Abstract
Thirteen endophytic fungal isolates, inhabiting Citrus macroptera growing in Assam, India, were studied for their ability to produce bioactive metabolites. Ethyl acetate extracted secondary metabolites of endophytic Talaromyces assiutensis, exhibited the highest antimicrobial activity against Staphylococcus epidermidis followed by Acremonium falciformae which inhibited Sclerotinia sclerotiorum. Taxonomic identity of T. assiutensis was confirmed by internal transcribed spacer r-DNA sequence analysis. Quantitative estimation of phytochemicals of the ethyl acetate extracted secondary metabolites produced by T. assiutensis, revealed that flavonoid content was the highest followed by alkaloids, saponin and terpenoids. FT-IR spectrophotometry of the extract showed the presence of alcohol, alkane, acid anhydride, alkene, aromatic, alkyl halide and amine as functional groups. Nine major cmpounds, viz., 3-undecene-5-methyl-; 2,5-dihydroxy-3-methyl-2,5-cyclohexadiene-1,4-dione; 3-n-hexylthiolane, S,S-dioxide; Methyl 8-Methylnonanoate; (4H)4a,5,6,7,8,8a-Hexahydrobenzopyran-5-one-3-carboxamide,2; Cyclopentane undecanoic acid, methyl ester; Trans-2,4-Dimethylthiane, S,S-dioxide; 5-Hydroxy-4-hydroxymethyl-1-(1-hydroxy-1-isopropyl)-Cyclohex-3-ene and 3-Nonyn-1-ol were identified from the bioactive metabolites of T. assiutensis using GC–MS. The results indicate that the antimicrobial compounds produced by T. assiutensis may be used in pharmaceutical as well as agricultural industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During recent years, the synthetic chemical compounds used in different sectors are being replaced by a wide range of natural metabolites extracted from plants as well as microorganisms. Use of plants in large numbers for industrial purposes, however, has decreased plant population consequently, making them rare, threatened, endangered and finally extinct. Microorganisms may play an important role as an alternative source of different metabolites and can help in replacing plants and consequently conserving them (Teiten et al. 2013). The microorganisms inhabiting a special niche like the healthy plant tissues (e.g. endophytes) might produce novel metabolites as their survival strategy inside host tissues. Endophytic fungi have been observed to produce metabolites having applications in therapeutics, industry and agriculture (Teiten et al. 2013). Many endophytes have been isolated from medicinal plants, which are able to produce bioactive compounds or metabolites identical to the one produced by the host plants. Taxol, an anticancer compound, extracted from endophytic fungi, Taxomyces andreanae, inhabiting Pacific yew, which produces taxol as the endophyte does (Stierle et al. 1993). Application of endophytes for these purposes will reduce the pressure on plants thereby helping in their conservation (Strobel and Daisy 2003).
Endophytic fungi produce extensive therapeutic substances like antimicrobial, antioxidant, anticancerous, herbicidal, insecticidal, immunomodulatory compounds (Kaaniche et al. 2019). Endophytic fungi can produce antimicrobial secondary metabolites containing aliphatic compounds, alkaloids, flavonoids, saponins, peptides, phenols, quinones, tannins, terpinoids, lactones etc. that may be effective against different bacteria, fungi and actinomycetes (Kaaniche et al. 2019). Compounds such as antineoplastic paclitaxel, anticancerous podophyllotoxin, deoxypodophyllotoxin, camptothecin, antidepressant hypericin, insecticidal azadirachtin A and B etc. have been extracted from different endophytic fungi (Kusari et al. 2012). Findings of this nature have led researchers to work more belligerently on endophytic fungi. However, it is necessary to select host plants pragmatically if one wishes to isolate the endophytic fungi capable of producing novel secondary metabolites. Strobel and Daisy (2003) have advocated that plants for isolation of endophytes be selected from those growing in great biodiversity areas and having ethnobotanical history. Therefore, much emphasis is given to less explored, endemic, rare, endangered or threatened plants for isolation of endophytes and biological activity of their metabolites for future applications in different fields.
Citrus macroptera, commonly known as “Satkara” in Assam and “Wild orange” in English, is an endangered medicinal plant growing in the biodiversity hot spot region of North East India (Malik et al. 2006). The indigenous people of N.E. India traditionally use different parts like fruits, leaves, stems etc. of this plant for treatment of various diseases. The metabolites produced by this plant show antioxidant, cytotoxic, antimicrobial, thrombolytic, hypoglycemic, anxiolytic, antidepressant, cardioprotective, antihypertensive, antipyretic, appetite stimulant potentials and hepato-protective activities (Aktar 2017). Not much work has been done on isolation of endophytic fungi from C. macroptera and biological activities of metabolites produced by these fungi. The present study, therefore, was carried out to isolate endophytic fungi for production of biologically active secondary metabolites.
