Abstract
The aim of this study was to determine if three cereal crops differed in their behavior to take up soil and fertilizer P, with emphasis on the relationship between phosphatase activity and P fractionation. We used a vertical rhizobox experiment with wheat, oat, and barley sown on Chilean Andisol (Barros Arana Series) with low P availability under greenhouse conditions. Plants were fertilized with the equivalent of 100 kg P ha−1 of triple superphosphate (TSP) or rock phosphate (RP). Plant biomass was determined for each of the three cereal plant species. Additionally, phosphatase (P-ase) activity in roots, soil in presence of roots (soil+R), and soil in absence of roots (soil−R) after 60-day growth were evaluated, and soil P fractionation was determined using the Hedley procedure. Fertilizer increased both P uptake and biomass production, particularly in shoots. The P uptake efficiency (∆P uptake between fertilized and unfertilized treatment/P input) was low (4.6%) and similar for both fertilizers for oat, but RP was more efficient for wheat (> 30%) and even more so for barley (nearly threefold), due the higher shoot P concentration of RP fertilized plants, which could be attributable to a major P-ase activity in plants fertilized with RP. Despite, fertilizer P was most clearly identified in labile inorganic soil fractions with Olsen P being greater after TSP addition than RP. In particular, plants showed contrasting soil+R P-ase activity inducing differences in soil+R P speciation, increasing labile NaHCO3-Pi and NaOH-Pi fractions with TSP. Strong relationships were found between the sum of labile Pi fractions and P uptake. We conclude that slower release of RP has a positive impact on P-ase activity and leads to better fertilizer efficiency than TSP, especially for barley.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus (P) is an essential element required for plant growth, with very low concentration in soil solution, which is seldom adequate for optimal plant growth (Balemi and Negisho 2012). The low soluble P concentration is a consequence of the large reactivity of phosphate ions with numerous soil constituents, including Al and Fe hydro(oxides) leading to a strong P retention in soil. Particularly, low P availability has been reported for soils rich in those minerals, such as those developed from volcanic ash as Andisol. Consequently, P availability for plants depends on their capability to influence its concentration gradient and diffusivity in the soil near the roots (Cabeza et al. 2017). Therefore, root-soil interactions in the rhizosphere noticeably affect the availability of P to plants. In consequence, plants have developed adaptations to access soil P, e.g., rhizosphere pH changes, root structure, exudation of organic anions, microbial reclutation and root mycorrhizal associations (Aguilera et al. 2015; Martinez et al. 2015), and root secretion of enzymes, that are the main effectors of metabolic pathways occurring in living organisms (Gianfreda 2015). In particular, acid phosphatases (P-ase) as P-solubilizing enzymes are typically more abundant in the rhizosphere and are responsible for the mineralization of organic P to give available inorganic P (George et al. 2017). In addition, plant roots secrete organic compounds that stimulate microbial activity in the rhizosphere and hence P availability (Crème et al. 2016; Mora et al. 2017). Microbial activity may change the chemistry or biochemistry of the rhizosphere allowing for P uptake from insoluble P forms (George et al. 2017). Some species develop more extensive root systems and root hairs allowing the plants to explore a larger volume of soil (Balemi and Negisho 2012).
Various methods for studying chemical changes in the rhizosphere have been developed for annual crops, grasses, and legumes (Hedley et al. 1994). Among these methods, rhizoboxes have been used to determine changes in P availability induced by roots (Zoysa et al. 1999). Furthermore, sequential P fractionation using chemical extractions (Hedley procedure) has been used widely for determining soil P availability as Pi and Po fractions differing in chemical extractability (Hedley et al. 1982). This procedure has been also used for P characterization in Andisols (e.g., Redel et al. 2008, 2016; Velasquez et al. 2016a). It has been shown that a high proportion of P is present as residual recalcitrant P (Borie and Zunino 1983), mainly attributed to mineral-associated organic P compounds. However, recently, it was shown that residual P consists of a mixture of organic and mineral compounds (Velasquez et al. 2016b). The plant availability of such compounds is unclear. Some investigations indicated that there is a relationship between P fractions and phosphatase activities (George et al. 2002), suggesting that plants may increase root exudation in low soil P availability conditions. Studies performed on agroforestry species showed that phosphatase activities increased in the rhizosphere of agroforestry species, either directly by secretion or indirectly by stimulation of microbial activity and/or depletion of Pi (George et al. 2002). However, no clear relationships were found between the activities of both root-associated and exuded phosphatases and the P concentration and content of plants (Machado and Furlani 2004).
