilayer Membranes. Langmuir 28:12744–12752
Donatello S (2009) Characteristics of incinerated sewage sludge ashes: potential for phosphate extraction and re-use as a pozzolanic material in construction products. https://doi.org/10.13140/RG.2.2.33926.63040
Donatello S, Freeman-Pask A, Tyrer M, Cheeseman CR (2010) Effect of milling and acid washing on the pozzolanic activity of incinerator sewage sludge ash. Cement Concr Compos 32:54–61
Donatello S, Cheeseman CR (2013) Recycling and recovery routes for incinerated sewage sludge ash (ISSA): a review. Waste Manage 33:2328–2340
Dueñas JF, Alonso JR, Rey ÀF, Ferrer AS (2003) Characterisation of phosphorous forms in wastewater treatment plants. J Hazard Mater 97:193–205
Fang L, Li JS, Donatello S, Cheeseman CR, Wang QM, Poon CS, Tsang DCW (2018a) Recovery of phosphorus from incinerated sewage sludge ash by combined two-step extraction and selective precipitation. Chem Eng J 348:74–83
Fang L, Li JS, Guo MZ, Cheeseman CR, Tsang DCW, Donatello S, Poon CS (2018b) Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere 193:278–287
Fang Z, Liu F, Li Y, Li B, Yang T, Li R (2021) Influence of microwave-assisted pyrolysis parameters and additives on phosphorus speciation and transformation in phosphorus-enriched biochar derived from municipal sewage sludge. J Clean Prod 287:125–550. https://doi.org/10.1016/j.jclepro.2020.125550
Galey B, Gautier M, Kim B, Blanc D, Chatain V, Ducom G, Dumont N, Gourdon R (2022) Trace metal elements vaporization and phosphorus recovery during sewage sludge thermochemical treatment - A review. J Hazard Mater 424:127360
Geng YK, Wang Y, Pan XR, Sheng GP (2018) Electricity generation and in situ phosphate recovery from enhanced biological phosphorus removal sludge by electrodialysis membrane bioreactor. Bioresource Technol 247:471–476
Gerardo ML, Zacharof MP, Lovitt RW (2013) Strategies for the recovery of nutrients and metals from anaerobically digested dairy farm sludge using cross-flow microfiltration. Water Res 47:4833–4842
Gerardo ML, Lord AM, Lovitt RW (2015) An investigation of pH mediated extraction and precipitation of phosphorus from sludge using microfiltration: processing and costs. Separ Sci Technol 50:2155–2163
Gorazda K, Kowalski Z, Wzorek Z (2012) From sewage sludge ash to calcium phosphate fertilizers. Pol J Chem Technol 14:54–58
Gorazda K, Tarko B, Werle S, Wzorek Z (2018) Sewage sludge as a fuel and raw material for phosphorus recovery: combined process of gasification and P extraction. Waste Manag 73:404–415
Guedes P, Couto N, Ottosen LM, Ribeiro AB (2014) Phosphorus recovery from sewage sludge ash through an electrodialytic process. Waste Manage 34:886–892
Guedes P, Mateus EP, Almeida J, Ferreira AR, Couto N, Ribeiro AB (2016) Electrodialytic treatment of sewage sludge: current intensity influence on phosphorus recovery and organic contaminants removal. Chem Eng J 306:1058–1066
Guilayn F, Braak E, Piveteau S, Daumer ML (2017) Sequencing biological acidification of waste-activated sludge aiming to optimize phosphorus dissolution and recovery. Environ Technol 38:1399–1407
Guo Y, Li YY (2020) Hydroxyapatite crystallization-based phosphorus recovery coupling with the nitrogen removal through partial nitritation/anammox in a single reactor. Water Res 187:116444
He Z-W, Tang C-C, Wang L, Guo Z-C, Zhou A-J, Sun D, Liu W-Z, Wang A-J (2017) Transformation and release of phosphorus from waste activated sludge upon combined acid/alkaline treatment. Rsc Adv 7:35340–35345
Herzel H, Kruger O, Hermann L, Adam C (2016) Sewage sludge ash–a promising secondary phosphorus source for fertilizer production. Sci Total Environ 542:1136–1143
Hu D, Zhang C, Zhang Y (2021) Comparison of different pretreatment methods on phosphorus release and recovery as struvite from excess sludge. Environ Technol 1–9