Materials and methods
Isolation and culture conditions
Leaf, bark and root samples from three healthy C. maroptera plants were collected aseptically for the isolation of the endophytic fungi, from three different localities, viz., (1) Medicinal Plant Garden of Diphu (Recreation Park), Karbi Anglong, Assam; (2) Doldoli Reserved Forest, Karbi Anglong, Assam and (3) Dr. H.K. Baruah Regional Botanical Resource Centre, Gauhati University, Assam, India. Freshly collected and washed samples were cut into a total of 1008 segments of about 2 cm long and 0.5 cm broad with sterile knife, comprising 336 each from leaves, barks and roots. Segments were sequentially immersed in 70% ethanol for 3 min, 4% aqueous solution of sodium hypochlorite (NaClO) for 5 min and 70% ethanol for 1 min and finally in 0.1% mercuric chloride (HgCl2) for 3 min (Strobel et al. 2002). The segments, thereafter, were rinsed with sterile distilled water and then surface sterilization efficiency was determined for every fragment following the imprint method of Schulz et al. (1993). The segments were then inoculated in petriplates (90 × 15 mm) containing different media viz., Czapeck-Dox-Agar, Potato-Dextrose-Agar, Malt Extract Agar, Sabourad Dextrose Agar, V8 juice agar, Nutrient Agar, water agar media and media amended with plant extracts separately and incubated at 25 ± 1 °C until the mycelium or the colony appeared surrounding the segments. All the media were procured from HiMedia Laboratories Pvt. Ltd., India. Plant extraction was done by boiling 500 g of the bark and leaf separately in 250 ml of distilled water for 10–15 min (Tayung 2008). The preparation was cooled and filtered through sterile Whatman No.1 filter paper to obtain the crude extract. Streptomycin sulphate (50 µg mL−1 concentration) was used in all the media to prevent bacterial contamination.
The colonization frequency (CF%) of endophytic fungi was calculated by using the following formula given by Hata and Futai (1995):
where, NCOL = number of leaf/bark/root segments colonized by specific fungus, Nt = total number of leaf/bark/root segments plated
Frequency of dominant endophytes was calculated as percentage of colony frequency divided by sum of percentage of colony frequency of all endophytes × 100 (Carroll and Carroll 1978).
Similarity co-efficient (SC = 2w/a + b+c) was calculated by using the formula given by Carroll and Carroll (1978) and was expressed as percentage, where, a = the sum of colonization frequency for all fungal species in a tissue; b, c = the similar sum for another tissue and w = the sum of lower colonization frequencies for fungal endophytes in common between the tissues.
Identification of isolates
Morphological identification The isolates were identified on the basis of their vegetative and reproductive characters as observed by Labomed (ATC2000) microscope. They were identified using manuals of Nagamani et al. (2006).
Molecular identification The taxonomical identity of the potent isolate T. assiutensis was confirmed using molecular tools. The genomic DNA of the fungal isolate was extracted from 7 days old mycelium growing in Potato dextrose broth, following the protocol of Keith et al. (2006). For amplification of ITS-rDNA, the whole genomic DNA was amplified in thermo-cycler (Bio-Rad C-1000) using universal primer pair of ITS1 (5ʹ-TCCGTAGGTGAACCTGCGG-3ʹ) and ITS4 (5ʹ-TCCTCCGCTTATTGATATGC-3ʹ) (White et al. 1990a, b) which amplified the ITS region of the isolate. The conditions for polymerase chain reaction for ITS-rDNA amplification was 94 °C for 5 min for initial denaturation, amplification at 30 cycles of 94 °C for 30 s, 53 °C for 30 s, 72 °C for 2 min, final elongation at 72 °C for 5 min and after completion the reaction was kept at 4 °C. The final product was 630 bp, which was run in 1% agarose gels by electrophoresis and observed in gel documentation system. Then the product was purified using PCR purification kit (Fermentas, Lithuania) as per the manufacturer’s protocol and PCR product was sequenced by Sanger’s Dideoxy method on applied Biosystem 3730XL (HKP Scientific, Bhubaneswar). To search the similar sequences from the GenBank sequence database for the 5.8S-ITS sequence of fungi the BLAST search (http://www.ncbinlmgov/BLAST) with NCBI database program was used. The sequences were aligned with representative sequences in the NCBI database using MEGA7 software, the phylogenetic analysis of the alignment was performed with Maximum Likelihood method.
Mass multiplication and crude secondary metabolites production
For secondary metabolites extraction, all the isolates were grown in 1000 mL Erlenmeyer flask containing 500 mL potato dextrose broth media and incubated at 25 ± 1 °C for 3–4 weeks under a stationary condition (Phongpaichit et al. 2007). To separate the mycelia, the broth culture was filtered, blended thoroughly and centrifuged at 3600 rpm for 10 min. The supernatant was then extracted three times with ethyl acetate and concentrated to dryness by using rotary vacuum evaporator (Model: EYELA/NVC-2100) at 40 °C. The resulting extracts from each isolate was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mg mL−1. The solution was sterilized by filtration through 0.4 µm cellulose acetate (hydrophilic) filter and was examined for antimicrobial activity against some microbes as given below.
Microbial test organisms
The antimicrobial activity was tested against two Gram-negative bacteria, viz., Escherichia coli (MTCC 443) and Klebsiella pneumoniae (MTCC 109), two Gram-positive bacteria, viz., Bacillus subtilis (MTCC 441) and Staphylococcus epidermidis (MTCC 435) and two fungi, Sclerotinia sclerotiorum (MTCC 8785) and Fusarium oxysporum (MTCC 284). The test organisms except S. epidermidis and the fungi, were obtained from the Institute of Microbial Technology (IMTECH), Chandigarh, India. S. epidermidis was obtained from Regional Institute of Medical Sciences (RIMS), Imphal, India and fungi were collected from Defence Research Laboratory (DRL), Tezpur, India.
