Abstract
Invasive fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is a species native to the Americas which has spread to Africa in 2016. This insect has been reported in Benin as a major pest of maize causing important economic losses and putting at risk food and nutritional security. This study evaluated the damage caused by this pest to maize in different cropping system and management practices. It also assessed predatory ants presence and diversity and their potential in controlling FAW. Results showed that 50% of farmers grow maize in a mixed cropping systems in association with sorghum, cassava and cowpea and also used biopesticides. FAW larval population and damage in maize fields varied accros villages. Surprinsingly FAW larval population was higher in maize field sprayed with insecticides than untreated field. Seven species of predatory ants were recorded in maize field. Ants' population was higher in untreated field (1043 ants per hectare) than treated field (806 ants per hectare). In the laboratory, ants species exhibits great predatory potential. Further studies are needed to discuss uses of ants in FAW management in Benin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Fall armyworm (FAW) Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) is mentioned on more than 353 plant species across the globe (Montezano et al. 2018; CABI 2020). This species is a major pest in the Americas, where its known preferred host plants are Poaceae, including economically important crops such as maize, millet, sorghum, rice, wheat and sugarcane. Damage of FAW are also observed on other major crops such as cowpea, groundnut, potato, soybean and cotton (FAO 2017).
This pest is a newly invasive one in Africa (Goergen et al. 2016; Cock et al. 2017; Day et al. 2017; Sisay et al. 2018; Kumela et al. 2019). Probably introduced accidentally, major outbreaks were reported in South West Nigeria and Ghana in 2016 and shortly after in Benin, Sao Tome and Togo (Goergen et al. 2016).
On maize, damage can be observed on all plant parts depending on the development stage (Sena et al. 2003). Larger caterpillars act as cutworms by entirely sectioning the stem base of maize plantlets. During the maize vegetative phase, constant feeding results in skeletonized leaves and heavily windowed whorls loaded with larval frass. On grown maize plants, larvae also attack reproductive organs feeding on tassels or boring into the ears (Goergen et al. 2016). In Western Africa, the maize fields areas attacked are estimated to 39,540,160 hectares, leading to a probable loss of 41,517,168 tons, or 30% of production (Maïga et al. 2017). In Benin, damages to maize have been reported on around 38,000 hectares in the northern region (Goergen et al. 2016; IPPC 2016). This threatens the main source of calories for rural populations and places millions of people in famine and food insecurity.
Farmers in Benin have relied on synthetic insecticides such as deltametrin and chlorpyrifos-ethyl (Allaba-Boni et al. 2016) to protect their maize when first damage were reported in 2016. Later on additional insecticides such as emamectin benzoate or methylamino abamectin benzoate were introduced in the country. Given the history of pesticides uses by farmers in Benin, overuses and misuse can be expected (de Bon et al. 2014). Also as observed in other countries, use of synthetic chemicals can lead very early to cases of FAW resistance (Pavela 2005; Belay et al. 2012; Omoto et al. 2016). The use of bio-pesticides based on plant extracts such as neem Azadirachta indica A. Juss (Meliaceae) against FAW can be an alternative (Tavares et al. 2010; Bateman et al. 2018; Midega et al. 2018; Shaiba et al. 2019). However standartized biopesticides are either not available or costly as compared to synthetic insecticides (Popp et al. 2013). Another alternative is the use of natural enemies for Biological control of FAW. It is an economical and environmentally friendly approach which has been used against the FAW in the Americas (Hruska 2019). However the development of a biological control approach requires a thorough knowledge of the cropping system and the exisiting natural enemies. In Benin, since the detection of FAW, no study has yet been conducted on FAW in maize-based cropping systems and their natural enemies.