Wheat, oat, and barley crops are among the dominant cereal crop species in southern Chile, with over 370,000 ha sown each year, 236,500, 107,500, and 26,000, respectively (ODEPA 2015), many of them growing on Andisol, thus requiring high fertilizer inputs. Alternative P sources need to be found due to the high cost of conventional superphosphate fertilizer, and their application to soil may be adapted to different plant species. Studies on root surface activity (root P-ase) of these crops have not indicated significant differences among them (Sarapatka et al. 2004). However, contrasting results have been observed for root P-ase in different plant species at short-term greenhouse study for Andisols, for example, greater P-ase activity was found for barley as compared to oat and wheat plants (Paredes et al. 2011; Martinez et al. 2015).
Our overall aim is to find efficient phosphorus fertilizer strategies for the production of cereal crops in P-deficient acid Andisols. Since these soils are acid, rock phosphate may be expected to be efficient, so we compared rock phosphate and soluble triple super phosphate as sources of P. However, since the synthesis of phosphatases is known to be one of the strategies used by plants to improve P acquisition, we hypothesized that P-ase activities might be affected reduced by P fertilizer addition. This would reduce the supply to plants of P from soil organic P and thus increase the reliance on fertilizer P. We expected that fertilizer P would increase pools of inorganic P in soils and hoped to detect greater depletion of soil P in unfertilized soils. We used two-compartment culture device to compare soil directly influenced by the presence of plant roots (soil+R), and unfertilized soil separated from roots (soil−R). There may be some root influence in the soil−R, but previous studies have detected significant changes in chemical properties for only a few millimeters, and so after homogenizing 5-cm depth of soil, this may be considered to be “bulk” soil. We expected that P-ase activity and its effect on P fractions will be greater in soil in presence of roots (soil+R) than in soil in absence of roots (soil−R). We have compared three cereal plant species (wheat, oat, and barley) that differ in their strategies to take up P from fertilizer and soil, and focus on the relationship between P-ase and P fractionation in order to understand how plants optimize the fertilization in an Andisol with low P availability.
2 Materials and Methods
Andisol samples collected from grassland pasture soil (0–10-cm depth), with low available P (4 mg kg−1 Olsen P), were used for this study. The soil is located in the IX Region of southern Chile, near Freire in the Central valley, 39° 06′ 12″ S, 72° 37′ 42″ W, corresponding to “Barros Arana” soil series, typic Hapludands, with silt loam texture. Other soil characteristics are given at Table 1. The soil samples were air dried, sieved (< 2 mm), and stored until analysis.
