Abstract
Tannins, non-toxic natural polyphenol biomolecules widely recognized as secondary plant metabolites categorized under polyphenols and are potential candidates for biosorbent manufacturing. The acquit of toxic wastewater from commerce to open habitat causes relentless unceasing pollution of the environment, air, water resources due to the latency of soluble metal ions. Adsorption is the most developed and efficient process for extracting a multitude of substances from aqueous solutions. Tannin based adsorbents (TBAs) are highly proficient in the removal of heavy metals (density > 5 g/cm3), dyes, surfactants, and chemical products from polluted waters. TBA exploits the extraction and removal of heavy metals (mercury, uranium, lead, and chromium) under its proficient natural affinity to uptake heavy metals by forming chelates with metals due to the presence of a high number of adjacent hydroxyl groups in its molecule. Electrostatic attraction schemed to be the underlying mechanism, cationic heavy metals assimilated on to the anionic surface grabbed into the porous framework with a low surface area and toxic heavy metal ion permutation effectively attained at HNO3 or HCL (0.1 mol/L) solution. Moreover, tannins upon chemical modification/inclusion with different substances produce adsorbents with superior adsorption capability towards targeted metal ions. The removal of cationic contaminants favored at basic pH levels, and while analyzing mimosa bark tannin foams reveals 12.5% of Cu(II), and pine bark tannin foam 20.1% of Pb(II) adsorption. The TBA adsorption rate remained unlateralized even after four consecutive cycles. Herein this review provides an in-depth study on tannin-based adsorbents capable of removing the toxic heavy metal ions from wastewater and their formulation, extraction methods, effect if the interference of competitive ions, possibility of lixiviation of constituent particle to water and some possible future prospects regarding their efficient separation.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polyphenols, ubiquitous [1] class of non-volatile secondary plant metabolites [2] (not polymers of phenol) featured by the subsistence of one or more hydroxyl groups coupled to an aromatic ring and deprived of nitrogen-based functions [3] generally in the defense against ultraviolet radiation or aggression by pathogens [3], showing unique physical and chemical properties equivalent to their immense molecular weight(upper value-800 Dalton) and torrent phenolic substructures adept of protein precipitation, hasty diffusion through the cell membrane [4]. Polyphenols attracted attention from worldwide scientists and can be relevant in an application such as traditional dye, originator in green chemistry [5], chelated trace metal ions, chain breaker, or radical scavengers [6].
The blooming industrialization and motorization [7] of the world have led to a steep rise in adulteration and harmful pollutants like heavy metal ions, dyes where the estrangement of toxic engineering wastewater is an imperious issue for the environment [8]. Polluted water contains abundant cationic metals like nickel, arsenic, zinc, lead, and cadmium that pose hazards to human health when invaded beyond tolerance daily limit (TDI) [9], which ultimately leads to tissue, and cellular damage leads to an array of conflicting effects and human epidemics [10]. Advanced wastewater treatment approaches, such as membrane separation, adsorption, ion exchange, and precipitation, have been explored by the researchers [9, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. The supreme hazardous metals involve cadmium (Cd2+), arsenic (As3+/5+), mercury (Hg2+), copper (Cu2+) along with these vast radioactive elements such as uranium (U6+) [41] and thorium (Th4+) were also dumped into the water streams by nuclear power plants. Oil spilling another promising problem even leads to the death of marine as well as humans. Industrial dye compromises the aesthetic quality of water bodies and its disposal for ensuring a necessary quality life for humankind. Sources of heavy metal ion and its adsorption schematically represented in Fig. 1.
Arsenic desired as a doping agent in semiconductors (gallium arsenide), also used in pyrotechnics bronzing, wood preservatives, metal adhesives, medical fields and in ancient cave portrait for red stain and elemental mercury was spotted in pre-historic Greece where it (as well as white lead) was handled as a beautifier for shiny skin. This heavy metal waste dumped into water bodies, upon consumption results in relentless disorders. Mercury chiefly crowdsourced from mineral cinnabar and cannot be detected in the wild as a free state which is widely applicable as a liquid electrolyte, in dental amalgams, and often in making scientific instruments such as a thermometer and its electrical conductivity is used to create switches [42]. Streetlights, fluorescent lamps were mercury in vapor state were utilized. Significant use cadmium of found to be in batteries (mainly rechargeable nickel–cadmium–NiCad batteries), control rods of nuclear reactors, also composed of cadmium, protective plate on other metals.