Natural enemies of FAW included entomopathogenic nematodes, fungi, bacteria and virus (Maniania and Fargues 1985; Negrisoli et al. 2010; Polanczyk et al. 2000; Akutse et al. 2019; Gichuhi et al. 2020) and arthropods (Harrison et al. 2019). Among arthopods, several egg and larval parasitoids of FAW (Röse et al. 1997) have been reported in Africa (Sisay et al. 2018; Kenis et al. 2019; Agboyi et al. 2019 and 2020; Koffi et al. 2020). So far only Koffi et al. (2020) reported predators on FAW in Africa. Predators especially ants play important roles in the structure and functioning of agroecosystems and its studies on FAW control at community level are missing.
Ants are the most abundant hymenoptera predators in tropical cropping systems and have a major influence on diverse habitats (Way and Khoo 1992). Their potential as pest regulators has been widely demonstrated. For example in banana-based cropping systems ants such as Pheidole spp., Camponotus spp., and Odontomachus mayi Mann have been identified as predators of banana weevil Cosmopolites sordidus Germar (Dassou et al. 2016). Maize associated with other crops such as banana increases the abundance of predatory ants (Dassou et al. 2015). Predatory ants contribute to the biological control of FAW (Hruska 2019) in maize-based cropping systems in Honduras (Wyckhuys and O’Neil 2006).
This study aimed to evaluate the importance of FAW population and damage in maize the central region of Benin and assess the potential of predation by ant species. Specifically, we: i) determined abundance of FAW and their damage on maize in farmers’ fields; ii) assessed FAW larval population and damage in untreated and treated field; iii) inventoriated predatory ant species and population abundance in untreated and treated maize field; and iii) assessed potential of predation of FAW larvae by predatory ants in the laboratory.
Material and methods
Study site
The on-farm study was conducted in the district of Dassa-Zoume (center of Benin) in 2018. Dassa-Zoume is one of six districts of the Department of Zou-Collines with an area of 1711 km2 (Fig. 1). Dassa-Zoume belongs to the subequatorial climatic zone with two seasons, a dry season (from November to March) and a rainy season (from April to October). The average annual rainfall is around 1,100 mm. The distribution of rains is fairly regular with the maximum rainfall recorded generally in July. Temperature variations are relatively high and ranged between 25 and 38°C (Adomou et al. 2006). The soils are usually of tropical ferruginous type, but hydromorphic soils and vertisols type can also can be found.
Assessment of FAW abundance and damage in Farmers’ field
This study was carried in 6 villages (Ayedero, Kere, Kpingni, Moumoudji, Vedji and Zongo) in which maize is produced in monoculture or intercropped with other commodities (Fig. 1). In each village, from March to August 2018, 10 maize fields (each farm belongs to one farmer) were selected for assessment of FAW prevalence and damage. The maize fields were visited thrice, 1 month after planting (vegetative stage), and at flowering and fruiting stage. In each field, an area of 1 hectare was delimited, in which 50 maize plants were randomly selected at each date of observation. The number of FAW larvae were counted on selected plants to assess FAW abundance followed by the number of perforated and unperforated leaves to determine damaged leaves. The number of galleries dug on each stem were also counted and all the damage were appreciated using a scores scale (Davis et al. 1996; Aguire et al. 2016). The scores ranged from 1 to 9 with 1 showing no visible foliar damage and 9 showing plants with the whorl destroyed and ear damaged (Davis et al. 1996; Aguire et al. 2016). The scores were assigned to each of the 50 plants selected on a 1 hectare field. These scores were transformed into damage percentages during data processing to perform statistical analyzes. The scores used correspond to the following percentages: 0% for the score 1 (used for plants showing no damage), 0 to 10% for the score 2, 10 to 25% for the score 3, 25% to 40% for the score 4, 40% to 55% for the score 5, 55% to 70% for the score 6, 70% to 85% for the score 7, 85% to 99% for the score 8 and 100% for the score 9 (severe damage). The number of FAW larvae was obtained as the number of FAW individuals collected from the 50 maize plants in each field.