2.1 Experimental Device Preparation
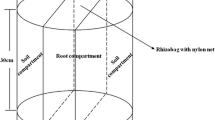
We prepared experimental devices for a greenhouse trial, consisting of a two-compartment device (Fig. 1), made up of two PVC cylinders, the upper compartment having an internal diameter of 12-cm and 10-cm depth and the lower compartment having an internal diameter of 11.5-cm and 5-cm depth, covering 113 cm2 of the surface area. The two compartments were separated by a 30-μm pore-diameter nylon mesh (Nuruzzaman et al. 2006). The upper compartment represents the soil in presence of roots (soil+R) and lower compartment represent the soil in absence of roots (soil−R). Soil was weighed separately into each device zone, and the upper compartment was amended with the corresponding fertilizer for each treatment (565-mg Triple superphosphate (TSP), 20% P or 1570 mg rock phosphate (RP), Bifox (8.3% P, 90% solubility in 2% citric acid) corresponding to the equivalent of 100 kg P ha−1 or 108 mg kg−1) in order to mimic a superficial P fertilization at field (and calculated taking into account the internal surface of the experimental device), and 50 kg N ha−1 as urea. Thereafter, the soil was maintained at field capacity and the fertilizers were mixed in a bag separately for each pot. The upper compartment was packed with 1050 g of soil (density of 0.90 Mg m−3) and the lower compartment with 470 g of soil (dry matter basis). Six seeds of wheat (Triticum aestivum L.) cv. Caluga-Baer, oat (Avena sativa L.) cv. Supernova-INIA, or barley (Hordeum vulgare L.) cv. Sebastián-Baer plants were sown, thinned to four plants after 5 days to leave the most representative plants into the upper compartments of each experimental devices. These varieties are widely used in the Region of La Araucania, the center of southern Chilean cereal production. The experiment design corresponded to a randomized complete block design, with three fertilizer treatments (no fertilizer, TSP, and RP) and three crop species (wheat, oat, and barley), with three replicates for each treatment, representing a total of 27 experimental units.
2.2 Greenhouse Trial
The experimental devices were maintained under greenhouse conditions (22 °C ± 2 °C) and their position randomized each week. Every day, water loss was determined by weighing and replaced by pipetting the required volume of distilled water homogeneously across the pot surface. At the end of the 60 days, plants were harvested. Shoots and roots were carefully separated, and the soil adhering to the roots were returned and homogenized into soil+R. Roots were washed with distilled water for future analyses. Shoot and root material was dried at 65 °C for 48 h to determine dry weight (DW) and P content. Fresh samples of the soil of the upper and lower compartments (soil+R and soil−R, respectively) of each experimental device were taken for P-ase determination. The rest was air dried, homogenized and sieved (< 2 mm) for P fractionation.
2.3 Plant and Soil Analyses
Total P (PT) in soil samples was extracted with NaOBr (Dick and Tabatabai 1977) and determined by the method of Murphy and Riley (1962). Total C (CT) was determined by elemental analyzer (CHN NA1500; Carlo Erba Elemental Analyzer, Stanford, CA, USA). pH was determined in soil:water solution 1:2.5. Olsen P was determined by soil extraction with 0.5 M NaHCO3 adjusted to pH 8.5, according to the Olsen and Sommers (1982) method. Exchangeable cations (Ca, Mg, K, and Na) were extracted with 1 M CH3COONH4 and Al with 1 M KCl solution.
Phosphorus fractions (NaHCO3-Pi, NaHCO3-Po, NaOH-Pi, NaOH-Po, and HCl-Pi) in soil were determined according to a modified Hedley fractionation method (Hedley et al. 1982). Briefly, 0.5 g of soil was extracted with 30 mL 0.5 M NaHCO3 pH 8.5 during 16 h followed by extraction with 30 mL 0.1 M NaOH for 16 h and finally with 30 mL 0.5 M HCl-Pi for 16 h. The samples were centrifuged and filtered. In all extracts, inorganic phosphorus (Pi) was measured spectrophotometrically by the method of Murphy and Riley (1962) at 820 nm and pH 5.0, whereas organic phosphorous (Po) in all extracts was calculated as the difference between PT determined for each fraction by alkaline oxidation of the residue with NaOBr (Dick and Tabatabai 1977) and the Pi determined in the extracts. Thus, this fractionation procedure yielded five analytical fractions differing in lability: NaHCO3-Pi, NaHCO3-Po, NaOH-Pi, NaOH-Po, and HCl-Pi. Residual P was determined in the residual soil by alkaline oxidation with NaOBr (Dick and Tabatabai 1977).
