Abstract
Biological nitrogen fixation (BNF) varies in different soils and is impacted by rice planting. This study was conducted to investigate the extent of BNF in acidic soil planted with rice and to explore if the light and flood layer thickness affected the BNF. Soil and rice together were incubated in a 15N2-labeled closed chamber for whole growth period, and light and water depth effects were measured by labeling aboveground parts of rice plants in a plastic bag and labeling air in bottle without rice plants, respectively. The results showed that the total N fixation of soil and plant was 11.65 kg ha−1; nevertheless, it was only 2.11 kg ha−1 under fallow soil. In planted soil, plants and soil accounted for 25.1% and 74.9% of total 15N fixed, correspondingly. Soil NH4+-N concentration decreased due to uptake by the rice plants. There was higher available iron (Fe2+) in soil with rice plantings than in the fallow soil which was beneficial for BNF. Furthermore, 15N atom% in the roots was found higher than in the aboveground plant parts or leaves whether from experiment with whole rice-soil labeled in 15N2-enriched closed chamber or from that only rice aboveground parts labeled by 15N2, whereas water depth above the soil surface insignificantly influenced BNF without rice planting. Rice planting may significantly increase the amount of N fixation in acidic paddy soils. Further work regarding the role of rice plant and thickness of the water layer in BNF is highly important to gain an improved understanding of the N cycle in the rice ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrogen gas (N2) accounts for almost 80% of the volume of air. However, plants cannot use it directly, and it can only be absorbed and utilized by plants through biological or industrial fixation. The biological nitrogen fixation (BNF) and biological reduction of N2 to ammonium can merely be carried out by nitrogenase enzymes, which exist exclusively in prokaryotes (Canfield et al. 2010). The BNF can be symbiotic when there are mutualistic associations between plant species and N fixing microorganisms (mainly rhizobia) or can be asymbiotic when it is carried out by free-living fixing microorganisms (do Figueiredo et al. 2013). Free-living N fixing microorganisms can accomplish BNF through autotrophic fixation, in which required energy is attained from photosynthesis. However, free-living heterotrophic N fixers in soil fuel up their activity by using energy from organic matter decomposition. The influence of several factors on autotrophic and heterotrophic BNF and their differential role have been focused by several researchers (Das et al. 2011; Bei et al. 2013; Perakis et al. 2017; Coyne et al. 2020). BNF occurs in soil by rhizobia and heterotrophic bacteria living in the rhizosphere of rice plants (Ladha and Reddy 2003). The phyllosphere bacteria in the plant also have been found efficient for BNF (Venkatachalam et al. 2016; Syiem et al. 2017).
It is reported that about 12% of the N in terrestrial plants comes from biological fixation and the remaining 88% enters through internal circulation or organic N decomposition in the soil (Ussiri and Lal 2013), while the global BNF rates are ranging from 108 to 148 Tg N per year (Meyerholt et al. 2016), and the industrial production of N fertilizer is around 140 Tg of N per year (Canfield et al. 2010). Furthermore, studies have shown that the non-symbiotic BNF contributes around 25% of the N harvest for agriculture (corn, rice, and wheat planting systems), of which BNF adds nearly 22 kg N per hectare per year to the N harvest of rice (Ladha et al. 2016). Therefore, BNF serves as a great supply of N in paddy fields, which can reduce the environmental problems resulting from chemical N fertilizer applications.
Rice planting enhances both phototrophic and heterotrophic BNF in a flooded rice-soil system with pH (H2O) 8.0 (Bei et al. 2013). There is a higher N percentage computed under non-symbiotic BNF in the rice roots than other parts, such as leaves or stem (Wang et al. 2019). Zheng et al. (2019) found that N additions inhibited but micronutrient could stimulate BNF in terrestrial ecosystems by compiling a global database of free-living N fixation and symbiotic N fixation in response to N, P, and micronutrient addition across different biomes. It is well known that, mostly, BNF is carried out by the activity of the Fe protein and MoFe protein (Rubio and Ludden 2008; Bellenger et al. 2014). Thus, the higher availability of Fe or Mo increases BNF (Wurzburger et al. 2012; Winbourne et al. 2017; Zheng et al. 2019; Ma et al. 2019). Electrons are transferred between the reduced and the oxidized states of Fe protein or MoFe protein to accomplish the BNF. Soil available forms of Mo as MoO42− (Wurzburger et al. 2012) and available forms of Fe as Fe3+ in proteins are more critical for accepting electron to be reduced. Furthermore, the increasing available carbon (C) encouraged BNF under flooding than non-flooding conditions (Das et al. 2011). Therefore, BNF would change in different soil types and environments. Yet, there is no clear information available about the extent of BNF in acidic soils with higher iron contents.