Assessment of FAW and ants prevalence in treated and untreated maize fields
This study was carried out in two fields of 0.5 hectare each selected randomly in the village of Kpingni (Fig. 1). One of the 2 fields was sprayed (treated) once per month during the vegetative stage by farmers with emamectin benzoate at the dose of 220 g/hectare, with a total of two applications. The second field was not sprayed with any pesticides (untreated). Once per week, in the morning (from 7 to 11 am), FAW larvae were counted on 50 randomly selected maize plants in each of the two fields during eleven weeks. In addition, the number of damaged leaves (including fully opened youngest leaves and leaves of the whorl) were also counted on the same 50 maize plants to determine the percentage of plant damaged by larvae. In the same two fields, ant countings were carried out 3 times during the study period, using the the method described by Dassou et al. (2015, 2016, 2017). Bait traps with a few drops of honey mixed with tuna were put on ceramic plates and placed in the field between 7 and 11 am. A total of 100 baited traps were randomly placed within each field. After 30 min of field exposition, ants were captured with an aspirator and kept in 70% alcohol and identified using Bolton’s key (Bolton 1973). All FAW individuals from the different larval stages were grouped together to perform the statistical analyzes. After identification, all the ant individuals were grouped per species.
Laboratory evaluation of FAW larvae predation by ants
Five individuals of each identified ant species was given five 4th instar and five 5th instar larvae together. The ants and FAW larvae were confined for 2 h in a Petri dish (∅ = 9 cm; h = 1.5 cm). The petri dish lid has mosquito net to allow ventilation. The experiment was replicated 6 times for each ant species. The number of FAW larvae attacked and consumed by each ant species was counted.
Data analysis
For the first step, Generalized Linear Models (GLMs) with the Poisson family and Analyses of Variance (ANOVA) were used to determine the variation in the FAW number and also Davis’s scores noted by field across the selected villages. In this step, GLMs with ANOVA were also used to determine the variation of other damage types such as the number of perforated leaves and stems damage (galleries number) across the selected villages. In the second step, GLMs were used to determine the effect of ant species on the number of FAW larvae consumed. The Student t-test was used to compare the number of FAW and the number of leaves per plant and also the number of ants between treated and untreated plots. GLMs were tested against a null model using a Likelihood Ratio Test (LRT) (Bolker et al. 2009). The Student test was used to test the difference between treated and untreated plots according to the numbers of FAW larvae. All GLMs were estimated using the ‘lme4′ package (Bates et al. 2012), in which the maximum likelihood of parameters is approximated by the Laplace method (Bolker et al. 2009). Collected data were processed and analyzed using the software R version 3.4.2 (R Core Team 2016).
Results
Management of FAW by Farmers and abundance and damage of FAW in Farmers’ field
Two types of maize cropping systems were observed in studied areas. Half of the farmers (50%) practiced monoculture and the others associate maize with either sorghum, cassava or cowpea. Fertilizers were not used in the studied areas. About 50% of farmers sprayed neem oil to protect their maize from FAW while 33% of them applied emamectin benzoate instead. The other farmers (17%) did not use any pest management control (Table 1).
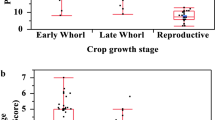
FAW average number varied significantly across the villages (df = 5, P = 0.012). The villages Kere, Moudji and Zongo were those with an average number > 40 individuals /50 plants (Fig. 2). There was no variation in the Davis score scales noted per infested plant from selected fields across villages (df = 5, P = 0.102). The village Moudji had the highest score followed by Kere and Zongo (Fig. 3). There was a significant variation of leaves damage (leaf perforation) (df = 5, P = 0.0001) and stems damage (galleries in stems) according to studied villages (df = 5, P = 0.03).
FAW larval densities and damages in treated and untreated maize fields
The FAW larval population dynamics follows the same pattern during the season in both treated and untreated fields (df = 1, P = 0.38) (Fig. 4). During the season, the larval population peaks three times, in the 14 and 21 days, in the 42 and 49 days and in the 77 days after planting. No significant difference was noted between treated and untreated fields for damaged plants (df = 1, P = 0.515) (Fig. 5).
