Abstract
Background and aims
Biological nitrogen fixation (BNF) in paddy systems is impacted by nitrogen application levels and irrigation strategies, but the extent to which these factors influence BNF and its distribution in soil and rice is largely unclear. This study investigates this influence.
Methods
An airtight, transparent growth chamber based 15N-labelling system was used to investigate how different nitrogen application levels (0, 125, 187.5 and 250 kg N ha−1) and irrigation strategies (flooding irrigation or intermittent irrigation) impact the amount of BNF and its distribution in soil and rice.
Results
Nitrogen application at 125–250 kg N ha−1 reduced the amount of BNF by 81–86%. The inhibition effect of nitrogen application on BNF at a soil depth of 1–15 cm was greater than that at 0–1 cm. Relative to the continuous flooding irrigation, intermittent irrigation enhanced rice growth and promoted the transfer of fixed nitrogen from 0-1 cm soil layer to rice, but it did not change the total amount of BNF.
Conclusions
This study indicated that BNF supplied little nitrogen for rice production at the high nitrogen application levels, but the intermittent irrigation could promote utilization of biologically fixed nitrogen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen-fertilizer and irrigation practices are key factors affecting rice yields; however, they also have direct and indirect effects on biological nitrogen fixation (BNF) in paddy fields, which is a naturally-occurring process that can also affect rice yields. BNF itself can lead to moderate but constant yields when no chemical nitrogen is added (Roger and Watanabe 1996); making utilization of BNF an important process for sustainable agriculture. Since the invention of Haber Bosch process in 1910, nitrogen application has greatly increased crop yields. However, overuse of synthetic nitrogen fertilizers in farms has led to several health and environmental problems, from fresh water eutrophication to global warming (Zhang et al. 2015). Therefore, increased utilization of naturally occurring BNF is of increasing interest, in order to reduce the usage of chemical nitrogen to ensure grain yield (Rosenblueth et al. 2018).
Nitrogen-fixing microorganisms can not only utilize nitrogen from air through BNF, but also absorb existing nitrogen sources from the external environment (Reed et al. 2011). Due to the high energy consumption of the nitrogen-fixation process, nitrogen-fixing bacteria prefer to use exogenous nitrogen (Bodelier et al. 2000). Some studies have reported that nitrogen application significantly changes the nitrogen-fixing microbial community structure in the rice field (Knauth et al. 2005; Tan et al. 2003). By using the nitrogen balance method in a pot experiment planted with rice, Santiago-Ventura et al (1986) found that the low rate of N fertilizer (5.64 mg N kg−1 soil) did not depress BNF, while a high rate of N fertilizer (99.72 mg N kg−1 soil) eliminated BNF. By using the acetylene (C2H2) reduction method, a range of lab-incubation studies showed that free-living nitrogen fixation in environments with low nitrogen availability, whether soil, rhizosphere, or moss, is generally higher than that in environments with high nitrogen availability (Kox et al. 2016; Patra et al. 2007; Hobbs and Schimel 1984). However, under field conditions during the whole rice season, the extent of BNF reduction induced by different nitrogen fertilizer application rates has not been studied, nor has the resulting distribution of fixed nitrogen in the soil layer and rice plants. We hypothesize that the amount of BNF could be gradually increased by reducing the amount of nitrogen fertilizer in the field environment.
Diazotrophs are known to be moisture sensitive (Smercina et al. 2019). Among these organisms, phototrophic bacteria and heterotrophic bacteria are the main contributors to BNF in the case of rice planting, and the contribution of these two types of microorganisms is about 50%, each (Bei et al. 2013). Phototrophic bacteria (e.g. blue green alga) usually inhabit flooded waters and soil surfaces, while heterotrophic bacteria live in the rhizosphere of rice (Ladha and Reddy 2003). Nostoc, which is a genus of cyanobacteria with the strongest nitrogen fixation capacity in paddy soil (Ma et al. 2019b; Wang et al. 2020), can survive drought for two years, and once there is a small amount of precipitation, they can restart respiration and activate nitrogen fixation (Gao 1998; Scherer et al. 1984). The activity of nitrogen-fixing enzymes in rhizosphere soil was found to increase when the paddy soil was shifted from dry to wet soil conditions, but it would decrease from wet to dry soil conditions (Rao and Rao 1984). We hypothesize in this study therefore that the amount of BNF under a flooding-moist irrigation practice pattern (intermittent irrigation) would be lower than that of continuous flooding irrigation.
BNF is a process in which gaseous N2 is converted into ammonia (NH3) via the enzyme nitrogenase of the diazotrophs. The amount of BNF is therefore affected by the number and species of diazotrophs (Tadakatsu et al. 2017; Nag et al. 2019). Application of nitrogen fertilizer can not only directly change soil microbial activity and community structure (Wang et al. 2009; Zheng et al. 2008), but also indirectly affect soil microbial community structure by changing soil fertility and affecting rice growth (Lu et al. 2006; Bodelier et al. 2000). The alternation of water status can make the soil in the anaerobic, aerobic or facultative aerobic habitat, which also has an important impact on the soil microbial abundance and community structure (Yoshida 1971; Lu 2006). Therefore, in order to know how nitrogen application levels and irrigation strategies affect nitrogen fixation, it is crucial to know how nitrogen application levels and irrigation strategies affect nitrogen-fixing bacteria and nitrogenase activities.
