Abstract
This study investigated the effects of biochar, produced from plantain peel, on the yield of potatoes (Solanum tuberosum L.) irrigated with wastewater in two consecutive seasons. Potatoes were grown in 2015 and 2016 in nine lysimeters (1.0 m × 0.45 m), packed with sandy soil to a bulk density of 1.35 Mg m−3. The lysimeters were arranged in a completely randomized design with three replicates. The treatments were (i) wastewater with biochar, (ii) wastewater without biochar, and (iii) freshwater without biochar. The soil with biochar treatments was amended in 2015 with an application rate of 1% (w/w) on the top 0.1 m of soil. After 33 days of planting, the potatoes were irrigated 8 times, on a 10-day irrigation interval, with freshwater or wastewater that was synthesized to represent a typical wastewater in developing countries. Plant health parameters (e.g., photosynthetic rate) were measured. After 120 days of planting, the potato tubers were harvested; the fresh weight was measured and the tubers were counted. The plant health parameters (e.g., photosynthesis rate) varied with time but were not affected by biochar amendment. Also, the total fresh tuber weights as well as the total number of tubers were similar in all treatments although the biochar showed a significant positive effect (p < 0.05) on the pH and the cation exchange capacity of the soil. Thus, it was concluded that application of the plantain peel biochar as soil amendment showed no significant effect on the yield of potatoes irrigated with wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With a current value of 7.3 billion, the world population is expected to reach 11.2 billion by 2100 (Teytelboym 2016; United Nations 2017). Accordingly, more water will be required to produce more food for the growing population. This will potentially exert more pressure on freshwater, especially in the agricultural sector, since 70% of its withdrawal is currently used in agriculture, worldwide, to sustain food production (Aquasat 2014). Therefore, there is an urgent need to seek for alternative sources of water (for agriculture) that will reduce the burden on the current freshwater resources and still increase crop yield.
Wastewater could be a viable option to supply supplemental irrigation water given that it is quite available and, in addition, is reportedly rich in macronutrients such as N, P, and K (Qian and Mecham 2005; Raschid-Sally and Jayakody 2009; Singh et al. 2012), which could reduce cost of inorganic fertilizers. For instance, wastewater irrigation increased soil available N, P, and K, as well as organic carbon (Singh et al. 2012; Mojid et al. 2019). Despite its benefits, wastewater irrigation could have detrimental effects on soil properties such as pH (Mojid et al. 2019), which could affect the availability of plant essential nutrients in soil solution and consequently reduce crop yield. Furthermore, wastewater irrigation could release antagonistic heavy metals in soil (Angin et al. 2005), depending on the wastewater source. Therefore, incorporating soil conditioners, such as biochar, is important as they could bound wastewater-laden contaminants and reduce their uptake by crops (Puga et al. 2016; Nzediegwu et al. 2019); biochar could also increase soil pH and CEC leading to higher crop yield (Gao et al. 2017).
Biochar, a solid product of organic waste pyrolysis, is reportedly rich in soluble salts, which translates to high soil pH (Lehmann and Joseph 2009; Singh et al. 2010). When incorporated into soil (acidic), biochar raised the pH of soil (Alling et al. 2014). For instance, hardwood hickory-derived biochar increased soil pH (15%) as compared to no-biochar-amended soil (Laird et al. 2010); the study also reported that biochar significantly increased the surface area of the soil. However, establishing clear relationships between soil physiochemical properties (e.g., pH) and biochar application (as a soil amendment) is difficult due to the variability in the properties of biochar and the site-specific interactions with soil and vegetation (Alburquerque et al. 2013; Xie et al. 2014).
Variability in the characteristics of biochar often results from different feedstocks used for its production (Alburquerque et al. 2013). A few studies have shown conflicting results—either positive (Artiola et al. 2012; Butnan et al. 2015; Rodríguez-Vila et al. 2015) or neutral (Borchard et al. 2014; Jay et al. 2015)—with respect to crop yield when different biochars were used. For instance, Artiola et al. (2012) reported that biochar derived from pine forest waste increased the pH of soil and subsequently increased the production of Bermuda grass. Likewise, Alburquerque et al. (2013) showed that when wheat straw and olive tree pruning biochar were added to soil, available P as well as the yield of wheat grain increased. On the contrary, biochars derived from wood (Fagus species and Picea species) had no effect on the yield of maize (Borchard et al. 2014), and biochar from another wood (Castanea sativa) also had no effect on the yield of potatoes (Jay et al. 2015).
Plantain peel (40% of plantain fruit (Rubatzky and Yamaguchi 1997)) could be a sustainable feedstock for transforming into biochar since it ends up as a waste in most countries where plantain is produced (Tchango Tchango et al. 1999), thereby adding value to the waste, while protecting the environment from nuisance. In a previous study (Nzediegwu et al. 2019), plantain peel biochar (mixed with sandy soil under potato cultivation) adsorbed wastewater-laden heavy metals (Cd and Zn) as compared to no-biochar soil; it is also known that the effect of biochar is feedstock- and crop-specific. Therefore, an investigation on the effect of plantain peel biochar on a given crop like potato is valuable because potato ranks first among other tuber crops (Consortium 2011) and its demand (globally) is increasing possibly due to its nutritional value (Brown 2005; FAO 2017). A few biochars have been tested for growing potatoes (Akhtar et al. 2015; Collins et al. 2013; Hien et al. 2017; Koga et al. 2017; Liu et al. 2017; Liu et al. 2014; Walter and Rao 2015); biochars from bamboo, wood, rice husk, and kunai grass have shown either positive (Hien et al. 2017; Liu et al. 2014; Walter and Rao 2015), neutral (Jay et al. 2015; Koga et al. 2017), or negative (Liu et al. 2017) impact on the yield of potatoes. It needs to be investigated whether plantain peel biochar affects crop yield. Moreover, it is not clear what would be the interaction of wastewater irrigation and this biochar in soil, and consequently the effect on potato yield. Therefore, our goal was to understand the effect of plantain peel biochar on the yield of potatoes, cultivated in a lysimeter field soil and irrigated with synthetic wastewater for two seasons. Specifically, different yield parameters such as tuber weight and number of tubers were measured for the two seasons (2015 and 2016).
