Abstract
A glauconitic siltstone sample with a potash content of 10% is investigated as a potential potash source. The fine grain structure comprises potash bearing phases of glauconite, muscovite, and microcline. The as-received samples showed poor dissolution ~ 5%. Planetary ball milling was attempted for the dissociation of the underlying silicate structure for the potash release, and ~ 60% potash was obtained after 12-h milling. Mechanical activation (8 h, 60% NaOH) prior to heat treatment (600 °C, 30 min) was found successful in recovering more than 95% K values from glauconite. The final residue consists mainly of quartz, indicating a complete breakdown of the silicate matrix. The mechanical activation process followed is simple, adaptable, and less energy-intensive compared to existing methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fertilizers comprising nitrogen (N), phosphorus (P), and potassium (K) play an important role in soil replenishment for agriculture. The world K demand is projected ~ 39.4 MT by 2024. The major soluble potash deposits comprising sylvite, sylvinite, or carnalite are found in the United States, Canada, Belarus, and Russia [1]. Potash is widely used in agriculture (~ 95%), and the rest is being used in allied industries. Agriculture-dependent countries, i.e., Brazil and India, possess limited soluble K-bearing deposits and therefore depend heavily on imports [2]. In the last decade, silicate sources, feldspar, nepheline syenite, mica, and glauconitic siltstone comprising K2O ~ 5–10% were investigated as alternative potash deposits. The extraction of K as KCl or another soluble phase from such sources is sluggish because of the crystalline structure of alumina-silicate minerals, complexity, and economic constraints. The processing routes comprise leaching with highly concentrated acids [3,4,5,6,7], fusion processing using alkali salts or limestone [8,9,10,11,12,13,14,15,16], and hydrothermal processing [17, 18]. The potash volatilization at high-temperature, excessive flux/strong acids, and secondary residue generation is key issues related to the above-mentioned processing routes. The key findings in the literature related to the recovery of potash from glauconitic rocks are exclusively shown in Table 1. In literature, the extraction of K from glauconite-rich silicate rocks is limited to heat treatment with Ca/Na-based fluxes, coke, and sulfuric acid leaching in the presence of fluoride ion. These processes have specific environmental concerns regarding the reagents involved and subsequently generate additional waste residue. It is worth mentioning that the feed used in these studies is significantly different from the feed of the present study. Very recently, planetary ball milling of glauconite sample (85% glauconite and 12% quartz) up to 240 min is reported, which resulted in a 30-fold increase in cation exchange capacity for K+ ion [20].
Rock flour minerals comprising glauconite, chlorite, biotite, and feldspathoids are reported in agricultural use [21, 22]. Based on our previous work comprising planetary ball milling of sericite samples consisting of muscovite, phlogopite, orthoclase, and quartz phases, it was found that more than 90% K can be recovered within 10–12 h milling [23]. The K-dissociation from these silicate deposits is primarily a surface reaction and therefore requires substantial grinding for the generation of reactive surface area, which can accelerate the K-release [24]. The structural disordering of an aluminosilicate matrix can be employed to release K+ ion present in the interlayer position [25, 26]. Significant changes in particle size, morphology, microstructure, and sometimes particle agglomeration due to fine particle adhesion, which impedes leaching [26, 27]. Mechanical activation is reported beneficial for numerous concentrates [28]; however, it is limited for potash extraction from silicate rocks [23]. Kleiv and Thornhill [25] reported increased reactivity of the milled product of K-feldspar and nepheline syenite. In this work, glauconitic siltstone is investigated as an alternative potash source. Glauconite is a group of hydrous, iron, potassium sheet silicates with a compositional range between smectite type glauconite and mica type glauconite end members and empirical chemical formula: K0.6Na0.05Fe3+1.3Mg0.4Fe2+0.2Al0.3Si3.8O10(OH)2 [29]. In this work, the glauconitic siltstone sample containing 40% glauconite is mechanically activated using planetary ball mill both with and without chemical additive. Dissociation behavior and mineral phase transformation of different K-bearing phases were investigated systematically using different parameters such as specific surface area, particle size, and morphology followed by leaching to recover potash values.
2 Materials and Methods
2.1 Sample
The glauconitic siltstone sample with K2O grades between 8 and 12%, was prepared using stage wise jaw and roll crushing, followed by grinding in a laboratory ball mill for 30 min using different ball sizes followed by sieving and riffler sampling. The percentages of minus 200 mesh (75 micron) for crushing product and ball milling product are 5% and 80%, respectively.