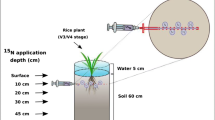
In this study, an airtight, transparent growth chamber based 15N-labelling system was used (Bei et al. 2013) in the field environment to determine BNF under different nitrogen levels and irrigation practices over an entire growing season. The nifH gene of soil DNA was amplified by real-time fluorescent quantitative PCR to study the number of nitrogen-fixing bacteria and its nitrogen fixation activity. The community composition of diazotrophs in each treatment was assessed by nifH gene sequencing. The study is expected to reveal how nitrogen levels and water management impact biological nitrogen fixation, its soil-to-rice distribution, and diazotrophic communities in a paddy field system.
Material and methods
Site description, soil collection and soil properties
The experiment site is located in Xiaoji town, Jiangdu City, Jiangsu province, China (119° 42′ E, 32° 35′ N). The soil studied can be classified as Inceptisol based on the US soil taxonomy (or Shajiang Aquic Anthrosol based on Chinese soil taxonomy). The soil samples were randomly collected at several points along an S shape path (i.e. within a rectangular field, samples are taken from one corner of the rectangle to the opposite corner along an S-shaped route) from the plough layer (0–15 cm) at the end of April in 2016. Visible plant materials in the soil were removed, and then the soils were air-dried, thoroughly homogenized and sieved (< 5 mm). The soil had a pH value of 6.1 (soil:water = 1:5, w:v), and it contained 7.4% sand, 78.0% silt and 14.6% clay. More physio-chemical properties of the soil samples can be found in Wang et al. (2019).
Field 15N2 labeling experiment
A field 15N2 labeling experiment was conducted in the paddy field located in Xiaoji town, Jiangdu city, Jiangsu province, China from 23rd July to 23rd October in 2016. The airtight chambers (length × width × height = 1160 × 680 × 890 mm) (ITIGCN Crop., Ltd, Nanjing, China) in 3 replicates were treated with 15N2 and installed in parallel in the rice field. All the joints on the chambers are connected by gas-sealed joints or waterproof cable joints. In order to be gastight, the outside of each connection joint was sealed with silica gel. Air tightness was checked by two procedures before the start of the incubation. First, air tightness of each joint was checked with soapy water by air circulation. Second, a pure CO2 (99.99%) was injected into the chambers to 800 to 1000 μL L−1 for several times, and the change of CO2 concentration in the chambers was monitored to judge whether there was air leakage. Once the experiment started, the chambers were not opened until the end of the incubation. The temperature and CO2 concentration in the chambers were automatically controlled to keep in line with the ambient air temperature (ambient temperature ± 1 °C) and CO2 concentration (400 ± 20 μL L−1). Excessive O2 generated by rice photosynthesis in the chambers was removed using a gas (butane) lighter to keep the O2 concentration at 21%. The oxygen concentration was measured by an oxygen meter. When the oxygen concentration exceeded 22%, the lighter was turned on, and when the oxygen concentration dropped to about 21%, the lighter was turned off. In addition, three replicates of all treatments were placed outside the chambers as unlabeled controls. More detailed information about the design and control system of this airtight 15N2-labeled chamber is described by Bei et al. (2013).
In this experiment, there were six treatments as follows: (1) no nitrogen application with continuous flooding irrigation (0 N), (2) 125 kg N ha−1 with continuous flooding irrigation (125 N), (3) 187.5 kg N ha−1 with continuous flooding irrigation (187.5 N), (4) 250 kg N ha−1 with continuous flooding irrigation (250 N), (5) no nitrogen application with intermittent irrigation (0 N + II), and (6) 250 kg N ha−1 with intermittent irrigation (250 N + II) (250 kg N ha−1 is the local application level). Air-dried and sieved (< 5 mm) soil (1.4 kg) was filled in each pot (length × width × height = 9 × 9 × 20 cm) to a depth of 15 cm. All treatments had three repetitions with each replication in each chamber, respectively. Before seedling transplantation, each pot was filled with water for 7 days, and the water surface was 1–2 cm above the soil surface. Two rice seedlings (Oryza sativa L., cultivar Wuyunjing 23) were transplanted to each pot. 116.37 mg KH2PO4 and 32.55 mg KCl (equivalent to 75 kg P2O5 ha−1 and 75 kg K2O ha−1, which is the common amount locally), was added (July 18th in 2016 (day 1)) to each pot before rice transplantation. Nitrogen (N) was applied as urea according to the local N fertilization pattern: 50% before seedling transplantation (July 18th in 2016 (day 1)), 10% at the tilling stage (August 4th in 2016 (day 17)), and 40% at the heading stage (August 23th in 2016 (day 36)). In the continuous flooding irrigation treatments, the water surface was kept at 1–2 cm above the soil surface until 10 days before rice sampling. In the intermittent irrigation treatments, the water was 1–2 cm above the soil surface at the first 10 days, and then was kept moist. The strategy for keeping a constant moisture pattern was practiced as follows: stop watering until small cracks appear between the soil and the wall of the pot, then add water to the soil to 1–2 cm above the soil surface. This moisture protocol was repeated until 10 days before sampling.
Right after the preparation of each air-tight chamber with the rice pots of different treatments inside, 40 L of the air in each chamber was replaced by 40 L of 15N2 (99 atom% 15N). 15N2 was produced and purified following the method of Ohyama and Kumazawa (1981). The process was as follows. 15N2 was generated by adding sodium hypobromite (NaBrO) to 15N-labelled ammonium sulphate (15NH4)2SO4 (99.14 15N atom%, Shanghai Engineering Research Center of Stable Isotopes), and the products were passed through a liquid N2 cold trap, a KMnO4–KOH solution and a Na2SO4–H2SO4 solution to remove the 15N-labelled nitrogen oxides and ammonia (Ma et al. 2019b). The 15N enrichment of N2 within the three chambers was monitored by taking gas samples once a week during the 90-day labeling period. A MAT 253 stable isotope ratio mass spectrometer (Thermo Fisher Scientific Inc., Bremen, Germany) was used for analysis of the 15N-enrichment of N2 in the 15N2-labeled chamber.
