Abstract
The introduction of fall armyworm (FAW), Spodoptera frugiperda (Lepidoptera: Noctuidae) into Ghana is a threat to maize production. This study determined the severity of this pest on maize production subjected to Bacillus thuringiensis (Bt), emamectin benzoate (Eb) sprayed and unsprayed farms under farmers’ practices in Ghana. At least one farm per treatment was selected in each Agro-Ecological Zone (AEZ) for data collection throughout the maize phenology during three production seasons. Percent damaged plants and ears were determined, the proportion of feeding damage on leaves and ears was scored, and yields measured on each farm. Ear damage was most severe in the Guinea Savannah Agroecological Zone with a correspondingly lower yield. The highest yield was recorded from the Tropical Rain Forest zone. The damage levels decreased when plants aged, but the scoring of damage level on attacked ears was greater than that on leaves. Maize plant damage was highest with corresponding lowest yields on unsprayed farms compared to sprayed farms which recorded similar results for both insecticides. Bt and Eb based insecticides (applied at 50g/15L H2O/ha and 75mL H2O/ha, respectively) are effective on FAW larvae and are therefore recommended for FAW management in Ghana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), is a major pest of maize and many crops of economic importance in the Americas (Luginbill 1928; Bass 1978; Pitre 1979; Sparks 1979; Young 1979; Pitre and Hogg 1983; Wiseman et al. 1983; Pitre et al. 1983; Nagoshi and Meagher 2004; Hernández-Mendoza et al. 2008; Goergen et al. 2016). It has spread throughout Sub-Saharan African countries and recently to Asia (Nagoshi et al. 2017, 2018; IITA 2018; ICAR-NBAIR 2018; IPPC 2018, 2019; FAO 2019). The damaging stage of this insect is limited to the larval stage which feeds on crops and causes damage that results in yield losses and eventually impacts the livelihoods of farmers as well as the economy of countries (Casmuz et al. 2010). The larvae of FAW attack all the phenological stages of the maize plant by feeding on the leaves, inside whorl, and tender tissues of the tassel and ears. At the early stages of the maize plants, the young larvae firstly feed on the tender portions of leaves leaving intact the membranous epidermis on the other side (Capinera 2000, 2017), whereas mature larvae feed on the leaf by making holes or may destroy small plants and strip larger ones (Cruz 1995). Inside the whorl, larvae are protected from external factors and are free to cause severe leaf-damage. Depending on the developmental stage of the maize plant infested by the FAW, pest density, management strategies, capacity of natural factors to reduce the pest population, and agronomic practices, the yield losses may vary from mild to severe (Perdiguero et al. 1967; Carvalho 1970; Cruz and Turpin 1982, 1983; Williams and Davis 1990; Willink et al. 1991; Cruz et al. 1996; De Almeida Sarmento et al. 2002).
To minimize damages caused by the FAW, different management methods have been developed. The resistant maize hybrids with antibiosis and non-preference as key mechanisms have successfully produced greater yields compared to susceptible hybrids at similar infestation levels (Wiseman et al. 1981, 1983; Sparks 1986; Wiseman and Davis 1990). Biological control of FAW has been considered an important management method since the identification of 53 species of parasitoids that infest the FAW globally (Ashley 1979; Sparks 1986). However, when the infestation level is above the economic threshold, insecticides are the most effective method applied at the egg masses or early larval instar stages for population reduction (Beuzilin et al. 2014). Management of the FAW has thus become heavily reliant on insecticides, but unfortunately, incorrect and indiscriminate application of insecticides has induced resistance in the pest as well as affecting non-target organisms including native natural enemies (Tinoco and Halperin 1998; Gómez-Valderrama et al. 2010).
A State of Emergency for agriculture was declared in Ghana in 2017 after the invasion and severe infestation of this pest on maize (Koffi et al. 2020a). The Government as part of the response assisted farmers with different type of insecticides, training, and awareness creation among actors. However, due to the invasive nature of the pest, and rapid spread, the effectiveness of pesticides was not tested. This study therefore aimed at evaluating the two most commonly used pesticide active ingredients (Bacillus thuringiensis and emamectin benzoate) by farmers to manage this pest. The damage levels on maize plants at different phenological stages and ears as well as yield were studied in the AEZs of Ghana.