Acid phosphatase (orthophosphoric-monoester phosphomonoesterase) activity (P-ase) in both soil+R and soil−R was determined by the para-nitrophenylphosphate (p-NPP) method of Tabatabai and Bremner (1969) with modifications as described Rubio et al. (1990) and Redel et al. (2008) for soils with high organic matter content. Briefly, soil samples (1 g) were incubated with 1 mL 50 mM p-NPP and 4 mL 0.1 M tris buffer pH 5.5 for 1 h at 20 °C. At the end of the incubation period, 1 mL 0.5 M CaCl2 was added, and the solution was quickly filtered (Whatman 5C Filter paper). Then, the filtrate was treated with 4 ml 0.5 M NaOH. Samples were homogenized and centrifuged at 2500g for 10 min. Para-nitrophenol (p-NF) released was determined spectrophotometrically by measuring the absorbance of the supernatant at 400 nm. As p-NF is adsorbed by soils, the results were corrected with a soil sample incubated with p-NPP and buffer to determine p-NF adsorption onto soil to avoid underestimation of the final amount of free p-NF. Another sample was incubated with soil and buffer only (control). Furthermore, root surface phosphatase (root P-ase) was determined using ρ-nitrophenylphosphate (p-NPP, Sigma-Aldrich, St. Louis, USA), according to the procedure modified by Rubio et al. (1990) and Paredes et al. (2011). Acid phosphatase activity was expressed with respect to root dry weight.
To determine shoot and root P content, the shoot and root samples were ashed at 500 °C for 12 h and dissolved in 2.0 M HCl solution. P concentration was analyzed by spectrophotometry at 820 nm and pH 5.0 (Murphy and Riley 1962). Phosphorus uptake was calculated as the product of dry biomass and P concentration of shoot + root (P uptake = shoot DW × shoot P concentration + root DW × root P concentration). P uptake efficiency was calculated according to: P uptake efficiency = ∆P uptake / P input as fertilizer × 100.
2.4 Statistics
Data were analyzed using analysis of variance (two-way ANOVA) to identify significant differences among treatments. Kolgomorov test was applied for testing data normality. We tested the strength of the relationship between selected parameters carried out using Pearson’s correlation coefficient (P ≤ 0.05 or P ≤ 0.01). Furthermore, to group and determine significant differences between samples based on cereal crop P-ase activity and P fractionation, data were imported into the PRIMER 7 software (PRIMER-E Ltd., Ivybridge, UK), transformed by a Xþ1 (multipliying data by log 10), and normalized (Durán et al. 2017). Then, a distance matrix was generated based on Euclidean distances, and then data was submitted to a principal component analysis (PCA) and hierarchical clustering used to define groups of factors in order to discern relationships or associations based on weighting of their simultaneous contributions.
3 Results
3.1 Soil-Plant Parameters and Phosphatase (P-ase) Activities
Soil pH was significantly higher in soil+R (5.56 ± 0.03) than in the soil−R (5.17 ± 0.01). In soil+R, significant differences were observed between P treatments with P fertilizer causing alkalinization of soil and the effect being greater for TSP than RP for both wheat and oat. P fertilizer addition, both RP and TSP, increased soil pH, and the effect was larger for RP in soil+R (Table 2). However, this tendency was not observed in soil−R (Table 3).
Root P-ase activity was greater in wheat roots than in roots of oat or barley for each of the treatments (Table 4). In the presence of wheat or barley, soil showed a larger P-ase activity than soil−R when fertilized with either RP and TSP (P = 0.001**, P = 0.02*), but this trend was not observed for barley (P = 0.388). P addition increased in root P-ase activity, except for TSP in wheat where the effect was not significant. In root-affected soil, TSP led to a decrease in P-ase activity for wheat whereas RP led to increases in soil+R for oat and barley (Table 4). Fertilizer addition increased shoot biomass, shoot P content, and P uptake (Table 5). P uptake was similar for wheat and oat, but the response to RP was markedly better than TSP for barley. Shoot P concentration was significatively correlated with root P-ase (r = 0.62, Fig. S1a) due to the species differences (wheat having both greatest P uptake and root P-ase activity), whereas root P concentrations differed a little between treatments (Table 5).