2 Materials and Methods
2.1 Biochar Characterization
Detailed description of the biochar used in this study has been documented elsewhere (Nzediegwu et al. 2019). Briefly, the biochar has a pH of 10.3, determined following dissolution of the biochar (1:30 w/w) in deionized water, shaken for 4 h, and measured with an electrode type pH meter (Accumet AB 15); it has a mineral ash content of 77.45%, determined following ASTM 7582. The total C and total N, determined as per ASTM D5373, were 18.1 and 0.6%, respectively, resulting in a C:N ratio of 30.2. The total metal (essential and non-essential; Table 1) contents were determined by nitric acid digestion method (Kargar et al. 2013), where 0.16 g of the oven-dried biochar was digested with 2 mL of 70% nitric acid on a block digester with the temperature gradually increased to 120 °C and then held for 5 h; afterwards, the digested solution was diluted (50 times) with double deionized water. The soluble metals were analyzed using inductively coupled plasma mass spectrometry (Varian ICP820-MS or Analytik-Jena).
2.2 Experimental Setup
The experiment was conducted in 2015 (the first season) and 2016 (the second season) between the months of June and October, when outside temperature was above 10 °C (Food and Agriculture Organization FAO 2008). A BIOS thermometer/hydrometer (Model: tr415) was installed outside to record temperature for the experimental period. Sandy soils were packed to a bulk density of 1.35 Mg m−3 in nine outdoor lysimeters, having four 10-mm holes drilled radially at 0.15-m depth for soil sampling. Properties of the soil are presented in Table 1. The lysimeters were arranged in a completely randomized design with 3 replicates. The three treatments studied were wastewater and biochar (WW+B), wastewater with no biochar (WW-B), and freshwater without biochar (FW-B). The WW-B served as control for the biochar treatment (WW+B), while FW-B served as control for the wastewater treatment (WW-B). The soil was brought to field capacity 1 day before biochar was incorporated. The biochar was mixed in the three lysimeters under WW+B at the top soil profile (0- to 0.1-m depth) at a rate of 13.5 t ha−1. Afterwards, potatoes (Solanum tuberosum L., cv. Russet Burbank) were procured and planted. The procedures for potato procurement, planting, fertilization, and irrigation have been documented elsewhere (Nzediegwu et al. 2019). Five weeks after planting, when the potatoes had emerged and stabilized, as visualized by new leaf development, synthesized wastewater (Table 2) as well as freshwater was applied (11.5 L lysimeter−1) at a 10-day interval for eight times. Soil samples were collected from the surface and 0.1-m depth 2 days after irrigation and stored in − 24 °C freezer for further analysis.
Plant health parameters were monitored from when the plant canopy had developed (~ 5 weeks after planting) till the end of growing season. All foliage measurements were taken from the fourth petiole (Stark et al. 2004) on days 1, 3, and 9 after each irrigation, which correspond to when the potatoes were either water stressed or not. With the photon flux density set to 800 μmol m−2 s−1 (i.e., twice the value used in greenhouse studies (Robredo et al. 2007)), photosynthesis rate was measured using LI-COR portable photosynthesis system (LI-6400, LI-COR Inc., Lincoln, Nebraska). Greenness, a measure of leaf chlorophyll level, was measured with Minolta chlorophyll meter (SPAD-502, MINOLTA Co. Ltd., Japan). The leaf temperature, a measure of water stress, was measured using Hylogy Infrared Digital thermometer (Model: MD-H4); typically, a clearance of 0.03 m was allowed between the leaf surface and the infrared thermometer. Plant reflectance (normalize difference vegetative index (NDVI)), which serves as a growth monitoring parameter (Prasad et al. 2006), was measured with an active canopy crop sensor (Crop Circle, ACS-430, Holland Scientific, Lincoln Nebraska USA); for each lysimeter, a clearance of 0.25 m was maintained between the plant canopy and the sensor.
After 120 days of planting, the potatoes were harvested. The root weight, shoot length, number of tubers, above ground biomass weight, and total tuber weight were recorded. The yield per lysimeter (alias yield per plant) for each treatment was calculated as the average tuber weight. With a row spacing of 0.9 m and a within row spacing of 0.3 m (Bohl et al. 1995), a plant density (i.e., number of plant per hectare) of 35,880 plant ha−1 (Canadian Food Inspection Agency CFIA 2017) was used to calculate the total yield (t ha−1) (i.e., plant density × yield (t) per plant ). The potato tubers were graded by passing them, one after the other, through a 50-mm diameter hole (Shiri-e-Janagard et al. 2009; USDA 1983). The gravimetric moisture content of the soil samples, collected depth-wise (0.0 m and 0.1 m), 2 days after each irrigation, were determined following the standard oven-dry method (Hollinger and Isard 1994). The wet soil samples were weighed and dried in an oven at 105 °C for 24 h; the moisture content was calculated as ((weight of wet soil − weight of dry soil)/weight of wet soil) × 100 % . The exchangeable cations and cation exchange capacity (CEC) of the soil samples were determined following BaCl2 method (Carter and Gregorich 2008), while the pH of the soil was determined following the standard soil survey method (Rayment and Higginson 1992), where air-dried soil was bathed with deionized water (1:5 w/w soil:water), shaken for 1 h, and the suspension measured with an electrode type meter (Accumet AB 15).