2.2 Analysis Methods
2.2.1 Liberated K Evaluation
The extent of liberated K was evaluated by leaching, and the experiments were conducted in a 250-mL glass beaker placed on a magnetic stirrer (IKA) at ambient conditions, solid-liquid ratio, and stirring speed of 1:10 (g/mL) and 900 rpm. The leach solution was subjected to vacuum filtration followed by oven-drying at 110 °C. Flame Photometer (Systronic) was used to determine the K dissolution and was calculated using, % K dissolution = Ksolution/KFeed × 100. The experiments were repeated twice, and the mean value is reported.
2.2.2 Characterization and Analytical Analysis
The chemical analysis was carried out using wet chemical analysis (WCA) in aqua regia digestion. The sample was also characterized by the electron probe microanalyzer (EPMA) for chemical analysis. X-ray diffraction (XRD) was carried out (Rigaku make) using Cu-Kα radiation, 10°–60° at 2°/min to identify decomposition of phases. The amorphization degree was determined using quantitative XRD analysis with formula; (I/Io) × 100 where I and Io are peak phase intensities before and after treatment [14, 16]. The mineralogical analysis, morphology, or chemical composition was evaluated using optical microscopy and scanning electron microscopy (SEM) equipped with the electron dispersive X-ray technique (EDS).
Specific surface area at different time intervals was measured using Brunauer, Emmett, and Teller (BET) analysis with the N2 adsorption technique [30]. Thermo-gravimetric analysis (TGA) of feed and NaOH mixture was studied at 20–1250 °C, airflow rate of 200 mL/min at 10°/min for thermal behavior. All the chemicals used are of reagent grade purity and procured from Merck Millipore Company.
2.3 Experimental Procedure
2.3.1 Planetary Ball Milling
Planetary ball mill (Retsch, PM 100) was used for studying the mechanical activation and milling behavior of the feed sample at different time intervals with a ball-powder ratio of 8:1 and milling speed 200 rpm, respectively. The feed was placed into tungsten carbide jar (capacity 125 mL) with tungsten carbide balls of different sizes (5 mm, 10 mm) and was tightly closed using a clamp. Milling was continued for every 30 min and paused alternately for 30 min, to avoid heat generation and milling induced dehydroxylation. The milled products were recovered using a spatula and were stored in a desiccator.
2.3.2 Experiment Flow Chart
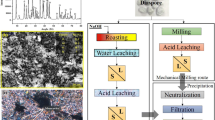
The complete experimental flowsheet adopted in this study is shown in Fig. 1. Two different routes were followed: (1) planetary ball milling → leaching and (2) planetary ball milling with NaOH → heat treatment → leaching. The mechanical activation route was followed by milling glauconite for a duration of 4, 8, 12, and 16 h. The milled samples were also leached in the lixiviants as mentioned earlier to determine the amount of K available after milling. XRD analysis was carried out at the different duration of milling and the extent of crystallinity of different K-bearing phases was studied with milling time. Instead of measuring particle size, BET surface area measurement was preferred considering very fine particle size. Furthermore, glauconite sample was mechanically activated with NaOH for 8 h followed by heat treatment. Heat treatment was carried out in a muffle furnace in silica crucible covered with a lid for 30 min at different temperatures (200–600 °C). The heat-treated mass is then leached in water to remove water-soluble sodium silicate phase. The residue obtained after filtration is further leached in 2% citric acid.
3 Results and Discussion
3.1 Mineralogy
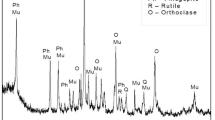
The chemical composition of the feed is shown in Table 2, and XRD analysis is shown in Fig. 2a that depicts glauconite (K(Fe,Al)2(Si,Al)4O10(OH)2), muscovite (KAl3Si3O10(OH)2), microcline (KAlSi3O8), and quartz (SiO2) as the main phases. It was found that glauconite (21.7%), muscovite (33.1%), and microcline (30.3%) are the main potash bearing phases, and stoichiometric calculations for K yielded 9.15% K in the feed. The optical microscope image shown in Fig. 2b shows uniform texture green, golden grains. The rock appears laminated due to the intercalation of glauconite-rich sheets and quartz-feldspar. The SEM micrograph shown in Fig. 2c depicts flaky particles of ~ 8 μm average size. The EDS analysis of glauconitic siltstone over the selected area in Fig. 2c showed similar fractions of O, Si, Al, Fe, and K as 42.58, 27.19, 9.04, 6.97, and 9.92 wt%, respectively, and is in line with the other results.