Ants prevalence in treated and untreated maize fields
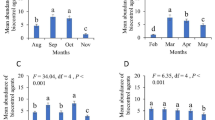
A total of 12,946 ants belonging to 8 major species were observed in both treated (5644 individuals) and untreated (7302 individuals) plots. Camponotus sp. was the most abundant taxon (2088 individuals in untreated plot and 1420 individuals in treated plot) and Megaponera sp. (198 to 344 individuals) and Pheidole megacephalla (32 to 294 individuals) the less abundant one. The overall ant density per hectare was higher in untreated plot as compared to treated plot (t = 2.8325, df = 23, P = 0.005448). When considering ant species, Brachyponera sennaarensis and Pheidole megacephalla density per hectare were higher in treated plot than untreated field while the opposite was observed for Megaponera sp. (Fig.6a). The insecticide treatment had a significant effect on all ant species except Camponotus sericeus (Table 2). A significant difference was observed between treated and untreated plot for Oecophylla longinoda (t = 4.0466, df = 12, P < 0.00001), Camponotus sp. (t = 2.5008, df = 12, P = 0.01385) but not for Camponotus sericeus (t = 0.1827, df = 12, P = 0.8541) and Pheidole sp. (t = 1.1715, df = 12, P = 0.2438) (Fig. 6b).
FAW larvae predation of by ants
There was a significant difference between ant species for numbers of preys attacked and consumed (df = 6, P < 0.00001). The ant species Camponotus sericeus, Camponotus sp., Pheidole sp. and Pheidole megacephalla consumed more FAW larvae than others (Fig. 7).
Discussion
The current study is the first one in Benin that focused on the FAW management in maize cropping systems and the potential of predatory ants. Our results on FAW populations and leaf damage showed a significant variation accros villages. Similar findings have been reported in other Africa countries (FAO 2017; Chimweta et al. 2019). This could be explained by the agricultural and control methods used by farmers (Barros et al. 2010). For example, Prasanna et al. (2018) showed that weeding could affect FAW abundance, since some weeds such as Agrotis spp., Digitaria spp., Sorghum halepense and Cenchrus tribuloides harbor the pest. Crop and varietal rotation may also affect FAW population in the field (Omoto et al. 2016). We did not collect data on farmers different practices (planting date, manure and compost use, herbicide and pesticide use) from one village to another that could explain variation between villages observed in this study.
Our study on effect of insecticide application on FAW larval population, did not reveal any significant difference between field treated with emamectin benzoate and untreated fields. This is suprising because emamectin benzoate is proven effective against FAW (Sparks and Nauen 2015; Wu et al. 2016; Zhao et al. 2020). However given that farmers in our case spayed it at a low frequency, only once per month it could have affect the performance of the treatment. This showed that emamectin benzoate should be sprayed twice according to the infestation level for effective control of FAW. However, previous studies have showed that some pests, such as diamondback moth (Wang et al. 2016) and mites (Kwon et al. 2010) have developed resistance to abamectin.
We have recorded 8 ant species in maize fields. These ant species were the most abundant and diversified of maize-based cropping systems. Very few studies focused on the ant community in African cropping systems. The few studies carried out focused on one to two dominant ant species in cropping systems. In this context, Pheidole megacephalla has been mentioned among the natural enemies of FAW of maize cropping systems in Ghana (Koffi et al. 2020). A diversity of 7 ant species was also found of which Monomorium sp., Paratrechna longicornis and Pheidole spp. were most abundant when maize was intercropped in plantain-based cropping systems in Cameroon (Dassou et al. 2015). In Nicaragua, ants were found to significantly reduce FAW abundance as well as damage by FAW to maize plants (Perfecto 1991). In addition, Wyckhuys and O'Neil (2006) have investigated relationships between the population dynamics of FAW and associated natural enemies within maize fields in the Honduran highlands and have showed high abundances of ants especially Solenopsis geminata Fabricius, Brachymyrmex spp., Camponotus spp., Crematogaster spp. and Pheidole spp. However, several ant species are among the major predators of FAW and should be conserved in maize fields for the integrated pest management.
