Abstract
Boron (B) is an essential micronutrient for plant growth and development, but in soil of arid and semiarid environments, B frequently exceeds the plant requirements. B toxicity hampers plant performances and productivity even though there is a lack of information about changes in leaf sugar metabolism and nutrient partitioning provoked by B excess, especially in tree sugar alcohol-producing species where B is highly mobile. In current experiment, hydroponically grown loquat seedlings were subjected to increasing B levels (25, 50, 100, 200, and 400 μΜ) in the nutrient solution for 69 days. B excess caused visible symptoms in the upper part of loquat shoots (leaves and stem), typical symptoms usually found in species where B is highly phloem mobile. Furthermore, Β excess caused significant (i) reduction of plant growth, leaf number, and stem diameter; and (ii) alterations in macro- and micronutrient allocation patterns in different plant organs, e.g., decrease of K, P, Mn, and Mg concentration in roots. Younger fully expanded leaves of B-treated seedlings showed a decline of sucrose paralleled by increments of glucose and fructose concentration in leaves, alteration of leaf pigment composition, and increased peroxidation of lipid bilayers (higher malondialdehyde by-products). Our observations suggest that loquat is very sensitive to B excess and B toxicity can affect dramatically the plant physiology and biochemistry, thus leading to changes in sugar patterns, a reduced growth and, eventually, a reduced productivity of this species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Boron is a micronutrient critical to plant growth, with a window between essential and toxic concentrations extremely narrow (Nable et al. 1997). The presence of B in the environment derives primarily from the weathering of B-containing minerals, but also from geothermal steams which significantly contribute to natural enrichment of B in soil and water (Chatzissavvidis et al. 2004; Pennisi et al. 2006). However, the most impactful source of high-concentrated B, with an average of 4.5 mg L−1 B dissolved as boric acid, is certainly the seawater which can contaminate fresh water in coastal areas following tidal cycle (Kabay et al. 2010). Differently to other nutrient elements, environmental release of B directly or indirectly attributable to human activities plays a minor role compared to the amplitude of the environmental B enrichment deriving from natural sources, though global change is predicted to increase arid/semiarid environments (Landi and Benelli 2016), therefore exacerbating B accumulation in the soil. In a general way, edaphic concentrations of B in the soil solution ranging from 5 to 100 mg L−1 are toxic for many species, though a large degree of tolerance can be observed among species (Maas 1990; Ferreyra et al. 1997) and even genotypes (Papadakis et al. 2003; Papadakis et al. 2004; Cervilla et al. 2007; Landi et al. 2013a, 2013b; Pardossi et al. 2015; Meriño-Gergichevich et al. 2017; Sarafi et al. 2018).
In soil, B is found primarily as boric acid B(OH)3, which can either diffuse passively into root hair cells or else is taken up through channels (Tanaka and Fujiwara 2008). Boric acid is a weak acid at cytoplasm pH (about 7.0–7.5) and, under physiological conditions, B(OH)3 can easily bind to molecules with mono-, di-, and polyhydroxyl groups such as ribose, apiose, sorbitol, mannitol, and other polyalcohols (Ralston and Hunt 2001). Under conditions of excessive B supply, B is principally absorbed passively by roots and translocated to leaves via non-living cells of the xylem, driven by the evapotranspiration flux (Brown et al. 2002; Tanaka and Fujiwara 2008). Because xylem provides nutrients to mature leaves, which have the highest transpiration rates, it is expected that the older leaves exhibit more severe symptoms of B toxicity than younger ones (Tanaka and Fujiwara 2008). Until recently, it has generally been accepted that B is an immobile nutrient via the phloem and therefore it tends to accumulate in highly transpiring mature leaves (Brown and Shelp 1997). Differently, in some plant species (e.g., Pyrus, Malus, Prunus, Allium, and Brassica) where sugar alcohols (i.e., mannitol and sorbitol), in place of sucrose, are predominant for the phloem translocation of photosynthates, B has been found to be uniformly distributed within plants or even at a higher concentration in young tissues than in mature leaves (Brown and Shelp 1997; Camacho-Cristóbal et al. 2008). These results demonstrated the capacity of B to be moved by phloem flux in some plant species due to its ability to bind with cis-hydroxyl groups of sorbitol and mannitol giving the origin to diol-B complexes (Reid et al. 2004). Phloem translocation does not follow the evapotranspiration stream and it supplies the major proportion of nutrient requirements for actively growing areas such as young leaves and fruit, organs which do not readily transpire (Brown and Shelp 1997) and in which B symptoms occur in sugar alcohol-translocating species, such as loquat [Eriobotrya japonica (Thunb.) Lindl].
