Abstract
Fungal endophytes are known to be diverse and establish a mutualistic relationship with the host plant and protect them from the soil borne pathogens. In this study, we isolated a total of 283 fungal strains belonging to 8 different genera and 16 morphotypes from 450 tissue segments (Leaf, stems and flowers) of bengle sage (Meriandra bengalensis Benth.) plants during different (winter, summer and rainy) seasons, grown under natural conditions of Manipur and identified them on the basis of cultural morphology. The percentage of isolation rate (IR %) and colonization rates (CR %) of endophtic fungi communities were high in leaf segments (51% and 66%) and winter season (47% and 75%), respectively. The highest relative abundance (RA %) and isolation frequency (IF %) were recorded with Colletotrichum sp.2 and diversity indices of fungal endophytes were also calculated. The bioactive potential of Phoma sp. MBS13 was evaluated in vitro by dual culture tests against Aspergillus flavus, Aspergillus niger, Alternaria solani and Fusarium oxysporum isolates, which showed strong (Class 1and Class 2) antagonistic activity. In addition, the spectral (FTIR and GC–MS) analysis of ethyl acetate extracted metabolites of Phoma sp. MBS13 revealed the presence of various volatile compounds, and showed a good inhibitory activity against some human pathogenic bacteria i.e. Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Salmonella typhi. Maximum inhibitory zone (15.3 ± 0.15 mm) was recorded with E. coli. Thus, the present findings reveal the importance of endophytic fungi for their role in antimicrobial activity and biocontrol potential against pathogenic microorganisms that could be exploited by the biotechnological and agricultural industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endophytic fungi have either symbiotic or asymbiotic association with the majority of the plant species exist in the environment without causing any discernible infectious symptom (Suryanarayanan et al. 2002). Further, endophytic fungi are beneficial to the host plants by modulating a variety of basic functions like primary productivity, alleviating abiotic stress tolerance, disease resistance and absorption of nutrients by promoting plant growth (Rodriguez et al. 2009). In recent years, the endophytic fungi have attracted increasing attention due to its potential secondary metabolites productions (Palanichamy et al. 2018) and that can be used as promising substance for antifungal and anticancer activity as a biological control agents in modern medicines, agriculture and industry such as novel antibiotics, antimycotics, immunosuppressants, and anticancer compounds (Strobel and Daisy 2003). Several studies revealed that around 3 lakhs plant species are colonized by endophytic fungi in almost every organ (leaves, stem, flower, root, rhizome and seed) of host plants (Wearn et al. 2012; Gautam 2014), and among them very few were studied to analyze the production of bioactive metabolites (Palanichamy et al. 2018; Kim et al. 2019) from these associated fungi. The diversity and distribution of endophytic fungal communities in medicinal plant species were outlined by different researchers in various ecosystems and niches (Krishnamurthy et al. 2008; Huang et al. 2008; Gautam 2014). However, the seasonal and organal diversity of endophytic fungi in ethanomedicinal plants are limited (Göre and Bucak 2007; Mishra et al. 2012; Chowdhary and Kaushik 2015).
Meriandra bengalensis Benth. (Family: Lamiceae) is an aromatic perennial shrub and frequently found in abandoned farm lands in the valley and highlands areas of Manipur, North East (NE) India. The plant (M. bengalensis) possesses immense medicinal property. Both fresh leaves and inflorescences extract are used as medicine by local communities to treat colds and stomach ache, tonsillitis, reducing high blood pressure, and also considered as tonic, carminative, astringent and antiseptic etc. (Bruno et al. 1985; Sinha 1996). In addition, M. bengalensis plant oil and their essential constituents were documented by GC–MS analysis (Rana and Blazquez 2009). However, their endophytic fungi diversity was not yet explored till date.
Generally, in India, the endophytic fungi associated with common medicinal plant such as Azadirachta indica A. Juss. (ver. Neem) was well studied (Rajagopal and Suryanarayann 2000). Whereas, in Lamiaceae family, Gangadevi and Muthumary (2007), Banerjee et al. (2009), Debbab et al. (2009), Jayanthi et al. (2014), Chowdhary and Kaushik (2015), Masaumi et al. (2015), Zafari-Shayan et al. (2016) and Katoch et al. (2017) studied the endophytic fungal diversity in some medicinal plants i.e. Ocimum sanctum, O. bacilicum, Leucas aspera, Anisomeles malabarica, Thymus sp., Monarda citriodora, Phlomis bruguieri, Phlomis olivieri and Salvia officinalis. Besides the enormous diversity of medicinal plant species found in NE India, very few investigations had been made as far as their associated endophytic fungi are concerned (Nirjanta Devi and Singh 2013; Deka and Jha 2020).