However ant densities were higher in untreated plot (7302 ants/hectare) as compared to treated plot (5644 ants/hectare). This was expected as reported by Choate et al. (2013). This is in line with the work of Cruz et al. (1997) who emphasized that the chemical use is a new threat both for the environment and for the recrudescence of food poisoning. As an integral part of terrestrial ecosystems, ants have been widely used as biological indicators in the study of arthropod biological diversity and in habitat rehabilitation studies. Thus, in other studies such as Matlock and de la Cruz (2003), the effects of pesticides on arthropods were evaluated and the ant diversity and abundance were determined in banana plantations treated with pesticides and in low-input banana plantations with reduced applications. They showed that pest treatments have reduced ant feeding but have not upset the dominance relationships of the species. These results confirmed that the use of chemical pesticides in maize cropping systems would have reduced the ant density with no effect on the FAW density. In addition, the analysis showed a significant difference between the treated plot and untreated plot for Oecophylla longinoda, Pheidole sp. and Camponotus sp. But the effect is not significant for Camponotus sericeus. This confirms that the predators Camponotus sp., Oecophylla longinoda and Pheidole sp. are the most abundant in cropping systems and could be used in biological control programs.
Our results showed a significant difference between ant species for their potential to attack and consume the FAW larvae. The ant species Camponotus sp., Camponotus sericeus, Pheidole sp., Pheidole megacephalla and Oecophylla longinoda attacked and consumed most larvae. These ant species were shown to be a pest predator in many cropping systems including banana (Dassou et al. 2015), mango (Van Mele et al. 2007) and cashew (Anato et al. 2015). For years, the Charpenter ants Camponotus sp. have been proven to be predators of FAW larvae (Ashley et al. 1980). Regarding Pheidole spp., it was shown in a study in the Honduran highlands that among arthropods associated with FAW there are ant species including Pheidole spp (Wyckhuys and O’Neil 2006).
Conclusion
In summary, the density of the S. frugiperda larvae varied from one village to another. However, no variation of FAW density was observed between treated and untreated fields. The non-use of chemicals against this pest does not increase its density and this may result from natural regulation by general predators including ants.
Data availability
Data generated during this study are available from the corresponding author.
References
Adomou AC, Sinsin B, van der Maesen LJG (2006) Phytosociological and chorological approaches to phytogeography: a meso-scale study in Benin. Systematics and geography of plants 76:155–178
Agboyi LK, Mensah SA, Clottey VA, Beseh P, Glikpo R, Rwomushana I, Kenis M (2019) Evidence of Leaf Consumption Rate Decrease in Fall Armyworm, Spodoptera frugiperda. Larvae Parasitized by Coccygidium luteum Insects 10(11):410
Agboyi LK, Goergen G, Beseh P, Mensah SA, Clottey VA, Glikpo R, Rwomushana I (2020) Parasitoid complex of fall armyworm, Spodoptera frugiperda. Ghana and Benin Insects 11(2):68
Aguirre LA, Hernández-Juàrez A, Flores M, Cerna E, Landeros J, Frías GA, Harris MK (2016) Evaluation of foliar damage by Spodoptera frugiperda (Lepidoptera: Noctuidae) to genetically modified corn (Poales: Poaceae) in Mexico. Fla Entomol 99(2):276–281