Loquat is considered to be very sensitive to high B levels (López-Gómez et al. 2007), given that, according to Keren and Bingham (1985), loquat plants are sensitive to B in irrigation water which ranges from 0.3 to 1.0 mg L−1. Nevertheless, the knowledge on how B excess affects the yield and metabolism of loquat plants is still limited. In particular, there is a lack of information about changes in leaf sugar metabolism and nutrient partitioning provoked by increasing concentrations of B in loquat plants. Previous research highlights that B excess seriously perturbed loquat metabolism by altering several morpho-anatomical, physiological, and biochemical parameters, even before the appearance of typical symptoms of B toxicity over the leaf lamina (Papadakis et al. 2018). In this experiment, increasing levels of B supply (25—as control—50, 100, 200, or 400 μΜ) were imposed in the nutrient solution during the hydroponic cultivation of loquat seedlings until to the appearance of typical symptoms of B toxicity (yellow-brown necrotic areas over the margin of the leaf lamina). Plant biomass and nutrient allocation patterns, chlorophylls, carotenoids, proline, soluble sugars, and polyols (mannitol and sorbitol) as well as the estimation of lipid peroxidation were assessed to generate knowledge on the physiological/biochemical effects induced by B excess in loquat seedlings.
2 Materials and Methods
2.1 Plant Material and Growing Conditions
Six-month-old seedlings of E. japonica, uniform in stem diameter, leaf area, and height (data not shown), were grown in a glasshouse located at the arboretum of the Agricultural University of Athens (latitude 37.981907, longitude 23.705639). Initially, the plants were grown in plastic pots containing a mixture of peat/perlite (2:1, v/v). Afterwards, the plants were singularly transplanted into black plastic bags containing 2 L of a mixture of sand/perlite (1:2, v/v) for 21 days (acclimation period). During transplanting, the seedling roots were thoroughly washed firstly with tap water and then with distilled water in order to remove residuals of the initial mixture. During the acclimation period, loquat seedlings were irrigated three times per week with tap water. Afterwards, each plant, in each plastic bag, was fertigated with a full-strength Hoagland’s nutrient solution, differing in B concentration (25, 50, 100, 200, or 400 μΜ Β). Each fertigation event supplied enough solution (400–600 mL per plant) to fill the pores (field capacity). Furthermore, with this procedure, the B concentrations in the planting media were maintained identical to the original solution (see further details in Papadakis et al. 2003). The control treatment was considered the solution containing 25 μΜ B, given that in previous experiments, it was a safe and non-limiting concentration (Papadakis et al. 2018). A total of 25 plants was used (five replicates per each B treatment). Climatic parameters were monitored by a weather station located inside the glasshouse. The minimum, maximum, and averaged air temperature during the cultivation period were 18, 28, and 24.8 °C, respectively. Maximum temperature reached up to 30–32 °C in sunny hours. Daily global radiation averaged 11.5 MJ m−2.
Sixty-nine days after the beginning of B treatments, about 10 days after the appearance of the first B toxicity symptoms, the seedlings were harvested and separated into leaves, stems, and roots. After the measurement of the number of leaves per plant as well as the length and mean diameter of the stems, each plant part was weighted (fresh weight), washed initially with tap water, and afterwards twice with distilled water. Finally, all plant parts were oven-dried at 75 °C till constant weight (dry weight). For biochemical analyses, the first and second fully expanded mature leaves (from the top) were used, where B principally accumulates in polyol-translocating species as loquat.
2.2 Macro- and Micronutrient Determination
Dried plant parts were milled to fine powder. The concentrations of B, P, K, Ca, Mg, Fe, Mn, Zn, and Na were determined in leaves, stems, and roots. B concentration was determined colorimetrically (420 nm; Helios Gamma UV-Vis Spectrophotometer 9423 UVG, Unicam, UK) by the azomethine-H method (Wolf 1971). For measurements of P, K, Ca, Mg, Mn, Zn, Na, and Fe, dried powder (0.5 g) of each sample was also ashed for 5.5 h at 550 °C, dissolved in 3 mL 6 N HCl, and diluted with deionized water up to 50 mL. Concentration of P was determined colorimetrically (470 nm; Helios Gamma UV-Vis Spectrophotometer 9423 UVG, Unicam, UK) by the vanado-molybdo-phosphate yellow color method (Page et al. 1982). Ca, Mg, Mn, Zn, and Fe were quantified using an atomic absorption spectrophotometer (Varian SpectrAA 20, Varian Ltd., Victoria, AU), whereas K and Na using a flame spectrophotometer (PGI 2000, PG Instruments Ltd. Alma Park-Leicestershire, UK) standard method (Lagunen 1992).