Materials and methods
Study area and sampling schedule
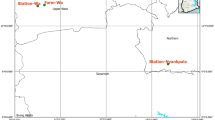
Inspections as well as data collections were done on maize farms at different phenological stages from different localities of the major AEZs of Ghana (Fig. 1) during three consecutive cropping seasons from 2017 to 2018. During each cropping season, five maize farms were selected within each AEZ. The first data collection covered the period from 5th May to 22nd July 2017, the second from 29th August to 27th November 2017, and the third from 12th July to 20th October 2018. The first period (Per 1) was during the major cropping season in the South and the beginning of the cropping season in the North. The second period (Per 2) was in the minor cropping season in the South and covered the end of the cropping season in the North. The third period (Per 3) covered the last half of cropping season in the north and the first half of minor cropping season in the south.
Survey and data collections
Among 90 maize farms randomly selected from all the six AEZs of Ghana, 33 farms were sprayed with emamectin benzoate (at 75mL/15L H2O/ha), 27 farms with Bacillus thuringiensis (at 50g/15L H2O/ha), and 30 farms as control or unsprayed. Farms were sprayed three times at vegetative four-six leaves, eight-ten leaves, and tasseling stages. All The farms were inspected once at each phenological stage which included vegetative V4-8, vegetative V8-12, tassel, and mature stages to record damage parameters on 100 plants. Sampling followed the diagonal quadrant method with 20 plants sampled from each of four corners of the farm plus the intersection of the two diagonals to obtain five sampling plots. The number of plants or ears damaged by fall armyworm as described by Capinera (2000, 2017) was recorded for each phenological stage and the percentage of damaged plants or ears calculated using Eq. (1). Scoring of damage severity on leaves and ears followed the scale of Davis and Lewis. At harvest, yields were estimated as total grain weight per hectare.
where: Dl - Percentage of damaged plants or ears, Ndp - number of damaged plants or ears, and Np = total number of inspected plants or ears. The proportion of control with a specific control method was calculated using Eq. (2):
where: Cl – control level of a specific control method, ncf – number of farms controlled with a specific method, nf – total number of inspected farms.
Data analysis
All computations were carried out with Excel and grouped into AEZs, plant phenological stages, and types of management methods adopted during the three periods of this study. The percentage values were arcsine ARSIN (SQRT(X)) transformed before analysis. All data were subjected to a Schapiro test in GenStat Twelfth Edition (GenStat Procedure Library Release PL20.1) for normality. Normally distributed data were further subjected to ANOVA that carried out the interaction effects among the factors and means separated with Tukey at a 5% error level. In the absence of normality, data were subjected to a non-parametric test (Kruskal-Wallis) at the 5% confidence level.
Results
Damage across cropping periods
Significant interactions were observed between the cropping periods and AEZs distributed within the two cropping seasons of the south and a cropping season of the north by analyzing the percent damaged plants (F2, 10=3.58; P<0.001), the score of damaged leaves (F2, 10=5.80; P<0.001), and percent damaged ears (F2, 10=2.09; P=0.036). This interaction was not significant for the damage score on ears (F2, 10=0.47; P=0.902), and yield (F2, 4=0.13; P=0.969). Interactions were significant between the cropping periods and management practices regarding the percent damaged plants (F2, 4=3.44; P=0.009), damage score on leaves (F2, 4=7.04; P<0.001) but not for the percent damaged ears (F2, 4=1.34; P=0.263), damage score on ears (F2, 4=1.82; P=0.133) and yield (F2, 4=0.13; P=0.969).