Antimicrobial activity assay
Kirby-Bauer disc diffusion method was followed for determination of antimicrobial activity (Bauer et al. 1966) of crude secondary metabolite. In the experiment, 0.2 ml of each test bacteria (1 × 108 CFU mL−1) was spread evenly over the nutrient agar media by sterile cotton swab. Similarly, plates with PDA medium were swabbed with 0.2 ml of cultured test fungi (1 × 108 CFU mL−1). Sterile paper discs (6 mm in diameter) soaked in 0.5 ml of each crude metabolite solution (10 mg mL−1 DMSO) was used to determine the antimicrobial activity. Sterilized discs immersed in DMSO (100%) were used as negative control while tetracycline (10 µg/disc) and fluconazole (10 µg/disc), the standard antibacterial and antifungal agents respectively were used as positive control. Zone of inhibition was measured after 24 h of incubation at 30 °C for bacteria and after 48–72 h at 25 ± 2 °C for fungi. For each test three replicates were maintained.
Determination of minimum inhibitory concentration (MIC) of the of the extracted metabolite
The lowest concentration of an antimicrobial agent that inhibits the visible growth of a microorganism is known as the MIC. The minimum inhibitory concentration (MIC) of the ethyl acetate extracted metabolite of the most potent isolate was tested against the test microorganisms. The MIC value was determined by micro broth dilution assay as described by Santos and Filho (2003). The assay was carried out in sterile 96-well plates and total volume per well was 100 µl. The inoculum was prepared to give approximately 10−4 to 10−5 CFU per well and the compound was tested at concentration from 1000 µg to 35 µg mL−1 in twofold step dilution. Three wells were inoculated for a given concentration. The plates were incubated at 26 ± 1° C for 5 days.
Phytochemical analysis of ethyl acetate extracted secondary metabolite
Preliminary qualitative phytochemical analysis The ethyl acetate (EA) extract of Talaromyces assiutensis, was concentrated to dryness by using rotary vacuum evaporator (Model: EYELA/NVC-2100) at 40 °C. Dried crude extract were checked for the presence of some of the secondary metabolites such as alkaloid, flavonoids, phenols, saponins, steriods, cardiac glycosides, tannins, terpinoids by standard procedures (Bhardwaj and Agrawal 2015).
Alkaloids The fungal crude extracts were dissolved in 2 N HCl. The mixture was filtered using 0.4 µm cellulose acetate (hydrophilic) filter and the filtrate was treated with a few drops of Mayer’s reagent (3 ml of potassium iodide solution mixed with 2 mL mercuric chloride solution). The creamish precipitate indicated the presence of alkaloids (Bhardwaj and Agrawal 2015).
Flavonoids 0.5 mg of crude extract was added to 5 ml of DMSO (dimethyl sulphoxide) in a test tube. To this solution, 5–10 drops of dilute HCl and small pieces of magnesium were added. The solution was boiled for few minutes. In the presence of flavonoids, dirty brown colour was produced (Bhardwaj and Agrawal 2015).
Phenols Ferric chloride test was done to determine the phenolic content of the crude extracts. Fifty milligram crude dry extract was dissolved in 5 mL of distilled water. To this solution, a few drops of neutral 5% ferric chloride solution were added. Formation of a blackish green colour indicated the presence of phenolic compounds (Handunnetti et al. 2009).
Tannins A bluish black colour was formed on addition of alcoholic FeCl3 reagent to the fungal dry crude extract. This colour disappeared on addition of a little dilute H2SO4 was followed by the formation of yellowish brown precipitate (Handunnetti et al. 2009).
Cardiac glycosides Keller-Kiliani’s test was performed to assess the presence of cardiac glycosides (Handunnetti et al. 2009). The fungal dry crude extract was treated with 1 ml of FeCl3 reagent (mixture of 1 ml of 5% FeCl3 solution and 99 ml of glacial acetic acid). To this solution a few drops of concentrated H2SO4 was added. Appearance of greenish blue colour within a few minutes indicated the presence of cardiac glycosides (Handunnetti et al. 2009).
Saponins The presence of saponins was determined by Frothing test. The crude dry extract of fungi was vigorously shaken with distilled water and was allowed to stand for 10 min. Absence of froth indicated absence of saponins and stable froth of more than 1.5 cm indicated their presence (Handunnetti et al. 2009).
Terpenoids Salkowski’s test was done to evaluate the terpenoid presence. In 2 ml of chloroform, 1 ml of fungal crude extract was added. To this solution, 3 ml of concentrated H2SO4 was added to form a layer. Formation of a reddish-brown coloured precipitate at the interface indicated the presence of terpenoids (Bhardwaj and Agrawal 2015).
Quantitative phytochemicals analysis After preliminary qualitative screening, the phytochemicals found in the EA extracted metabolites of T. assiutensis were tested for quantitative estimation.