2.3 Chlorophyll and Carotenoid Concentration
Fresh leaf samples (75 mg) were grinded in a cold mortar with 10 mL of cold acetone (80%; v/v), transferred in falcon tubes, kept in the dark for 1 h, and in the meantime vortexed at 15 min intervals. After that, samples were centrifuged at 4400g for 5 min at 4 °C. Chlorophyll and carotenoid concentrations were determined spectrophotometrically (Helios Gamma UV-Vis Spectrophotometer 9423 UVG, Unicam, UK) by collecting extract absorbance at 470 nm, 647 nm, and 663 nm and using the equation described by Lichtenthaler and Buschmann (2001):
2.4 Proline Estimation
Fresh leaves (0.1 g) were cut into small pieces and grinded in a cold mortar with 10 mL of 80% (v/v) ethanol. The homogenate was placed in falcon tubes and then centrifuged at 4400g for 5 min at 4 °C. One milliliter of the supernatant was transferred into test tubes containing 2 mL of acid ninhydrin. After vortexing, test tubes were maintained at 95 °C for 25 min in a water bath, then transferred to an ice bath, and finally, they were allowed to cool down at room temperature. After centrifugation (5 min, 4400g, 4 °C), the absorption of the supernatant was recorded at 520 nm and proline was quantified as described by Bates et al. (1973).
2.5 Carbohydrate Determination
For soluble carbohydrate determination, 30 mg of freeze-dried leaf tissue was mixed to 2 mL of HPLC-grade water (Carlo Erba Reagents S.A.S, France) and vortexed for 20 s. Then, extraction of the water-soluble carbohydrates was performed in a microwave oven for 2 min at 400 W. After centrifugation (4400g for 10 min at 4 °C), the supernatant was removed and the process was repeated twice. The two supernatants were pooled together and filtered by using syringe filters (0.2 μm pore size). HPLC analyses were conducted using a HPLC pump (Waters, model 510) equipped with an HP refractive index, RI (HP 1047A). The mobile phase consisted of HPLC-grade water. A 20-μl aliquot of the extract was injected into an Agilent HI-PLEX Ca2+ column. The temperature of the column was 80 °C and the flow was constant at 0.6 mL min−1. The processing of the chromatograms was done by means of a specific program on the computer (Peak Simple Chromatography Data System, SRI Model 302). Sucrose, glucose, fructose, sorbitol, and mannitol quantitative determinations were derived by a specific reference curve made for each sugar (or polyol).
2.6 Determination of Lipid Peroxidation
Leaf tissue (0.5 g FW) was homogenized in 10 mL of 0.1% trichloroacetic acid at 4 °C. A centrifugation at 4400g for 15 min at 4 °C was then carried out. Lipid peroxidation was estimated by the quantification of malondialdehyde (MDA) by-products determined by reaction of the extract with 0.5% 2-thiobarbituric acid (TBA) in 20% TCA (w/v) as described in Heath and Packer (1968).
2.7 Statistical Analysis
Homogeneity of variance was assessed by Bartlett’s test and then the data were subjected to one-way analysis of variance (ANOVA) using the “PASW Statistics 18” statistical package (SPSS INC., Chicago, USA) followed by LSD post hoc test (P ≤ 0.05). Percentage values were arcsine transformed prior analyses. For mean separation, the Duncan multiple range test for P ≤ 0.05 was applied. Correlations between B concentration in the nutrient solution and studied parameters were examined and the corresponding correlation coefficients (r) were calculated using Pearson’s correlation test. The experiments were arranged following a completely randomized experimental design. Experiments were repeated twice with similar results and a representative dataset is reported herein.
3 Results
3.1 Effects of B Treatments on Plant Growth, Symptom Appearance, and B Plant Partitioning
Increasing concentrations of B in the nutrient solution (from 50 to 200 μM) caused a proportionate reduction of loquat organ biomass (Fig. 1a). Conversely, the highest B concentration induced a stronger decline of leaf biomass (− 74%) with respect to that of stem (− 56%) and root (− 46%). Concentration of B and loss of biomass of each plant organ were always significantly positively correlated: r was − 0.733 (P < 0.01), − 0.690 (P < 0.01), and − 0.459 (P < 0.05) for leaves, root, and stem, respectively. Diameter of stem (r = − 0.639, P < 0.01) as well as the number of leaves per plant (r = − 0.594, P < 0.01) were affected negatively by the increment of B concentration in the nutrient solution (Fig. 1b, c, respectively), with a similar extent from 50 to 200 μM (~− 15% on average) whereas the most severe was the effect of 400 μM B (~− 47%). At the end of the experiment, symptoms of B toxicity appeared in top leaves (as wilting and necrosis) and upper portion of plant stem (browning, drying, and necrosis of the apex) even in plants irrigated with 50 μΜ Β (Fig. S1).