However, previous studies have reported that the fungal endophytes isolated from native medicinal plants were the excellent producers of strong bactericidal, cytotoxic, fungicidal and herbicidal metabolites (Wang et al. 2012; Katoch et al. 2017; Kim et al. 2019). There is a need to explore the fungal endophytes from medicinal plants because it has been hypothesized that medicinal plants harbour distinct and rare microbes that mimic the chemistry of their respective hosts and synthesize identical bioactive products (Strobel and Daisy 2003; Nirjanta Devi and Singh 2013). Therefore, an attempt has been made to understand the ecological diversity of endophytic fungi colonized in M. bengalensis plants and biological activities of metabolites produced by these fungi. We investigated (1) the occurrence, distribution and species diversity of endophytic fungi during three different seasons and plant tissues associated with this plant and (2) assessed their bioactive potential using extracted fungal metabolites against common plant pathogenic fungi and human pathogenic bacteria.
Materials and methods
Study site and sample collection
The bengle sage (Meriandra bengalensis Benth.) a well known perennial medicinal plant, is broadly distributed in tropical and sub-tropical regions of North East India. The study was conducted in abandoned cultivated fields located on Thounaojam village, Bishnupur district (24° 18′ 13.1″ N; 93° 43′ 18.6″ E; 754 m a.s.l.), Manipur. The mean annual temperature of the study site ranged between 10.8 to 30.4 °C, and the mean annual rainfall is about 1552 mm. The climate of the study site was tropical humid type.
The present investigation was carried out during October 2015 to September 2016 with three different seasons like, winter (October to January), summer (March to June) and rainy (July–September), and with various plant parts (Leaf, stem and flower). Fifteen randomly selected (five each season), disease free plants were chosen for this study. The different parts of the plant were randomly collected from each season and plant, respectively. These fresh plant materials were immediately placed in poly bags, labeled, and taken to the laboratory for further processing and isolation of fungal endophytes.
Isolation and identification of endophytic fungi
Symptomless and fresh plant parts (leaf, stem and flower) were initially surface sterilized by the modified method of Paul et al. (2012) as follows, 70% (v/v) ethanol for 2 min, 5% (w/v) sodium hypochlorite solution for 5 min and 70% ethanol for 30 s followed by two rinses in sterile distilled water. The above process was repeated twicely. For control, the 1 mL final sterile water rinse was plated and observed post-incubation period. Absence of fungal growth indicate the leaf surface was sterile. The surface-sterilized plant fragments were kept in dry autoclaved plot paper for 10 min to reduce moisture and cut into 0.5–1.0 cm lengths fragments using a sterile surgical blade, and then transferred into Petri dish (90 mm diam.) containing potato dextrose agar (PDA, includes 200 g L−1 fresh potato extract, 20 g L−1 glucose, and 20 g L−1 agar, pH 7.0) that are very common medium used for isolating endophytic fungi. To inhibit bacterial contamination in PDA, streptomycin sulphate antibiotics were added to a final concentration of 150 mg/L−1 (Gangadevi and Muthumary 2007). In total, 450 plant fragments/pieces (includes, leaf (50), stem (50), flower (50) in each season) were used to isolate the associated fungal endophytes from field grown medicinal plants. Generally, maximum 5 pieces of plant segments was accommodate per petri plates and 10 replicates were used for each plant part and seasons. Further, inoculated plates were incubated at room temperature (28 ± 2 °C) and checked daily for 7 days. The actively growing fungal colony from the leaf, stem and flower tips was removed and transferred into fresh PDA plates. Each colony was transferred at least three more times until a visually uniform culture was obtained. After, the pure form of the isolated fungal cultures was generated on PDA slants, which were maintained at 4 °C. The pure cultures of each fungal endophyte were identified based on morphological features of fungi with the support of National Fungal Culture Collection of India (NFCCI), Agharkar Research Institute, Pune, India.
Evaluation of endophytic fungal diversity
The occurrence and diversity of endophytic fungi communities from different plant parts and seasons were analyzed by Paul et al. (2012) and Yu et al. (2018). Initially, the number of isolates (n) and the absolute frequency (AF) of each fungal taxon was measured. The isolation rate (IR %) was calculated by dividing the total number of isolates recovered from each plant segments by overall total number of endophytic isolates obtained × 100; the relative abundance (RA %) was calculated by dividing the total number of isolates representing a single taxon by the total number of taxa obtained from each plant tissues × 100; the isolation frequency (IF %) was calculated by dividing the total number of isolates representing a each taxon by the total number of plant segments incubated × 100. Moreover, the colonization rate (CR %) of fungal infection was calculated by dividing number of plant segments colonized by fungal species by total number of plant segments used per sample × 100. In addition, to study the similarity of fungal diversity between the different plant parts and seasons were also estimated by ecological diversity indices (Shannon and Weaver 1963; Simpson 1964) such as Species richness (S) which is defined by: S = ∑n, where n is number of fungal species associated with bengle sage plant, Shannon–Weaver index (H′) was used to show diversity of the endophytic fungal species and was calculated as: H′ = − Ʃpi lnpi. Evenness (E) was calculated as H′/Hmax, where Hmax = ln(S), S is the total number of taxa in the subsample. Simpson’s index of diversity (1-D) was measured as D = 1 − Ʃpi2, where pi is the proportion of isolates assigned to the ith taxa, that is Pi = ni/N) was calculated to compare the species richness. A Simpson diversity index close to 1 means that the sample is highly diverse. Diversity parameters were calculated for each season, and tissue type using PAST software (Hammer et al. 2001).