It is well known that aboveground water layer thickness adjusts the optimal environment for cyanobacteria and photosynthetic bacteria that inhabit floodwaters and soil surfaces, as well as heterotrophic or free-living bacteria that resides in the root zone (Ladha and Reddy 2003). Syiem et al. (2017) reported that rice plants might fix N in water or aboveground parts. Furthermore, Zheng et al. (2019) examined whether environmental factors (mean annual temperature, mean annual precipitation, and background N deposition) affect the extent of BNF in response to nutrient addition. However, the impact of water layer thickness on BNF has not been reported yet. Even, it was unclear if the light had a significant influence on soil BNF under rice plantings.
In this study, we hypothesized that planting rice could increase soil BNF, which might be influenced by light or water layer thickness. Thus, we carried out the incubation experiment to investigate the BNF for an acidic paddy soil in the growth chamber laid in the rice field ecosystem. We conducted this experiment with two treatments, i.e., planted with rice (SR) and soil fallow (SF). During the whole rice growing period, N2 was labeled with 15N in the air. At the same time, these two treatments were applied without 15N2 labeling to observe the changes in soil nutrients and carbon status in the field. Furthermore, the role of aboveground rice parts in BNF under the paddy ecosystem was investigated by 15N2 labeling only rice part before heading, and the differential effects of water layer thickness above soil surface in the field were taken into account for the present research. This study aimed to evaluate the role of rice planting on BNF in red soil and that of light and water depth.
2 Material and Methods
2.1 Soil Used
The test soil in 0–20 cm was taken from Pucheng County (27° 32′ N, 118° 11′ E) in Nanping City, Fujian Province, China, where the climate was subtropical with monsoon having a frost-free period of 240–330 days with annual average temperature 20 °C and annual precipitation was 800–1900 mm with mild, moist, and rainy climate. It was a typical acidic paddy soil (Aquic Hapludults, USDA soil taxonomy, Soil Survey Staff 2014) with pH (H2O) value of 5.26 coupled with total N 1.81 g kg−1, total C 16.98 g kg−1, total phosphorus 1.45 g kg−1, available phosphorus 3.62 mg kg−1, sand 31.1%, silt 52.5%, and clay 16.4%.
2.2 Experimental Design
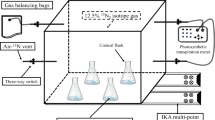
Rice cultivation and planned experiments were carried out in Xiaoji Town, Jiangdu District, Yangzhou City, Jiangsu Province (32° 35′ N, 119° 42′ E), China. Some pots, a diameter of 9 cm with a height of 15 cm, were prepared with 1.1 kg soil and used for experimentation. Two treatments, planted with rice (SR) and soil fallow (SF), were considered in a growth chamber laid in field in situ to explore the role of rice planting on BNF by 15N2 labeling method. A local variety named “Wu Yun japonica rice 23” was selected for experimental rice type, and thereafter, seedlings were transplanted into pots for SR treatment. There were three seedlings in a single hole of one pot and a water layer thickness of 2 to 3 cm was maintained above the soil surface. The seedlings were cleaned with water before transplanting. The chamber system was constructed and operated precisely (patent number: ZL 200910232184.3) and 15N-N2 in the air of the chamber labeling method was accomplished according to Bei et al. (2013). At the same time, the two treatments were applied in the field without 15N2 labeling by randomization with three replicates, to observe the changes of soil nutrients and carbon status. The experimental duration started from rice tillering and ended up at the grain filling stage. Consequently, a total of 92 days experiment was maintained with 15N2 label gas and was carried out from July 20 to October 20 in 2016 under the controlled chamber condition. During the growth and development of rice, there was not any application of N fertilizer. Phosphate and potassium as inorganic fertilizers were applied on the soil surface in solid form before planting at the recommended rate that was 60.8 mg pot−1 (75 kg ha−1) for P2O5 and K2O as compound fertilizer. As the rice reached maturity, soil samples from the pot in the chamber were collected at 0–1 and 1–15 cm. Rice plants were collected and divided into the aboveground part and root. These samples were used to analyze 15N abundance and computation of 15N fixation. Soil samples from the pots in the field was collected at 0–15 cm every week and were used to analyze NH4+-N, NO3−-N, soil soluble organic C, available iron, and available molybdenum concentration.