The percentages of damaged plants during the Per1 and Per2 were nearly three times higher than those of Per3. When the total surface of damaged leaves by the FAW larvae was scored, they were all under half of the total leaf surfaces. The scores during the Per1 and Per2 were similar but higher than the score of Per3. The levels of damage due to the FAW feeding on maize ears also showed similarities between Per1 and Per2 which were approximately three times higher than the percentage recorded during the Per3. Concerning the feeding scores of ears by the FAW larvae, a considerable decrease was observed during the Per3 compared to the previous periods. The yield estimation for the three seasons showed similar and low yields in Per1 and Per2 compared to the Per3.
Impact of FAW on maize production within and across AEZs
The AEZs and the management practices did not show significant interactions for the percent damaged plants (F5, 9=0.18; P=0.996), damage score of leaves (F5, 9=1.01; P=0.436), percent damaged ears (F5, 9=0.80; P=0.618), damage score on ears (F5, 9=0.83; P=0.590), and yield (F5, 9=1.16; P=0.333).
During three periods of study, the percentage of damaged plants was higher in the Guinea Savannah (GS) zone which is bordered by the Sudan Savannah (SS) zone where the lowest damaged plants was recorded. Within all the AEZs, the percentages of damaged plants were higher during the Per1 and Per2 of 2017 than the Per3 in the 2018 cropping season (Table 1). Fall armyworm larval damage severity on leaves was higher in GS than the other AEZs during Per1 and Per2. However, during Per3, the highest scores were registered in SS. Within all the AEZs, damage severities were higher during the Per1 and Per2 than Per3 (Table 1).
The percentages of damaged ears were higher in CS and GS than the other AEZs. In terms of seasonal trends, the percentages of damaged ears were higher during the first two periods than the Per3 in all the AEZs (Table 1). No significant difference was established for damage severity among the AEZs during the study period (Table 1). Within the AEZs, the damage scores were high during the Per1 and Per2 than the Per3 in all AEZs except SDF and SS (Table 1).
The yields recorded during the Per1 and Per2 showed no significant difference across the AEZs, while the yields were higher in Tropical Rain Forest (TRF) and Semi-Deciduous Forest (SDF) than other AEZs during the Per3. Within the AEZs, only the SDF presented significant differences among the three periods with the highest yield in Per3 (Table 1).
Damage variations among maize plant phenological stages
Between the management practices and plants stages, the interactions were not significant for the percentage of damaged plants (F2, 4=0.10; P=0.982), and damage score of leaves (F2, 4=1.60; P=0.175).
The damages of FAW larvae on maize plants and ears were most severe during the early vegetative growth stages (V4-12). During the three cropping seasons of this study, the percentage of damaged plants decreased as plants advanced in age. However, the difference in FAW damage between the maize plants at V4-8 and V8-12 stages was not significant but differences were observed between these stages and the older (tassel and mature) stages. Larval feeding scores on maize leaves, though similar between the V4-8 and V8-12, decreased with the age of maize plants as observed during the Per1 and Per2. During the Per3, no difference was observed between the V4-8, V8-12, and tassel stages. But the damage scores on ears due to the FAW larvae were more severe than the damage score to leaves during the vegetative stages.
Impact of management practices on fall armyworm damage severity
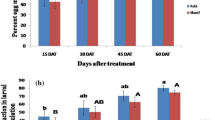
Farms on which FAW was managed with insecticides containing the entomopathogenic bacteria B. thuringiensis and avermectin, emamectin benzoate as active ingredients, were compared to control farms where no insecticides were used. Under natural FAW infestation pressures, a higher percentage of damaged plants were observed on the control farms than both insecticide-treated farms during the three periods of this study. Farms treated with both insecticides recorded a closer percentage of damaged plants (Table 2). A similar trend was observed for FAW damage severity on leaves among the three treatments during three periods of study (Table 2).
The percentage of damaged ears was lower in the insecticide-treated farms than the control farms during the Per1, but the Per2 and Per3 showed no significant differences for the percent damaged plant from the different treatments (Table 2). When the damage scores on ears were compared among the treatments, it showed a significantly higher damage proportion on ears in control farms than sprayed farms during the Per1 and Per2. While no significant difference was observed among the three treatments during Per3 (Table 2). The farms sprayed with the entomopathogen and avermectin insecticide registered closer yields per hectare, which were higher than the control farms (Table 2).