a Fresh weight (g) of root (black), stem (gray), leaves (white), b stem diameter (mm), and c leaf number of Eriobotrya japonica seedlings in relation to increasing B concentrations in the nutrient solution (25–400 μM). Means (n = 5; ±SE) keyed with similar lowercase letters are not statistically different among B treatments (Duncan’s post hoc; P ≤ 0.05), while bars keyed with different capital letters indicate that average of total plant biomass is statistically different (P ≤ 0.05). Linear correlations between a each plant portion, b stem diameter, and c leaf number against B concentration in the nutrient solutions were tested and the correlation coefficients (r) were calculated using Pearson’s correlation test (ns = P ≥ 0.05; * = P ≤ 0.05; ** = P ≤ 0.01)
The increase of B levels in the nutrient solution resulted in a notable increase of its levels in all studied organs (Table 1). Interestingly, different patterns of fluctuation of B accumulation were observed in roots (non-significant linear correlation coefficient, r), stems (r = 0.678, P < 0.01), and leaves (r = 0.853, P < 0.01). In roots, even the moderate concentration of 50 μΜ of B in the nutrient solution resulted in remarkable increase of B accumulation when compared to the controls (25 μM B) and no significant differences were recorded between the treatments 50–400 μM B. The concentration of B in stems was higher in (but similar between) 50–100 μM and 100–200 μΜ B, whereas in leaves, B concentration increased in a dose-dependent manner.
3.2 Allocation Pattern of Macro- and Micronutrients in Roots, Stems, and Leaves
The effects of B excess on the concentrations of the most macro- and micronutrients were mainly evident in roots, where the levels of K, P, Mg, and Mn were significantly suppressed by all the treatments 50–400 μM B. Constrained Na accumulation in the root was only found in the range 50–200 μΜ Β (Table 1). The effects of B excess on macro- and micronutrients in stems and in leaves were not uniform as those found in roots. In leaves, B only caused a consistent increment of Mn and Mg (excluded 400 μM B) (Table 1). In loquat stems, in some cases, significant decrease was observed, such as in the levels of Mg (100–400 μΜ Β) and Zn (50–100 μΜ B), while in some other cases, B toxicity increased the accumulation of minerals such as K and Na (50–400 μΜ Β) (Table 1). The concentrations of Ca and Fe were not affected at all by the B concentration in the nutrient solution nor in leaves or stems and roots (25–400 μΜ Β) (Table 1).
3.3 Sugar and Sugar Alcohol Patterns, Chlorophyll, Carotenoid, Proline Content, and Lipid Peroxidation in Loquat Leaves Under B Excess
In our experiment, B concentrations higher than 50 μM caused a severe reduction of total sugars sensu lato (including sugar alcohols mannitol and sorbitol), and translocating sugars (sucrose, mannitol, and sorbitol), though in both the cases, the decline was only attributable to the B-induced decrease of foliar sucrose (Table 2). Differently, a build-up of glucose and fructose (and therefore their sum: non-translocating sugars) was observed, again only in the range 100–400 μM B, whereas no changes were found in plants subjected to 50 μM B (r = 0.635 for fructose, P < 0.01; r = 0.569 for glucose, P < 0.01; Table 2). Sucrose level was suppressed significantly under the effect of increasing abundance of B especially over 100 μM B (r = − 0.871, P < 0.01). Sorbitol levels remained rather constant in all treatments (25–400 μΜ Β), whereas relatively higher levels of mannitol were found at 100 μΜ B and a trend to the increment was found also at 50 and 200 μΜ Β.
Incremented concentrations of total chlorophylls (ChlTOT) were found in plant treated with 50–200 μM B (Fig. 2a) and then decreased again at 400 μM B. The increment was principally attributable to higher levels of Chl b found in those plants, which induced in turn a reduction of Chl a/b (Fig. 2b). No changes of carotenoid content were found, irrespectively of treatments (Fig. 2c).
a Content of total chlorophyll (ChlTOT; mg g−1 FW) as the sum of Chl a (gray bar) and Chl b (white bar). b Chl a/b ratio. c Total carotenoid content (μg g−1 FW) in leaves of Eriobotrya japonica seedlings in relation to increasing B concentration in the nutrient solution (25–400 μM). Means (n = 5; ±SE) keyed with similar lowercase letters are not statistically different according to Fisher’s protected LSD (P ≤ 0.05). Means keyed with different capital letters indicate that values of ChlTOT are statistically different according to Fisher’s protected LSD (P = 0.05). Linear correlations between stem diameter (b) and leaf number (c) and B concentration in the nutrient solutions were tested and correlation coefficients (r) were calculated using Pearson’s correlation test. (ns = P ≥ 0.05)
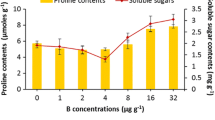
Malondialdehyde by-product and proline levels followed similar patterns in treated plants (Fig. 3a and b, respectively). In particular, a noteworthy increase of MDA by-products was detected in all the plants treated with elevated B levels (50–400 μM B) when compared to controls.