2.3 Data Analysis
All analyses were performed using SAS-JMP® Pro 13.2.0 (Copyright © 2016 SAS Institute Inc.). The greenness, leaf temperature, photosynthetic measurement, and NDVI data were subjected to repeated measures analysis of variance using the compound multivariate approach; the two repeated factors were irrigation interval and days after irrigation (i.e., when the measurements were taken). The soil moisture data was subjected to one-layer repeated measures analysis, while the yield related data, exchangeable cations, and CEC were subjected to one-way analysis of variance.
3 Results
3.1 Leaf Greenness
The greenness values, measured at the bulking stage in the first season (2015), are presented in Fig. 1 along with the greenness values in the second season (2016), segmented by three levels, 1, 3, and 9 days after irrigation, for the vegetative season of the potatoes. Although with no significant treatment effect (p > 0.05), the SPAD readings in the first season revealed a slight decrease in greenness from about 35 at the beginning to about 31 towards the end of the season. Likewise, in the second season, after the second split N fertilization was applied on day 51, the greenness increased for about 2 weeks and then gradually decreased with the growing season. Irrigation with wastewater showed no significant difference (p > 0.05) in greenness as compared to the corresponding greenness in the freshwater treatment (Fig. 1). Overall, biochar amendment did not affect the leaf greenness for both growing seasons.
Greenness measured using SPAD for both growing seasons in 2015 and 2016. For the first season, Day 93-IR7 and Day 103-IR8 indicate irrigations 7 and 8, respectively; the error bars represent standard error for three replicates. FW-B is freshwater without biochar, WW+B is wastewater with biochar, and WW-B is wastewater without biochar
3.2 Leaf and Ambient Temperature
The leaf and ambient temperature values, measured in the second season and segmented according to days after irrigation, are presented in Fig. 2. For the entire monitoring period, the leaf temperature ranged from 17.5 to 30.6 °C. There was effect of days, driven mainly by the ambient air temperature (Fig. 2). Overall, neither irrigation water quality nor biochar amendment showed any significant effect on the leaf temperature.
Leaf temperature measured in the second season and segmented by levels of days after irrigation; ambient is the ambient air temperature in the tent; the error bars represent standard error of three replicates. FW-B is freshwater without biochar, WW+B is wastewater with biochar, and WW-B is wastewater without biochar
3.3 Photosynthesis Rate
The photosynthesis rates of the potatoes, measured in the second season, are presented in Fig. 3. Photosynthesis indicated a general trend of decrease with time for all three measurement days, although statistically not significant (p > 0.05). It ranged from 14.16 μmol CO2 m−2 s−1 few days after the first irrigation to 2.21 μmol CO2 m−2 s−1 towards the end of the season; however, there was no treatment effect.
3.4 Normalized Difference Vegetative Index
The normalized difference vegetative index (NDVI), measured during the vegetative period in 2016 and segmented according to days after irrigation, is presented in Fig. 4. Overall, the NDVI ranged from 0.61 (day 53) to 0.86 (day 73) and there was no treatment effect.
3.5 Soil Moisture
Figure 5 illustrates the moisture content (gravimetric) of the soil sampled at the surface and 0.1 m below (i.e., \( {\left[\mathrm{MC}\right]}_{\mathrm{soil}}^{\mathrm{surf}} \) and\( {\left[\mathrm{MC}\right]}_{\mathrm{soil}}^{0.1} \), respectively) for both seasons (2015 and 2016), whereas Table 3 presents the repeated measures analysis. In the first season, the lowest \( {\left[\mathrm{MC}\right]}_{\mathrm{soil}}^{\mathrm{surf}} \)of 7.1% was measured in the WW-B after the first irrigation, whereas after eight irrigations, the highest moisture of 11.5% was measured, depicting an increase in moisture with irrigation. In the second season, a similar trend was noticed, where \( {\left[\mathrm{MC}\right]}_{\mathrm{soil}}^{\mathrm{surf}} \) increased with irrigation. This was true also for the biochar-amended treatment (WW+B), mainly in the second season. Interestingly, it seems that in the first season, while the WW-B held more water at the surface, the WW+B held more water at the 0.1-m depth, where water is mostly required by plant roots for metabolism (Clothier and Green 1994). At 0.1-m depth, irrigation had no effect on the soil moisture as \( {\left[\mathrm{MC}\right]}_{\mathrm{soil}}^{0.1} \) in WW-B remained almost the same for the both seasons.
3.6 Exchangeable Cations and Cation Exchange Capacity
Table 4 presents the exchangeable cations, cation exchange capacity (CEC), and base saturation of the soil, with or without biochar. The CEC of the surface soil for both treatments was apparently similar. However, at the 0.1-m depth, the CEC significantly increased (p < 0.05) by 65% in WW+B (vs. WW-B). The base saturation on the other hand was significantly higher at both depths in the WW+B treatment as compared to the WW-B treatment.
3.7 Potato Yield
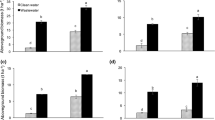
With 28 mm < potato size ≤ 50 mm, irrespective of the treatments (WW-B, WW+B, and FW-B), the potatoes were considered as marketable in line with Shiri-e-Janagard et al. (2009). As such, the potatoes were pooled together, regardless of the size, for tuber weight measurement. Figure 6 presents the yield parameters of the potatoes under wastewater (with biochar (WW+B) or without biochar (WW-B) amendment) and freshwater (FW-B) irrigation for the two seasons. Being a measure of the yield, the tuber weight per plant varied in both seasons. More specifically, in the first season, the tuber weight in FW-B was 0.83 kg/plant, whereas in the second season, the tuber weight was 0.26 kg/plant, showing a 69% decrease. This observation was not peculiar to FW-B treatment as WW-B showed almost similar (70%) decrease as well. There was no significant difference in tuber weight between the wastewater irrigated potatoes and their freshwater counterpart for both seasons. For the biochar-amended treatment (WW+B), the tuber weight in the first season was quite less as compared to the WW-B treatment. In the second season, no treatment effect on the potato yield was observed.