3.2 Mechanical Milling and Activation
Glauconitic siltstone feed showed limited K-dissolution in different lixiviants due to a locked silicate structure. Mechanical activation and milling were attempted for the liberation of the locked K values, and dissolution with a variation of milling time is shown in Fig. 3. Water leaching yielded only 6 %K dissolution, whereas, in citric acid (organic acid) leaching, K dissolution increased with milling up to 12 h and saturated up to 16 h, and thereby contradicts extensive milling. Very similar results were obtained and favor citric acid because of low toxicity compared to inorganic acids and also showed a high affinity towards K+ to form stable complex [31]. The specific surface area (SSA) of milled samples shown in Fig. 3 increased from 10.04 to 17.45 m2/g within milling of 4 h and further decreased because of the agglomerates formation.
Mechanical activation caused alterations in the mineral structure such that interlayer K in the glauconite and muscovite is released and leads to enhanced K dissolution [20]. The extensive milling (> 16 h) leads to fine agglomeration due to the exposure of hydrogen bonds in the hydroxyl group in the mineral. Therefore, metal dissolution tends to decrease with an increase in the agglomeration, and 20 h milling time resulted in a 5% decrease in K dissolution. It is worthwhile to mention that a milling time of 12 h is sufficient to break the silicate matrix resulting in ~ 60% K dissolution.
3.3 Characterization of the Product
XRD analysis shown in Fig. 4a reveals that with increasing milling time, the amorphic character increases as reflected by low-intensity peaks. XRD of leach residue-milled sample (16 h) shown in Fig. 4a comprises mainly quartz. The dissociation behavior or the change in the crystallinity degree with time is shown in Fig. 4b with muscovite (2Ѳ: 26.84), microcline (2Ѳ: 27.74), and glauconite (2Ѳ: 25.88). Within 4 h of milling, crystallinity falls ~ 63% for glauconite, and with subsequent 4-h milling, crystallinity reduced to 29% and was found almost similar with additional milling. The crystallinity of the microcline phase decreased by 70% within 4 h of milling, followed by a 5% drop in the next 4 h and further saturated. Muscovite showed a maximum crystallinity drop of 80% after 4 h. The rate of crystallinity decrease becomes sluggish with milling time indicated restricted or slow milling because of high energy micro-events through the milling process and may also be due to the embedded small solid crystal in the underlying amorphous background [23]. Therefore, it can be concluded that glauconite is more stable than microcline, followed by muscovite.
The micrographs of glauconitic siltstone feed, milled product (4, 6, 12, 16 h), and residue (12 h milled) are given in Fig. 5. The particles are dispersed in the matrix with an average particle size of 8 μm. After 4-h milling, the particles are spread in an agglomerated form with an average size of 4 μm. Coalesced particles with a mean size of ~ 3.5 μm were observed after 12 h of milling. Further milling of 4 h revealed spherical agglomerates with an average particle size of ~ 7 μm. The particles corresponding to 12-h milled samples leached on the surface of agglomerates and irregularly shaped particles of size ~ 9 μm were obtained. The EDX analysis of 16-h milled leach residue is shown in Fig. 5g and contains approximately 2.5% K compared to 9.9% in the feed. The atomic % of silicon and oxygen of the leach residue confirmed SiO2 as the major phase and is in agreement with the XRD results. Ti and Fe could be associated with titanium oxide and goethite, stable minerals identified in the feed.