Adsorption establishes to be beneficial in removing metal-cations from wastewater [43]. The adhesion of ions, atoms, or molecules from a liquid or gaseous phase confined either by physical or chemical means onto the solid surface. Two different types of adsorptions are (i) physisorption caused by the Van der Waals force, (ii) chemisorption by the chemical reaction amongst adsorbent surface, and adsorbate encompasses more substantial effects and improved adsorption [44]. Tannins being a class of polyphenols easily extracted from natural resources shows an excellent affinity towards the metal ions in aqueous solutions that can use as a material of biosorbents in wastewater treatment.
Since 1994, struggles on rigid foams based on pine tannin have made without any decisive triumph. Formulation of environmentally friendly rigid tannin/furanic foams of fire resistance and often equitable to pine tannin foams has been emerged recently [45]. Tannin foams can be used in wooden doors for internal and external insulation rather than used in metal ion adsorption. Polycondensation of tannin powder, formaldehyde and furfuryl alcohol in 70%, 5% and 25% (w/w) respectively engenders tannin rigid foams (TRF) [46]. Hydrolysis of sugar from crops yields furfuryl alcohol [47] and also through the catalytic reduction of furfural [48]. Resin mix upon crosslinking and boiling of physical blowing agent and a solvent of low boiling point carried out for the determination of foam density. The chemical rudiments lie on the gelation of tannin [49], which is a rationally established practice. The immobilization of tannin executed as a sequence by the solvent blowing agent within a range of 10–30 s after weaving gelation peripherals, which develops the main disparity with gelation to a delicate process [50]. Significant drawbacks reported by virtue of the TRF potential did not achieve high levels and the low efficiency that measured as q capacities [8].
This review aims to describe polyphenols and their efficiency in removing cationic heavy metal ions and dyes within polluted industrial wastewater. The literature survey disclosed that researchers are very much inquisitive about tannin and its properties. Toxic heavy metal ions are being a prominent constituent in water owing to diversely affect health and longevity enlightened from this point tannin-based adsorbents were selected for review along with a brief note about the heavy metals, and their noxiousness were comprised within the review. It also includes the structure, application, and future scopes of polyphenols (tannins). Adsorption isotherms and thermodynamics of adsorption are also described along with other extraction methods.
2 Heavy metal ion noxiousness in humans
Arsenic, which inducts carcinogenic include epigenetic alterations, bruise dynamic DNA maintenance system and the generation of ROS as they have proven to produce hydrogen peroxide (H2O2), superoxide (O .−2 ), singlet oxygen (1O2), nitric oxide (NO·) free radicals [51]. Acute arsenic affects the heart, brain which may ruin of blood vessels and the gastrointestinal tissue. Chronic arsenic leads to irreversible alterations in the active organs, bladder cancer, skin cancer liver, and ultimately lead to death [52]. As per WHO, the safe level of arsenic in the body is 0.02 mg/L. Cadmium and its conglomerations exhibit various damage to the health of humankind and are worsened pursuant to the incapability of humans to eliminate cadmium and abstain by the kidney, consequently limiting its excretion. 0.06 mg/l is the optimum amount of cadmium in adults beyond the limit drives to ailment, namely, damages to lungs and respiratory irritation [53]. A higher level engenders stomach irritation, vomiting, and diarrhoea, proximal tubular cells, bone demineralization, either directly or indirectly (renal dysfunction) [54]. Chromium has the propensity to get corroded and cause allergic reactions to the body. Chromium has the habit of getting corroded and trigger allergic reactions to the body. Beyond significant dose induce severe respiratory, cardiovascular, neurological and haematological effects, and possibly death [55, 56].
Mercury (Hg) natural chemical element known as quicksilver found in environs (soil, air and water) exist in 3 forms—elemental/metallic, inorganic and as organic Hg. Hg exposure can be accompanied by water-soluble forms (methyl mercury or mercury chloride), ingestion of any kind or through mercury vapor inhalation. Humans, mostly exposed to methyl mercury, builds up in fish, shellfish [56]. Hg consumption during pregnancy will adversely affect the foetus bypassing its toxicity through diet and breast milk to infants [57]. Inhalation of mercury vapor enters the brain as red blood cell adherent Or as serum [58]. Exposure of mercuric vapor in higher levels may cause neurological dysfunction, anorexia, gastrointestinal disturbance and disruption in repairing of DNA [59]. Exposure towards elemental mercury induces hypertension [60], excessive anger, carotid atherosclerosis, anxiety, even depression [61]. Lead in the human body can interact with normal body function leads to irreversible health disorder [62]. In infants and children, soil, water, dust and paints are the most likely sources of lead [63]. Excessive lead levels in the blood affect the functioning of the nervous system [64]. The effect of chronic lead in blood (40–60) is persistent vomiting, convulsions and even coma. Increase in the fragility of the cell membrane, which reduces erythrocyte life span [65]. Exposure of lead in higher amounts (> 60) causes renal dysfunction. Bone is the largest site for the accumulation of lead in the body [66] {exposure on tap}. Prolonged exposure to lead may cause reproductive impairment, poor pregnancy outcome and blood pressure [64].