Study of antifungal activity by dual culture method
The selected endophytic fungus (Phoma sp. MBS13) was screened for their ability to act against the common plant pathogenic fungi species by a dual culture method (Yu et al. 2018). Further, the phytopathogenic fungal isolates i.e. Aspergillus flavus, Aspergillus niger, Alternaria solani and Fusarium oxysporum were procured from Department of Plant Pathology, Central Agricultural University (CAU), Imphal. The mycelia discs (5 mm dia.) were removed from the margins of actively growing colonies of the both test pathogens and endophytic fungus placed 5 cm away from each other on opposite sides of 90 mm dia. Petri plate, containing about 20 mL of PDA medium. The paired cultures were incubated at 25 ± 1 °C for 5–7 days and then scored for degree of antagonism on a scale of class 1–5 (Bell et al. 1982). Class 1: Endophytic fungi completely overgrow the pathogen and covered the entire medium surface (highly antagonistic). Class 2: Endophytic fungi overgrow at least two-third of the medium surface (antagonistic). Class 3: Endophytic fungi and the pathogen each colonized approximately one half of the medium surface and neither organism appeared to dominate the other (moderately). Class 4: The pathogen colonized at least two third of the medium surface and appeared to withstand encroachment by the Endophyte fungi (poor antagonist). Class 5: The pathogen completely overgrows the endophytic fungi and occupied the entire medium surface (non antagonist).
Mass cultivation and crude metabolite production
For metabolite extraction, the fungal strain Phoma sp. MBS13 was inoculated in 1000 mL of potato dextrose broth (PDB) media and incubated at 25 ± 1 °C for 3–4 weeks under a stationary condition (Deka and Jha 2020). To separate the mycelia, the broth culture was filtered and supernatant was extracted three times with equal volumes of ethyl acetate (EtOAc) and concentrated to dryness by using vacuum evaporator at 40 °C and the brown coloured dried crude extract was stored for further analysis.
Spectral characterization of ethyl acetate extracted Phoma sp. MBS13 metabolite
Fourier transform infrared spectroscopy (FTIR) analysis
The crude extract of Phoma sp. MBS13 was analyzed by FTIR (THERMO NICOLET iS10 FT-IR SPECTROMETER) to know the different functional groups present in the fungal extracts. The sample was grounded with potassium bromide (KBr) pellet (1:10) and pressed into disks under vacuum using a Spectra Lab Pelletizer. Spectral analysis was performed in the study ranged between 4000–450 cm−1. Compounds contained in the extract were compared and interpreted for the shifting of functional peaks (Deka and Jha 2020).
Gas chromatography mass spectrometry (GC–MS) analysis
The composition of volatile bioactive compounds from crude extract of Phoma sp. MBS13 was determined by GC Clarus 500 Perkin Elmer using software Turbomass 5.2 equipped with mass detector Turbo mass gold Perkin Elmer. 2 μL sample was introduced via an all-glass injector working in the split mode, with He as the carrier gas with a linear velocity of 32 cm s−1. The HP-5 fused silica capillary column (length—30 m; film thickness—25 μm I.D—0.2 mm) was used (Nirjanta Devi and Singh 2013). The identification of components present in the metabolite was accomplished using computer searches on National Institute of Standard and Technology (NIST) Ver.2.1 MS data library and structures of the major compounds obtained through GC–MS analysis have been collected from National Institute of Standards and Technology Chemistry WebBook, US (https://webbook.nist.gov/chemistry/).
Antibacterial activity
Antibacterial activity of the fungal metabolite, extracted from Phoma sp. MBS13, was tested by modified agar well diffusion method (Deka and Jha 2020) against the human pathogenic bacterial strains i.e. Bacillus subtilis and Staphylococcus aureus (Gram positive) and Escherichia coli and Salmonella typhi (Gram negative), obtained from Regional Institute of Medical Sciences (RIMS), Imphal. The respective bacterial isolates were sub-cultured on Nutrient agar (NA) medium and an overnight culture of each bacterial strain (107 cells mL−1) was swabbed uniformly onto the individual Muller-Hinton agar (MHA) plates using sterile cotton swabs and different concentration of (20, 40 and 60 µg mL−1) of the fungal extract suspension in dimethylsulfoxide (DMSO) was poured into each well on the Petri plates. The antibiotic stranded (Streptomycin Sulphate, 1 mg mL−1) was used as a positive control and DMSO (10 mg mL−1) was used as negative control. After incubation at room temperature for 24 h, the zones of inhibition on the bacterial growth were measured. For each test, three replicates were maintained.