Total alkaloids content The total alkaloid content was determined using spectrophotometer at 470 nm by following the method of Shamsa et al. (2008). The standard atropine solution was prepared by dissolving 1 mg pure atropine (Sigma Chemical) in 10 mL distilled water (Shamsa et al. 2008). The test was performed in triplicate.
Total flavonoid content Total flavonoid content was determined by a colorimetric method reported by Shamsa et al. (2008). Absorbance of the final solution was measured at 510 nm. Catechin (Sigma-Aldrich Chemical) was used to prepare the standard curve (5-100 µg mL−1) to calculate the total flavonoid content. The test was performed in triplicate.
Total saponin content Total saponin content was determined spectrophotometrically at 544 nm, by following the method described by Shamsa et al. (2008). Diosgenin equivalents (DE) was used as standard to calculate the saponin concentrations.
Total terpenoid content About 1 g of the crude extract was weighed and dripped in 50 mL of 95% ethanol for 24 h. The extract was filtered and the filtrate was extracted with petroleum ether (60–80 °C) and concentrated to dryness. The dried extract was estimated as total terpenoids (Ferguson 1956).
Characterization of ethyl acetate extracted bioactive metabolite
FT-IR analysis Several drops of the extracted metabolite were taken onto an KBr aperture plate and it was sandwiched under another aperture plate, such that no gas bubbles are trapped. The KBr plates are translucent to the infrared light and therefore, no lines onto the spectra are introduced. Then the plates were inserted into the photo acoustic cell which is then sealed for the measurement. The spectra obtained through those samples were compared and interpreted for the shifting of functional peaks (Pervez et al. 2015).
GC–MS analysis For GC–MS analysis of the crude metabolite the sample solution was prepared in HPLC grade methanol and then used for GC–MS analysis as per the protocol proposed by Huang et al. (2007).
The temperature program for GC–MS analysis of the extract was as follows:
Initial temperature 50 °C for 5 min, ramp 5 °C/min to 150 °C, hold 5 min, ramp 10 °C/min to 280 °C, hold 10 min, InjAuto = 260 °C, Volume = 0 µl, split = 1:1, Carrier Gas = He, Solvent delay = 8.00 min, Transfer Temp = 180 °C, Source Temp = 160 °C, Scan = 50 to 600 Da, Column 60.0 m × 250 µm
The identification of components present in the metabolite was accomplished using computer searches on National Institute of Standard and Technology (NIST) Ver.2.1 MS data library and comparing the spectrum of the unknown compounds obtained through GC–MS. Structures of the major compounds obtained through GC–MS analysis have been collected from National Institute of Standards and Technology Chemistry WebBook, US (https://webbook.nist.gov/chemistry/).
Statistical analysis
The results were expressed in terms of mean ± standard deviation. All data presented are mean values of triplicate measurements (n = 3), obtained from three separate readings. Statistical analyses were performed by One-way analysis of variance (ANOVA) followed by least significant difference (LSD) test using Microsoft office excel 2016 to analyse the differences among mean values of different assays. Significance of the differences were measured statistically at p < 0.05.
Results
Endophytic fungal isolates
A total of 890 isolates (311 from leaf, 302 from bark, 277 from root) were obtained from 1008 segments (336 segments each from healthy leaf, bark and root samples) of C. macroptera. The isolates were identified based on their vegetative and reproductive characters, 12 different species and one sterile mycelia which could not be identified because of its sterile nature (Table 1) were isolated. The isolates belonged to Ascomycota, Oomycota and mycelia sterilia. Out of total isolates 75.28% belonged to Ascomycota (26.40% from leaf, 25.28% from bark and 23.60% root), 14.27% to Oomycota (5.39% from leaf and 4.94% from bark and 3.93% from root) and 10.45% were unidentified mycelia sterilia (3.15% from leaf, 3.71% from bark and 3.60% from root). Talaromyces assiutensis showed the highest colonization frequency (15.18% in leaf, 13.10% in bark, 10.42% in root) and frequency of dominance (16.57% in leaf 14.57% in bark, 12.64% in root) (Table 1). T. assiutensis exhibited maximum bioactivity. The taxonomic identity of this fungus was confirmed on the basis of the phylogenetic tree (Fig. 1) drawn based on 18S rDNA sequence of ITS region. The sequence was deposited to the GeneBank vide accession number MH843734.
The isolates sporulated profusely on Potato Dextrose Agar as compared to the other media used. Difference in isolation rate of the endophytic fungi was observed between amended (with bark and leaf extracts) and un-amended media. Out of 890 isolates 593 were isolated using media amended with plant extracts. Alternaria sp., Ceratocystis sp. and Aspergillus candidus were isolated only from leaf and bark samples and not from root samples, while Fusarium sp. was isolated only from root. Similarity co-efficient between Leaf-Bark-Root of C. macroptera was 48.72%.