Light impacts plant growth and activity of microorganism on plant. To explore the role of light in affecting BNF in leaves, the treatments for shaded light (SL) and the natural light treatment (NL) were considered by covering rice aboveground parts with a transparent and black plastic bag, respectively. Each treatment was carried out in three replications. Rice plants were transplanted in the pots carrying 1.5 kg soil, having an upper, lower diameter and a height of 17.7, 14.4, and 17.8 cm, correspondingly. When the labeling of rice leaves in the air with 15N-N2 was done at the later stage of jointing, the transparent or black plastic bag together with a nylon tube was tied up with the rice plant stem below the flooded surface, which avoided 15N2 gas leaking out of bag. The nylon tube was used to inject the labeling 15N2 gas into the bag. The first labeling was made on August 24, 2017, with 50 mL of an equal volume of 15N2 gas (abundance 99.14%) that was introduced into the bag through the nylon tube. After 10 days of labeling (September 4), we collected all gas from the bag and removed the labeling bag to resume the natural growth of the plants. On September 11, rice leaves were labeled for the second phase until the end of the labeling on September 27. After finishing the incubation, the rice plants were sampled for root, stem, and sheath ear (collectively called the stem) and leaf. Then, the soil was divided into soil layer 1 (0–1 cm) and soil layer 2 (> 1 cm) below the soil surface. These samples were used to analyze 15N abundance and assess the role of light in BNF.
Water layer thickness impacts soil condition and relationship between soil and air. To study its effect on BNF, 250-g air-dried soil was put into a 500 mL incubation bottle with an appropriate amount of ultrapure water for outdoor incubation. After preparing for a couple of weeks, two treatments with different submerged layers, i.e., 2 cm water layer thickness (W-2 cm) and anhydrous layer (W-0 cm), were designed for labeling incubation, where each treatment was replicated thrice. To experiment, 20 mL gas in the bottle was drawn out and an equal volume of 15N2 gas with an abundance of 99.14% was injected to complete the first labeling. The second labeling was made after 22 days and was maintained for 55 days. Upon finishing the incubation, the soil was divided into two layers according to the above-described method.
2.3 Physical-Chemical Analysis of Soils and Plant
Soil pH was measured on a DMP-2 mV/pH detector (Quark Ltd., Nanjing, China) after mixing with water (1:5; v/v). Soil texture was analyzed using a laser diffraction analyzer (Mastersizer 2000, the United Kingdom). The soil was extracted with 0.5 mol L−1 K2SO4 solution to determine NH4+-N and NO3−-N concentrations. NH4+-N was analyzed by the indophenol blue colorimetric method, and NO3−-N was analyzed by dual-wavelength spectrophotometry, while, soil soluble organic C was determined by potassium dichromate oxidation method. Soil available iron (Fe2+ and Fe3+) was extracted with 0.1 mol L−1 hydrochloric acid and determined by phenanthroline colorimetric method described in the Magazine of Soil and Agro-Chemistry Analysis (Bao 2000). Similarly, available soil molybdenum was extracted with oxalic acid-ammonium oxalate and analyzed by ICP (X SERIES 2, USA). Available phosphorus was extracted with 0.1 mol L−1 hydrochloric acid and determined by the molybdenum blue colorimetric method in an analyzer (SKALAR SAN++, Netherlands). Total soil C and N were measured using an element analyzer (Elemantar Vario MAX CN, Germany). The 15N abundance was determined through an isotopic mass spectrometer (Thermo Scientific MAT 253-Flash HT, USA).
2.4 Calculations
Biological nitrogen fixation in the field was estimated by using the method of Bei et al. (2013) and Naher et al. (2011), for 15N fixed amount (mg pot−1) in soil and plant samples: 15N fixed = TNsample × AT% 15Nexcess sample, where TNsample represents the total N (g) of the sample, AT% 15Nexcess sample = AT% 15Nlabeled − AT% 15Nun-labeled. AT% 15Nlabeled is the abundance of the sample in the chamber. AT% 15Nun-labeled is the abundance of the sample in the field.
The proportion of biologically fixed N in the rice-soil system: %N Fixation = AT% 15Nexcess sample/AT% 15Nexcess gas, where AT% 15Nexcess gas = AT% 15Ngas − 0.3663%.