a Malondialdehyde (MDA; nmol g−1 FW) by-products. b Proline levels (μmol g−1 FW) in leaves of Eriobotrya japonica seedlings in relation to increasing B concentrations in the nutrient solution (25–400 μM). Means (n = 5; ±SE) keyed with similar lowercase letters are not statistically different according to Fisher’s protected LSD (P ≤ 0.05). Linear correlations between each parameter against B concentration in the nutrient solutions were tested and the correlation coefficients (r) were calculated using Pearson’s correlation test (ns = P ≥ 0.05)
4 Discussion
Toxic effects of B were proportionate among different plant organs and were evident even at 50 μΜ B. In most cases, the impact of 50 μΜ B supply on the plant biometric parameters was not dissimilar to that induced by 100 and 200 μΜ B. Differently, the highest level of B (400 μM) constrained more severely the leaf biomass than that of stem and roots of the loquat seedlings. Accordingly, it has been observed in tomato (Kayaa and Ashraf 2015) and citrus (Shah et al. 2017) a similar proportionate reduction of all the plant organs due to excess B.
The appearance of symptoms in younger tissues, even at the first concentration of B excess applied in our experiment (50 μΜ B), is supportive for the extremely narrow “window” which exists between safe and toxic concentrations of this microelement (Nable et al. 1997). Symptoms of B excess recorded in the present experiment in actively growing loquat organs are typical of plant species characterized by high B mobility in phloem (Brown and Shelp 1997; Chatzissavvidis and Therios 2010) due to the translocation of polyols (sorbitol and mannitol in loquat). From a pomological point of view, the fact that symptoms of B toxicity were present at the apical-growing portions of shoots and that loquat trees produce fruit panicles from apical flower buds suggests that B toxicity can severely affect the productivity of loquat trees.
The severely decremented uptake of K, P, Mn, and Mg induced by B excess in roots of loquat can be attributed to both the toxic effect induced by B at root level, as well as an increased competition for the uptake of these elements caused by excessive presence of borate anions in the nutrient solution. Even though this reduced nutrient uptake (together with the B-triggered oxidative stress) can be on the bases of reduced plant biomass production, it is well evident that loquat seedlings were able to adjust the leaf metabolism in order to maintain the proportions among microelements similar to controls (unchanged levels of most nutrients in leaves). This is in agreement with previous experiment in which the mineral content of leaves was not affected by B excess (Papadakis et al. 2003; Yermiyahu et al. 2008). Other experiments suggest that no pattern exists between B supply and the levels of mineral elements in various plant parts of different plant species, such as broccoli (Shelp 1988), tomato (Gunes et al. 1999), pepper (Eraslan et al. 2007), kiwifruits (Sotiropoulos et al. 1999), olive (Chatzissavvidis and Therios 2010), and apple tree (Sotiropoulos et al. 2006; Paparnakis et al. 2013). Inconsistencies on the effects of B excess on uptake of other nutrients are probably an indication of the different mobility of nutrients in various species, different demands of nutrients, but principally to biochemical/physiological mechanisms adopted by different plant species/cultivars to counteract luxury availability of B in leaves. For this reason, alteration on some physiological parameters, such as pigment profile and composition, sugar metabolism, and reactive oxygen species production, is more consistently observed in plants exposed to toxic B levels (Papadakis et al. 2004; Sotiropoulos et al. 2006; Eraslan et al. 2008; Han et al. 2009; Landi et al. 2013a, 2013c; Landi et al. 2014).
Sugar metabolism is extremely complex and contributes to a plethora of mechanisms that orchestrates the plant defenses against different stressors (Rosa et al. 2009). Boron caused a reduction of total sugar content in loquat leaves, which was principally dependent to severely decremented level of sucrose. This effect was likely dependent to both the decline of photosynthetic rate of top mature leaves, which was compromised by B toxicity and the reduction of phloem integrity, which can cause downregulation of the biosynthesis of sucrose by the mature source leaves (Han et al. 2009; Lemoine et al. 2013). Higher level of glucose and fructose, which are substrates for sucrose biosynthesis, accumulated, in turn, in top mature leaves of plants grown with B excess, according to other works (Cervilla et al. 2007; Han et al. 2009). Fructose is involved in the biosynthesis of several defensive metabolites (Rosa et al. 2009) and this can explain the increment of phenolic compounds (i.e., anthocyanins: Landi et al. 2013a), ascorbic acid, and glutathione (Cervilla et al. 2007; Landi et al. 2013a, 2013c) found in leaves of plant subjected to B stress. In particular, the biosynthesis of powerful antioxidant compounds is useful to counteract B-triggered oxidative stress, which is a response found in our experiments (increment of MDA by-products) as well as by several other authors (reviewed by Landi et al. 2012).