3.2 Influence of P Source and Crop Species on Soil P Speciation
The amount of P taken up by plants was always small with respect to soil P, and the increments in P taken up from the fertilized soils were also very small (less than 6%). Olsen P was lower in soil+R than in soil−R for the controls of wheat and oat showing that this fraction was depleted (Tables 2 and 3). Olsen P and the sum of labile inorganic P contents (NaHCO3-Pi + NaOH-Pi + HCl-Pi) in soil+R were increased by fertilizer application and showed a strong positive correlation with both shoot DW and P uptake (Fig. S2) led by both fertilizer addition and species differences (Table 2). NaHCO3-Po contents in soil+R showed no consistent trend with P additions, with increases, decreases, and no significant effects, whereas NaOH-Po were decreased when control and P-amended soils or soil+R and soil−R were compared (Tables 2 and 3). Furthermore, soil+R P-ase was significatively correlated with NaHCO3-Pi with r = 0.62. This correlation comprised a scatter of points for control soils and an inverse relation between P-ase activity and NaHCO3-Pi (Fig. S1b) for P-amended soils.
The effect of P source on soil P forms was confirmed by principal component analysis (PCA) (Fig. 2). The most striking feature is that wheat is distinct from the other two cereal crops and displays a greater separation between the two fertilizers. For barley and oat, RP was more strongly associated with organic forms of soil P, whereas TSP fertilizer led to a strong association with inorganic P forms (NaHCO3-Pi and NaOH-Pi). Regarding P sources, both TSP and RP were grouped together at eucleidean distance of 3 but different behavior in wheat plants was found. Soil P-ase activity did not appear to be important for any of the treatments as shown by Anova analysis (Table 6).
Cereal plant species also affected the soil P distribution in NaHCO3-Pi and Po fractions. Looking at Tables 2 and 3, P speciation was affected by fertilization only in soil under wheat, which presented a reduction of the NaHCO3-Po fraction in soil+R. This tendency was not observed in the rest of species. However, barley presented the highest Pi fractions (NaOH-Pi and HCl-Pi) at soil+R in both fertilizer treatments. On average, barley showed an 18% higher NaOH-Pi concentration compared to wheat (Tables 2 and 3). Soil under barley had the highest residual P. PCA analysis confirms the different behavior of the three cereal plant species (Fig. 2).
4 Discussion
4.1 Phosphatase Activity and P Utilization
It is widely reported that plants may increase available Pi by the phosphatase mineralization of organic P (Balemi and Negisho 2012). Our results indicate greater soil P-ase activity in soil+R compared to soil−R except in barley, as reported by several authors for various plant species (reviews by Balemi and Negisho 2012). There are two factors that contribute to this, firstly the release of phosphatases from roots and secondly the presence of microbial phosphatases reflecting the greater microbial activity in the rhizosphere. There was a weak positive relationship between root and soil (soil+R) phosphatase activity. The relationship is weak because root P-ase activity may vary during the plant and root life-time whereas the soil activity results from the accumulation of root and microbial enzymes throughout the culture period. Therefore, our study underlines the role of plants for controlling the P-ase activity and hence P availability to moderate Pi deficiency and increase the plant productivity in Andisol (Ciereszko et al. 2017).