3.4 Effect of Mechanical Activation on Heat Treatment
Heat treatment of glauconite with NaOH flux was performed to dissociate the silicate structure and liberate K values. Microcline and muscovite react with NaOH as per Eqs. (1, 2) to form kalsilite (KAlSiO4) and sodium silicate (Na2SiO3). Sodium silicate is soluble in water that can be easily separated by a water wash step, and further leaching of solid to recover K. TGA-DTA analysis of glauconite and NaOH mixture is shown in Fig. 6. In DTA curve, there are four endothermic peaks at 68 °C, 138 °C, 236 °C, and 296 °C, first two endothermic peaks are due to volatilization of physically absorbed water in NaOH, corresponding weight loss can also be seen in TG curve, weight loss is also due to the dehydration of mineral. Optimization of heat treatment parameters (flux dosage, temperature, and time) for hand-mixed glauconite and NaOH sample was carried out by conducting regular experiments, and the highest K recovery of 78% was achieved at 600 °C for 30 min with 60% NaOH dose by weight. The influence of mechanical activation on thermal treatment was evaluated by mixing 60 wt% NaOH with a respective amount of feed followed by mechanical activation for 8 h at 200 rpm. The mechanically activated mixture was further treated in a muffle furnace up to 600 °C for 30 min and leached to recover K values. The effect of mechanical activation can be clearly seen in Fig. 7. Thermal treatment at 200 °C of mechanically activated samples yielded similar results compared to heat treatment at 600 °C of hand-mixed samples, thereby decreasing the required temperature by 400 °C. The heating of the mechanically activated sample at 600 °C was found to be successful in recovering more than 95% K values.
3.5 Characterization of Heat Treatment and Leaching Products
The XRD analysis of the heat-treated sample (600 °C, 30 min), residue after water wash (LR1), and final residue after oxalic acid leaching (LR2) is shown in Fig. 8. As per XRD analysis, primary K-bearing phases (microcline, muscovite, and glauconite) converted to sodium silicate and kalsilite after heat treatment. Furthermore, sodium silicate peak disappeared in the water-washed residue, and kalsilite became the major phase along with quartz. Kalsilite was further removed from the washed sample after citric acid leaching, and the final residue constitutes quarts as a major phase. Similar phase transitions in XRD analysis were observed for both mechanically activated and hand-mixed samples during heat treatment and leaching. SEM (EDS) analysis of a mechanically activated sample at different stages is shown in Fig. 9. SEM micrograph of the heat-treated sample shown in Fig. 9a shows particles fused together. After the water wash of the heat-treated sample, the particle becomes dispersed throughout the matrix, as shown in Fig. 9b. Irregularly shaped particles were observed in the SEM micrograph (Fig. 9c) of the final residue. EDS analysis further confirmed the Si (42 wt%), indicating SiO2 in the final residue.
4 Conclusion
In the present study, the glauconitic siltstone sample was evaluated as a potential potash source. The fine grain structure comprises the potash bearing phase of glauconite, muscovite, and microcline with very fine liberation size. The feed sample showed minimal K dissolution because of the locked underlying silicate structure. The complete dissociation of the structure was not observed, and 12-h milling time was found adequate for the K-release of ~ 60%. It was observed that excessive milling does not aid the K-release because of the agglomerate formation. Mechanical activation was found to have a positive impact on K recovery via the heat treatment route. Mechanical activation (8 h, 60% NaOH) prior to heat treatment (600 °C, 30 min) was found successful in recovering more than 95% K values from glauconite. The leach residue comprises mainly quartz depicting complete dissociation of the underlying silicate structure. The mechanical milling-activation process followed is simple, adaptable, and less energy-intensive compared to existing methods.
References
Ciceri D, Dac M, Allanore A (2015) Historical and technical developments of potassium resources. Sci Total Environ 502:590–601
IBM, Indian Minerals Yearbook 2017 (Part- III: Mineral Reviews) 56th edition: Potash
Carroll D, Starkey HC (1971) Reactivity of clay minerals with acids and alkalis. Clay Clay Miner 19:321–333
Tomita K, Dozono M (1972) Formation of an interstratified mineral by extraction of potassium from mica with sodium tetraphenylboron. Clay Clay Miner 20:225–231
Varadachari C (1997) Potash fertilizer from biotite. Ind Eng Chem Res 36:4768–4773