Similar to the tuber weight, the average number of tubers varied in both seasons. Results indicated a 64% decrease in the second season in FW-B treatment where the average number of tubers dropped from 14 to 5 in the second season. A similar trend was observed in WW-B. Additionally, statistical analysis showed no significant difference (p > 0.05) between the number of tubers that received either freshwater or wastewater. Although the number of tubers in biochar treatment was significantly less in the first season, similar values of the number of tubers were obtained in WW+B as compared to the other treatments (WW-B and FW-B) for the second season. Overall, the green biomass and root weight, which represent the non-edible part of the potatoes, indicated no significant differences among treatments even though WW+B showed significantly less yield than WW-B, in the first season (Fig. 6). The shoot length, representing the length of the longest stem, remained unchanged for both seasons implying no effect of the cultivation year or the treatments (Fig. 6).
4 Discussion
Plant health parameters (e.g., leaf greenness and photosynthesis rate) are used in real-time to study the growth performance of plants (Patil et al. 2014). In the present study, the plant health parameters (leaf greenness, leaf temperature, photosynthesis rate, and NDVI) responded differently with time, but were neither affected by the treatment nor the irrigation water. For both seasons, a decrease in the greenness values was observed (Fig. 1). This could be attributed to the decrease in N concentration which decreases with the increase in biomass (Bélanger et al. 2001). Similar observations were reported by Minotti et al. (1994) and Vos and Bom (1993) who studied the foliar N of potatoes using SPAD data. Although it was expected that irrigation with wastewater (vs. freshwater) should increase the greenness, given that wastewater contains multiple sources of nutrients (Table 2). It was assumed that nutrients were not readily available, possibly due to the high presence of contaminants (e.g., Fe) in the wastewater (Table 2) or due to less time for the nutrient mineralization (da Fonseca et al. 2005). Similar observation indicated that the chlorophyll content (a measure of greenness) of maize leaf was not affected by wastewater (sewage effluent) irrigation applied for 2 years (da Fonseca et al. (2005). The insignificant effect of the biochar could be explained by the insufficient improvement of N availability in the soil. Moreover, Nelson et al. (2011) reported that biochar addition to soil either suppressed or did not affect N availability when applied at 2% and 0.2% (w/w) rates, respectively. On the other hand, leaf temperature showed no treatment effect (Fig. 2); leaf temperature is an indication of water stress in plants with higher values suggesting more water stress (Loveys et al. 2008). Data showed the effect of the ambient temperature in line with the findings of Leuzinger et al. (2010), who observed similarity between ambient and leaf temperatures of Scots pine (Pinus sylvestris); this similarity is not unusual since the experiment was conducted in the field. The photosynthesis rate decreased with the development of the potatoes within the range reported by Chandra et al. (2008) for cannabis. Evidently, a slight color change was noticed on the potato leaves, especially towards maturity; this was expected because photosynthesis is associated with stomatal opening that occurs throughout the growing season of plants, including potatoes (Meidner and Mansfield 1968). The no treatment effect (Fig. 3) signifies that neither the irrigation water nor the biochar amendment influenced the growth parameters of potatoes. Since all the treatments received the same level of fertilization as recommended for potatoes, it is suggested that the soil condition necessary for the biochar to show its agronomic effectiveness was buffered. Moreover, biochar showed agronomic benefits mainly in nutrient-depleted soils (Hussain et al. 2017; Kimetu et al. 2008), whereas not much agronomic benefits were noticed in nutrient-rich soils; this confirms the obtained results which are supported by the findings of Hussain et al. (2017). The potato plants grew vigorously as suggested by the NDVI values in line with Patil et al. (2014); similar values have been reported for potatoes grown under rain-fed and irrigated water management (Shamal and Weatherhead 2014). The no treatment effect implies that wastewater irrigation as well as freshwater irrigation had similar impact on potatoes’ vigor; this is important as wastewater can serve as an alternative for potato irrigation in arid and semi-arid regions where freshwater is scarce. Amendment with biochar (WW+B) did not alter the NDVI (Fig. 4), as in the case of the other plant health parameters; all the potatoes grew vigorously and thus, the effect of the biochar could have been buffered by the nutrient-rich soil.
The soil properties (moisture content, CEC, and base saturation) were affected by both the treatment and depth, but not by the irrigation days, particularly for the moisture content (Fig. 5). The lack of effect of irrigation days on the soil moisture was expected given the nature of the sandy soil, which had low water retention potential (Rawls et al. 1982), thereby drains water quickly. However, \( {\left[\mathrm{MC}\right]}_{\mathrm{soil}}^{0.1} \) was slightly altered by biochar amendment (WW+B), especially in 2015, suggesting an improvement in the soil water retention properties, which could potentially result in higher potato yield. Also, the abundance of exchangeable cations (indicated by CEC) in soil is vital as it plays an active role in the bioavailability of nutrients which exist mostly in their ionic forms for plant uptake. The increase in the CEC in WW+B signifies a great potential for the biochar to elevate the cation exchange activities in the exchange complex of the soil, resulting in higher availability of soil nutrients. Accordingly, the base saturation, which reflects the percentage of base cations that occupies the exchange complex, was significantly higher (p > 0.05) in WW+B as compared to WW-B (Table 4); this is in line with the higher base cations (Ca, Mg, K, and Na) measured in WW+B as compared to WW-B (except Mg; Table 4). The increase in base cations results in increase in pH (Ste-Marie and Paré 1999), which controls nutrient availability and affects crop yield.