Watering, fertilizer application and gas collection during the incubation period were carried out as follows. A 4 × 6 mm (inner diameter × outer diameter) PU (polyurethane) pipe was installed on the upper edge of each pot in the chamber, and a medical three-way valve was installed on the other end of the pipe, which was placed in the water (the water depth was about 15–20 cm) (Supplementary material: Fig. S1). A water tank was placed (2 m above the ground) next to the chamber. One end of the other PU pipe was connected to the water tank, and a two-way valve was installed on the other end. the two-way valve was closed at the intervals between watering. When watering was needed, the two-way valve was opened and connected to the three-way valve. When fertilization was needed, the weighed fertilizer was dissolved in water, and a syringe was used to connect the three-way valve to add the dissolved fertilizer solution to the pot. The pipe was then flushed with pure water to avoid fertilizer residue on the pipe wall. Gas samples were taken through a silicon septum fixed on the top of the chamber by a syringe with a needle.
Sampling and 15N determination
After 90 days of 15N2 continuous labelling, the growth chambers were opened for soil and plant sampling. In each pot, rice plants were separated into aboveground and belowground parts (roots). The soil samples were divided into 0–1 cm and 1–15 cm depths. Soil subsamples were stored at − 80 °C for further molecular analysis. The remaining soil sample was dried and ground using a Retsch MM 400 mixer mill (Retsch, Haan, Germany). A Thermo Finnigan Delta plus Advantages Mass Spectrometer coupled with an elemental analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used for analysis of total N content and 15N-enrichment in soil and plants.
DNA extraction, real-time quantitative PCR (qPCR) and sequencing library construction
Soil microbial DNA from the growth chamber was extracted using the FastDNA Spin Kit for Soil (MP Biomedicals, Cleveland, OH, USA). The concentration and purity of the extracted DNA were analyzed using a Ultramicro ultraviolet visible spectrophotometer (NanoVue Plus, USA). The primer pairs polF (TGCGAYCCSAARGCBGACTC)/polR (ATSGCCATCATYTCRCCGGA) (Poly et al. 2001) were used for quantifying nifH gene copies, the size of the PCR products was 362 bp. The PCR thermal cycling conditions were set as follows: an initial denaturation step of 10 min at 95 °C, followed by 35 cycles of amplification (30 s at 95 °C, and 35 s at 58 °C, 45 s at 72 °C), and a final extension step of 10 min at 72 °C. The copy number of the target gene was quantified by qPCR analysis with a CFX96 Optimal Real-time system (Bio-Rad Laboratories, Inc. Hercules, CA). For sequencing library construction, the nifH gene was amplified using the same primer set polF and polR. A sample tagging approach was used to recognize each sample in one run of MiSeq Sequencing. Each tag (12 bases), synthesized by Generay (Generay Biotech, China), was added to the 5′-end of the forward primer. The PCR procedure were as follows: an initial denaturation step of 3 min at 95 ℃, was followed by 35 cycles of amplification (30 s at 95 °C, and 30 s at 57 °C, 30 s at 72 °C), and a final extension step of 5 min at 72 °C. The PCR products were verified on 1.5% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Bio-sciences, USA). Equimolar amounts of different PCR amplicons were added to a tube and evenly mixed. The mixed products were sequenced on the Illumina MiSeq platform (Illumina, USA). More detailed information about the Real-time qPCR and sequencing library construction can be found in Ma et al. (2019b).
Processing of sequence data
The QIIME I was used to process and analyze the raw FASTQ sequences (Caporaso et al. 2010). Reads were assigned to each sample according to their unique tag. The detailed sequence data processing method can be found in Ma et al. (2019a). Then, a R package named microeco was used for further data mining and mapping (Liu et al. 2020).
Calculation and statistical analysis
The amount of 15N fixed by BNF in the rice soil system during the experiment was calculated as:
where %Nfixed is the percentage of N derived from BNF in rice plant and soil. Ni sample is the amount of N in rice plant and soil in the 15N2-labeled chamber.
Statistical differences of the data were analyzed using the one-way ANOVA and Tukey’s honestly significant difference (HSD) test in SPSS. Values with P < 0.05 were recognized as significantly different.
Results
15N2-labelling growth chamber performance
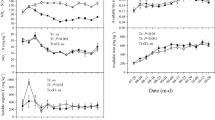
The abundance of 15N2 in the growth chambers decreased gradually from 8.26 to 1.99% over the rice season, with an average value of 5.44% (Fig. 1a). The CO2 concentration in the chambers remained at 400 ± 20μL L−1 during daytime, but it accumulated to a maximum of approximately 2000 μL L−1 at night due to lack of photosynthesis (Fig. 1b). The temperature in the growth chambers was consistent with the field temperature (Fig. 1c). The relative humidity in the chambers was between 50 and 90% (Fig. 1d). The oxygen produced by photosynthesis in the chambers was eliminated by a gas lighter, and the concentration was maintained at about 21% (Fig. 1e).