Akutse KS, Kimemia JW, Ekesi S, Khamis FM, Ombura OL, Subramanian S (2019) Ovicidal effects of entomopathogenic fungal isolates on the invasive Fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). J Appl Entomol 143(6):626–634
Allaba-Boni O, Carmelle B, Adjakpa JB, Chougourou D, Agbaka A, Tamo M (2016) Distribution temporelle et spatiale de Spodoptera frugiperda (JE Smith) sur les cultures de maïs au sud Bénin. Rapport / EPAC / UAC - Benin
Anato FM, Wargui RB, Sinzogan AA, Offenberg J, Adandonon A, Vayssières JF, Kossou DK (2015) Reducing losses inflicted by insect pests on cashew, using weaver ants as a biological control agent. Agr Forest Entomol 17(3):285–291
Ashley TR, Mitchell ER, Leppla NC, Grissell EE (1980) Parasites attacking fall armyworm larvae, Spodoptera frugiperda, in late planted field corn. Fla Entomol 136–142
Barros EM, Torres JB, Ruberson JR, Oliveira MD (2010) Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol Exp Appl 137(3):237–245
Bateman ML, Day RK, Luke B, Edgington S, Kuhlmann U, Cock MJ (2018) Assessment of potential biopesticide options for managing fall armyworm (Spodoptera frugiperda) in Africa. J Appl Entoml 142(9):805–819
Bates D, Maechler M, Bolker BM (2012) lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-39
Belay DK, Huckaba RM, Foster JE (2012) Susceptibility of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), at Santa Isabel, Puerto Rico, to different insecticides. Fla Entomol 95(2):476–479
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol & Evol 24(3):127–135
Bolton B (1973) The ant genera of West Africa: a synonymic synopsis with keys (Hymenoptera: Formicidae). Bull br Mus nat Hist Entomol 27:319–366
CABI (2020) Spodoptera frugiperda (fall armyworm) Datasheet. Invasive species compendium. https://www.cabi.org/isc/datasheet/29810. (Accessed on 24 April 2020)
Chimweta M, Nyakudya IW, Jimu L, Bray Mashingaidze A (2019) Fall armyworm [Spodoptera frugiperda (JE Smith)] damage in maize: management options for flood-recession cropping smallholder farmers. Int J Pest Manage 1–13
Choate B, Francis A, Drummond, (2013) The Influence of Insecticides and Vegetation in Structuring Formica Mound Ant Communities (Hymenoptera: Formicidae) in Maine Lowbush Blueberry. J Eco Entomol 106(2):716–726. https://doi.org/10.1603/EC12273
Cock MJ, Beseh PK, Buddie AG, Cafá G, Crozier J (2017) Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Scie rep 7(1):4103
Cruz I, Figueiredo ML, Valicente FH, Oliveira AC (1997) Application rate trials with a nuclear polyhedrosis virus to control Spodoptera frugiperda (Smith) on maize. An Soc Entomol Bras 26(1):145–152
Dassou AG, Carval D, Dépigny S, Fansi GH, Tixier P (2015) Ant abundance and Cosmopolites sordidus damage in plantain fields as affected by intercropping. Biol Control 81:51–57
Dassou AG, Dépigny S, Canard E, Vinatier F, Carval D, Tixier P (2016) Contrasting effects of plant diversity across arthropod trophic groups in plantain-based agroecosystems. Basic Appl Ecol 17:11–20
Dassou AG, Tixier P, Dépigny S, Carval D (2017) Vegetation structure of plantain-based agrosystems determines numerical dominance in community of ground-dwelling ants. PeerJ 1–13
Davis FM, Wiseman BR, Williams WP, Widstrom NW (1996) Insect colony, planting date, and plant growth stage effects on screening maize for leaf-feeding resistance to fall armyworm (Lepidoptera: Noctuidae). Fla Entomol, 317–317.