Data analysis
All the experimental results related to antimicrobial activity were expressed as mean ± SD.
Results
Identification of the endophytic fungi
A total of 283 fungal endophytes (leaf-145, stem-86, flower-52) were isolated from 450 tissue segments of M. bengalensis plants during three (winter, summer and rainy) seasons, among which 16 endophytic morphotypes belonging to 8 different genera i.e. Cladosporium, Colletotrichum, Fusarium, Nigrospora, Penicillium, Phoma, Phomopsis and Scytalidium were identified on the basis of morphological features (Fig. 1) and deposited in NFCCI (Table 1). Out of all, the genera Colletotrichum found to highest with 5 species, followed by Phoma and Phomopsis represented 2 species each. Whereas, the single species, were also recovered from each genera like Cladosporium, Fusarium, Nigrospora, Penicillium and Scytalidium, respectively. The identified fungal endophytes from M. benglanesis as follows, Cladosporium sp., Colletotrichum gleosporioides 1, C. gleosporioides 2, Colletotrichum sp.1., Colletotrichum sp.2., Colletotrichum sp.3., Fusarium oxysporium, Nigrospora oryzae, Penicillium sp., Phoma sp.1, Phoma sp.2, Phomopsis sp.1., Phomopsis sp.2., Scytalidium lignicola and non sporulating dematiaceous form 1 and 2 (Table S1).
Colony morphology of isolated fungal endophytes from different plant segments of Meriandra bengalensis. a Cladosporium sp. (MBS1); b Colletotrichum gleosporioides 1 (MBS2); c C. gleosporioides 2 (MBS3); d Colletotrichum sp.1 (MBS4); e Colletotrichum sp.2 (MBS5); f Colletotrichum sp.3 (MBS6); g Fusarium sp. (MBS7); h Nigrospora oryzae (MBS8); i, j non sporulating dematiaceous form (MBS9) and (MBS10); k Penicillium sp. (MBS11); l Phoma sp.1 (MBS12); m Phoma sp.2 (MBS13); n Phomopsis sp.1 (MBS14); o Phomopsis sp.2 (MBS15); p Scytalidium lignicola (MBS16)
Distribution and diversity of the endophytic fungi
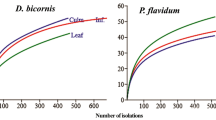
Differences in the fungal isolation rate (IR %) and colonization rate (CR %) in various plant tissues during different seasons were calculated, with general IR (63%) and CR (47.3%), which revealed the medicinal plant were highly colonized by endophyte fungi (Figs. 2, 3). However, the isolation rate (IR %) in three different tissue segments (leaf, stem and flower) and seasons (winter, summer and rainy) of M. benglanesis were 51%, 31% and 18% (Fig. 2a) and 47%, 30% and 23% (Fig. 2b), respectively. Similarly, the colonization rate (CR %) were showed as 66%, 44% and 32% (Fig. 3a) and 75%, 39% and 28% (Fig. 3b), respectively. Our results confirmed that the highest IR and CR were recorded in leaf segments and winter seasons. The examined plant tissues and different seasons were found to harbour diverse number of endophytic fungi. The relative abundance (RA) of endophytic fungi in M. benglanesis tissues with different season varied from 4.17–60.87% (Table 2). Among all, the endophytic fungi Colletotrichum sp.3 found to be dominant with high relative abundance (RA) and while Colletotrichum sp.2 in isolation frequency (IF). However, the lowest percent of IF was recovered from the species of Phomopsis sp.1. Furthermore, the differences in the species distribution of endophytic fungi isolated from various plant tissues and seasons were also observed. The fungi Colletotrichum sp. was commonly recorded with all examined plant tissues i.e. leaf, stem and flower. Whereas, Fusarium sp. and Nigrospora oryzae were only recovered from leaf and stem tissues. Penicillium sp. was found with both leaf and flower, while Phomopsis sp. was recorded exclusively from the flowers part of the plant. Likewise, Phoma sp. was present only in winter season (Table 2).
To characterize the diversity of the isolated endophytic fungi, we calculated the Species richness (SR), Simpson’s index of diversity (1-D), Shannon–Wiener index of diversity (H), and evenness (E) (Table 3). Maximum SR of endophytic fungi was recorded with leaf segments (8.33) and winter season (9.33), compared to that of the plant tissues flower (5.33) and rainy (4.67) in M. benglanesis. Similarly, Simpson’s (1-D) and Shannon–Wiener (H) index were highest in leaf and winter, 8.40 and 8.67 and 1.960 and 2.115, respectively. However, the evenness (E) was maximum in flower and winter season, 0.923 and 0.910, respectively (Table 3).