Hu PS, Liu JY, Bao HX, Wu L, Jiang L, Zou LP, Wu YJ, Qian GR, Li YY (2018) Enhancing phosphorus release from waste activated sludge by combining high-voltage pulsed discharge pretreatment with anaerobic fermentation. J Clean Prod 196:1044–1051
Hu D, Zhang C, Zhang Y (2023) Comparison of different pretreatment methods on phosphorus release and recovery as struvite from excess sludge. Environ Technol 44(2):161–169. https://doi.org/10.1080/09593330.2021.1967459
Jeon S, Kim D-J (2018) Enhanced phosphorus bioavailability and heavy metal removal from sewage sludge ash through thermochemical treatment with chlorine donors. J Ind Eng Chem 58:216–221
Jupp AR, Beijer S, Narain GC, Schipper W, Slootweg JC (2021) Phosphorus recovery and recycling - closing the loop. Chem Soc Rev 50:87–101
Kleemann R, Chenoweth J, Clift R, Morse S, Pearce P, Saroj D (2017) Comparison of phosphorus recovery from incinerated sewage sludge ash (ISSA) and pyrolysed sewage sludge char (PSSC). Waste Manag 60:201–210
Li R, Zhao W, Li Y, Wang W, Zhu X (2015) Heavy metal removal and speciation transformation through the calcination treatment of phosphorus-enriched sewage sludge ash. J Hazard Mater 283:423–431
Li JS, Tsang DCW, Wang QM, Fang L, Xue Q, Poon CS (2017) Fate of metals before and after chemical extraction of incinerated sewage sludge ash. Chemosphere 186:350–359
Li R-h, Wang X-m, Li X-y (2018) A membrane bioreactor with iron dosing and acidogenic co-fermentation for enhanced phosphorus removal and recovery in wastewater treatment. Water Res 129:402–412
Li S, Zeng W, Ren Z, Jia Z, Peng X, Peng Y (2021a) A novel strategy to capture phosphate as high-quality struvite from the sewage sludge ash: process mechanism and application. J Clean Prod 322:129–162. https://doi.org/10.1016/j.jclepro.2021.129162
Li X, Shen S, Xu Y, Guo T, Dai H, Lu X (2021b) Application of membrane separation processes in phosphorus recovery: a review. Sci Total Environ 767:144346
Liu J, Deng S, Qiu B, Shang Y, Tian J, Bashir A, Cheng X (2019) Comparison of pretreatment methods for phosphorus release from waste activated sludge. Chem Eng J 368:754–763
Loganathan P, Vigneswaran S, Kandasamy J, Bolan NS (2014) Removal and recovery of phosphate from water using sorption. Crit Rev Environ Sci Technol 44:847–907
Ma P, Rosen C (2021) Land application of sewage sludge incinerator ash for phosphorus recovery: a review. Chemosphere 274:129–609. https://doi.org/10.1016/j.chemosphere.2021.129609
Magrí A, Company E, Gich F, Colprim J (2021) Hydroxyapatite formation in a single-stage anammox-based batch treatment system: reactor performance, phosphorus recovery, and microbial community. Acs Sustain Chem Eng 9:2745–2761
Morse GK, Brett SW, Guy JA, Lester JN (1998) Review: phosphorus removal and recovery technologies. Sci Total Environ 212:69–81
Nanzer S, Oberson A, Huthwelker T, Eggenberger U, Frossard E (2014) The molecular environment of phosphorus in sewage sludge ash: implications for bioavailability. J Environ Qual 43:1050–1060
Nowak B, Wegerer H, Aschenbrenner P, Rechberger H, Winter F (2012) Sewage sludge ash to phosphate fertilizer by chlorination and thermal treatment: residence time requirements for heavy metal removal. Environ Technol 33:2375–2381
O’Loughlin EJ, Boyanov MI, Flynn TM, Gorski CA, Hofmann SM, McCormick ML, Scherer MM, Kemner KM (2013) Effects of Bound phosphate on the bioreduction of lepidocrocite (γ-FeOOH) and maghemite (γ-Fe2O3) and formation of secondary minerals. Environ Sci Technol 47:9157–9166
Petzet S, Peplinski B, Bodkhe SY, Cornel P (2011) Recovery of phosphorus and aluminium from sewage sludge ash by a new wet chemical elution process (SESAL-Phos-recovery process). Water Sci Technol 64(3):693–699. https://doi.org/10.2166/wst.2011.682
Pokhrel SP, Milke MW, Bello-Mendoza R, Buitrón G, Thiele J (2018) Use of solid phosphorus fractionation data to evaluate phosphorus release from waste activated sludge. Waste Manag 76:90–97