Differently to fructose, compiling evidences show that glucose acts as osmoprotectant under stress conditions (for a review, see Gupta and Kaur 2005), but there is no evidence to support the hypothesis that B toxicity in leaves is due to osmotic stress induced by foliar accumulation of B (Reid et al. 2004). According to other studies that observed increased level of proline under B toxicity (e.g., Eraslan et al. 2008), it is presumable, therefore, that also proline accumulation is not a mean to increase the osmotic potential of the leaf and maintain an active xylem flux, but it seems more realistic that proline biosynthesis represents another additional way to increase the antioxidant pool of the cell (Hayat et al. 2012). Glucose can both represent the substrate for cellular respiration as well as a key signaling molecule in plants under stress (Gupta and Kaur 2005; Sheen 2014). In particular, several genes related to the photosynthetic process are inhibited by glucose-induced phosphorylation (Lemoine et al. 2013; Sheen 2014). Accordingly, Guo et al. (2014) observed that B toxicity in citrus decreased the CO2 assimilation and the expression of genes involved in photosynthesis and carbohydrate metabolism, such as Rubisco and ADP-glucose pyrophosphorylase, whereas Han et al. (2009) observed a strong increment of fructose and glucose and a decline of sucrose in citrus leaves. Strong accumulation of hexose can lead to premature leaf senescence (Landi et al. 2015) and this partially explains the lower Chl a/b ratio found in loquat leaves under B excess. Different papers have reported mixed results about ChlTOT content under B excess (Han et al. 2009; Landi et al. 2013a, 2013c; Shah et al. 2017), but the reduction of Chl a/b ratio has been consistently found as consequence of B excess. In our experiments, changes of Chl a/b ratio are mainly dependent to increased biosynthesis of Chl b rather than a downregulation of Chl a biosynthesis under increasing B concentrations. Higher level of Chl b, prevalently connected to photosystem I, could be an adaptation response, transferring “workload” from photosystem II to photosystem I, which is usually less prone to photoinhibition (Tikkanen et al. 2014). Even though the photosynthetic process is usually hampered by B toxicity, in B symptomatic leaves, the maintenance of level of ChlTOT in healthy areas as similar as those recorded in control leaves might be necessary to compensate for the loss of chlorophylls in necrotic areas. Differently, no significant effect on carotenoid content was found in loquat leaves as well as in other species (Keles et al. 2004; Shah et al. 2017), suggesting a minor role of these accessory pigments under B stress.
5 Conclusions
Our dataset offers new evidences about physiological/biochemical adjustments adopted by loquat seedlings subjected to boron excess (summarized in Fig. S2). Loquat plants result highly sensitive to boron excess and in this species, boron-induced symptoms occurred firstly in younger fully expanded leaves, leaf petioles, and top part of the stem, as typically observed in other polyalcohol-translocating species. Roots accumulated high level of boron and offered a first barrier against boron translocation to the shoot, but when the concentration of boron in the medium increased strongly, boron accumulation increased dramatically, even in leaves and stems. B hampered the biosynthesis of sucrose in the leaves which correlates to accumulation of its intermediates (glucose and fructose). This, in turn, can promote early senescence in leaves, as indicated by lower level of chlorophyll a/b ratio and malondialdehyde by-product accumulation. Conversely, an impairment of translocating sugars can seriously compromise the development of sink organs, such as young leaves and fruits. Overall, these affects pose serious concerns about the possible effect of boron excess to fruit production and further research is necessary to investigate the interplay between nutrient partitioning, sugar metabolism, and progression of leaf senescence in loquat leaves under B excess and to understand whether these processes can significantly compromise the yield of this economically important tree fruit species.