Discussion
This study was conducted to assess the severity of FAW on maize production subjected to Bacillus thuringiensis and emamectin benzoate sprays which were compared to unsprayed control farms during three cropping seasons across the AEZs of Ghana. The invasion of FAW in Ghana was reported in 2016 (Cock et al. 2017; Koffi et al. 2020a) and by 2017, the pest had established in all the AEZs of the country (Koffi et al. 2020a, c). In the absence of appropriate control measures and under favourable natural conditions, the new pest rapidly multiplied and increased in population leading to outbreaks with severe impact on maize production in 2017 as observed during the Per1 and Per2 of this study. Considering the agricultural emergency, urgent control measures were taken with considerable involvement of the Government of Ghana and its partners to assist farmers. Efforts towards finding sustainable solutions included of the identification of seven species of larval parasitoids and three species of larval predators of FAW which were collected at Ghana in 2017 (Koffi et al. 2020b), and later egg and larval parasitoids in 2018 (Agboyi et al. 2020). Other efforts to manage the new pest, combined with the presence of natural enemies reduced this pest population, and therefore its impact on maize in 2018 as evident during the Per3 of this study (Koffi et al. 2020c). Koffi et al. (2020a) in their study on maize infestation of FAW within AEZs of Togo and Ghana in West Africa three years after this pest invasion, reported higher infestation in 2016 and 2017 than in 2018 in both countries. The high damage impacts on maize plants and ears during the Per1 and Per2 affected the yields which were lower than the yield of Per3.
The highest damage on maize plants and ears was recorded at the GS which is traditionally considered as a high maize production zone (MoFA 2016). It was also reported among the three zones forming a boundary where high infestations of FAW were reported in 2016 and 2017 (Koffi et al. 2020a). However, the high damages recorded did not result in low yield as expected, indicating that other factors may affect maize yield across the AEZs. These factors can be associated with the type of soils, rainfall patterns, and agronomic practices. But the Savannah zones are characterized by sandy soil that can be found in SS, GS, and CS. If maize yield is only depending on the type of soil, the yields from these three zones should be similar with or without similar damages of FAW on plants and ears. The damages from GS were however higher than the two other zones, thus eliminating the assumption of the effect of type of soils on the yield during this study. Southern Ghana, which includes CS, TRF, SDF, and TZ has bimodal rainfall while the North covered by GS and SS has unimodal rainfall. If the similarities of yields observed during this study should be affected by the rainfall pattern, the yield from GS may be lower than the SS due to high damages in GS. This assumption should also be eliminated for the results of this study. The most probable factor that may have lowered the yields in other AEZs to the level observed in GS should be the agronomic practices that vary among farmers. These practices include ploughing, weeding, ridging, and fertilizer applications that were not taken into consideration by this study.
The tissues of young maize plants are much succulent and preferred for feeding by FAW larvae. This also attracts females to lay eggs and since food is available, newly hatched larvae survive and disperse to the adjacent plants (Ali et al. 1989, 1990) to increase the number of damaged plants and the feeding proportion on leaves of the early-stage plants. As plants age, the leaf tissues become hard and unsuitable for larvae which are exposed to high mortality and progressively reduce the percentage of damaged plants and the damage severity on leaves. During the vegetative period, however, plants continue to produce new leaves that are suitable and available for the FAW larvae. This accounts for a similar percentage of plants damaged and damage severity on the V4-8 and V8-12 stages. During the tassel stage, larvae move to the axis of the leaves and are more exposed to mortality factors such as parasitoids and predators that reduce the number of damaged plants. At this stage, feeding is limited to the tassel and ears. At the maturity stage, which covers the ear formation and ear filling up to harvest, the tender tissues suitable for FAW larval feeding are limited to the ears which are usually too tough for young larvae, considerably reducing ear damage. However, when 234 the larva force ear-boring, it gets into the newly formed cob and causes severe damage. Once larvae find their way into the ear, they stay protected and cause significant feeding damage to the kernel with a consequence for grain yield.