Performance of the 15N2-labelling growth chamber in the whole rice growing season. (a Dynamics of 15N-abundance in the 15N2-labelling growth chambers, b Hourly variations of the CO2 concentration in the 15N2-labelling chambers during daytime, c Hourly variations of the air temperature in the 15N2-labelling growth chambers and the surrounding rice field during the daytime, d Hourly variations of the relative humidity in the 15N2-labelling chambers during daytime, e variations of the O2 concentration in the 15N2-labelling chambers)
Nitrogen application levels on nitrogen fixation
The 15Nexcess sample in the 0–1 cm and 1–15 cm soil layers of the 0 N treatment was significantly higher than that in nitrogen fertilizer application treatments, also in each treatment, the 15Nexcess sample in the 0–1 cm soil layer was significantly higher than that in the 1–15 cm soil layer (Table 1). The Nfixed in the 0–1 cm and 1–15 cm soil layers in the 0 N treatment were significantly higher than those in the nitrogen application treatments, with the total Nfixed in soil in 0 N treatment being 6.5–8.3 times higher than those of the N application treatments (125, 187.5 and 250 kg N ha−1) (Table 1). Among the treatments of 125 N, 187.5 N and 250 N, there were no significant differences of the Nfixed in soil between each other either in the 0–1 cm or the 1–15 cm soil layers, respectively. The Nfixed was also mainly in the 0–1 cm soil layer for these nitrogen application treatments just as the 0 N treatment. The proportion of the Nfixed in the 0–1 cm soil layer to that in the whole soil were 70%, 82%, 88%, 98% in the 0 N, 125 N, 187.5 N, 250 N treatments, respectively (Table 1).
The Nfixed in the rice plant in the 0 N treatment was 2.8–4.3 times higher than that in the 125 N, 187.5 N and 250 N treatments (Table 1). Nitrogen application significantly reduced Nfixed in the whole rice plant and the aboveground plant, but there was no significant difference in the roots. With the increase of nitrogen application rate, the 15Nexcess sample of rice roots and the aboveground biomass decreased, but there was no statistical significance among the different N application treatments. No significant difference was observed in the whole rice biomass and the total nitrogen contained in rice among all the treatments (Table 1).
Irrigation practices on nitrogen fixation
In the 0 N treatment (Table 2), the Nfixed in the 0–1 cm soil layer, 1–15 cm soil and the total soil from the continuous flooding treatments was higher than those from the intermittent irrigation treatment, but was not statistically significant. While under the 250 N treatment, the Nfixed in the 0–1 cm soil layer and the total soil from the continuous flooding treatments was significantly higher than those from the intermittent irrigation treatment. In each treatment, the total nitrogen of 0–1 cm soil layer was significantly lower than that of 1–15 cm soil layer, while the Nfixed and the 15Nexcess sample in 0–1 cm soil layer were significantly higher than those in 1–15 cm soil layer.
The Nfixed in rice root, aboveground plant and the whole rice plant under intermittent irrigation was significantly higher than those under continuous flooding irrigation (Table 2). In the 0 N treatment, as compared with the flooding irrigation, the intermittent irrigation did not significantly change the 15Nexcess sample and total nitrogen of rice, but it significantly increased the dry weight of the whole rice plant. In the 250 N treatment, the intermittent irrigation either did not significantly change the 15Nexcess sample. But the intermittent irrigation significantly increased the total nitrogen of the aboveground part and the whole plant, and it also significantly increased the dry weight of the root, aboveground part and the whole plant. In each treatment, the Nfixed and dry weight of the aboveground plant were significantly higher than those in rice root, but there was no significant difference of 15Nexcess sample between the aboveground plant and rice roots (Table 2).
Proportion and distribution of fixed nitrogen in soil and rice
The nitrogen application significantly decreased the proportion of Nfixed to total nitrogen in soil and rice plant (Table 1), however, the water management didn’t significantly change the ratio of the Nfixed to total nitrogen in soil and rice plant (Table 2). The nitrogen application increased the proportion of Nfixed in the total rice to the total Nfixed (Table1). The proportion of Nfixed in the 0–1 cm soil layer to the total Nfixed increased with increasing nitrogen application rates. The ratio for the 250 N treatment was significantly higher than that in the 0 N and 125 N treatments (Table 1). Along with the increase of nitrogen application rate, the proportion of Nfixed in the 1–15 cm soil layer to the total Nfixed, as well as the proportion of Nfixed in the whole soil to the total Nfixed decreased, with the proportion in the 0 N treatment significantly higher than that in the 187.5 N and 250 N treatments. As compared to the 0 N treatment, nitrogen application didn’t change the proportion of Nfixed in root to the total Nfixed, but it increased the proportion of Nfixed in aboveground plant to total Nfixed, as well as the proportion of Nfixed in whole rice to total Nfixed. Intermittent irrigation increased the proportions of Nfixed in the total rice to the total Nfixed (Table 2). As compared with the continuous flooding irrigation, intermittent irrigation reduced the proportions of the Nfixed in 0–1 cm soil or in the whole soil to total Nfixed. On the contrary, intermittent irrigation increased the proportions of Nfixed in root, aboveground plant and whole rice plant to the total Nfixed (Table 2).
Abundance of nifH gene and nitrogen fixation activity
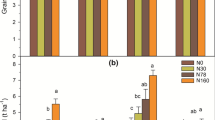
The total Nfixed of the 0 N, 125 N, 187.5 N, 250 N treatments were 15.59, 2.61, 2.93, and 2.16 mg N pot−1 (equivalent to 19.25, 3.22, 3.61, 2.67 kg N ha−1), respectively (Fig. 2a). Nitrogen application reduced the total Nfixed at 12.66–13.43 mg N pot−1(equivalent to 15.63–16.58 kg N ha−1), which accounted for 81.21–86.15% of total Nfixed in the 0 N treatment. On the contrary, the nifH gene copy numbers increased with the increase of nitrogen application rate, ranging from 1.16 × 105 to 3.23 × 105 copies g−1 dry soil. The average nitrogen fixation activity of 0 N treatment was significantly higher than those of nitrogen fertilizer treatments, which was by 15.49–10.76 times (Fig. 2b). The total Nfixed of the 0 N, 0 N + II, 250 N, 250 N + II treatments was 15.59, 16.21, 2.93, and 1.53 mg N pot−1 (equivalent to 19.25, 20.02, 2.67, 1.89 kg N ha−1), respectively (Fig. 3a). There was no significant difference in total Nfixed and nifH gene copy number between continuous flooding irrigation and intermittent irrigation. Similarly, there was no significant difference in the average nitrogen fixation activity between the continuous flooding irrigation and intermittent irrigation regimes (Fig. 3b).