Antifungal activity
Dual cultures of endophytic fungus Phoma sp. MBS13 from M. benglanesis was depicted (Fig. 4) and this exhibited significant potential antifungal activity against studied phytopathogens ranged from class 1 (highly antagonistic) to A. flavus and class 2 (antagonistic) to A. niger, A. solani and F. oxysporum.
Metabolite production and spectral analysis
The average yield of crude EtOAc extract (metabolite) in 1L of PDB with Phoma sp. MBS13 was recorded in 836 ± 1.16 mg of dry weight.
FT-IR analysis
The FT-IR spectrum of EtOAc extracted bioactive metabolite Phoma sp. MBS13 showed bands at 2961.79 cm−1, 2923.74 cm−1, 2852.38 cm−1, 1742.02 cm−1, 1463.67 cm−1, 1261.35 cm−1, 1097.79 cm−1, 1022.14 cm−1 and 802.26 cm−1 (Fig. 5). Bands at 2961.79 cm−1, 2923.74 cm−1 and 2852.38 cm−1 that corresponded to the stretching vibrations of alkanes; 1742.02 cm−1 to that of acid anhydride; 1463.67 cm−1 to aromatics; 1261.35 cm−1 to alkyl halide; 1097.79 cm−1 and 1022.14 cm−1 to amine (C–N) and 802.26 cm−1 to alkene.
GC–MS analysis
GC–MS analysis of EtOAc extracted metabolite produced by Phoma sp. MBS13 revealed the presence of volatile bioactive compounds viz., E-11,13-Tetradecadien-1-ol, 2-Trifluoroacetoxytridecane, N-Hexadecanoic acid, Octadecanoic acid, 2-(2-hydroxyethoxy)-ethyl ester, Decane, 1-fluoro-, 3-Heptafluorobutyroxy-tetradecane, Pentafluoropropionic acid-tetradecyl ester, 3-Acetoxytridecane, Oleic acid, Z-8-Methyl-9-tetradecenoic acid, 4-Fluoro-1-methyl-5-carboxylic acid, ethyl(ester), Undecanoic acid, 10-bromo-, Erucic acid, 2-Piperidinone, n-[4-bromo-n-butyl]-, Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)-ethyl ester, 1,1,1-Trifluoroheptadecen-2-one, 4-Tetradecanol, 4-Acetoxy-1-decene, 2,6,6-Trimethyl-bicyclo[3.1.1]hept-3-ylamine, 9-Octadecenal, 3-Decyn-2-ol, Cyclohexane, (1-hexadecylheptadecyl)-, and Sulfurous acid, dodecyl 2-propyl ester. The chemical formula, structure, molecular weight and RT (min) of these identified compounds have been presented in Table S2 and GC–MS chromatogram is depicted in Fig. 6.
Antibacterial activity
The antibacterial activity of EtOAc extracted metabolite from Phoma sp. MBS13 exhibited increased inhibitory activity with increasing concentrations towards both Gram-positive (B. subtilis, S. aureus) and Gram-negative (E. coli, S. typi) bacteria (Fig. 7, Table 4). Maximum inhibitory zone (15.3 ± 0.15 mm) was recorded with E. coli.
Antibacterial activity of bioactive crude EtOAc extract of metabolite produced by Phoma sp. MBS13 against human bacterial pathogens. a Bacillus subtilis, b Staphylococcus aureus, c Escherichia coli, d Salmonella typhi. The metabolite concentration was 20, 40 and 60 µg mL−1, respectively. −ve: DMSO; +ve: standard antibiotic (Streptomycin sulphate)
Discussion
There are no reports available regarding to the endophytic fungi diversity in M. benglanesis from Manipur. Several studies revealed that the endophytic fungi communities could increase the plant fitness by improving the tolerance to heavy metals and drought and also by reducing the phytopathogen settling (Suryanarayanan et al. 2002) in various environmental conditions. In addition, endophytic fungal communities associated with different kinds of host plants (herb, shrub and trees), plant fragments (leaves, stem, flower, root, rhizomes and seed) and seasons (summer, rainy and winter) from various ecosystems such as tropical, sub-tropical, temperate and Arctic were investigated by earlier workers and found a number of fungi (Suryanarayanan et al. 2002; Göre and Bucak 2007; Rosa et al. 2010; Jayanthi et al. 2014). Rajagopal and Suryanarayann (2000), Gautam (2014), and Chowdhary and Kaushik (2015) investigated the occurrence of endophytic fungi from different tissue parts of Indian medicinal plant species. However, Banerjee et al. (2009) isolated number of endophytic fungi species especially from medicinal plants of Lamiaceae family which were recorded as significant diversity of endophytic fungi. For that reason, we choose the unexplored medicinal plant M. benglanesis, for our experiment.