Rao BR, Rao LS, Mazumder AK, Rao TC (1993) Fluoride aided potassium extraction from glauconitic sandstone for liquid fertilizer. Miner Eng 6:405–413
Wang H, Feng Q, Tang X, Zuo K, Liu K (2017) Insights into alkali-acid leaching of Sericite: dissolution behaviour and mechanism. Minerals 196:1–12
Mazumder AK, Sharma T, Rao TC (1993) Extraction of potassium from glauconitic sandstone by the roast-leach method. Int J Miner Process 38:111–123
Jena SK, Dhawan N, Rao DS, Das B (2015) Extraction of potassium values from pyrophyllite mine waste. Sep Sci Technol 51:269–277
Santos WO, Mattiello EM, Costa LM, Abrahao WAP, Novais RS, Cantarutti RB (2015) Thermal and chemical solubilization of verdete for use as potassium fertilizer. Int J Miner Process 140:72–78
Rudmin M, Banerjee S, Mazurov A, Makarov B, Martemyanovd D (2017) Economic potential of glauconitic rocks in Bakchar deposit (S-E Western Siberia) for alternate potash fertilizer. Appl Clay Sci 150:225–233
Shekhar S, Mishra D, Agarwal D, Sahu KK (2017) Physico-chemical treatment of glauconitic sandstone to recover potash and magnetite. J Clean Prod 147:681–693
Shekhar S, Mishra D, Agarwal A, Sahu KK (2017) Physical and chemical characterization and recovery of potash fertilizer from glauconitic clay for agricultural application. Appl Clay Sci 143:50–56
Kumar A, Tanvar H, Pratap Y, Dhawan N (2019) Evaluation of Mica as a source of potash. Mining Metall Explor 36(3):547–555
Rudmin M, Oskina Y, Banerjee S, Mazurov A, Soktoev B, Shaldybin M (2018) Roasting-leaching experiments on glauconitic rocks of Bakchar ironstone deposit (Western Siberia) for evaluation their fertilizer potential. Appl Clay Sci 162:121–128
Tanvar H, Dhawan N (2019a) Kinetic and thermodynamic study of potassium recovery from silicate rocks. Miner Process Ext Metall:1–13. https://doi.org/10.1080/25726641.2019.1699360
Ciceri D, Oliveira MD, Allanore A (2017) Potassium fertilizer via hydrothermal alteration of K-feldspar ore. Green Chem 9:5187–5202
Liu SK, Han C, Liu JM, Li H (2015) Hydrothermal decomposition of potassium feldspar under alkaline conditions. RSC Adv 5:93301–93309
Shekhar S, Sinha S, Mishra D, Agrawal A, Sahu KK (2020) A sustainable process for recovery of potash fertilizer from glauconite through simultaneous production of pigment grade red oxide. Sustain Mater Technol 23:e00129
Singla R, Alex TC, Kuma R (2020) On mechanical activation of glauconite: physicochemical changes, alterations in cation exchange capacity and mechanisms. Powder Technol 360:337–351
Harley AD, Gilkes RJ (2000) Factors influencing the release of plant nutrients from silicate rock powders: a geochemical overview. Nutr Cycl Agroecosyst 56:11–36
Gautneb H, Bakken AK (1995) Crushed rocks, minerals and mine tailings as sources of potassium in agriculture. Nor Geol Unders 108:1–89
Singh YP, Tanvar H, Kumar G, Dhawan N (2019) Investigation of planetary ball milling of sericite for potash recovery. Powder Technol 351:115–121
Ciceri D, Oliveira M, Stokes RM, Skorina T, Allanore A (2017) Characterization of potassium agrominerals: correlations between petrographic features, comminution and leaching of ultrapotassic syenites. Miner Eng 102:42–57
Kleiv RA, Thornhill M (2007) Production of mechanically activated rock flour fertilizer by high intensive ultrafine grinding. Miner Eng 20(4):334–341
Sanchez CE, Torres ME, Diaz C, Saito F (2004) Effects of grinding of the feldspar in the sintering using a planetary ball mill. J Mater Process Technol 152:284–290
Kohobhange SPK, Manoratne CH, Pitawala HMTGA, Rajapakse RMG (2018) The effect of prolonged milling time on comminution of quartz. Powder Technol 330:266–274
Baláž P (2003) Mechanical activation in hydrometallurg. Int J Miner Process 72:341–354
Dooley JH (2006) Glauconite. Pp. 495–506 in: Industrial Minerals & Rocks: Commodities, Markets, and Uses (J. Kogel, N. Trivedi, J. Barker, and N. Krukowski, editors). Society for Mining, Metallurgy, and Exploration, Littleton, Colorado, USA
Brunauer S, Emmet PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Williams PJ, Cloete TE (2010) The production and use of citric acid for the removal of potassium from the iron ore concentrate of the Sishen Iron Ore Mine South Africa. S Afr J Sci 106:1–5
Funding
The funding was received from the Science Engineering Research Board via EMR/2016/000505 for carrying out the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pratap, Y., Tanvar, H., Moreira, D. et al. Processing of Glauconitic Siltstone for Potash Recovery. Mining, Metallurgy & Exploration 37, 1231–1239 (2020). https://doi.org/10.1007/s42461-020-00218-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-020-00218-5