When damage is above the economic threshold level, control measures need to be applied to the FAW larvae. In Ghana, insecticides remain the main tools for managing this new pest on maize farms though the effectiveness of some commonly used insecticides is questionable (Abrahams et al. 2017). The significance of this pest has been further enhanced by the inconsistent reliance on foliar insecticide sprays (Adamczyk et al. 2001). Some farmers do not spray their maize farms against FAW and the effectiveness of insecticide applications was not scientifically proven in Ghana. To test the field effectiveness of some insecticides, products based on two active ingredients Bacillus thuringiensis and emamectin benzoate, which are most widely used in Ghana against the FAW larvae were selected. The choice of these two insecticides is to compare the avermectin insecticides, emamectin benzoate to an alternative entomopathogen-based biopesticide (Bacillus thuringiensis). The bioefficacy of the two insecticides was similar, resulting in higher yields compared to the unsprayed farms. However, owing to the adverse effects of synthetic insecticides such as being harmful to humans and non-targeted organisms, as well as the environment, (Yu 1983), Bacillus thuringiensis based insecticides are recommended for FAW management in Ghana. Alternatively, since both insecticides have completely different modes of action, the two can be alternated as a mean of insecticide resistance management.
References
Abrahams P, BatemanM, Beale T, Clotte V, Cock M et al (2017) Fall armyworm: impacts and implications for Africa. CABI Evidence Note (2), September 2017. Report to DFID. https://www.invasive-species.org/Uploads/InvasiveSpecies/Fall%20Armyworm%20Evidence%20Note%20September%202017.pdf (accessed 13 August 2018)
Adamczyk JJ Jr, Adams LC, Hardee DD (2001) Field efficacy and seasonal expression profiles for terminal leaves of single and double Bacillus thuringiensis toxin cotton genotypes. J Econ Entomol 94:1589–1593
Agboyi LK, Goergen G, Beseh P, Mensah SA, Clottey VA, Glikpo R, Buddie A, Cafá G, Offord L, Day R, Rwomushana I, Kenis M (2020) Parasitoid complex of fall armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects 11:68. https://doi.org/10.3390/insects11020068
Ali A, Luttrell RG, Pitre HN, Davis FM (1989) Distribution of fall armyworm (Lepidoptera: Noctuidae) egg masses on cotton. Environ Entomol 18:881–885
Ali A, Luttrell RG, Pitre HN (1990) Feeding sites and distribution of fall armyworm (Lepidoptera: Noctuidae) larvae on cotton. Environ Entomol 19:1060–1067
Ashley TR (1979) Classification and distribution of fall armyworm parasites. Fla Entomol 62:114–122
Bass MH (1978) Fall armyworm: Evaluation of insecticides for control. Auburn Univ Agric Exp Stn Leaflet 93:7
Beuzelin J, Brown S, Davis J, Foil LD, Hall M, Huang F, Kerns D, Morgan A, Pollet D, Reed D et al (2014) Cotton insect pest management, p. 7– 12. In 2014 Louisiana Insect Pest Management Guide. LSU Ag Center [Online] (http://www.lsuagcenter.com/NR/rdonlyres/B733BE31-7DEA-4264-93A6-AF6FED7023D3/96796/pub1802InsectMgmtGuide2014completebook247pages.pdf) (accessed 15 Sep 2014)
Capinera J L (2000) Fall armyworm, Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae). The University of Florida, Institute of Food and Agricultural Sciences. (UF/IFAS), Gainesville, FL
Capinera JL (2017) Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Insecta: Lepidoptera: Noctuidae). Available online: http://edis.ifas.ufl.edu/in255 (accessed on 10 Oct 2017)
Carvalho RPL (1970) Danos, Ilutuação de população, controle e comportamento de Spodoptera frugiperda (J.E. Smith, 1794) susceptibilidade de dilerentes genótipos de milho em condições de campo. (Piraci aba: Imprensa ESALQ) PhD Thesis, pp 170