The occurrence and distribution of endophytic fungi in plant-associated habitats may affect the microbial communities that influence them colonize leaves, stem, fruits and roots etc. (Rosa et al. 2010). Previous reports also revealed that endophytes were isolated from different plant tissues/organs or seasons (Göre and Bucak 2007; Paul et al. 2012; Yu et al. 2018). In this study, we isolated endophytic fungi from leaves, stem and flowers of M. benglanesis plant during three different seasons. It was a new idea which was not previously investigated and we found a total 283 endophytic strains containing 16 morphospecies. Most endophytic fungal species obtained herein were consistent with previous studies related to other Lamiaceae members (Ocimum sanctum, O. bacilicum and Leucas aspera) by Banerjee et al. (2009) and Thymus sp. by Masaumi et al. (2015) and Anisomeles malabarica by Jayanthi et al. (2014). Whereas, Gautam (2014) represented only 8 species from the leaves of Ocimum sanctum grown in Himachal Pradesh, India, which is comparatively low to our study.
Among the isolated endophyte species, the genera Colletotrichum represented five different species and commonly distributed to all three seasons and tissue fragments (leaf, stem and flower) with high rate relative abundance (RA %) and isolation frequency (IF %). Similar results were also observed by Paul et al. (2012) in leaves of chili cultivar i.e. Capsicum annum from Korea. Whereas, the lowest percent of IF% was recovered from the species of Phomopsis sp.1., it was not frequently occurred endophytes. The presence of Colletotrichum sp. is common to majority of studies, but not as species diversity (Banerjee et al. 2009; Paul et al. 2012). This results revealed the diversity and species occurrence of particular endophytes is not evenly distributed, it is always influenced by plant species, climate and different age of host plants (Göre and Bucak 2007). Similarly, the association of other fungal endophytes presents in the study, like Cladosporium sp., Fusarium oxysporium, Nigrospora oryzae, Penicillium sp., Phoma sp., Phomopsis sp. and Scytalidium sp. were also studied by different authors from time to time (Gangadevi and Muthumary 2007; Mishra et al. 2012; Katoch et al. 2017). Further, association of sterile mycelia (non sporulating dematiaceous form) were also prevalent in most of the endophytic research studies (Paul et al. 2012).
Moreover, the present results revealed that the isolation and distribution of endophytic fungi in M. benglanesis plant was organ-specific and season specific. The number of endophytic fungi isolated from leaf segments (IR %) were highest accounting 51% of all isolates; 31% and 18% isolates were obtained from the stem and flower tissues, respectively. Similarly, the endophytic fungi isolated from different seasons, were higher in winter (47%), summer (30%) and rainy (23%), respectively. Masaumi et al. (2015) and Yu et al. (2018) confirmed that the present finding were as similar to earlier reports. The similar trend of results were also recorded in study of colonization frequency (CR %) as well. The species richness (SR), the Simpson’s index (I-D) and Shannon–Wiener index (H) indicated that the highest species diversity was in the leaf and winter season, followed by the stem and summer, accordingly. Yu et al. (2018) also find similar observation in C. oleifera species.
Previous results suggesting that these endophytic fungi could play an important role in the plant development by its potential antifungal activity against phytopathogenic fungi (Paul et al. 2012). In this study, we found that Phoma sp. (MBS13) inhibited the mycelia growth activity of pathogenic Aspergillus flavus, Aspergillus niger, Alternaria solani and Fusarium oxysporum in dual culture assay. Rajagopal et al. (2008) reported that endophytic Phoma sp. isolated from Neem trees could inhibit the growth of plant pathogenic fungal species such as Aspergillus niger.
Endophytic fungi are recognized as a potential source of antimicrobial secondary metabolites that could be used in biomedicinal industries (Strobel and Daisy 2003). Different researchers have characterized the compounds present in the EtOAc extracted bioactive metabolites produced by different endophytic fungi (Palanichamy et al. 2018; Deka and Jha 2020), using FT-IR and GC–MS analysis. In the present study, FTIR results of EtOAc extracted metabolite of Phoma sp. MBS13 showed bands corresponding to the stretching vibrations of alkanes, acid anhydride, aromatics, alkyl halide, amine (C-N) and alkene. However, Deka and Jha (2018) also observed the (alkanes, acid anhydride, amine etc.) type of compounds present in the EtOAc crude metabolite of an endophytic fungus i.e. Talaromyces verruculosus isolated from Citrus macroptera plant.