Overall, the yield parameters (e.g., tuber weight) were neither affected by the treatment nor the irrigation water although there was treatment effect in the first season mainly due to germination delay, but the delay was not noticed in the second year. Similarity in the yield from both freshwater and wastewater irrigated potatoes is quite important given the scarcity of freshwater, especially in arid and semi-arid regions. Irrigating with wastewater would obviously serve as an alternative option while maintaining the same yield. Delayed germination in the first season in all the biochar-amended treatments resulted in transplanting of the potatoes (planted on same day in extra lysimeters) to the biochar-amended treatments. This could be the reason for the reduced yield recorded in the first season. Given the relatively high ash content of biochar (77.45%) and the application rate (1% w/w of soil) which corresponds to < 8 g (ash) kg−1 (soil), it is assumed that biochar has no negative effect on the germination; this is supported by Nabeela et al. (2015) where similar application rate of wood ash was applied for rapeseed (Brassica napus L.) without delay in the germination. Moreover, in the second season, the delayed germination was not observed as all the potatoes germinated and emerged typically at the same time; there was no treatment effect in the tuber weight across the treatments. Thus, the delayed germination in the first season could be considered as a coincidence and it probably explains the less yield in WW+B as compared to the other treatment (WW-B). When crops, including potatoes, are transplanted, they are subjected to shock, which affects their agronomic cycle (Rowell et al. 1986; Shonnard and Peloquin 1991). Table 4 indicates noticeable increase of soil CEC which possibly favored contaminant adsorption over increasing the yield (Nzediegwu et al. 2019). Biochar showed significant agronomic benefits for sandy soils such as increasing CEC, pH, and water holding capacity compared to organic soils (Liu et al. 2016). This is in line with Koga et al. (2017) who reported the improvement of soil porosity in the presence of wood-derived biochar while no significant effect on potato yield. Similar to the tuber weight, the delayed germination in the first season affected the number of tubers in this season, but there was no treatment effect in the subsequent season.
The no treatment effect on the non-edible parts for the both seasons is explained by the delayed germination, which perhaps shortened the cultivation days (120) of the potatoes (Russet Burbank) used in this experiment. Delayed germination could have affected the potato growth stages, especially tuber initiation, bulking, and maturation, which occur towards the later part of potato growth (He et al. 2012). Potatoes, after planting, have five developmental stages, i.e., sprout development, vegetative growth, tuber initiation, tuber bulking, and maturation (Johnson and Powelson 2008). Shortened cultivation days—as noticed in this study—had no effect on potato biomass production, which occurs at the early growth stage (He et al. 2012). Moreover, as stated previously, in 2016 with no germination delay, the green biomass and root weight as well as tuber weight were typically similar across treatments (i.e., FW-B, WW-B, and WW+B). The similarity in the shoot length, for the two seasons, confirmed the lack of direct relationship with the tuber production.
Although some studies have revealed that biochar improves crop yield (Uzoma et al. 2011a), however, our yield results in addition to the plant health parameters (e.g., greenness and photosynthesis rate) did not indicate any effect. Since the agronomic effects of biochar are feedstock specific (Alburquerque et al. 2013), plantain peel biochar did not improve potato yield. Plantain peel biochar is more likely to improve the yield of potatoes with increase in application rate as observed by Uzoma et al. (2011b). Although mixing the biochar with soil increased the pH of the soil by 0.7 and then maintained it for 2 years, it did not increase the yield. On the other hand, the C:N ratio, the high mineral ash of the biochar coupled with the elevated CEC of the biochar-amended soil confirmed the potential agronomic benefits of plantain peel biochar for sandy soils.
According to Nzediegwu et al. (2019), plantain peel biochar amendment has reduced heavy metal uptake in potato tubers (flesh and peels) irrigated with wastewater. The application of biochar can contribute to the production of relatively safe potatoes when irrigated with wastewater comparable to those irrigated with freshwater. Although no yield differences (with or without biochar amendment) was obtained, farmers could underestimate the risks associated with wastewater irrigation as the potatoes contain significantly higher levels of heavy metals and they could be unsafe to eat.
5 Conclusions
After two seasons of field investigations, wastewater irrigated potatoes grown in a sandy soil and amended with plantain peel biochar in the top 0.1 m showed no significant differences in yield compared to the un-amended soils either irrigated with wastewater or freshwater. Total fresh tuber weights as well as the total number of tubers were similar in all treatments. This was further confirmed by the no significant difference observed in the plant health parameters such as leaf greenness, plant canopy reflectance, and photosynthesis rates measured in the second season. On the other hand, the biochar showed a positive effect on the pH and the cation exchange capacity of the soil. This could be of great interest given that biochar as environmentally friendly material provides no detrimental effect on potato production and reduces uptake of wastewater borne contaminants in potatoes. More investigations are needed to further evaluate the efficiency of biochar application rate as a tool to maximize the environmental benefits of biochar.