Soil N fixation per unit soil area (kg ha−1) = (TNsoil × %N Fixation soil + TNplant × %N Fixationplant)/S, where S represents the soil area (ha).
2.5 Statistical Analysis
One-way ANOVA and LSD methods were used for the analysis of variance to examine the differences among various rice samples, while the significant differences among treatments were determined by repeated measurement analysis of variance. Independent samples and one-tailed T test were used to analyze the significant differences between treatments. The data in tables and figures were showed as mean ± standard deviation (n = 3).
3 Results
3.1 Total Accumulation of 15N in Rice Plants and Soil During the Growth Period
The biomass as well as total N of the aboveground parts of the plants was higher than those of the roots (Table 1); however, the value of 15N atom% excess was significantly lower than the roots. The amount of 15N fixed in aboveground organs of plants at SR accounted for 78.2% of the total fixed amount of 15N in the whole plant. Further, there was a significantly higher 15N abundance in the first layer (0–1 cm) than the second layer (1–15 cm) in both SR and SF (Table 2), while there was a non-significant difference of 15N in fixed amount between two soil layers in SR; nevertheless, it was higher at 0–1 cm than at 1–15 cm in SF treatment. The total amount of soil 15N fixed per pot in SR was 4.1 times higher than in SF (P < 0.05). In the treatment planted with rice, plant and soil accounted for 25.1% and 74.9% of total 15N fixed per pot, individually, whereas soil nitrogen fixation was recorded as 11.65 and 2.11 kg ha−1 in SR and SF, correspondingly.
3.2 Dynamic Changes in Soil Nutrient Status During Rice Growth
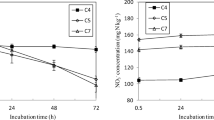
NH4+-N was significantly higher by 31.5–296% at SF than SR (Fig. 1); nevertheless, NO3−-N contents at both treatments decreased with time. A higher soluble organic C in SF than SR was observed only on August 4. Thereafter, the soluble organic C was similar between SR and SF. The available iron and molybdenum contents of paddy soils increased with rice growth, ranging from 206 to 681 mg kg−1 and from 0.123 to 0.236 mg kg−1, respectively. A non-significant difference was found between SF and SR for NO3−-N, soluble organic C, and available molybdenum contents.
Changes in soil (0–15 cm) nutrient contents in different treatments with growing rice (month-day). The significant differences between treatments of soil planted with rice (SR) and soil fallow (SF) with incubation duration were determined by repeated measurement analysis of variance. Tr, treatment; Ti, time linked with rice growth; ns, non-significant difference
3.3 Biological Nitrogen Fixation in Rice Leaves as Affected by Light and Flooding Layer
When the aboveground parts of plants were merely exposed to 15N2, the 15N atomic percentage in the plant exceeds the natural 15N atomic percentage (0.3663) (Fig. 2). Under the shaded light treatment (SL), the 15N atomic percentage of leaves was found significantly lower than that of the stem, while in the natural light treatment (NL), the 15N atomic percentage was recorded significantly different in the order of, roots > stems > leaves. There was a significant influence of the two treatments on 15N atomic percentage in all parts of the rice plants, in which the 15N atomic percentage was computed higher at SL than NL for stem and leaves. However, it was higher at NL than SL for root.
15N abundance in rice plants and soil with experiment of 15N labeling on plant. Different lowercase letters indicate significant (P < 0.05) differences in different rice organs or soil layer under the same treatment. Different capital letters indicate significant (P < 0.01) differences under different treatments of the same rice plant organ or soil layer. SL, shaded light; NL, natural light; Tr, treatment; RO, rice organ; ns, non-significant difference
The 15N atomic percentage in two soil layers also exceeded the natural 15N atomic percentage (Fig. 2). The considerably higher 15N atomic percentage was found only at NL than SL at layer 1, while it was observed non-significant between them at layer 2; even, it was recorded higher at NL than SL. The rice flooding layers with different thicknesses (W-0 cm and W-2 cm) exhibited a non-significant effect on the 15N atomic percentage in different soil layers (Fig. 3).