Day R, Abrahams P, Bateman M, Beale T, Clottey V, Cock M, Gomez J (2017) Fall armyworm: impacts and implications for Africa. Outlooks on Pest Manage 28(5):196–201
de Bon H, Huat J, Parrot L, Sinzogan A, Thibaud M, Malezieux E, Vayssières JF (2014) Pesticides risks from fruits and vegetable pest management by small farmers in sub- saharan Africa. A review Agron Sustain Dev 34:723–736
FAO (2017) Fall armyworm outbreak, a blow to prospects of recovery for southern Africa. Rome, Italy: FAO. http://www.fao.org/africa/news/detail-news/en/c/469532/
Gichuhi J, Sevgan S, Khamis F, Van den Berg J, du Plessis H, Ekesi S, Herren JK (2020) Diversity of fall armyworm, Spodoptera frugiperda and their gut bacterial community in Kenya. PeerJ 8:e8701
Goergen G, Kumar PL, Sankung SB, Togola A, Tamo M (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PloS One 11(10):e0165632
Harrison RD, Thierfelder C, Baudron F, Chinwada P, Midega C, Schaffner U, van den Berg J (2019) Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: providing low-cost, smallholder friendly solutions to an invasive pest. J Environ Manage 243:318–330
Hruska AJ (2019) Fall armyworm (Spodoptera frugiperda) management by smallholders. CAB Reviews 14(043):1–11
IPPC (2016) Les dégâts causés par Spodoptera frugiperda. (The damage caused by Spodoptera frugiperda.) IPPC Official Pest Report. Rome, Italy: FAO. https://www.ippc.int/
Kenis M, du Plessis H, Van den Berg J, Ba MN, Goergen G, Kwadjo KE, Baoua I, Buddie A, Cafà G (2019) Offord L (2019) Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already present on the continent. Insects 10:92
Koffi D, Kyerematen R, Eziah VY, Agboka K, Adom M, Goergen G, Meagher RL Jr (2020) Natural enemies of the fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) in Ghana. Fla Entomol 103:85–90
Kumela T, Simiyu J, Sisay B, Likhayo P, Mendesil E, Gohole L, Tefera T (2019) Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int J Pest Manage 65(1):1–9
Kwon DH, Yoon KS, Clark JM, Lee SH (2010) A point mutation in a glutamategated chloride channel confers abamectin resistance in the two-spotted spider mite. Tetranychus urticae Koch Insect Mol Biol 19:583–591
Maiga I, Ndiaye M, Gagare S, Oumarou G, Oumarou S (2017) La chenille d’automne Spodoptera frugiperda, nouveau ravageur du maïs en Afrique de l’Ouest, a atteint le Niger. Bulletin spécial, Centre Régional AGRHYMET/CILSS, Niger
Maniania NK, Fargues J (1985) Susceptibility of the Fall Armyworm, Spodoptera frugiperda, to the Fungal Pathogens Paecilomyces fumoso-roseus and Nomuraea rileyi. Fla Entomol 178–183.
Matlock RB Jr, de la Cruz R (2003) Ants as indicators of pesticide impacts in banana. Environ Entomol 32(4):816–829
Midega CA, Pittchar JO, Pickett JA, Hailu GW, Khan ZR (2018) A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (JE Smith), in maize in East Africa. Crop Prot 105:10–15
Montezano DG, Sosa-Gómez DR, Roque-Specht VF, (2018) Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Faculty Publications: Department of Entomology 718. http://digitalcommons.unl.edu/entomologyfacpub/718
Negrisoli AS Jr, Garcia MS, Negrisoli CRB (2010) Compatibility of entomopathogenic nematodes (Nematoda: Rhabditida) with registered insecticides for Spodoptera frugiperda (Smith, 1797)(Lepidoptera: Noctuidae) under laboratory conditions. Crop Prot 29(6):545–549
Omoto C, Bernardi O, Salmeron E, Sorgatto RJ, Dourado PM, Crivellari A, Head GP (2016) Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manage Sci 72(9):1727–1736
Pavela R (2005) Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia 76(7–8):691–696