Copy numbers of nifH gene, total nitrogen fixation (a) and average nitrogen fixation activity (b) under different nitrogen application rates. Error bars represent standard error (n = 3). In each figure, the same letters above the same color bars indicate non-significant (Tukey’s test, P > 0.05) difference among the treatments
Copy numbers of nifH gene, total nitrogen fixation (a) and average nitrogen fixation activity (b) under different irrigation practices. Error bars represent standard error (n = 3). In each figure, the same letters above the same color bars indicate non-significant (Tukey’s test, P > 0.05) difference among the treatments
Community compositions of diazotrophs
Under different nitrogen rate application treatments, the number of sequences per sample ranged from 82,420 to 112,997. 82,420 sequences were used to make the sequence number equal for each sample. After the data were filtered, 55,478 OTUs were maintained for further analysis. The nifH gene taxonomic analysis showed that Proteobacteria, Cyanobacteria, Actinobacteria and Firmicutes were dominant phyla, representing 86.8–96.8% of the diazotrophs in each treatment (Table 3). At the class level, the most abundant 10 classes are displayed in Table 3. Alphaproteobacteria was the most abundant class in the 125 N, 187.5 N and 250 N treatments, its relative abundance in the 125 N and 187.5 N treatments was significantly higher than that in the 250 N treatment, and the relative abundance of Alphaproteobacteria in the 250 N treatment was significantly higher than in the 0 N treatment. While the absolute abundance of Alphaproteobacteria in the 250 N treatment was significantly higher than that in the 0 N and 125 N treatments, but there is no statistical difference between 250 and 178.5 N. Gammaproteobacteria was the most abundant class in the 0 N treatment, and its relative abundance was significantly higher than that in the nitrogen application treatments, but there was no significant difference in absolute abundance among treatments. Both in terms of the absolute abundance and the relative abundance, Oscillatoriophycideae of the 250 N treatment were significantly higher than those of the other treatments. Compared with 0 N treatment, nitrogen application significantly increased the absolute abundance of Betaproteobacteria and Clostridia, but significantly decreased the absolute abundance of delta/epsilon subdivisions. The absolute abundance of Nostocales and Actinobacteria in the 250 N treatment were significantly higher than that in other treatments.
In different irrigation practice treatments, the number of sequences per sample ranged from 77,617 to 112,997. 77,617 sequences were used to make the sequence number equal for each sample. After the data were filtered, 71,875 OTUs were maintained for further analysis. The nifH gene taxonomic analysis showed that Proteobacteria, Cyanobacteria, Actinobacteria and Firmicutes were dominant phyla, representing 87.9–96.2% of the diazotrophs in each treatment (Table 4). At class level, intermittent irrigation significantly increased the relative abundance of Alphaproteobacteria, but it only increased the absolute abundance of Alphaproteobacteria under 0 N application. Intermittent irrigation significantly decreased the absolute abundance of Gammaproteobacteria but it only decreased the relative abundance of Gammaproteobacteria under 0 N application. Intermittent irrigation significantly decreased the relative and absolute abundance of Oscillatoriophycideae, Nostocales and Stigonematales under 250 N application, but it didn’t change the relative and absolute abundance of Oscillatoriophycideae, Nostocales and Stigonematales under 0 N application.
Discussion
Nitrogen application greatly reduced the total Nfixed by 81.21–86.15%, but along with the increase of nitrogen application from 125 to 250 N, there was no further significant decrease of total Nfixed (Fig. 2a). The nitrogen application also significantly decreased the contribution of Nfixed to total nitrogen in soil and rice plant (Table 1). These results were broadly in accordance with our hypothesis that nitrogen application decreases the amount of BNF, however, the inhibition of BNF was not gradual but decreased rapidly with increasing N application, and as nitrogen application levels increased further, no more significant reduction was observed. This implies that under 125 kg N ha−1 (or greater than 125 kg N ha−1) applications, BNF contributes much less nitrogen than anticipated 22 kg ha−1 to rice fields (Ladha et al. 2016). In this experiment, if there were levels lower than125 kg N ha−1 (such as 60 kg N ha−1) of nitrogen fertilizer application, a gradual decreasing trend might be observed. BNF has been found to be suppressed in rhizosphere samples after ammonium sulphate treatments at 40 kg N ha−1, and even more so at 80 kg N ha−1 (Charyulu et al. 1981). This may be due to nitrogen applications significantly reducing the activity of nitrogenase (Fig. 2b). Nitrogen application also affects the distribution of fixed nitrogen in soil. The proportion of Nfixed in the 0–1 cm soil layer to total BNF increased with increasing nitrogen application rate, but the proportion of Nfixed in the 1–15 cm soil layer to total Nfixed decreased with increasing application rate (Table 1). This may be related to the distribution and retention time of the urea added to soil. Firstly, the NH4+ derived from the urea on the soil surface can easily diffuse into the atmosphere through the water layer (Inubushi and Watanabe, 1986). Therefore, as compared with the 1–15 cm soil layer, the retention time of NH4+ in 0–1 cm soil layer is relatively shorter, thus the nitrogen fixation activity of nitrogen-fixing bacteria in the 0–1 cm soil layer may be less inhibited by nitrogen application. In addition, ammonia deposition in this closed system may lead to an underestimation of the amount of BNF in the 0 N treatment. Secondly, there is still a certain oxidation environment on the surface of soil (Knauth et al. 2005; Irisarri et al. 2001), where part of NH4+ may be transformed into NO3− by nitrifying bacteria (Yamamuro et al. 1986). Cyanobacteria was reported to be the main nitrogen-fixing bacteria on the surface soil of rice field (Ma et al. 2019b; Wang et al. 2020). Großkopf (2012) found that the presence of high nitrate concentrations (up to 800 mM) had no inhibitory effect on growth and nitrogen fixation activity of cyanobacteria. In this study, the 250 N nitrogen fertilizer application significantly increased the relative and the absolute abundance of cyanobacteria in the surface (0–1 cm) soil, and also increased the number of Oscillatoriophycideae, Nostocales and Stigonematales (Table 3). The increased relative abundance of cyanobacteria did not do contribution to the BNF.