Screening for antimicrobial activity
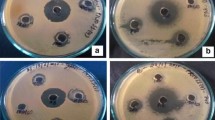
Crude secondary metabolites of all the isolates were tested for antimicrobial activity against two Gram-negative viz., E. coli and K. pneumoniae; two Gram-positive bacteria viz., B. subtilis, S. epidermidis and two fungi S. sclerotiorum and F. oxysporum. Crude metabolites of Talaromyces assiutensis, Aspergillus candidus, Acremonium falciformae and mycelia sterilia showed antagonistic activity against all the six test organisms tested (Table 2). The crude metabolite of T. assiutensis showed highest zone of inhibition (18.17 ± 0.76 mm) against Staphylococcus epidermidis (Table 2, Fig. 2a). Growth of B. subtilis was inhibited by all isolates (Table 2). Acremonium falciformae showed highest antifungal activity (16.19 ± 0.73 mm zone of inhibition) against S. sclerotiorum (Table 2, Fig. 2b).
a Antibacterial activity of bioactive crude secondary metabolite produced by Talaromyces assiutensis (Ta) against Staphylococcus epidermidis and b antifungal activity of bioactive crude secondary metabolite produced by Acremonium falceformae (Af) against Sclerotinia sclerotiorum. Positive Control-(a) Tetracycline (Tc-30 µg/disc) and (b), Fluconazole (Fn-10 µg/disc), Negative Control-Dimethyl sulfoxide (DMSO)
MIC of the ethyl acetate extracted crude metabolite
The minimum inhibitory concentration of the ethyl acetate extracted crude metabolite of Talaromyces assiutensis against different test microbes are shown in Table 3. The lowest MIC value of T. assiutensis extract was 113 ± 1.5 µg mL−1 against S. epidermidis followed by 230.55 ± 0.9 μg mL−1 against K. pneumoniae (Table 3). The highest MIC 480 ± 2.19 µg mL−1 was recorded against E. coli (Table 3).
Phytochemical analysis of ethyl acetate extracted secondary metabolite
Qualitative phytochemicals analysis: The ethyl acetate (EA) extracted metabolite of Talaromyces assiutensis was evaluated for the presence of phytochemicals as it showed highest antimicrobial activity. The results of phytochemical analysis of the extracts revealed the presence of flavonoids, terpenoids, alkaloids and saponin in the EA extracted metabolite of T. assiutensis. Phenol, tannin and cardiac glycosides were absent in the extracts.
Quantitative phytochemicals analysis
The EA extracted metabolite of T. assiutensis showed different amount of phytochemicals. The extract contained the highest content of flavonoids (6.53 ± 0.17 µg mg−1) was highest in the extract followed by alkaloids (5.19 ± 0.12 µg mg−1), saponins (3.26 ± 0.07 µg mg−1) and terpenoids (3.35 ± 0.1%) (Fig. 3). The flavonoid content was significantly different from that of the alkaloid and saponin contents (p < 0.05).
Characterization of ethyl acetate extracted bioactive metabolite
FT-IR analysis The FT-IR spectrum of EA extracted bioactive metabolite Talaromyces assiutensis showed bands at 3437 cm−1, 2956.87 cm−1, 2924 cm−1, 2852.72 cm−1, 1836 cm−1, 1637.56 cm−1, 1552.70 cm−1, 1462 cm−1, 1413.82 cm−1, 1298 cm−1, 1242 cm−1, 1188 cm−1, 1080 cm−1, 1039.63 cm−1, 962 cm−1, 721 cm−1, 650 cm−1 (Fig. 4). Bands at 3437 cm−1 that corresponded to the stretching vibrations of alcohol; 2956.87 cm−1, 2924 cm−1 and 2852.72 cm−1 corresponded to the stretching vibrations of alkanes; 1836 cm−1 to that of acid anhydride; 1637.56 cm−1 to alkene; 1552.70 cm−1, 1462 cm−1, 1413.82 cm−1 to aromatics; 1298 cm−1, 1242 cm−1, 1188 cm−1 to alkyl halide; 1080 cm−1 to amine (C-N); 1039.63 cm−1 to ether; 962 cm−1 and 721 cm−1 to alkene and 650 cm−1 to alkyl halide.
GC–MS analysis of ethyl acetate extracted metabolite: GC–MS analysis of secondary metabolites produced by T. assiutensis revealed the presence of nine probable major volatile compounds, viz., 3-undecene-5-methyl-; 2,5-dihydroxy-3-methyl-2,5-cyclohexadiene-1,4-dione; 3-n-hexylthiolane, S,S-dioxide; Methyl 8-Methylnonanoate; (4H)4a,5,6,7,8,8a- Hexahydrobenzopyran-5-one-3-carboxamide,2; Cyclopentane undecanoic acid, methyl ester; Trans-2,4-Dimethylthiane, S,S-dioxide; 5-Hydroxy-4-hydroxymethyl-1-(1-hydroxy-1-isopropyl)-Cyclohex-3-ene; and 3-Nonyn-1-ol with peak-area respectively 128,601.2; 474,942.6; 320,973.8; 454,450.2; 1,437,319,040; 172,088.6; 277,639.3; 1,473,169 and 1,451,447.6. The chemical formula, structure etc. of these probable major compounds of EA extracted bioactive metabolites have been presented in Table 4. GC–MS chromatogram is presented in Fig. 5. In the chromatogram the first major peak corresponded to the compound 3-undecene-5-methyl with retention time (RT) 34.879 min, second major peak corresponded to the compound 2,5-dihydroxy-3-methyl-2,5-cyclohexadiene-1,4-dione with RT 37.91 min, third major peak corresponded to the compound 3-n-hexylthiolane, S,S-dioxide with RT 38.35 min, fourth major peak corresponded to the compound Methyl 8-Methylnonanoate with RT 40.08 min, fifth major peak corresponded to the compound (4H)4a,5,6,7,8,8a-Hexahydrobenzopyran-5-one-3-carboxamide,2 with RT 40.87 min, sixth major peak corresponded to the compound Cyclopentane undecanoic acid, methyl ester with RT 42.22 min, seventh major peak corresponded to the compound Trans-2,4-Dimethylthiane, S,S-dioxide with RT 42.83 min, eight major peak corresponded to the compound 5-Hydroxy-4-hydroxymethyl-1-(1-hydroxy-1-isopropyl)-Cyclohex-3-ene with RT 43.99 min and ninth major peak corresponded to the compound 3-Nonyn-1-ol with RT 44.99 min (Fig. 5).