Casmuz A, Juárez ML, Socias MG, Murúa MG, Prieto S, Medina S, Willink E, Gastaminza G (2010) Revisión de los hospederos del gusano cogollero del maíz, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev Soc Entomol Arg 69:209–231
Cock MJW, Beseh PK, Buddie AG, Cafá G, Crozier J (2017) Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci Rep 7:4103. https://doi.org/10.1038/s41598-017-04238-y
Cruz I (1995) A lagarta-do-cartucho na cultura do milho. Embrapa/CNPMS. Circ Téc 21:45
Cruz I, Oliveira LJ, Oliveira AC, Vasconcelos CA (1996) Eleito do nível de saturação de alumínio em solo ácido sobre os danos de Spodoptera frugiperda (J. E. Smith) em milho. An Soc Entomol Brasil 25:293–297
Cruz I, Turpin FT (1982) Eleito da Spodoptera frugiperda em dilerentes estágios de crescimento da cultura de milho. Pesqui Agropecu Bras 17:355–359
Cruz I, Turpin FT (1983) Yield impact of larval infestation of the fall armyworm Spodoptera frugiperda (J. E. Smith) to mid-whorl growth stage of corn. J Econ Entomol 76:1052–1054
De Almeida Sarmento R, de Souza Aguiar RW, Vieira SMJ, de Oliveira HG, Holtz AM (2002) Biology review, occurrence and control of Spodoptera frugiperda (Lepidoptera, Noctuidae) in corn in Brazil. Biosci J 18:41–48
Food And Agriculture Organization (FAO) (2019) FAO Statement on Fall Armyworm in Sri Lanka. Rome, Italy: FAO. http://www.fao.org/srilanka/news/detail-events/en/c/1177796/. Accessed 29 Aug 2020
Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One 11(10):e0165632. https://doi.org/10.1371/journal.pone.0165632
Gómez-Valderrama EJ, Guevara-Agudelo EJ, Barrera-Cubillos GP, Cotes-Prado AM, Villamizar-Rivero LF (2010) Aislamiento, identificación y caracterización de Nucleopoliedrovirus nativos de Spodoptera frugiperda en Colombia. Rev Fac Nac Agron Medellín 63(2):55115520
Hernández-Mendoza JL, López-Barbosa EC, Garza-González E, Pérez MN (2008) Spatial distribution of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize landraces grown in Colima. México. Int J Trop Ins Sci 28:126–129
Indian Council of Agricultural Research, National Bureau of Agricultural Insect Resources (ICAR-NBAIR) (2018) Spodoptera frugiperda (J. E. Smith). Insects in Indian Agrosystems. ICAR-National Bureau of Agricultural Insect Resources (NBAIR), India. http://www.nbair.res.in/insectpests/Spodoptera_frugiperda.php
International Institute of Tropical Agriculture (IITA) (2018) Fall armyworm has reached the Indian subcontinent! Ibadan, Nigeria: IITA. http://www.iita.org/news-item/fall-armyworm-has-reached-the-indian-subcontinent
International Plant Protection Convention (IPPC) (2018) First detection of Fall Army Worm on the border of Thailand. IPPC Official Pest Report, No. THA-03/1. FAO: Rome, Italy. https://www.ippc.int
International Plant Protection Convention (IPPC) (2019) First Detection Report of the Fall Armyworm Spodoptera frugiperda (Lepidoptra: Noctuidae) on Maize in Myanmar. IPPC Official Pest Report, No. MMR-19/2. Rome, Italy: FAO. https://www.ippc.int
Koffi, D, Agboka K, Adenka KD, Osae M, Tounou AK, Adjevi MKA, Fening KO, Meagher Jr RL (2020a) Maize infestation of fall armyworm (Lepidoptera: Noctuidae) within agro-ecological zones of Togo and Ghana in West Africa 3 Yr after its invasion. Environ Entomol XX(XX):1–6. https://doi.org/10.1093/ee/nvaa048
Koffi, D, Kyrematen R, Eziah YV, Agboka K, Adom M, Goergen G, Meagher RL (2020b) Natural enemies of fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in Ghana. Fla Entomol 103(1):85–90
Koffi D, Kyrematen R, Eziah YV, Osei-Mensah OY, Afreh-Nuamah K, Aboagye E, Osae M, Meagher RL (2020c) Assessment of impacts of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) on maize production in Ghana. J Integr Pest Mang 11(1):1–7. https://doi.org/10.1093/jipm/pmaa015
Luginbill P (1928) The fall armyworm. US Dept Agric Tech Bull 34(1–91):2
Ministry of Food and Agriculture (MoFA) (2016) Agriculture in Ghana. Statistics, Research and Information Directorate, Accra, Ghana, 4 pp.