References
Bates LS, Waldren RP, Teare JD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Brown PH, Shelp BJ (1997) Boron mobility in plants. Plant Soil 193:83–101
Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Römheld V (2002) Boron in plant biology. Plant Biol 4:205–223
Camacho-Cristóbal JJ, Rexach J, González-Fontes A (2008) Boron in plants: deficiency and toxicity. Int J Plant Biol 50:1247–1255
Cervilla LM, Blasco BA, Ríos JJ, Romero L, Ruiz JM (2007) Oxidative stress and antioxidants in tomato (Solanum lycopersicum) plants subjected to boron toxicity. Ann Bot 100:747–756
Chatzissavvidis C, Therios I (2010) Response of four olive (Olea europaea L.) cultivars to six B concentrations: growth performance, nutrient status and gas exchange parameters. Sci Hortic 127:29–38
Chatzissavvidis C, Therios I, Antonopoulou C (2004) Seasonal variation of nutrient concentration in two olive (Olea europaea L.) cultivars irrigated with high boron water. J Hortic Sci Biotechnol 79:683–688
Eraslan F, Inal A, Gunes A, Alpaslan M (2007) Boron toxicity alters nitrate reductase activity, proline accumulation, membrane permeability, and mineral constituents of tomato and pepper plants. J Plant Nutr 30:981–994
Eraslan F, Inal A, Pilbeam DJ, Gunes A (2008) Interactive effects of salicylic acid and silicon on oxidative damage and antioxidant activity in spinach (Spinacia oleracea L. cv. Matador) grown under boron toxicity and salinity. Plant Growth Regul 55:207–219
Ferreyra RE, Aljaro AU, Ruiz RS, Rojas LP, Oster JD (1997) Behavior of 42 crop species grown in saline soils with high boron concentrations. Agric Water Manag 34:111–124
Gunes A, Alpaslan M, Cikili Y, Ozcan H (1999) Effect of zinc on the alleviation of boron toxicity in tomato. J Plant Nutr 22:1061–1068
Guo P, Qi YP, Yang LT, Ye X, Jiang HX, Huang JH, Chen LS (2014) cDNA-AFLP analysis reveals the adaptive responses of citrus to long-term boron-stress. BMC Plant Biol 14:284
Gupta AK, Kaur N (2005) Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J Biosci 30:761–776
Han S, Tang N, Jiang HX, Yang LT, Chen LS (2009) CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress. Plant Sci 176:143–153
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Kabay N, Güler E, Bryjak M (2010) Boron in seawater and methods for its separation—a review. Desalination 261(3):212–217
Kayaa C, Ashraf M (2015) Exogenous application of nitric oxide promotes growth and oxidative defense system in highly boron stressed tomato plants bearing fruit. Sci Hortic 185:43–47
Keles Y, Öncel I, Yenice N (2004) Relationship between boron content and antioxidant compounds in Citrus leaves taken from fields with different water source. Plant Soil 265:345–353
Keren R, Bingham FT (1985) Boron in water, soils, and plants. In: Advances in soil science, vol 1. Springer-Verlag Inc., New York, pp 229–276. https://doi.org/10.1007/978-1-4612-5046-3_7
Lagunen LHJ (1992) Spectrochemical analysis by atomic absorption and emission. Royal Society of Chemistry, Cambridge (United Kingdom)
Landi M, Benelli G (2016) Protecting crop species from biotic and abiotic constraints in the era of global change: are we ready for this challenge? Am J Agric Biol Sci 11:51–53
Landi M, Degl’Innocenti E, Pardossi A, Guidi L (2012) Antioxidant and photosynthetic responses in plants under boron toxicity: a review. Am J Agric Biol Sci 7:255–270
Landi M, Pardossi A, Remorini D, Guidi L (2013a) Antioxidant and photosynthetic response of a purple-leaved and a green-leaved cultivar of sweet basil (Ocimum basilicum L.) to boron excess. Environ Exp Bot 85:64–75
Landi M, Remorini D, Pardossi A, Guidi L (2013b) Sweet basil (Ocimum basilicum) with green or purple leaves: which differences occur in photosynthesis under boron toxicity? J Plant Nutr Soil Sci 176:942–951
Landi M, Remorini D, Pardossi A, Guidi L (2013c) Boron excess affects photosynthesis and antioxidant apparatus of greenhouse Cucurbita pepo and Cucumis sativus. J Plant Res 126:775–786
Landi M, Guidi L, Pardossi A, Tattini M, Gould KS (2014) Photoprotection by foliar anthocyanins mitigates effects of boron toxicity in sweet basil (Ocimum basilicum). Planta 240:941–953
Landi M, Tattini M, Gould KS (2015) Multiple functional roles of anthocyanins in plant-environment interactions. Environ Exp Bot 119:4–17
Lemoine R, La Camera S, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain JL, Laloi M, Coutos-Thévenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 24:272
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. Current protocols in food analytical chemistry. John Wiley & Sons Inc., New York (NY)