As compared with the continuous flooding irrigation, the intermittent irrigation had no significant effect on the total Nfixed and the ratio of the Nfixed to total nitrogen in soil and rice plant (Table 2), but it changed the distribution of Nfixed in soil and rice plants (Table 2). Intermittent irrigation reduced the proportion of Nfixed in the 0–1 cm soil layer and whole soil to total Nfixed, but it increased the proportion of Nfixed in root, aboveground plant and whole rice plant to total Nfixed. This may be due to the change of soil moisture status altering soil microbial abundance and community structure (Lu et al. 2006; Yoshida and Ancajas 1971). When the paddy soil has insufficient water, some anaerobic nitrogen-fixing bacteria will die and release nitrogen-containing compounds which will be absorbed by rice (Valiente et al. 2000). As compared with the 1–15 cm soil layer, the 0–1 cm soil layer is more likely to be subjected to the water shortage, but Nfixed is mainly concentrated in the 0–1 cm soil layer (Table 1) (Bei et al. 2013; Ma et al. 2019a). Therefore, more Nfixed was transferred from the 0–1 cm soil layer to rice than from the 1–15 cm soil layer (Table 1). For the whole growing season, intermittent irrigation did not change the total Nfixed, which may be due to the decrease of Nfixed during the water shortage period being offset by the potential increase of Nfixed in flooding period. When the paddy soil changed from a flooded state to a water deficient state, the death or deactivation of some anaerobic nitrogen-fixing bacteria and the inhibition of oxygen on nitrogen fixation could reduce the Nfixed (Valiente et al. 2000). When the paddy soil changed from a water deficient state to a flooded state, the soil oxygen content and redox potential (Eh) would decrease rapidly, the soil pH would tend to be neutral, and the soil available carbon may increase due to the potential increase of soil organic matter decomposition (Zeng et al. 2011). These changes provide better environmental conditions and energy sources beneficial for BNF than the continuous flood condition (Bais et al. 2006). Therefore, the BNF activity during the flooding period within the intermittent irrigation pattern was supposed to be higher than that of the continuous flooding pattern. In addition, no matter in 0 N or 250 N treatments, intermittent irrigation significantly increased the rice biomass, which in turn influenced the soil microbial activity and nutrient availability. A large amount of organic matter secreted by rice roots can provide an energy source for biological nitrogen fixation (Knauth et al. 2005). Furthermore, some nitrogen in the soil was absorbed by rice roots, which create a low nitrogen environment for nitrogen-fixing bacteria. Thus, the inhibition effect of ammonium in the process of biological nitrogen fixation was reduced (Reed et al. 2011). In general, as compared with the continuous flooding irrigation, although intermittent irrigation did not change the total Nfixed, it promoted the transfer of Nfixed from the 0–1 cm soil layer to rice, and therefore the nitrogen use efficiency.
Numerous PCR primers have been designed to amplify nifH, and different primers have different coverage for phylogenetic and environmental groupings in the nifH database (Gaby and Buckley 2012). In this study, two pairs of primers were used, polF (TGCGAYCCSAARGCBGACTC)/polR (ATSGCCATCATYTCRCCGGA) (Poly et al 2001) and nifHF (TGYGAYCCNAARGCNGA)/nifHRb (ADNGCCATCATYTCNCC) (Gaby and Buckley 2012; Fan et al. 2019). However, the concentration of the products that amplified by the pair of nifHF/nifHRb primers was too low. Even though the polF/polR primers theoretically have a lower recovery of nifH diversity than other primers such as the Zf/Zr pair (Zehr and McReynolds 1989; Gaby and Buckley, 2012), the polF/polR display a higher performance in vitro than in silico and can be used directly for qPCR (Poly et al., 2001; Mårtensson et al., 2009; Bouffaud et al., 2016). So only the pair of polF/polR primers was used herein. The nifH gene copy numbers increased with the increase of nitrogen application rate (Fig. 2a), although nitrogen application decreased the amount of BNF. Soil nitrogen-fixing bacteria can use a variety of nitrogen sources to support their growth. The preferred order of different forms of nitrogen fertilizer used by diazotrophs is as follows: (1) inorganic N, (2) low-molecular-weight organic N, (3) atmospheric N, and (4) high-molecular-weight organic N. Urea-N (low-molecular weight organic N) and its converted NH4+-N can be directly absorbed by nitrogen-fixing bacteria (Norman and Friesen 2017). Therefore, when the urea-N and inorganic N in soil is sufficient, there is no need for them to consume energy to obtain nitrogen source through biological nitrogen fixation. However, ammonium is well known to inhibit N fixation (Reed et al. 2011) and has been shown to inhibit nitrogenase synthesis at the genetic level through the regulation of nifA gene transcription (Dixon and Kahn 2004; Norman and Friesen 2017). On the other hand, Fan et al (2019) found that long-term fertilization reduces N fixation and specific groups of N fixers. In this study the soil used was collected from the paddy soil with a long-term nitrogen fertilization of 250 kg N ha−1, but we did not find that the reduction of nitrogen application increased the number of nitrogen-fixing bacteria. Nitrogen application enhanced the number of nitrogen-fixing bacteria, but it also inhibited nitrogen fixation activity (Fig. 2b). Irrigation practices did not change the nifH gene copy numbers (Fig. 3a). Cyanobacteria were expected to be the main nitrogen-fixing bacteria in the surface soil of paddy field (Ma et al. 2019b; Wang et al. 2020). Instead, the 250 N application significantly in this study increased the absolute abundance of Oscillatoriophycideae, Nostocales and Actinobacteria (Table 3). Intermittent irrigation significantly decreased the relative and absolute abundance of Oscillatoriophycideae, Nostocales and Stigonematales under 250 N application (Table 3), but the increase and decrease of cyanobacteria did not affect the total amount of nitrogen fixation.