A close correlation between root and soil P-ase is thus not necessarily to be expected, and so the absence of any strong correlation in this study is not surprising. For example, wheat showed the greatest root P-ase activity, but this was not reflected in P uptake. However, we observed a clear correlation between root P-ase and shoot P concentration (Fig. S1a), which could be attributed to plant adaptation to low P, enhanced root P-ase activity being related to P-ase root secretion followed by hydrolysis of organic P (Machado and Furlani 2004). The increase in root P-ase activity on addition of P fertilizer may reflect the role of P-ase in the cycling of stored P in plants, rather than a strategy to obtain P from soil. Thus, root P-ase plays an important role in the storage of P after root take-up and may not be synthesized primarily to mineralize soil P (Margalef et al. 2017). In general, the addition of RP to soil led to enhanced P-ase activity in roots, soil+R and soil−R of three cereal plant species. This may reflect the slow release of P that enhanced microbial activity thereby increasing P availability for plants and microbes (Margalef et al. 2017). Due to the slow release effect and consequently less P fixation by minerals (Calabi-Floody et al. 2012), P uptake in soil under barley was higher for RP fertilization as compared to TSP.
4.2 Interaction Between Crop Species and P Speciation in Soil
Soil under oat showed the highest Po/Pi ratio for the NaOH-P fraction in control soil, especially in soil−R, as reported for a previous study (Redel et al. 2007). Wheat was the only crop, which showed lower NaHCO3-Po with P fertilizer application than the other crops. This underlines the importance of root released P-ase for P mineralization in wheat. Similarly, we found that the fertilization had an effect on NaOH-Pi fractions in soil+R and soil−R, probably because of high Pi retention on Al and Fe compounds, particularly strong in Andisols (Redel et al. 2016).
4.3 Effect of Fertilizers on Soil P Speciation
As expected, P fertilization increased mineral P, as indicated by higher contents of NaOH-Pi fraction. The two fertilizer types showed contrasting effects with TPS showing greatest increases of the NaOH-Pi fraction (46% as compared to 29% for soils fertilized with RP, Table 2). Regarding the less available P fractions, RP fertilizer showed an important effect on the HCl-Pi accumulation. Both these effects could result from the acid solubility of remaining undissolved rock phosphate. A similar tendency was reported by Mutuo et al. (1999) for corn plants grown on acidic soils, where TSP was initially more effective than RP in increasing NaHCO3-Pi and NaOH-Pi fractions, while differences were no longer detectable after 18 months. Zoysa et al. (1997) also report differences between species in the soil P fractions depleted with and without rock phosphate fertilization; in general, the NaOH-Po fraction was least depleted, but in the case of tea plants, a net accumulation was observed. Additionally, we found an increase in the soil+R pH as compared to soil−R probably due to the influence of an excess of anions over cations because of root input in acidic soils (Álvarez et al. 2012) and the chemical dissolution of fertilizer P. Thus, the variation in the sum of P fractions was of the same order as the amount of fertilizer P and variation in P fractionation in soil+R respect to soil−R was observed. Zoysa et al. (1997) using a similar experimental device detected changes in P fractions over a distance of only about 3 mm of distance from the roots.
The understanding of cereal plant species behavior against fertilizer P application in soils with low P availability as an Andisol is needed in order to improve the fertilization strategies to sustainable agriculture.
5 Conclusions
In this study, we investigated the effect of TSP and PR fertilization on P-ase activity and P speciation in soil in the presence (soil+R) and absence of roots (soil−R) under three cereal plant species (wheat, oat, and barley). All three species showed different P-ase activity, with wheat showing the highest activity in roots. A close relation between P-ase activity and P uptake underlines the importance of this enzyme for P nutrition and for P dynamics within plants. Wheat was more sensitive to P nutrition (greatest values of root P-ase), whereas barley was most influenced by the type of fertilizer applied.
Application of RP, enhanced the P-ase activity in soils (soil+R and soil−R) and roots. Soil P fractionation was found to be different in soil fertilized with TSP and RP. RP appeared to be a better source of P than TSP as the fertilizer use efficiency was greater for barley and phosphatase activity was more often maintained or even enhanced. Significant differences between crop-fertilizer type combinations indicate that cereal plant species will be affected differently by management practices including fertilization due its different patterns of P-ase activity. Longer-term experiments and trails with different levels of P fertilization would be required to establish the best sustainable management system to optimize soil and fertilizer P availability.