Perfecto I (1991) Ants (Hymenoptera: Formicidae) as natural control agents of pests in irrigated maize in Nicaragua. J Econ Entomol 84(1):65–70
Prasanna BM, Huesing JE, Eddy R, Peschke VM (2018) Fall armyworm in Africa: a guide for integrated pest management. First edition, Feed the future 1–120
Polanczyk RA, Silva RFPD, Fiuza LM (2000) Effectiveness of Bacillus thuringiensis strains against Spodoptera frugiperda (Lepidoptera: Noctuidae). Braz J Microbiol 31(3):164–166
Popp J, Pető K, Nagy J (2013) Pesticide productivity and food security. A review Agron Sustain Dev 33(1):243–255
R Development Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Röse US, Alborn HT, Makranczy G, Lewis WJ, Tumlinson JH (1997) Host recognition by the specialist endoparasitoid Microplitis croceipes (Hymenoptera: Braconidae): Role of host-and plant-related volatiles. J Insect Behav 10(3):313–330
Sena DG Jr, Pinto FAC, Queiroz DM, Viana PA (2003) Fall armyworm damaged maize plant identification using digital images. Biosyst Eng 85(4):449–454
Shaiba Z, Amoore B, Amoore I, Renne E (2019) Assessing the impact of neem on fall armyworm damage to maize crops: a field-based study in Nabdam District, UER, Ghana. J Agr Sustain 12(2)
Sisay B, Simiyu J, Malusi P, Likhayo P, Mendesil E, Elibariki N, Tefera T (2018) First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J Appl Entomol 142(8):800–804
Sparks TC, Nauen R (2015) IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121:122–128
Tavares WS, Costa MA, Cruz I, Silveira RD, Serrao JE, Zanuncio JC (2010) Selective effects of natural and synthetic insecticides on mortality of Spodoptera frugiperda (Lepidoptera: Noctuidae) and its predator Eriopis connexa (Coleoptera: Coccinellidae). J Environ Sci Health B 45(6):557–561
Van Mele P, Vayssières J-F, Van Tellingen E, Vrolijks J (2007) Effects of an African weaver ant, Oecophylla longinoda, in controlling mango fruit flies (Diptera: Tephritidae) in Benin. J Econ Entomol 100(3):695–701
Wang X, Wang R, Yang Y, Wu S, O’Reilly AO, Wu Y (2016) A point mutation in the glutamate-gated chloride channel of Plutella xylostella is associated with resistance to abamectin. Insect Mol Biol 25:116–125
Way MJ, Khoo KC (1992) Role of ants in pest management. Annu Rev Entomol 37(1):479–503
Wu X, Zhang L, Yang C, Zong M, Huang Q, Tao L (2016) Detection on emamectin benzoate-induced apoptosis and DNA damage in Spodoptera frugiperda Sf-9 cell line. Pestic Biochem Physiol 126:6–12
Wyckhuys KA, O’Neil RJ (2006) Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot 25(11):1180–1190
Zhao YX, Huang JM, Ni H, Guo D, Yang FX, Wang X, Wu SF, Gao CF (2020) Susceptibility of fall armyworm, Spodoptera frugiperda (JE Smmith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pestic. Biochem, Physiol, p 104623
Acknowledgments
We thank maize farmers in Central Benin who unconditionally accepted to respond to interviews and to make available their fields for observations.
Funding
BIORAVE funded field activities including field materials and field visit travels.
Author information
Authors and Affiliations
Contributions
AGD, GAH, RI, AS, PS and AD participated in the study design; they analyzed and interpreted the data and drafted the manuscript. JH, AS and AGD carried out the field surveys. AGD, PS, RI, GAH, and AD corrected the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Dassou, A.G., Idohou, R., Azandémè-Hounmalon, G.Y. et al. Fall armyworm, Spodoptera frugiperda (J.E. Smith) in maize cropping systems in Benin: abundance, damage, predatory ants and potential control. Int J Trop Insect Sci 41, 2627–2636 (2021). https://doi.org/10.1007/s42690-021-00443-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00443-5