Conclusion
Increased nitrogen application decreased N2 fixation from 19.25 kg ha−1 for the 0 nitrogen application treatment to 3.22, 3.61, and 2.67 kg ha−1, respectively, for the 125, 187.5 and 250 kg ha−1 nitrogen application treatments. These results are broadly in accordance with our hypothesis that nitrogen application (equal or greater than 125 kg N ha−1) reduced the amount of BNF, however, the inhibition of BNF was not gradual but decreased rapidly with increasing N application, and as nitrogen application increased further, no further obvious reduction was observed. Inhibition of BNF by nitrogen application at the 1–15 cm soil horizon was greater than that in the 0–1 cm soil horizon. Intermittent irrigation increased the allocation of Nfixed in the rice. As compared with the continuous flooding pattern, the intermittent irrigation enhanced rice growth and promoted the transfer of Nfixed from 0–1 cm the soil layer to rice, but it did not change the total Nfixed. Increased nitrogen application promoted the nifH gene abundance, but it decreased their activity to fix atmospheric N2.
References
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266
Bei QC, Liu G, Tang HY, Cadisch G, Rasche F, Xie ZB (2013) Heterotrophic and phototrophic 15N2 fixation and distribution of fixed 15N in a flooded rice–soil system. Soil Biol Biochem 59:25–31
Bodelier PLE, Roslev P, Henckel T, Frenzel P (2000) Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 403:421–424
Bouffaud ML, Renoud S, Moënne-Loccoz Y, Muller D (2016) Is plant evolutionary history impacting recruitment of diazotrophs and nifH expression in the rhizosphere? Sci Rep 6:21690
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336.Charyulu PBBN, Nayak DN, Rao VR (1981) 15N2 incorporation as influence of rice variety, organic matter and combined nitrogen. Plant Soil 59:399–405
Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2:621–631
Fan KK, Delgado-Baquerizo M, Guo XS, Wang DZ, Wu YY, Zhu M, Yu W, Yao HY, Zhu YG, Chu HY (2019) Suppressed N fixation and diazotrophs after four decades of fertilization. Mirobiome 7:143
Gaby JC, Buckley DH (2012) A comprehensive evaluation of PCR primers to amplify the nifH gene of nitrogenase. PLoS ONE 7:e42149
Gao KS (1998) Chinese studies on the edible blue-green alga, Nostoc flagelliforme: a review. J Appl Phycol 10:37–49
Großkopf T, LaRoche J (2012) Direct and Indirect Costs of Dinitrogen Fixation in Crocosphaera watsonii WH8501 and Possible Implications for the Nitrogen Cycle. Front Microbiol 3:236
Hoobs NT, Schimel D (1984) Fire effects on nitrogen mineralization and fixation in mountain shrub and grassland communities. J Range Mamag 37:402–405
Inubushi K, Watanabe I (1986) Dynamics of available nitrogen in paddy soils. Soil Sci Plant Nutr 32:561–577
Irisarri P, Gonnet S, Monza J (2001) Cyanobacteria in Uruguayan rice fields: diversity, nitrogen-fixing ability and tolerance to herbicides and combined nitrogen. J Biotechnol 91:95–103
Knauth S, Hurek T, Brar D, Reinhold-Hurek B (2005) Influence of different Oryza cultivars on expression of nifH gene pools in roots of rice. Environ Microbiol 7:1725–1733
Kox MAR, Lüke C, Fritz C, Elzen E, Alen T, Camp H, Lamers L, Jetten MSM, Ettwig KF (2016) Effects of nitrogen fertilization on diazotrophic activity of microorganisms associated with Sphagnum magellanicum. Plant Soil 406:83–100
Ladha JK, Reddy PM (2003) Nitrogen fixation in rice systems: state of knowledge and future prospects. Plant Soil 252:151–167
Ladha JK, Tirol-Padre A, Reddy CK, Cassman KG, Verma S, Powlson DS, Kessel C, Richter DB, Chakraborty D, Pathak H (2016) Global nitrogen budgets in cereals: a 50-year assessment for maize, rice, and wheat production systems. Sci Rep 6: 19355
Liu C, Cui YM, Li XZ, Yao MJ (2020) Microeco: an R package for data mining in microbial community ecology. FEMS Microbiol Ecol 97(2): fiaa255
Lu Y, Rosencrantz D, Liesack W, Conrad R (2006) Structure and activity of bacterial community inhabiting rice roots and the rhizosphere. Environ Microbiol 8:1351–1360
Ma J, Bei QC, Wang XJ, Lan P, Liu G, Lin XW, Liu Q, Lin ZB, Liu BJ, Zhang YH, Jin HY, Hu TL, Zhu JG, Xie ZB (2019a) Impacts of Mo application on biological nitrogen fixation and diazotrophic communities in a flooded rice-soil system. Sci Total Environ 649:686–694
Ma J, Bei QC, Wang XJ, Liu G, Cadisch G, Lin XW, Zhu JG, Sun XL, Xie ZB (2019b) Paddy System with a Hybrid Rice Enhances Cyanobacteria Nostoc and Increases N2 Fixation. Pedosphere 29:374–387
Mårtensson L, Díez B, Wartiainen ZWW, EI-Shehawy R, Rasmussen U (2009) Diazotrophic diversity, nifH gene expression and nitrogenase activity in a rice paddy field in Fujian, China. Plant Soil 325:207–218