Nagoshi RN, Meagher RL (2004) Seasonal distribution of fall armyworm (Lepidoptera: Noctuidae) hoststrains in agricultural and turf grass habitats. Environ Entomol 33:881–889
Nagoshi RN, Koffi D, Agboka K, Tounou KA, Banerjee R, Jurat-Fuentes JL et al (2017) Comparative molecular analyses of invasive fall armyworm in Togo reveal strong similarities to populations from the eastern United States and the Greater Antilles. PLoS One 12(7):e0181982
Nagoshi RN, Goergen G, Tounou KA, Agboka K, Koffi D, Meagher RL (2018) Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern sub-Saharan Africa. Sci Rep 8:3710
Perdiguero JS, Barral JM, De Stacul M V (1967) Aspectos biologicos de plagas de maíz de la región chaqueña. Evaluatión de daño. INTA, Estación Experimental Agropecuaria
Pitre HN (1979) Fall armyworm on sorghum: other hosts. Bull. 876. Mississippi Agric For Exp Stn Mississippi State, MS
Pitre HN, Hogg DB (1983) Development of the fall armyworm on cotton, soybean and corn. J Georgia Entomol Soc 18:182–194
Pitre HN, Mulroony JE, Hogg DB (1983) Fall armyworm (Lepidoptera: Noctuidae) oviposition: crop preference and egg distribution on plants. J Econ Entomol 76:463–466
Sparks A (1979) A review of the biology of the fall armyworm. Fla Entomol 62:282–287
Sparks AN (1986) Fall armyworm (Lepidoptera: Noctuidae): Potential for area-wide management. Fla Entomol 69:603–614
Tinoco R, Halperin D (1998) Poverty, production and health: inhibition of erythrocyte cholinesterase through occupational exposure to organophosphate insecticides in Chiapas, México. Arch Enviro Health 53:29–35
Willlams WP, Davis FM (1990) Response 01 corn to artificial infestation with fall armyworm and southwestern corn borer larvae. Southwest Entomol 15:163–166
Willink E, Osores VM, Costilla MA (1991) EI gusano cogollero dei maiz. Avance Agroindustrial 3–7
Wiseman BR, Williams WP, Davis FM (1981) Fall armyworm: Resistance mechanisms in selected corns. J Eco Entomol 74:622–624
Wiseman BR, Davis FM, Williams WP (1983) Fall armyworm: Larval density and movement as an indication of non-preference in resistant corns. Prot Ecol 5:135–141
Wiseman BR, Davis FM (1990) Plant resistance to insects attacking corn and grain sorghum. Fla Entomol 73:446–458
Young JR (1979) Fall armyworm: control with insecticides. Fla Entomol 62:130–133
Yu SJ (1983) Age variation in insecticide susceptibility and detoxification capacity of fall armyworm (Lepidoptera:Noctuidae) larvae. J Econ Entomol 76:219–222
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
Authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koffi, D., Kyerematen, R., Osae, M. et al. Assessment of Bacillus thuringiensis and emamectin benzoate on the fall armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) severity on maize under farmers’ fields in Ghana. Int J Trop Insect Sci 42, 1619–1626 (2022). https://doi.org/10.1007/s42690-021-00683-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00683-5