References
Aboulhassan MA, Souabi S, Yaacoubi A, Baudu M (2006) Removal of surfactant from industrial wastewaters by coagulation flocculation process. Int J Environ Sci Technol 3:327–332
Ahmad A, Ghufran R, Zularisam AW (2011) Phytosequestration of metals in selected plants growing on a contaminated Okhla industrial areas, Okhla, New Delhi, India. Water Air Soil Pollut 217:255–266. https://doi.org/10.1007/s11270-010-0584-9
Akhtar SS, Andersen MN, Liu F (2015) Biochar mitigates salinity stress in potato. J Agron Crop Sci 201:368–378. https://doi.org/10.1111/jac.12132
Alburquerque JA, Salazar P, Barrón V, Torrent J, Del Campillo MDC, Gallardo A, Villar R (2013) Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron Sustain Dev 33:475–484
Alling V, Hale SE, Martinsen V, Mulder J, Smebye A, Breedveld GD, Cornelissen G (2014) The role of biochar in retaining nutrients in amended tropical soils. J Plant Nutr Soil Sci 177:671–680. https://doi.org/10.1002/jpln.201400109
Angin I, Yaganoglu AV, Turan M (2005) Effects of long-term wastewater irrigation on soil properties. J Sustain Agric 26:31–42. https://doi.org/10.1300/J064v26n03_05
Aquasat (2014) Water withdrawal by sector, around 2007. http://www.globalagriculture.org/fileadmin/files/weltagrarbericht/AquastatWithdrawal2014.pdf. Accessed 20th November, 2017
Artiola JF, Rasmussen C, Freitas R (2012) Effects of a biochar-amended alkaline soil on the growth of romaine lettuce and bermudagrass. Soil Sci 177:561–570
Bélanger G, Walsh JR, Richards JE, Milburn PH, Ziadi N (2001) Critical nitrogen curve and nitrogen nutrition index for potato in Eastern Canada. Am J Potato Res 78:355–364. https://doi.org/10.1007/bf02884344
Bohl WH, Nolte P, Kleinkopf GE, Thornton MK (1995) Potato seed management: seed size and age. University of Idaho, College of Agriculture, Cooperative Extension System, Agricultural Experiment Station, Moscow, ID
Borchard N, Siemens J, Ladd B, Möller A, Amelung W (2014) Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage Res 144:184–194
Brown CR (2005) Antioxidants in potato. Am J Potato Res 82:163–172
Butnan S, Deenik JL, Toomsan B, Antal MJ, Vityakon P (2015) Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 237:105–116
Canadian Food Inspection Agency CFIA (2017) Seed potato crop inspection. http://www.inspection.gc.ca/plants/potatoes/guidance-documents/pi-005/chapter-5/eng/1383761839133/1383762066614?chap=0. Accessed 4th November, 2017
Carter MR, Gregorich EG (2008) Soil sampling and methods of analysis. Canadian Society of Soil Science; CRC Press, [Pinawa, Manitoba]; Boca Raton, FL
Chandra S, Lata H, Khan IA, Elsohly MA (2008) Photosynthetic response of Cannabis sativa L. to variations in photosynthetic photon flux densities, temperature and CO2 conditions. Physiology and molecular biology of plants. An Int J Funct Plant Biol 14:299–306. https://doi.org/10.1007/s12298-008-0027-x
Clothier BE, Green SR (1994) Rootzone processes and the efficient use of irrigation water. Agric Water Manag 25:1–12. https://doi.org/10.1016/0378-3774(94)90048-5
Collins HP, Streubel J, Alva A, Porter L, Chaves B (2013) Phosphorus uptake by potato from biochar amended with anaerobic digested dairy manure effluent. Agron J 105:989–998. https://doi.org/10.2134/agronj2012.0363
Consortium PGS (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195. https://doi.org/10.1038/nature10158
da Fonseca AF, Melfi AJ, Montes CR (2005) Maize growth and changes in soil fertility after irrigation with treated sewage effluent. I. Plant dry matter yield and soil nitrogen and phosphorus availability. Commun Soil Sci Plant Anal 36:1965–1981. https://doi.org/10.1081/CSS-200062539
ElSayed EM, Prasher SO, Patel RM (2013) Effect of nonionic surfactant Brij 35 on the fate and transport of oxytetracycline antibiotic in soil. J Environ Manage 116:125–134. https://doi.org/10.1016/j.jenvman.2012.11.034
Food and Agriculture Organization FAO (2008) The potato: cultivation. http://www.fao.org/potato-2008/en/potato/cultivation.html. Accessed 30th September, 2017
Food and Agriculture Organization of the United Nations (2017) http://faostat.fao.org/default.aspx. Accessed 30th September, 2017
Gao L, Wang R, Shen G, Zhang J, Meng G, Zhang J (2017) Effects of biochar on nutrients and the microbial community structure of tobacco-planting soils. J Soil Sci Plant Nutr 17:884–896
He Z, Larkin RP, Honeycutt W (2012) Sustainable potato production: global case studies. Springer Science, Berlin. https://doi.org/10.1007/978-94-007-4104-1_11
Hien TTT, Shinogi Y, Mishra A, Viet DD (2017) The effect of bamboo biochar on crop’s productivity and quality in the field condition. J Fac Agric Kyushu Univ 62:477–482
Hollinger SE, Isard SA (1994) A soil moisture climatology of Illinois. J Clim 7:822–833. https://doi.org/10.1175/1520-0442(1994)007<0822:asmcoi>2.0.co;2
Huang X, Lin J, Yuan D, Hu R (2009) Determination of steroid sex hormones in wastewater by stir bar sorptive extraction based on poly (vinylpyridine-ethylene dimethacrylate) monolithic material and liquid chromatographic analysis. J Chromatogr 1216:3508–3511. https://doi.org/10.1016/j.chroma.2008.10.083