15N abundance in soil with different water layer depths. Different lowercase letters indicate significant (P < 0.05) differences under different treatments applied to the same soil layer. Different capital letters indicate significant differences in different soil layers under the same treatment (P < 0.05). W-2 cm, 2-cm water depth; W-0 cm, 0-cm water depth
4 Discussion
Bei et al. (2013) reported that rice planting enhanced both the phototrophic and heterotrophic BNF in a flooded rice-soil system. We found the total N fixation at SR was 11.65 kg ha−1, which was in the range of 2.2 to 20.1 kg N ha−1 during 42 days of 15N2 labeling duration reported by Wang et al. (2019). It is well known that NH4+-N is the dominant form of N in rice fields (Zhu et al. 2011). In this study, NH4+-N was significantly higher by 31.5–296% at SF than SR (Fig. 1) because the uptake and utilization of N by flooded rice gradually increased with the growth and development of rice (Hamoud et al. 2019). NH4+-N at SF did not decline with experimental duration due to a lack of plant growth to absorb N and the dissimilatory NO3−-N reduction to NH4+-N (DNRA). The NO3−-N contents in the soil at two treatments decreased with incubation time that might be due to DNRA or denitrification rather than leaching out of the soil. Volatilization and leaching losses of nitrogen from the soil (pH 8.03) as reported by Jadon et al. (2018) did not occur in this experiment because of soil pH is lower. Denitrification as testified by Friedl et al. (2018) did occur possibly, and denitrification and DNRA are thought to compete for available NO3−-N under anaerobic conditions (Friedl et al. 2018). Pandey et al. (2019) reported that DNRA exceeded denitrification in low N-fertilized paddies. In the present study, since there was not any N fertilizer application, therefore, BNF and DNRA might contribute to more NH4+-N at SR besides the uptake of NH4+-N. However, BNF was weak at SF because of high soil NH4+-N, which was consistent with the negative effects of N addition on BNF (Perakis et al. 2017; Zheng et al. 2019).
A higher soluble organic C in SF than SR was only observed on August 4. After 1 week, the soluble organic C did not fluctuate throughout the experimentation. It was reported that organic C from roots and other sources could provide a considerable amount of energy to enhance BNF capacity by the ARA method (Das et al. 2011). However, in the present study, we found a non-significant difference of soluble organic C between SF and SR (Fig. 1), which might be due to the utilization of soluble organic C for heterotrophic N fixation in rice-planted soil, or heterotrophic soil respiration promoting DNRA (Friedl et al. 2018). These results suggested that more studies about soil available C as one of the important factors regulating BNF are required.
Under flooded soil conditions, available iron increased with rice growth, which might be related to the occurred reduction of Fe3+ into Fe2+ under anaerobic condition (Akter et al. 2018). The higher soil available iron might contribute to BNF (Fig. 1), which is consistent with previous reports (Wurzburger et al. 2012; Zheng et al. 2019). Winbourne et al. (2017) also observed a threefold increase in BNF rates with Fe additions at the limestone site in the wet season. There was a significantly higher BNF in SR than in SF (Fig. 1). However, there was a non-significant difference in the available molybdenum between SR and SF (Fig. 1). In Mo-deficient paddy fields (≤ 0.068 mg kg−1), molybdenum application greatly increased BNF (Ma et al. 2019). Previously, it was reported that the positive effects on BNF had been only observed in the form of Mo and low P co-additions (Rousk et al. 2017). Experimental fertilization of organic horizon soil revealed widespread Mo limitation of heterotrophic N fixation, especially at sites where soil total Mo was scarce relative to total C (Perakis et al. 2017). It is pertinent from these results that multiple nutrient controls of heterotrophic N fixation were more common than single-nutrient effects.
When the aboveground parts of plants were merely exposed to 15N2, the 15N atomic percentage in the plant exceeded the natural 15N atomic percentage (Fig. 2). The 15N atomic percentage was higher at SL than NL for stem and leaves, but it was recorded inverse for roots. The low intensity of solar radiation induced stomatal closure (Aasamaa and Sõber 2011), blocking the communication between plant surfaces and internal mechanisms. Phyllosphere microbiology can enhance the multiple interactions between plant and microbial communities (Peñuelas and Terradas 2014). It was reported that the phyllosphere bacteria play a critical role in promoting biological nitrogen fixation (Venkatachalam et al. 2016; Syiem et al. 2017) and the interaction between plant and phyllosphere was considerably affected by light (Carvalho and Castillo 2018). Therefore, rice stomata opening or closing might be one of the factors to affect BNF by phyllosphere microbiology.