Prot T, Nguyen VH, Wilfert P, Dugulan AI, Goubitz K, De Ridder DJ, Korving L, Rem P, Bouderbala A, Witkamp GJ, van Loosdrecht MCM (2019) Magnetic separation and characterization of vivianite from digested sewage sludge. Sep Purif Technol 224:564–579. https://doi.org/10.1016/j.seppur.2019.05.057
Qiang ZM, Wang L, Dong HY, Qu JH (2015) Operation performance of an A/A/O process coupled with excess sludge ozonation and phosphorus recovery: a pilot-scale study. Chem Eng J 268:162–169
Qiu GL, Ting YP (2014) Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Biores Technol 170:221–229
Qiu GL, Zhang S, Raghavan DSS, Das S, Ting YP (2016a) Towards high through-put biological treatment of municipal wastewater and enhanced phosphorus recovery using a hybrid microfiltration-forward osmosis membrane bioreactor with hydraulic retention time in sub-hour level. Biores Technol 219:298–310
Qiu GL, Zhang S, Shankari D, Raghavan S, Das S, Ting YP (2016b) The potential of hybrid forward osmosis membrane bioreactor (FOMBR) processes in achieving high throughput treatment of municipal wastewater with enhanced phosphorus recovery. Water Res 105:370–382
Quist-Jensen CA, Sorensen JM, Svenstrup A, Scarpa L, Carlsen TS, Jensen HC, Wybrandt L, Christensen ML (2018) Membrane crystallization for phosphorus recovery and ammonia stripping from reject water from sludge dewatering process. Desalination 440:156–160
Quist-Jensen CA, Wybrandt L, Lokkegaard H, Antonsen SB, Christensen ML (2019) Pilot-scale study for phosphorus recovery by sludge acidification and dewatering. Environ Technol 1–7
Quist-Jensen CA, Wybrandt L, Løkkegaard H, Buch S, Morten A, Christensen L (2020) Pilot-scale study for phosphorus recovery by sludge acidification and dewatering. Environ Technol 41(22):2928–2934. https://doi.org/10.1080/09593330.2019.1588385
Rahman S, Chowdhury RB, D’Costa NG, Milne N, Bhuiyan M, Sujauddin M (2019) Determining the potential role of the waste sector in decoupling of phosphorus: a comprehensive review of national scale substance flow analyses. Resour Conserv Recycl 144:144–157
Rittmann BE, Mayer B, Westerhoff P, Edwards M (2011) Capturing the lost phosphorus. Chemosphere 84:846–853
Rothe M, Kleeberg A, Hupfer M (2016) The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth-Sci Rev 158:51–64
Roy ED (2017) Phosphorus recovery and recycling with ecological engineering: A review. Ecol Eng 98:213–227
Semerci N, Kunt B, Calli B (2019) Phosphorus recovery from sewage sludge ash with bioleaching and electrodialysis. Int Biodeterior Biodegradation 144:104–739. https://doi.org/10.1016/j.ibiod.2019.104739
Shi Y, Luo G, Rao Y, Chen H, Zhang S (2019) Hydrothermal conversion of dewatered sewage sludge: focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 228:619–628. https://doi.org/10.1016/j.chemosphere.2019.04.109
Söldner A, Zach J, König B (2019) Deep eutectic solvents as extraction media for metal salts and oxides exemplarily shown for phosphates from incinerated sewage sludge ash. Green Chem 21:321–328
Stemann J, Peplinski B, Adam C (2015) Thermochemical treatment of sewage sludge ash with sodium salt additives for phosphorus fertilizer production–analysis of underlying chemical reactions. Waste Manag 45:385–390
Tang S, Yan F, Zheng C, Zhang Z (2018) Novel calcium oxide-enhancement phosphorus recycling technique through sewage sludge pyrolysis. ACS Sustain Chem Eng 6(7):9167–9177. https://doi.org/10.1021/acssuschemeng.8b01492
Thong ZW, Cui Y, Ong YK, Chung TS (2016) Molecular design of nanofiltration membranes for the recovery of phosphorus from sewage sludge. Acs Sustain Chem Eng 4:5570–5577
Vasenko L, Bonnemain-Fernandes A, Malwade C, Qu H (2020) Phosphorus recovery from municipal wastewater via a two-step process of ozonation and crystallization: process development, optimization and upscaling. Environ Sci: Water Res Technol 6:817–828