Discussion
Medicinal plants have long been traditionally used in different parts of the world. Their indiscriminate use has resulted into destruction of species in their natural habitats. To reduce this, researchers have now focussed on bioactive metabolites produced by endophytic fungi. In the present study also endophytic fungi were isolated from Citrus macroptera, hoping that they will be capable of producing bioactive secondary metabolite similar to that of the host. A total of 13 morphologically distinct endophytic fungi were isolated from C. macroptera, most of which belonged to the phylum Ascomycetes. Except Alternaria sp., Ceratocystis sp., Aspergillus candidus and Fusarium sp., all endophytes colonized leaf, bark and root of the plants supporting the view of Dix and Webster (1995). Dix and Webster (1995) believed endophytes were generally not considered as organ-/site-specific microbes and it is likely that many of the species isolated from bark may also occur in leaves. In our study, Alternaria sp., Ceratocystis sp. and Aspergillus candidus colonized only leaf and bark samples, while Fusarium sp. colonized only the root. This indicated organ specificity of the isolates. Endophytic colonization was higher in leaves compared to barks or roots, which supports the findings of Maheswari and Rajagopal (2013) who also observed high percentage of frequency of colonization of the endophytic fungi. Difference in colonization rate of endophytic fungi in leaf, bark and root samples may be attributed to variation in phytochemical constituents and anatomical differences of these tissues (Maheswari and Rajagopal 2013).
Endophytic fungi are recognized as a potential source of antimicrobial secondary metabolites that could be used for various medicinal purposes (Tayung 2008). Results of the present work suggested that C. macroptera harboured endophytic fungi capable of producing antimicrobial secondary metabolites active against both bacteria as well as fungi. Among 13 isolates, 8 inhibited Staphylococcus epidermidis, 12 inhibited Escherichia coli, 10 inhibited Sclerotinia sclerotiorum and 8 inhibited Fusarium oxysporum. Deka and Jha (2018) isolated Acremonium falciformae from Litsea cubeba, which showed antagonistic activity against Staphylococcus epidermidis. Endophytic Talaromyces assiutensis isolated from Zingiber officinale in Indonesia, showed 26.3% inhibition against Fusarium oxysporum (Ginting et al. 2013). In our present study also, amongst all, T. assiutensis showed highest bacterial inhibition against S. epidermidis while A. falciformae showed highest antifungal activity against Sclerotinia sclerotiorum.
The phytochemical analysis of EA extracted metabolites of endophytic fungi were also carried out. Endophytic Penicillium sp. produced different phytochemicals (Dhankhar et al. 2012). Huang et al. (2007) reported that phenol and flavonoid compounds extracted from fungi possessed different bioactivities. Escherichia coli, Salmonella typhimurium, Bacillus subtilis and Staphylococcus aureus inhibiting active metabolites of the endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa, contained chemical groups such as phenols, flavonoids, terpenoids, alkaloids, tannins, carbohydrates and saponins (Sharma et al. 2016). Flavonoid content was highest in both of the crude extracts, followed by alkaloid and terpenoid. This active endophytic fungus has the ability to produce bioactive secondary metabolites containing various phytochemicals which will be useful for numerous plant and human ailments.
The antimicrobial metabolite of T. assiutensis showed bands corresponding to the stretching vibrations of alcohol, alkane, acid anhydride, alkene, aromatic, alkyl halide and amine. Different researchers have characterized the compounds present in the EA extracted bioactive metabolites produced by endophytic microbes, using Fourier-transform infrared (FT-IR) and gas chromatography-mass spectrometry (GC–MS) instrumental analysis. Using FT-IR, Miao et al. (2012) characterised the compounds present in the ethyl acetate extract of the antimicrobial secondary metabolite of Talaromyces verruculosus. They observed that the IR spectrum revealed the presence of OH groups, C=O group, benzene ring etc.