References
Aguilera P, Cornejo P, Borie F, Barea JM, von Baer E, Oehl F (2015) Diversity of arbuscular mycorrhizal fungi associated to Triticum aestivum L. plants growing in an andosol with phytotoxic aluminum levels. Agric Ecosyst Environ 186:178–184
Álvarez E, Fernández-Sanjurjo MJ, Núñez A, Seco N, Corti G (2012) Aluminium fractionation and speciation in bulk and rhizosphere of a grass soil amended with mussel shells or lime. Geoderma 173–174:322–329
Balemi T, Negisho K (2012) Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J Soil Sci Plant Nutr 12:547–561
Borie F, Zunino H (1983) Organic matter - phosphorus associations as a sink in P-fixation processes in allophanic soils of Chile. Soil Biol Biochem 15:599–603
Cabeza R, Myint K, Steingrobe B, Cs S, Schulze J, Claassen N (2017) Phosphorus fractions depletion in the rhizosphere of young and adult maize and oilseed rape plants. J Soil Sci Plant Nutr 17:824–838
Calabi-Floody M, Velásquez G, Gianfreda L, Saggar S, Bolan N, Rumpel C, Mora ML (2012) Improving bioavailability of phosphorous from cattle dung by using phosphatase immobilized on natural clay and nanoclay. Chemophere 89:644–655
Ciereszko I, Balwicka H, Żebrowska E (2017) Acid phosphatases activity and growth of barley, oat, rye and wheat plants as affected by Pi deficiency. Open Plant Sci J 10:110–122
Crème A, Rumpel C, Gastal F, Mora ML, Chabbi A (2016) Effects of grasses and a legume grown in monoculture or mixture on soil organic matter and phosphorus forms. Plant Soil 402:117–128
Dick WA, Tabatabai MA (1977) Determination of orthophosphate in aqueous solutions containing labile organic and inorganic phosphorus compounds. J Environ Qual 6:82–85
Durán P, Jorquera M, Viscardi S, Carrion VJ (2017) Screening and characterization of potentially suppressive soils against Gaeumannomyces graminis under extensive wheat cropping by chilean indigenous communities. Front Microbiol 8:1–16
George P, Gregory J, Robinson JS, Buresh RJ (2002) Changes in phosphorus concentrations and pH in the rhizosphere of some agroforestry and crop species. Plant Soil 246:65–73
George TS, Giles CD, Menezes-Blackburn D et al (2017) Organic phosphorus in the terrestrial environment: a perspective on the state of the art and future priorities. Plant Soil 427:191–208
Gianfreda L (2015) Enzymes of importance to rhizosphere processes. J Soil Sci Plant Nutr, pp 283–306
Hedley H, Stewart J, Chauhan B (1982) Changes in organic and inorganic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976
Hedley M, Kirk G, Santos M (1994) Phosphorus efficiency and the forms of soil-phosphorus utilized by upland rice cultivars. Plant Soil 158:53–62
Machado C, Furlani A (2004) Root phosphatase activity, plant growth and phosphorus accumulation of maize genotypes. Sci Agric 61:216–223
Margalef O, Sardans J, Fernández-Martínez M, Molowny-Horas R, Janssens IA, Ciais P, Goll D, Richter A, Obersteiner M, Asensio D, Peñuelas J (2017) Global patterns of phosphatase activity in natural soils. Sci Rep 7:1337
Martinez OA, Crowley DE, Mora ML, Jorquera MA (2015) Short-term study shows that phytase-mineralizing rhizobacteria inoculation affects the biomass, phosphorus (P) uptake and rhizosphere properties of cereal plants. J Soil Sci Plant Nutr 15:153–166
Mora L, Demanet R, Acuña JJ, Viscardi S, Jorquera M, Rengel Z, Durán P (2017) Aluminum-tolerant bacteria improve the plant growth and phosphorus content in ryegrass grown in a volcanic soil amended with cattle dung manure. Appl Soil Ecol 115:19–26