GC–MS analysis revealed the presence volatile compounds in the crude metabolite produced by Phoma sp. MBS13 isolated from M. benglanesis. The antimicrobial compounds like Sulfurous acid, dodecyl 2-propyl ester, Cyclohexane, (1-hexadecylheptadecyl)- and Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester were identified in the metabolite produced by the potent fungi (Phoma sp. MBS13). Similarly, Nirjanta Devi and Singh (2013) and Kanjana et al. (2019) also identified Cyclohexane and Hexadecanoic acid from different endophytic fungal species viz. Colletotrichum gloeosporioides, Chaetomium globosum and Cladosporium tenuissimum. Zafari-Shayan et al. (2016) also identified Octadecanoic acid, 2-(2-hydroxyethoxy) ethyl ester, Hexadecanoic acid and Z-8-Methyl-9-tetradecenoic acid from leaf extract of Phlomis bruguieri (Lamiaceae) plant. Nirjanta Devi and Singh (2013) and Deka and Jha (2020) suggested that the medicinal plant containing potential bioactive compounds may also be produced by the colonized endophytes of the same. Moreover, the different functional compounds i.e. E-11,13-Tetradecadien-1-ol, 2-Trifluoroacetoxytridecane, 3-Heptafluorobutyroxy-tetradecane, Oleic acid, 4-Fluoro-1-methyl-5-carboxylic acid, ethyl(ester), 2-Piperidinone, n-[4-bromo-n-butyl]-, 1,1,1-Trifluoroheptadecen-2-one, 4-Tetradecanol, 2,6,6-Trimethyl-bicyclo[3.1.1]hept-3-ylamine and 9-Octadecenal present in Phoma sp. MBS13 fungus extract showed the important active secondary metabolites for several industrial applications (Wang et al. 2012). In addition, results of the present work suggested that M. benglanesis harboured endophytic fungus Phoma sp. MBS13 metabolites are capable of producing antimicrobial activity against both gram (+ve) and (−ve) pathogenic bacteria. Similarly, Gomes da Silva et al. (2017) reported that the endophytic Phoma sp. URM 7221 extract positively inhibited the growth of Bacillus subtilis (35 ± 0.0 mm) and Staphylococcus aureus (22 ± 0.4 mm), which is comparatively higher to our study.
Conclusion
The present study concluded that the isolation of endophytic fungi from the different plant segments of studied medicinal plant M. benglanesis revealed the presence of diverse species of endophytes. The diversity and species richness of fungal endophytes were also varied with different plant tissues and seasons. The maximum fungal endophytes were recovered from leaf tissue and winter seasons. We showed that the endophytic fungus Phoma sp. MBS13 was able to inhibit the growth of common fungal phytopathogens and act as biocontrol. The EtOAc extracted bioactive metabolite of the studied fungus was analyzed by FTIR and GC–MS and it demonstrated the positive antibacterial activity against human pathogenic bacteria. Our study suggests that cultivated medicinal plants could be the interesting niche that harbors many different fungal species and the endophytic isolate Phoma sp. MBS13, can produce different compounds having antimicrobial properties.
References
Banerjee D, Manna S, Mahapatra S, Pati BR (2009) Fungal endophytes in three medicinal plants of Lamiaceae. Acta Microbiol Immunol Hung 56:243–250
Bell D, Wells KH, Markham CR (1982) In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology 72:379–382
Bruno M, Mellerio G, Piozzi F, Vita-Finzi P (1985) GC-MS analysis of the essential oil of Meriandra benghalensis. In: Svendsen AB, Scheffer JJC (eds) Essential oils and aromatic plants. Martinus Nijhoff/Dr. W. Junk Publishers, Dordrecht, pp 151–154
Chowdhary K, Kaushik N (2015) Fungal endophyte diversity and bioactivity in the Indian Medicinal plant Ocimum sanctum L. PLoS One 10:e0141444. https://doi.org/10.1371/journal.pone.0141444
Debbab A, Aly AH, Edrada-Ebel RA, Müller WEG, Mosaddak M, Hakiki A, Ebel R, Proksch P (2009) Bioactive secondary metabolites from the endophytic fungus Chaetomium sp. isolated from Salvia officinalis growing in Morocco. Biotechnol Agron Soc Environ 13:229–234
Deka D, Jha DK (2020) Bioactivity assessment of endophytic fungi associated with Citrus macroptera Montr.: an endangered ethnomedicinal plant used in folk medicines in North-East India. Indian Phytopathol 73:21–33. https://doi.org/10.1007/s42360-019-00179-w
Gangadevi V, Muthumary J (2007) Endophytic fungal diversity from young, mature and senescent leaves of Ocimum basilicum L. with special reference to taxol production. Indian J Sci Technol 1:1–15
Gautam AK (2014) Diversity of fungal endophytes in some medicinal plants of Himachal Pradesh, India. Arch Phytopathol Plant Protect 47:537–544