GC–MS analysis revealed the presence of 9 major compounds in the antimicrobial metabolite produced by Talaromyces assiutensis isolated from C. macroptera. 3-n-hexylthiolane, S,S-dioxide was identified in the bioactive metabolite produced by the potent fungi (T. assiutensis). Using GC–MS analysis, Nithyadevi and Sivakumar (2015) also identified 3-n-hexylthiolane, S,S-dioxide having antimicrobial activity in the methanolic leaf extract of Solanum torvum. Cyclopentane undecanoic acid, methyl ester was identified as a major compound in the antimicrobial secondary metabolite of T. assiutensis. Prasad et al. (2016) also identified cyclopentane undecanoic acid, methyl ester in the leaf and rind extract of Citrus maxima, which showed antimicrobial activity. Trans-2,4-dimethylthiane, S,S-dioxide which was present in the bioactive metabolite of Talaromyces assiutensis, was also identified from crude extract of Streptomyces werraensis isolated from soil of Tamil Nadu, India, showing antimicrobial activity against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Salmonella typhi, Bacillus cereus by Devi and Prabakaran (2014). A compound, (4H)4a,5,6,7,8,8a- hexahydrobenzopyran-5-one-3-carboxamide,2 was identified during the present study from antimicrobial secondary metabolite of T. assiutensis using GC–MS analysis. This compound was also identified by Hameed et al. (2016) using GC–MS analysis from antifungal and antibacterial methanolic bark extract of Cinnamomum zeylanicum. However, there is no report available related to the antimicrobial activity of T. assiutensis and bioactivity of the compounds 3-undecene-5-methyl-; 2,5-dihydroxy-3-methyl-2,5-cyclohexadiene-1,4-dione; methyl 8-methylnonanoate; 5-hydroxy-4-hydroxymethyl-1-(1-hydroxy-1-isopropyl)-Cyclohex-3-ene and 3-nonyn-1-ol which were identified in the EA extracted metabolite of T. assiutensis isolated during the present investigation from C. macroptera. Salvatore et al. (2018) characterized talarodiolide, a new 12-membered macrodiolide, using GC–MS analysis, isolated from the culture filtrate of Talaromyces sp. However, no such compound was observed in the metabolite of T. assiutensis during the present study.
Conclusion
Endophytic fungi are one of the very important microorganisms with high therapeutic value for pharmaceutical and agricultural applications. The results of this study represent that endophytic fungi may serve as a potential natural source of enzymes and other compounds having antimicrobial properties. This study provides a background for more comprehensive work on bioactive compounds produced by different endophytic fungi residing within C. macroptera and suggests that the isolates are potential source of enzymes and antimicrobial compounds, which might be used for controlling plant and human pathogens. Talaromyces assiutensis is one of the most active endophyte isolated from C. macroptera. The minimum inhibitory concentration of the bioactive metabolite of T. assiutensis was 113 ± 1.5 µg mL−1 against Staphylococcus epidermidis and the zone of inhibition was 17.11 ± 0.26 mm. On the other hand, the control (tetracycline) inhibited Staphylococcus epidermidis at 10 mcg concentration and the zone of inhibition was 19.67 ± 0.58 mm. The endophytic isolate T. assiutensis, can produce different compounds having antimicrobial as well as antioxidant properties. The bioactive metabolites of T. assiutensis should be studied in depth to find out the bioactive major and minor compounds.
References
Aktar K (2017) Foyzun T (2017) Phytochemistry and Pharmacological Studies of Citrus macroptera: a medicinal plant review. Evid Based Complem Altern Med 7:1–7. https://doi.org/10.1155/2017/9789802
Bauer AW, Kirby WMM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45:493–496
Bhardwaj A, Agrawal PK (2015) A review fungal endophytes: as a store house of bioactive compound. World J Pharma Sci 3(9):228–237
Carroll GC, Carroll FE (1978) Studies on the incidence of coniferous needle endophytes in the Pacific Northwest. Can J Bot 56(24):3034–3043. https://doi.org/10.1139/b78-367
Deka D, Jha DK (2018) Antimicrobial Activity of Endophytic Fungi from Leaves and Barks of Litsea cubeba Pers., a Traditionally Important Medicinal Plant of North East India. Jordan J Biol Sci 11(1):73–79
Devi NN, Prabakaran JJ (2014) Bioactive metabolites from an endophytic fungus Penicillium sp. isolated from Centella asiatica. Curr Res Environ App Mycol 4(1):34–43
Dhankhar S, Dhankhar S, Kumar M, Ruhil S, Balhara M, Chillar AK (2012) Analysis toward innovative herbal antibacterial & antifungal drugs. Recent Pat Anti-Infect Drug Discovery 7(3):242–248
Dix NJ, Webster J (1995) Fungal ecology. University of Michigan, Chapman & Hall, London
Ferguson NM (1956) A text book of pharmacognosy. Mac Milan Company, New Delhi, pp 969–973
Ginting RCB, Sukarno N, Widyastuti U, Darusman L, Kanaya S (2013) Diversity of Endophytic Fungi from Red Ginger (Zingiber officinale Rosc.) Plant and Their Inhibitory Effect to Fusarium oxysporum Plant Pathogenic Fungi. Hayati J Biosci 20(3):127-137. http://journal.ipb.ac.id/index.php/hayati