In the natural light treatment (NL), the 15N atomic percentage was recorded significantly different in the order of roots > stems > leaves, which suggested that 15N might be transferred from leaves to root when 15N fixed at leave. Under the shaded light treatment (SL), the 15N atomic percentage of leaves was found significantly lower than that of the stem, but not between root and stem or leaves, which suggested that 15N was transferred from leaves to stem, not to roots. Although rice plant has a critical role in promoting biological nitrogen fixation attributed to the phyllosphere bacteria (Venkatachalam et al. 2016; Syiem et al. 2017), it is apparent that more studies about the role of plant in rice ecosystem are needed concerning BNF. In addition, there was significantly higher 15N abundance and the total amount of soil 15N fixed per pot in SR than in SF (P < 0.05) (Table 1). Cyanobacteria and photosynthetic bacteria are used to inhabit in floodwaters and on soil surfaces (Ladha and Reddy 2003). Cyanobacteria had a significant role in triggering the growth and development of plants, contributing BNF in the rice fields (Singh 2014). In this study, there was a higher 15N abundance at soil thickness of 0–1 cm than at > 1 cm for SF, but not for SR (Table 2). This indicated that rice planting might be one of the vital factors for field BNF and this study highlights the role of leaves in BNF.
Research on weather water layers’ thicknesses (W-0 cm and W-2 cm) affecting soil N fixation was considered in this study, because cyanobacteria and photosynthetic bacteria inhabited in floodwaters (Ladha and Reddy 2003). Most free-living cyanobacteria can fix atmospheric nitrogen and flourish in rice fields as they provide optimum growth conditions for cyanobacteria in terms of water requirements (Syiem et al. 2017). However, the significant influence of watering layers on the 15N atom % in soil was not observed (Fig. 3), even though the 15N atom % in soil was higher than the natural 15N atom % (0.366). The rice fields exhibit a peculiar aquatic ecosystem in which the water layers are often very shallow, but relatively constant during a fraction of the year; because of that, the sediment-water interaction becomes highly important and likely plays a major role in the biological activities (Fernandez-Valiente and Quesada 2004). It is reported that there was a higher proportion of the heterotrophic N fixation in the rice-planted soils as compared to other crop cultivations (Bei et al. 2013) because of flooded irrigations in the rice ecosystem. Free-living heterotrophic N fixer activity in soil needs C energy, which might come from plant input. Therefore, although these results indicated that once the soil water is saturated, the thickness of the water layer (2 cm) might not affect BNF; more studies about the role of thickness of the water layer still are still needed for the rice planting systems.
5 Conclusions
Rice planting can substantially increase the extent of N fixation in the soils of paddy fields. The decrease of inorganic N due to plant uptake in rice fields could be helpful to promote biological nitrogen fixation (BNF). When only rice aboveground parts were under 15N2-labeled condition, the light induced a higher 15N atom% in rice roots than leaves, which suggested that 15N fixed in aboveground plant parts possibly transferred from leaves to the roots. Water depth above the soil surface did not influence BNF in this research. Rice plant has a critical role in BNF and we strongly argue to consider the regulation of light, soil available C, and water depth on BNF in the presence rice planting system.