López-Gómez E, San Juan MA, Diaz-Vivancos P, Mataix Beneyto J, Garcia-Legaz MF, Hernandez JA (2007) Effect of rootstocks grafting and boron on the antioxidant systems and salinity tolerance of loquat plants (Eriobotrya japonica L). Environ Exp Bot 60:151–158
Maas EV (1990) Crop salt tolerance. In: Tanji KK (ed) Agricultural salinity assessment and management. ASCE Manual Reports on Engineering Practices, New York (NY), pp 262–304
Meriño-Gergichevich C, Reyes-Díaz M, Guerrero J, Ondrasek G (2017) Physiological and nutritional responses in two highbush blueberry cultivars exposed to deficiency and excess of boron. J Soil Sci Plant Nutr 17(2):307–318
Nable RO, Banuelos GS, Paull JG (1997) Boron toxicity. Plant Soil 198:181–198
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis. Vol. 2. Soil Science Society Inc, Madison
Papadakis IE, Dimassi KN, Therios IN (2003) Response of two citrus genotypes to six boron concentrations: concentration and distribution of nutrients, total absorption, and nutrient use efficiency. Aust J Agric Res 54:571–580
Papadakis IE, Dimassi KN, Bosabalidis AM, Therios IN, Patakas A, Giannakoula A (2004) Boron toxicity in ‘Clementine’ mandarin plants grafted on two rootstocks. Plant Sci 166:539–547
Papadakis IE, Tsiantas PI, Tsaniklidis G, Landi M, Psychoyou M, Fasseas C (2018) Changes in sugar metabolism associated to stem bark thickening partially assist young tissues of Eriobotrya japonica seedlings under boron stress. J Plant Physiol 231:337–345
Paparnakis A, Chatzissavvidis C, Antoniadis V (2013) How apple responds to boron excess in acidic and limed soil. J Soil Sci Plant Nutr 13:787–796
Pardossi A, Romani M, Carmassi G, Guidi L, Landi M, Incrocci L, Maggini R, Puccinelli M, Vacca W, Ziliani M (2015) Boron accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves. Plant Soil 395:375–389
Pennisi M, Gonfiantini R, Grassi S, Squarci P (2006) The utilization of boron and strontium isotopes for the assessment of boron contamination of the Cecina River alluvial aquifer (central-western Tuscany, Italy). Appl Geochem 21:643–655
Ralston NVC, Hunt CD (2001) Diadenosine phosphates and S-adenosylmethionine: novel boron binding biomolecules detected by capillary electrophoresis. Biochim Biophys Acta (BBA) 1527:20–30
Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD (2004) A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ 25:1405–1414
Rosa M, Prado C, Podazza G, Interdonato R, González JA, Hilal M, Prado FE (2009) Soluble sugars—metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Signal Behav 4:388–393
Sarafi E, Siomos A; Tsouvaltzis P, Ioannis T, Chatzissavvidis C (2018) The influence of boron on pepper plants nutritional status and nutrient efficiency. J Soil Sci Plant Nutr 18(3):653–657
Shah A, Wu X, Ullah A, Fahad S, Muhammad R, Yan L, Jiang C (2017) Deficiency and toxicity of boron: alterations in growth, oxidative damage and uptake by citrange orange plants. Ecotoxicol Environ Saf 145:575–582
Sheen J (2014) Master regulators in plant glucose signaling networks. J Plant Biol 57:67–69
Shelp BJ (1988) Boron mobility and nutrition in broccoli (Brassica oleracea var. italica). Ann Bot 61:83–91
Sotiropoulos TE, Therios IN, Dimassi KN (1999) Calcium application as a means to improve tolerance of kiwifruit (Actinidia deliciosa L.) to boron toxicity. Sci Hortic 81:443–449
Sotiropoulos TE, Molassiotis A, Almaliotis D, Mouhtaridou G, Dimassi K, Therios I, Diamantidis G (2006) Growth, nutritional status, chlorophyll content, and antioxidant responses of the apple rootstock MM 111 shoots cultured under high boron concentrations in vitro. J Plant Nutr 29:575–583
Tanaka M, Fujiwara T (2008) Physiological roles and transport mechanisms of boron: perspectives from plants. Pflugers Arch 456:671–677
Tikkanen M, Mekala NR, Aro EM (2014) Photosystem II photoinhibition-repair cycle protects photosystem I from irreversible damage. Biochim Biophys Acta (BBA) 1837:210–215
Wolf B (1971) Determination of boron in soil extracts, plant materials, composts, manures, water and nutrient solutions. Commun Soil Sci Plant Anal 2:363–374
Yermiyahu U, Ben-Gal A, Keren R, Reid RJ (2008) Combined effect of salinity and boron on plant growth and yield. Plant Soil 304:73–87
Acknowledgements
We are thankful to three anonymous referees for their critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 7230 kb)
Rights and permissions
About this article
Cite this article
Tsiantas, P.I., Papadakis, I.E., Tsaniklidis, G. et al. Allocation Pattern, Nutrient Partitioning, Sugar Metabolism, and Pigment Composition in Hydroponically Grown Loquat Seedlings Subjected to Increasing Boron Concentrations. J Soil Sci Plant Nutr 19, 556–564 (2019). https://doi.org/10.1007/s42729-019-00054-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00054-7