Hameed IH, Altameme HJ, Mohammed GJ (2016) Evaluation of antifungal and antibacterial activity and analysis of bioactive phytochemical compounds of Cinnamomum zeylanicum (cinnamon bark) using gas chromatography–mass spectrometry. Orient J Chem 32(4):1769–1788
Handunnetti SM, Kumara RR, Deraniyagala SA, Ratnasooriya WD (2009) Anti-inflammatory activity of Ixora coccinea methanolic leaf extract. Pharmacog Res 1(2):80–90
Hata K, Futai K (1995) Endophytic fungi associated with healthy pine needles and needles infested by the pine needle gall midge, Thecodiplosis japonensis. Can J Bot 73(3):384–390
Huang WY, Cai YZ, Ling J, Corke H, Sun M (2007) A potential antioxidant resource: endophytic fungi from medicinal plants. Econ Bot 61(1):14–30
Kaaniche F, Hamed A, Abdel-Razek AS, WibbergD Abdissa N, El Euch IZ (2019) Bioactive secondary metabolites from new endophytic fungus Curvularia. sp isolated from Rauwolfia macrophylla. PLoS One 14(6):e0217627. https://doi.org/10.1371/journal.pone.0217627
Keith LM, Velasquez ME, Zee FT (2006) Identification and characterization of Pestalotiopsis spp. Causing scab disease of Guava, Psidium guajava. Plant Dis 90:16–23
Kusari S, Verma VC, Lamshoft M, Spiteller M (2012) An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J Microbiol Biotechnol 28:1287–1294
Maheswari S, Rajagopal K (2013) Biodiversity of endophytic fungi in Kigelia pinnata during two different seasons. Curr Sci 104:515–518
Malik SK, Chaudhury R, Dhariwal OP, Kalia RK (2006) Collection and Characterization of Citrus indica Tanaka and C. macroptera Montr.: wild endangered species of Northeastern India. Genet Resour Crop Evol 53(7):1485–1493. https://doi.org/10.1007/s10722-005-7468-7
Miao F, Yang R, Chen DD, Wang Y, Qin BF, Yang XJ, Zhou L (2012) Isolation, identification and antimicrobial activities of two secondary metabolites of Talaromyces verruculosus. Molecules 17(12):14091–14098. https://doi.org/10.3390/molecules171214091
Nagamani A, Kunwar SK, Monoharachary C (2006) Handbook of soil fungi. I. K. International Pvt. Ltd., New Delhi
Nithyadevi J, Sivakumar R (2015) Phytochemical screening and GC–MS, FT-IR analysis of methanolic extract leaves of Solanum torvum Sw. Int J Res Biosc 3(9):61–66
Pervez MR, Musaddiq M, Thakare PV, Kumar A (2015) Characterization of bioactive compound isolated from Myrothecium spp. with UV, FTIR and HPLC Analysis. Indian J Pharm Biol Res 3(1):1–5
Phongpaichit S, Nikom J, Rungjindamai N, Sakayaroj J, Hutadilok-Towatana N, Rukachaisirikul V, Kirtikara K (2007) Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol Med Microbiol 51:517–525
Prasad DA, Prasad BR, Prasad DK, Shetty P, Kumar KNS (2016) GC–MS Compositional analysis of essential oil of leaf and fruit rind of Citrus maxima (Burm.) Merr. from Coastal Karnataka, India. J App Pharm Sci 6(5):68–72
Salvatore MM, DellaGreca M, Nicoletti R, Salvatore F, Vinale F, Naviglio D, Andolfi A (2018) Talarodiolide, a new 12-membered macrodiolide, and gc/ms investigation of culture filtrate and mycelial extracts of Talaromyces pinophilus. Molecules 23:950–959
Santos H, Filho P (2003) Doenças de fruteiras tropicais de interesse agroindustrial. Embrapa Informacao Tecnologica Brasilia 11:435–491
Schulz B, Wanke V, Draeger S, Aust HJ (1993) Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 97:1447–1450
Shamsa F, Hamidreza G, Monsef RG, Mohammadreza V (2008) Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J Pharmaceutical Sci 32:17–20
Sharma D, Pramanik A, Agrawal PK (2016) Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D. Don. 3 Biotech 6:2-10
Stierle A, Strobel GA, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae. Science 260:214–216
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67(4):491–502
Strobel G, Ford E, Worapong J, Harper JK, Arif AM, Grant DM (2002) Isopestacin, a unique isobenzofuranone from Pestalotiopsis microspora possessing antifungal and antioxidant properties. Phytochem 60:179–183
Tayung K (2008) Studies on Endophytic Microorganisms Associated with Taxus sp. Ph.D. thesis, Gauhati University, Guwahati, Assam, India
Teiten MH, Gaascht F, Dicato M, Diederich M (2013) Anticancer bioactivity of compounds from medicinal plants used in European medieval traditions. Biochem Pharmacol 86(9):1239–1247. https://doi.org/10.1016/j.bcp.2013.08.007
White TJ, Bruns TD, Lee SB, Taylor JW (1990a) Amplification and direct sequencing of fungal ribosomal RNA. Gen Phylogenet 38:315–322
White TJ, Bruns TD, Lee SB, Taylor JW (1990b) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Acknowledgement
Deepanwita Deka is thankful to the University Grant Commission, New Delhi for providing Basic Scientific Research (UGC-BSR) fellowship under Special Assistance Program (UGC-SAP, DRS-I), Department of Botany, Gauhati University, Guwahati.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deka, D., Jha, D.K. Bioactivity assessment of endophytic fungi associated with Citrus macroptera Montr.: an endangered ethnomedicinal plant used in folk medicines in North-East India. Indian Phytopathology 73, 21–33 (2020). https://doi.org/10.1007/s42360-019-00179-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-019-00179-w