References
Aasamaa K, Sõber A (2011) Stomatal sensitivities to changes in leaf water potential, air humidity, CO2 concentration and light intensity, and the effect of abscisic acid on the sensitivities in six temperate deciduous tree species. Environ Exp Bot 71:72–78. https://doi.org/10.1016/j.envexpbot.2010.10.013
Akter M, Deroo H, De Grave E, Van Alboom T, Kader MA, Pierreux S, Begum MA, Boeckx P, Sleutel S (2018) Link between paddy soil mineral nitrogen release and iron and manganese reduction examined in a rice pot growth experiment. Geoderma 326:9–21. https://doi.org/10.1016/j.geoderma.2018.04.002
Bao SD (2000) Soil and agro-chemistry analysis. China Agricultural Press, Beijing (In Chinese)
Bei QC, Liu G, Tang HY, Cadisch G, Rasche F, Xie ZB (2013) Heterotrophic and phototrophic 15N2 fixation and distribution of fixed 15N in a flooded rice-soil system. Soil Biol Biochem 59:25–31. https://doi.org/10.1016/j.soilbio.2013.01.008
Bellenger JP, Xu Y, Zhang X, Morel FMM, Kraepiel AML (2014) Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2-fixing bacteria in soils. Soil Biol Biochem 69:413–120. https://doi.org/10.1016/j.soilbio.2013.11.015
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of earth’s nitrogen cycle. Science 330:192–196. https://doi.org/10.1126/science.1186120
Carvalho SD, Castillo JA (2018) Influence of light on plant–phyllosphere interaction. Front Plant Sci 9:1482. https://doi.org/10.3389/fpls.2018.01482
Coyne KJ, Parker AE, Lee CK, Sohm JA, Kalmbach A, Gunderson T, León-Zayas R, Capone DG, Carpenter EJ, Cary SC (2020) The distribution and relative ecological roles of autotrophic and heterotrophic diazotrophs in the McMurdo Dry Valleys, Antarctica. FEMS Microbiol Ecol 96:fiaa010. https://doi.org/10.1093/femsec/fiaa010
Das S, Bhattacharyya P, Adhya TK (2011) Impact of elevated CO2, flooding, and temperature interaction on heterotrophic nitrogen fixation in tropical rice soils. Biol Fertil Soils 47:25–30. https://doi.org/10.1007/s00374-010-0496-2
do Figueiredo MVB, do Mergulhão ACES, Sobral JK, de Junior MAL, de ASF A (2013) Biological nitrogen fixation: importance, associated diversity, and estimates. In: Arora NK (ed) Plant microbe symbiosis: fundamentals and advances. Springer, Delhi, pp 267–289. https://doi.org/10.1007/978-81-322-1287-4
Fernandez-Valiente E, Quesada A (2004) A shallow water ecosystem: rice-fields. The relevance of cyanobacteria in the ecosystem. Limnetica 23:95–107. https://doi.org/10.23818/limn.23.08
Friedl J, De Rosa D, Rowlings DW, Grace PR, Müller C, Scheer C (2018) Dissimilatory nitrate reduction to ammonium (DNRA), not denitrification dominates nitrate reduction in subtropical pasture soils upon rewetting. Soil Biol Biochem 125:340–349. https://doi.org/10.1016/j.soilbio.2018.07.024
Hamoud YA, Guo X, Wang Z, Shaghaleh H, Chen S, Hassan A, Bakour A (2019) Effects of irrigation regime and soil clay content and their interaction on the biological yield, nitrogen uptake and nitrogen-use efficiency of rice grown in southern China. Agric Water Manag 213:934–946. https://doi.org/10.1016/j.agwat.2018.12.017
Jadon P, Selladurai R, Yadav SS, Coumar MV, Dotaniya ML, Singh AK, Bhadouriya J, Kundu S (2018) Volatilization and leaching losses of nitrogen from different coated urea fertilizers. J Soil Sci Plant Nutr 18:1036–1047. https://doi.org/10.4067/S0718-95162018005002903
Ladha JK, Reddy PM (2003) Nitrogen fixation in rice systems: state of knowledge and future prospects. Plant Soil 252:151–167. https://doi.org/10.1023/A:1024175307238
Ladha JK, Tirol-Padre A, Reddy CK, Cassman KG, Verma S, Powlson DS, Kessel CV, Richter DB, Chakraborty D, Pathak H (2016) Global nitrogen budgets in cereals: a 50-year assessment for maize, rice, and wheat production systems. Sci Rep 6:19355. https://doi.org/10.1038/srep19355
Ma J, Bei QC, Wang XJ, Lan P, Liu G, Lin XW, Liu Q, Lin ZB, Liu BJ, Zhang YH, Jin HY, Hu TL, Zhu JG, Xie ZB (2019) Impacts of Mo application on biological nitrogen fixation and diazotrophic communities in a flooded rice-soil system. Sci Total Environ 649:685–694. https://doi.org/10.1016/j.scitotenv.2018.08.318