Gomes da Silva GBP, Silvino KF, Bezerra JDP, de Farias TGS, de Araújo JM, Stamford TLM (2017) Antimicrobial activity of Phoma sp. URM 7221: an endophyte from Schinus terebinthifolius Raddi (Anacardiaceae). Afr J Microbiol Res 11:1–7
Göre ME, Bucak C (2007) Geographical and seasonal influence on the distribution of fungal endophytes in Laurus nobilis. For Pathol 37:281–288
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:4–9
Huang WY, Cai YZ, Hyde KD, Croke H, Sun M (2008) Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers 33:61–75
Jayanthi G, Karthikeyan K, Muthumary J (2014) Pervasiveness of endophytic fungal diversity in Anisomeles malabarica from Aliyar, Western Ghats, South India. Mycosphere 5:830–840
Kanjana M, Kanimozhi G, Udayakumar R, Panneerselvam A (2019) GC-MS analysis of bioactive compounds of endophytic fungi Chaetomium globosum, Cladosporium tenuissimum and Penicillium janthinellum. J Biomed Pharm Sci 2:1000123
Katoch M, Phull S, Vaid S, Singh S (2017) Diversity, phylogeny, anticancer and antimicrobial potential of fungal endophytes associated with Monarda citriodora L. BMC Microbiol 17:1–13
Kim JW, Choi HG, Song JH, Kang KS, Shim SH (2019) Bioactive secondary metabolites from an endophytic fungus Phoma sp. PF2 derived from Artemisia princeps Pamp. J Antibiot 72:174–177. https://doi.org/10.1038/s41429-018-0131-2
Krishnamurthy YL, Shankar NB, Shashikala J (2008) Fungal communities in herbaceous medicinal plants, Malnad region, Southern India. Microbes Environ 23:24–28
Masaumi S, Mirzaei S, Zafari D, Kalvandi R (2015) Isolation, identification and biodiversity of endophytic fungi of Thymus. Prog Biol Sci 5:43–50
Mishra A, Gond SK, Kumar A, Sharma VK, Verma SK, Kharwar RN, Sieber TN (2012) Season and tissue type affect fungal endophyte communities of the Indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microb Ecol 64:388–398
Nirjanta Devi N, Singh MS (2013) GC-MS analysis of metabolites from endophytic fungus Colletotrichum gloeosporioides isolated from Phlogacanthus thyrsiflorus Nees. Int J Pharm Sci Rev Res 23:392–395
Paul NC, Deng J, Sang H, Choi YP, Yu SH (2012) Distribution and antifungal activity of endophytic fungi in different growth stages of chilli pepper (Capsicum annuum L.) in Korea. Plant Pathol J 28:10–19
Rajagopal R, Suryanarayann TS (2000) Isolation of endophytic fungi from leaves of neem (Azardirachta indica). Curr Sci 78:1375–1378
Rajagopal K, Kathireshan AK, Mahendran TS, Ananthi M, Seethalakshmi T (2008) Anti fungal activity endophytic and phellophytic fungi isolated from Neem (Azardirachta indica) against few pathogenic fungi and phylloplane fungi. Indian J Appl Microbiol 8:57–59
Rana VS, Blazquez MA (2009) Constituents of the essential oil of Meriandra bengalensis Benth. leaves from India. J Essent Oil Res 21:22–23
Rosa LH, Vieira MLA, Santiago IF, Rosa CA (2010) Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol Ecol 73:178–189
Shannon CE, Weaver W (1963) The mathematical theory of communication. University of Illinois Press, Urbana
Simpson GG (1964) Species density of North American recent mammals. Syst Zool 13:57–73
Sinha SC (1996) Medicinal plants of Manipur. Mass & Sinha, Imphal, India, p 114
Strobel GA, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbial Mol Biol Rev 67:491–502
Suryanarayanan TS, Murali TS, Venkatesan G (2002) Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Botany 80:818–826
Wang LW, Xu BG, Wang JY, Su ZZ, Lin FC, Zhang CL, Kubicek CP (2012) Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl Microbiol Biotechnol 93:1231–1239. https://doi.org/10.1007/s00253-011-3472-3
Wearn JA, Sutton BC, Morley NJ, Gange AC (2012) Species and organ specificity of fungal endophytes in herbaceous grassland plants. J Ecol 100:1085–1092. https://doi.org/10.1111/j.1365-2745,2012.01997.x
Yu J, Wu Y, He Z, Li M, Zhu K, Gao B (2018) Diversity and antifungal of endophytic fungi associated with Camellia oleifera. Microbiology. https://doi.org/10.1080/12298093.2018.1454008
Zafari-Shayan S, Moradkhani S, Dastan D (2016) Analysis of fatty acid composition of two selected Phlomis species. J HerbMed Pharmacol 5:153–156
Acknowledgements
The first author gratefully acknowledges the Head of the Department of Life Sciences for providing lab facilities, to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Devi, W.S., Surendirakumar, K. & Singh, M.S. Distribution of endophytic fungi associated with Meriandra bengalensis Benth. and assessment of their bioactive potential in vitro. Vegetos 35, 995–1006 (2022). https://doi.org/10.1007/s42535-022-00374-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00374-7