Hussain M et al (2017) Biochar for crop production: potential benefits and risks. J Soils Sediments 17:685–716. https://doi.org/10.1007/s11368-016-1360-2
Jay CN, Fitzgerald JD, Hipps NA, Atkinson CJ (2015) Why short-term biochar application has no yield benefits: evidence from three field-grown crops. Soil Use Manag 31:241–250. https://doi.org/10.1111/sum.12181
Johnson DA, Powelson ML (2008) Potato health management. APS Press, St. Paul, MN
Kargar M, Jutras P, Clark OG, Hendershot WH, Prasher SO (2013) Trace metal contamination influenced by land use, soil age, and organic matter in Montreal tree pit soil. J Environ Qual 42:1527–1533. https://doi.org/10.2134/jeq2013.02.0055
Kimetu JM, Lehmann J, Ngoze SO, Mugendi DN, Kinyangi JM, Riha S, Verchot L, Recha JW, Pell AN (2008) Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 11:726. https://doi.org/10.1007/s10021-008-9154-z
Koga N, Shimoda S, Iwata Y (2017) Biochar impacts on crop productivity and greenhouse gas emissions from an andosol. J Environ Qual 46:27–35. https://doi.org/10.2134/jeq2016.04.0156
Laird DA, Fleming P, Davis DD, Horton R, Wang B, Karlen DL (2010) Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 158:443–449. https://doi.org/10.1016/j.geoderma.2010.05.013
LaPara TM, Klatt CG, Chen R (2006) Adaptations in bacterial catabolic enzyme activity and community structure in membrane-coupled bioreactors fed simple synthetic wastewater. J Biotechnol 121:368–380. https://doi.org/10.1016/j.jbiotec.2005.07.013
Lehmann J, Joseph S (2009) Biochar for environmental management : science and technology. Earthscan, London, Sterling, VA
Leuzinger S, Vogt R, Körner C (2010) Tree surface temperature in an urban environment. Agric Forest Meteorol 150:56–62. https://doi.org/10.1016/j.agrformet.2009.08.006
Li D, Yang M, Hu J, Ren L, Zhang Y, Li K (2008) Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ Toxicol Chem 27:80–86
Liu Z, Chen X, Jing Y, Li Q, Zhang J, Huang Q (2014) Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 123:45–51. https://doi.org/10.1016/j.catena.2014.07.005
Liu Z, Dugan B, Masiello C, Barnes R, Gallagher M, Gonnermann H (2016) Impacts of biochar concentration and particle size on hydraulic conductivity and DOC leaching of biochar-sand mixtures. J Hydrol 533:461–472
Liu C, Liu F, Ravnskov S, Rubæk GH, Sun Z, Andersen MN (2017) Impact of wood biochar and its interactions with mycorrhizal fungi, phosphorus fertilization and irrigation strategies on potato growth. J Agron Crop Sci 203:131–145. https://doi.org/10.1111/jac.12185
Loveys BR, Theobald JC, Jones HG, McCarthy MG (2008) An assessment of plants-based measures of grapevine performance as irrigation scheduling tools. In, 2008. International Society for Horticultural Science (ISHS), Leuven, Belgium, pp 421-427. https://doi.org/10.17660/ActaHortic.2008.792.49
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Meidner H, Mansfield TA (1968) Physiology of stomata. Tata Mcgraw-Hill Publishing Company Limited, Bombay
Minotti PL, Halseth DE, Sieczka JB (1994) Field chlorophyll measurements to assess the nitrogen status of potato varieties. HortScience 29:1497–1500
Mojid MA, Hossain ABMZ, Wyseure GCL (2019) Impacts of municipal wastewater on basic soil properties as evaluated by soil column leaching experiment in laboratory. J Soil Sci Plant Nutr 19:402–412. https://doi.org/10.1007/s42729-019-00042-x
Nabeela F, Murad W, Khan I, Mian IA, Rehman H, Adnan M, Azizullah A (2015) Effect of wood ash application on the morphological, physiological and biochemical parameters of Brassica napus L. Plant Physiol Biochem 95:15–25. https://doi.org/10.1016/j.plaphy.2015.06.017
Nelson NO, Agudelo SC, Yuan W, Gan J (2011) Nitrogen and phosphorus availability in biochar-amended soils. Soil Sci 176:218–226. https://doi.org/10.1097/SS.0b013e3182171eac
Nopens I, Capalozza C, Vanrolleghem PA (2001) Stability analysis of a synthetic municipal wastewater, BIOMATH edn. Universiteit Gent Belgium
Nzediegwu C, Prasher S, Elsayed E, Dhiman J, Mawof A, Patel R (2019) Effect of biochar on heavy metal accumulation in potatoes from wastewater irrigation. J Environ Manag 232:153–164. https://doi.org/10.1016/j.jenvman.2018.11.013
Patil V, Al-Gaadi K, Wahb-Allah M, Saleh A, Marey S, Samdani M, Abbas M (2014) Use of saline water for greenhouse bell pepper (Capsicum annuum) production. Am J Agric Biol Sci 9:208
Prasad AK, Chai L, Singh RP, Kafatos M (2006) Crop yield estimation model for Iowa using remote sensing and surface parameters. Int J Appl Earth Obs Geoinf 8:26–33. https://doi.org/10.1016/j.jag.2005.06.002
Puga AP, Melo LCA, de Abreu CA, Coscione AR, Paz-Ferreiro J (2016) Leaching and fractionation of heavy metals in mining soils amended with biochar. Soil Tillage Res 164:25–33. https://doi.org/10.1016/j.still.2016.01.008
Qian YL, Mecham B (2005) Long-term effects of recycled wastewater irrigation on soil chemical properties on Golf Course Fairways. Agron J 97:717–721. https://doi.org/10.2134/agronj2004.0140
Raschid-Sally L, Jayakody P (2009) Drivers and characteristics of wastewater agriculture in developing countries: results from a global assessment vol 127. IWMI Research Reports H041686, International Water Management Institute, Colombo, Sri Lanka