Meyerholt J, Zaehle S, Smith MJ (2016) Variability of projected terrestrial biosphere responses to elevated levels of atmospheric CO2 due to uncertainty in biological nitrogen fixation. Biogeosciences 13:1491–1518. https://doi.org/10.5194/bg-13-1491-2016
Naher UA, Othman R, Shamsuddin ZHJ, Saud HM, Ismail MR, Rahim KA (2011) Effect of root exuded specific sugars on biological nitrogen fixation and growth promotion in rice (Oryza sativa). Aust J Crop Sci 5:1210–1217
Pandey A, Suter H, He JZ, Hu HW, Chen D (2019) Dissimilatory nitrate reduction to ammonium dominates nitrate reduction in long-term low nitrogen fertilized rice paddies. Soil Biol Biochem 131:149–156. https://doi.org/10.1016/j.soilbio.2019.01.007
Peñuelas J, Terradas J (2014) The foliar microbiome. Trends Plant Sci 19:278–280. https://doi.org/10.1016/j.tplants.2013.12.007
Perakis SS, Pett-Ridge JC, Catricala CE (2017) Nutrient feedbacks to soil heterotrophic nitrogen fixation in forests. Biogeochemistry 134:41–55. https://doi.org/10.1007/s10533-017-0341-x
Rousk K, Degboe J, Michelsen A, Bradley R, Bellenger JP (2017) Molybdenum and phosphorus limitation of moss-associated nitrogen fixation in boreal ecosystems. New Phytol 214:97–107. https://doi.org/10.1111/nph.14331
Rubio LM, Ludden PW (2008) Biosynthesis of the iron-molybdenum cofactor of nitrogenase. Annu Rev Microbiol 62:93–111. https://doi.org/10.1146/annurev.micro.62.081307.162737
Singh S (2014) A review on possible elicitor molecules of cyanobacteria: their role in improving plant growth and providing tolerance against biotic or abiotic stress. J Appl Microbiol 117:1221–1244. https://doi.org/10.1111/jam.12612
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. USDA-Natural Resources Conservation Service, Washington, DC
Syiem MB, Singh AK, Rai AN (2017) N2-fixing cyanobacterial systems as biofertilizer. In: Singh JS, Seneviratne G, Agro-environmental sustainability. Springer International Publishing, pp 43–61. https://doi.org/10.1007/978-3-319-49724-2_3
Ussiri D, Lal R (2013) Global nitrogen cycle. In: Ussiri D, Lal R (eds) Soil emission of nitrous oxide and its mitigation. Netherlands. Springer, Heidelberg, pp 29–62. https://doi.org/10.1007/978-94-007-5364-8_2
Venkatachalam S, Ranjan K, Prasanna R, Ramakrishnan B, Thapa S, Kanchan A (2016) Diversity and functional traits of culturable microbiome members, including cyanobacteria in the rice phyllosphere. Plant Biol 18:627–637. https://doi.org/10.1111/plb.12441
Wang XJ, Liu BJ, Ma J, Zhang YH, Hu TL, Zhang H, Feng YC, Pan HL, Xu ZW, Liu G, Lin XW, Zhu JG, Bei QC, Xie ZB (2019) Soil aluminum oxides determine biological nitrogen fixation and diazotrophic communities across major types of paddy soils in China. Soil Biol Biochem 131:81–89. https://doi.org/10.1016/j.soilbio.2018.12.028
Winbourne JB, Brewer SW, Houlton BZ (2017) Iron controls over di-nitrogen fixation in karst tropical forest. Ecology 98:773–781. https://doi.org/10.1002/ecy.1700
Wurzburger N, Bellenger JP, Kraepiel AML, Hedin LO (2012) Molybdenum and phosphorus interact to constrain asymbiotic nitrogen fixation in tropical forests. PLoS One 7:e33710. https://doi.org/10.1371/journal.pone.0033710
Zheng M, Zhou Z, Luo Y, Zhao P, Mo J (2019) Global pattern and controls of biological nitrogen fixation under nutrient enrichment: a meta-analysis. Glob Chang Biol 25:3018–3030. https://doi.org/10.1111/gcb.14705
Zhu YY, Zeng HQ, Di TJ, Xu GH, Shen QR (2011) Investigation on the mechanism of adaption of plasma membrane H+-ATPase to ammonium nutrition in rice. Chin J Rice Sci 25:112–118 (In Chinese). https://doi.org/10.3724/SP.J.1011.2011.00110
Acknowledgments
We are thankful to Liuming Yang and Yuanzhen Peng for their technical assistance. We also thank Zubin Xie for experimental assistance from State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31770659, 31570607) and the Open Fund Issues of State Key Laboratory of Soil and Sustainable Agriculture, Institute of Soil Science, Chinese Academy of Sciences (Y412201420).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, H., Mao, P., Imran, S. et al. Rice Planting Increases Biological Nitrogen Fixation in Acidic Soil and the Influence of Light and Flood Layer Thickness. J Soil Sci Plant Nutr 21, 341–348 (2021). https://doi.org/10.1007/s42729-020-00364-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00364-1