Venkiteshwaran K, McNamara PJ, Mayer BK (2018) Meta-analysis of non-reactive phosphorus in water, wastewater, and sludge, and strategies to convert it for enhanced phosphorus removal and recovery. Sci Total Environ 644:661–674
Wijdeveld WK, Prot T, Sudintas G, Kuntke P, Korving L, van Loosdrecht MCM (2022) Pilot-scale magnetic recovery of vivianite from digested sewage sludge. Water Res 212:118–131. https://doi.org/10.1016/j.watres.2022.118131
Wilfert P, Mandalidis A, Dugulan AI, Goubitz K, Korving L, Temmink H, Witkamp GJ, Van Loosdrecht MCM (2016) Vivianite as an important iron phosphate precipitate in sewage treatment plants. Water Res 104:449–460
Wollmann I, Möller K (2018) Phosphorus bioavailability of sewage sludge-based recycled fertilizers in an organically managed field experiment. J Plant Nutr Soil Sc 181:760–767
Wu H, Ikeda-Ohno A, Wang Y, Waite TD (2015) Iron and phosphorus speciation in Fe-conditioned membrane bioreactor activated sludge. Water Res 76:213–226
Xiao D, Huang HM, Jiang Y, Ding L (2017) Recovery of phosphate from the supernatant of activated sludge pretreated by microwave irradiation through chemical precipitation. Environ Sci Pollut R 24:26901–26909
Xie C, Zhao J, Tang J, Xu J, Lin X, Xu X (2011) The phosphorus fractions and alkaline phosphatase activities in sludge. Bioresour Technol 102:2455–2461
Xie M, Nghiem LD, Price WE, Elimelech M (2014) Toward resource recovery from wastewater: extraction of phosphorus from digested sludge using a hybrid forward osmosis-membrane distillation process. Environ Sci Tech Let 1:191–195
Xu HC, He PJ, Gu WM, Wang GZ, Shao LM (2012) Recovery of phosphorus as struvite from sewage sludge ash. J Environ Sci-China 24:1533–1538
Xu Y, Zhou Q, Wang X, Yang M, Fang Y, Lu Y (2021) An efficient strategy of phosphorus recovery: electrochemical pretreatment enhanced the anaerobic fermentation of waste activated sludge. Chemosphere 268:129391
Yuan Z, Pratt S, Batstone DJ (2012) Phosphorus recovery from wastewater through microbial processes. Curr Opin Biotechnol 23:878–883
Yu Y, Lei Z, Yuan T, Jiang Y, Chen N, Feng C, Shimizu K, Zhang Z (2017) Simultaneous phosphorus and nitrogen recovery from anaerobically digested sludge using a hybrid system coupling hydrothermal pretreatment with MAP precipitation. Bioresour Technol 243:634–640. https://doi.org/10.1016/j.biortech.2017.06.178
Yu B, Luo J, Xie H, Yang H, Chen S, Liu J, Zhang R, Li Y-Y (2021) Species fractions and characterization of phosphorus in sewage sludge: a critical review from the perspective of recovery. Sci Total Environ 786:147–437. https://doi.org/10.1016/j.scitotenv.2021.147437
Zhang C, Guisasola A, Baeza JA (2022) A review on the integration of mainstream P-recovery strategies with enhanced biological phosphorus removal. Water Res 212:118102
Zhang T, Wang Q, Deng Y, Jiang R (2018) Recovery of phosphorus from swine manure by ultrasound/H2O2 digestion, struvite crystallization, and ferric oxide hydrate/biochar adsorption. Front Chem 6:464
Zhao YZ, Ren QQ, Na YJ (2018) Phosphorus transformation from municipal sewage sludge incineration with biomass: formation of apatite phosphorus with high bioavailability. Energ Fuel 32:10951–10955
Funding
This research was supported by the Ecological environment scientific research project of Jiangsu Province (2021001) and the Major Science and Technology Project of Water Pollution Control and Management in China (2017ZX07202004-002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception and design. Data collection and Analysis were performed by Xiang Li, Shuting Shen, Yuye Xu and Ting Guo. The first draft of the manuscript was written by Xiang Li and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Shen, S., Xu, Y. et al. Mining phosphorus from waste streams at wastewater treatment plants: a review of enrichment, extraction, and crystallization methods. Environ Sci Pollut Res 30, 28407–28421 (2023). https://doi.org/10.1007/s11356-023-25388-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-25388-9