Rawls WL, Brakensiek DE, Saxtonn K (1982) Estimation of soil water properties. Trans ASAE 25:1316. https://doi.org/10.13031/2013.33720
Rayment G, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press Pty Ltd, Port Melbourne
Robredo A, Pérez-López U, de la Maza HS, González-Moro B, Lacuesta M, Mena-Petite A, Muñoz-Rueda A (2007) Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ Exp Bot 59:252–263. https://doi.org/10.1016/j.envexpbot.2006.01.001
Rodríguez-Vila A, Covelo EF, Forján R, Asensio V (2015) Recovering a copper mine soil using organic amendments and phytomanagement with Brassica juncea L. J Environ Manag 147:73–80
Rowell AB, Ewing EE, Plaisted RL (1986) Comparative field performance of potatoes from seedlings and tubers. Am Potato J 63:219–227. https://doi.org/10.1007/bf02853727
Rubatzky VE, Yamaguchi M (1997) Plantain, starchy banana, breadfruit, and jackfruit. In: World vegetables: principles, production, and nutritive values. Springer US, Boston, MA, pp 253–276. https://doi.org/10.1007/978-1-4615-6015-9_16
Shamal SAM, Weatherhead K (2014) Assessing spectral similarities between rainfed and irrigated croplands in a humid environment for irrigated land mapping. Outlook Agric 43:109–114. https://doi.org/10.5367/oa.2014.0168
Shiri-e-Janagard M, Tobeh A, Abbasi A, Jamaati-e-Somarin S, Hassanzadeh M, Zabihi-e-Mahmoodabad R (2009) Effects of water stress on water demand, growth and tuber grade of potato (Solanum tuberosum L.) crop. Res J Environ Sci 3:476–485
Shonnard GC, Peloquin SJ (1991) Performance of true potato seed families. 2. Comparison of transplants vs. seedling tubers. Potato Res 34:409–418. https://doi.org/10.1007/bf02360577
Sim W, Lee J, Shin S, Song K, Oh J (2011) Assessment of fates of estrogens in wastewater and sludge from various types of wastewater treatment plants. Chemosphere 82:1448–1453. https://doi.org/10.1016/j.chemosphere.2010.11.045
Singh B, Singh BP, Cowie AL (2010) Characterisation and evaluation of biochars for their application as a soil amendment. Aust J Soil Res 48:516–525
Singh PK, Deshbhratar PB, Ramteke DS (2012) Effects of sewage wastewater irrigation on soil properties, crop yield and environment. Agric Water Manag 103:100–104. https://doi.org/10.1016/j.agwat.2011.10.022
Singh KP, Rai P, Singh AK, Verma P, Gupta S (2014) Occurrence of pharmaceuticals in urban wastewater of north Indian cities and risk assessment. Environ Monit Assess 186:6663–6682
Stark JC, Westermann DT, Hopkins B (2004) Nutrient management guidelines for Russet Burbank potatoes. University of Idaho, College of Agricultural and Life Sciences, Mowsco, ID
Ste-Marie C, Paré D (1999) Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biol Biochem 31:1579–1589. https://doi.org/10.1016/S0038-0717(99)00086-3
Tchango Tchango J, Bikoï A, Achard R, Escalant J, Ngalani J (1999) Plantain: post-harvest operations Centre de Recherches Regionales sur Bananiers et Plantains, Cameroon (CRBP) Post Harvest Compendium:4–9
Teytelboym A (2016) Population growth NATURE 530
United Nations (2017) World population projected to reach 9.8 billion in 2050, and 11.2 billion in 2100 – says UN. http://www.un.org/sustainabledevelopment/blog/2017/06/world-population-projected-to-reach-9-8-billion-in-2050-and-11-2-billion-in-2100-says-un/. Accessed 20th November, 2017
USDA (1983) United States standards for grades of potatoes for processing. https://www.ams.usda.gov/sites/default/files/media/Potatoes_for_Processing_Standard%5B1%5D.pdf. Accessed 26th July, 2019
Uzoma KC, Inoue M, Andry H, Fujimaki H, Zahoor A, Nishihara E (2011a) Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag 27:205–212. https://doi.org/10.1111/j.1475-2743.2011.00340.x
Uzoma KC, Inoue M, Andry H, Zahoor A, Nishihara E (2011b) Influence of biochar application on sandy soil hydraulic properties and nutrient retention. J Food Agric Environ 9:1137–1143
Vos J, Bom M (1993) Hand-held chlorophyll meter: a promising tool to assess the nitrogen status of potato foliage. Potato Res 36:301–308. https://doi.org/10.1007/bf02361796
Walter R, Rao BKR (2015) Biochars influence sweet-potato yield and nutrient uptake in tropical Papua New Guinea. J Plant Nutr Soil Sci 178:393–400. https://doi.org/10.1002/jpln.201400405
Xie T, Reddy KR, Wang C, Yargicoglu E, Spokas K (2014) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol 45:939–969. https://doi.org/10.1080/10643389.2014.924180
Acknowledgments
The authors would also like to thank Sedigheh Zarayan, Lab Manager.
Funding
This study was funded by the Natural Sciences and Engineering Research Council of Canada, Tertiary Education Trust Fund, Nigeria, and the India-Canada Centre for Innovative Multidisciplinary Partnerships to Accelerate Community Transformation and Sustainability.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nzediegwu, C., Prasher, S., Elsayed, E. et al. Effect of Biochar on the Yield of Potatoes Cultivated Under Wastewater Irrigation for Two Seasons. J Soil Sci Plant Nutr 19, 865–877 (2019). https://doi.org/10.1007/s42729-019-00085-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00085-0