Nag P, Shriti S, Das S (2019) Microbiological strategies for enhancing biological nitrogen fication in nonlegumes. Journal of Applied Microbiology 1364–5072 Norman JS, Friesen ML (2017) Complex N acquisition by soil diazotrophs: how the ability to release exoenzymes affects N fixation by terrestrial free-living diazotrophs. ISME J 11:315–326
Ohyama T, Kumazawa K (1981) A simple method for the preparation, purification and storage of15N2gas for biological nitrogen fixation studies. Soil Sci Plant Nutr 27:263–265
Patra AK, Le Roux X, Abbadie L, Clays-Josserand A, Poly F, Loiseau P, Louault F (2007) Effect of microbial activity and nitrogen mineralization on free-living nitrogen fixation in permanent grassland soils. J Agron Crop Sci 193:153–156
Poly F, Ranjard L, Nazaret S, Gourbiere F, Monrozier LJ (2001) Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262
Rao VR, Rao JLN (1984) Nitrogen fixation (C2H2 reduction) in soil samples from rhizosphere of rice grown under alternate flooded and nonflooded conditions. Plant Soil 81:111–118
Reed SC, Cleveland CC, Townsend AR (2011) Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512
Roger PA, Watanabe I (1996) Technologies for utilizing biological nitrogen fixation in wetland rice: potentialities, current usage, and limiting factors. Fertilizer Res 9:39–77
Rosenblueth M, Ormeño-Orrillo E, López-López A, Rogel MA, Reyes-Hernández BJ, Martinez-Romero JC, Reddy PM, Martinez-Romero EM (2018) Nitrogen fixation in cereals. Front Microbiol 9:1794
Santiago-Ventura BM, Daez C, Ventura V, Watanabe I, App AA (1986) Effects of N-fertilizers, straw, and dry fallow on the nitrogen balance of a flooded soil planted with rice. Plant Soil 93:405–411
Scherer S, Ernst A, Chen TW, Böger P (1984) Rewetting of drought-resistant blue-green algae: time course of water uptake and reappearance of respiration, photosynthesis, and nitrogen fixation. Oecologia 62:418–423
Smercina DN, Evans SE, Friesen ML, Tiemann LK (2019) To fix or not to fix: controls on free-living nitrogen fixation in the rhizosphere. Appl Environ Microbiol 85(6):e02546-e2618
Tadakatsu Y, Junko TT, Kiwamu M (2017) Exploration of bacterial N2-fixation systems in association with soil-grown sugarcane, sweet potato, and paddy rice: a review and synthesis. Soil Sci Plant Nutr 63(6):578–590
Tan ZY, Hurek T, Reinhold-Hurek B (2003) Effect of N-fertilization, plant genotype and environmental conditions on nifH gene pools in roots of rice. Environ Microbiol 5:1009–1015
Valiente EF, Ucha A, Quesada A, Leganes F, Carreres R (2000) Contribution of N2 fixing cyanobacteria to rice production: availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant Soil 221:107–112
Wang XJ, Liu BJ, Ma J, Zhang YH, Hu TL, Zhang H, Feng YC, Pan HL, Xu ZW, Liu G, Lin XW, Zhu JG, Bei QC, Xie ZB (2019) Soil aluminum oxides determine biological nitrogen fixation and diazotrophic communities across major types of paddy soils in China. Soil Biol Biochem 131:81–89
Wang XJ, Bei QC, Yang W, Zhang H, Hao JL, Li Q, Feng YC, Xie ZB (2020) Unveiling of active diazotrophs in a flooded rice soil by combination of NanoSIMS and 15N2-DNA-stable isotope probing. Biol Fertil Soils 56:1189–1199
Wang YN, Ke XB, Wu LQ, Lu YH (2009) Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as affected by nitrogen fertilization. Syst Appl Microbiol 32(1):27–36
Yamamuro S (1986) Behavior of nitrogen in paddy soils. Jpn Agric Res Q 20:100–107
Yoshida T, Ancajas RR (1971) Nitrogen fixation by bacteria in the root zone of rice. Soil Sci Soc Amer Proc 35:156–158
Zehr JP, McPeynolds LA (1989) Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium thiebautii. Appl Environ Microbiol 55:2522–2526
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159:84–91
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015) Managing nitrogen for sustainable development. Nature 528(7580):51–59
Zheng Y, Zhang LM, Zheng YM, Di HJ (2008) Abundance and community composition of methanotrophs in a Chinese paddy soil under long-term fertilization practices. J Soils Sediments 8(6):406–414
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31870500 and 41501273), and the Special Project on the Basis of the National Science and Technology of China (2015FY110700).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Euan K. James.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Hu, T., Wang, H. et al. How do different nitrogen application levels and irrigation practices impact biological nitrogen fixation and its distribution in paddy system?. Plant Soil 467, 329–344 (2021). https://doi.org/10.1007/s11104-021-05093-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05093-7