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Mutuo P, Smithson P, Buresh R, Okalebo R (1999) Comparison of phosphate rock and triple superphosphate on a phosphorus- deficient Kenyan soil. Commun Soil Sci Plant Anal 30:1091–1103
Nuruzzaman M, Lambers H, Bolland M, Veneklaas E (2006) Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fraction in the rhizosphere of a cereal and three grain legumes. Plant Soil 281:109–120
ODEPA (2015) Cultivos anuales: superficie, producción y rendimientos. Oficina de Estudios y Políticas Agrarias (ODEPA), Santiago, Chile. Available at http://www.odepa.cl/cultivos-anuales-superficie-produccion-y-rendimientos-4/ (accessed december 2015)
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Deeney DR (eds) Methods of soil analysis. Part, vol 2. ASA, Madison, pp 403–430
Paredes C, Menezes-Blackburn D, Cartes P, Gianfreda L, Mora ML (2011) Phosphorus and nitrogen fertilization effect on phosphorus uptake and phosphatase activity in ryegrass and tall fescue grown in Chilean Andisol. Soil Sci 176:245–251
Redel Y, Rubio R, Rouanet J, Borie F (2007) Phosphorus bioavailability affected by tillage and crop rotation on a Chilean volcanic derived Ultisol. Geoderma 139:388–396
Redel Y, Rubio R, Godoy R, Borie F (2008) Phosphorus fractions and phosphatase activity in an Andisol under different forest ecosystems. Geoderma 145:216–221
Redel Y, Cartes P, Demanet R, Velásquez G, Poblete-Grant P, Bol R, Mora ML (2016) Assessment of phosphorus status influenced by Al and Fe compounds in volcanic grassland soils. J Soil Sci Plant Nutr 16:485–501
Rubio R, Moraga E, Borie F (1990) Acid phosphatase activity and vesicular-arbuscular infection associated with roots of four wheat cultivars. J Plant Nutr 13:585–598
Sarapatka B, Dudova L, Krskova M (2004) Effect of pH and phosphate supply on acid phosphatase activity in cereal roots. Biologia 59:127–131
Tabatabai M, Bremner Y (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–315
Velasquez G, Calabi-Floody M, Poblete-Grant P, Rumpel C, Demanet R, Condron L, Mora ML (2016a) Fertilizer effects on phosphorus fractions and organic matter in Andisols. J Soil Sci Plant Nutr 16:294–304
Velasquez G, Ngo P, Rumpel C, Calabi-Floody M, Redel Y, Turner B, Condron L, Mora ML (2016b) Chemical nature of residual fraction derived from Hedley fractionation in Andisols. Geoderma 271:27–31
Zoysa AKN, Loganathan P, Hedley MJ (1997) A technique for studying rhizosphere processes in tree crops: soil phosphorus depletion around camellia (Camellia japonica L.) roots. Plant Soil 190:253–265
Zoysa AKN, Loganathan P, Hedley MJ (1999) Phosphorus utilization efficiency and depletion of phosphate fractions in the rhizosphere of three tea (Camellia sinenesis L.) clones. Nutr Cycl Agroecosyst 53:189–201
Acknowledgments
The authors thank to Dr. Fernando Borie for his help in revising and reviewing this manuscript. We also thank the economic support of FONDECYT Iniciation No. 11121619 and ECOS-CONICYT C13U02 Grants.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 312 kb)
Rights and permissions
About this article
Cite this article
Redel, Y., Staunton, S., Durán, P. et al. Fertilizer P Uptake Determined by Soil P Fractionation and Phosphatase Activity. J Soil Sci Plant Nutr 19, 166–174 (2019). https://doi.org/10.1007/s42729-019-00024-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-019-00024-z