3 Polyphenols an overview
Polyphenols constitute a number of ubiquitous [67] compounds derived naturally mostly from the beverages, vegetables, fruits, and cereals, (~ 1 g/day) [68] and are plant metabolites of plants generally engaged in defending against ultraviolet rays or accumulation and have a crucial role in the inhibiting degenerative diseases like cancer and cardiovascular diseases [69] emerging by pathogens [3]. These compounds are embedded in the human diet [5], comprise a wide variety of molecules that have a polyphenol structure [70]. Figure 2 represents the general classification of polyphenols.
3.1 Structure of polyphenols
Polyphenols are a tier of chemical micronutrients in plants identified by multiple phenol structures and the number of phenolic moieties [71], where more than 5000 unique categories have been detected [3]. The classification relies on the strength of phenol rings and elements attached to these moieties to each other. The main categories comprise flavonoids, lignans, phenolic acids, tannins, and stilbenes (Fig. 3) [72]. Among these flavonoids are further classified into flavones, flavonols, flavonolds, isoflavones, and phenolic acid into hydroxybenzoic acid and hydroxycinnamic acid [26].
3.2 Extraction of polyphenols
Various methods have been established for the effective extraction of polyphenols from different natural resources. Furthermore, conventional extraction, microwave extraction (ME), and ultrahigh-pressure extraction were the manners commonly employed.
-
1.
Conventional heating
Extraction using the conventional heating process performs by heating in a water bath, the maximum extraction happens by slowly heating the plant usually done at 20–50 °C. After 50 °C, it undergoes a simultaneous decrease in the amount of polyphenols at the same time beyond 70 °C results in plant degradation.
-
2.
Microwave extraction (ME)
Microwave extraction (ME) performed in a glass extraction vessel (Fig. 4) [73] is one of the cost-effective methods for the extraction of polyphenols wherein the solvent and the sample heated directly by the interaction with the electromagnetic radiation (3000 MHz–300 GHz) [74]. Plants enriched with water in and massive amounts, radiated in a microwave oven to get heated under microwave radiation at regular intervals. The rapid heating of the solvent improves its solubility by enhancing the porosity, which results in better penetration of the solvent into the matrix [75]. Studies have proven that the shortest possible time for the extraction of tannin using ME is around 1–20 min [76]. The yield of tannins in the extracts strongly depends upon the volume and the radiation power [77]. An increase in the amount of solvents led to a rise in tannins being extracted up to a certain point (ratio 1:30) and then decreased. The thermal degradation of tannins can result in a reduced amount of tannin in the extracts normally due to the high irradiation values.
Various solvents (water, ethanol, methanol, ethyl acetate, acetone) can be employed for the extraction of polyphenol capable of absorbing microwaves, efficiently disrupt the cell structure. Ethanol and methanol are the most advisable solvents because tannins readily dissolve in them [78]. The optimum conditions for the extraction of polyphenols are using methanol (80%) along with 156 W power within 5 min. Tannin extraction from Moroccan Acacia mollissima barks proved to achieve better yields by using methanol as the solvent, even at room temperature. To evaluate the efficacy of solvent (ethanol and methanol) Rhazi et al. provided similar conditions throughout the extraction; methanol yields 19.09 mg/g of condensed tannins, 0.08 mg/g of hydrolysable tannins and 441.63 mg/g of total polyphenols whereas ethanol gives 18.50 mg/g of condensed tannins, 0.03 mg/g of hydrolysable tannins and 312.26 mg/g of polyphenols. ME process yields tannins 1.25 times more than the conventional method. The key benefits of this process are efficient extraction within a short span of time, consumption of a minimal amount of solvents [79] compared to conventional extraction methods, and finally, the agitation provides efficient mass transfer throughout the process [75].
-
3.
Ultrasound radiation extraction (URE)
The URE method is a simple, affordable, efficient, and alternative to conventional extraction techniques. Ultrasound can also alleviate operating temperature [80]. The ultrasound apparatus is affordable and accessible to operate relative to other unique extraction methods such as ME [81]. For extraction, effective penetration, cell disruption, and capillary impact, the detrimental behaviour of ultrasound was utilized. The ultrasound applied consist of high temperature, and the plant particles transmitted to the given solvent (diffusion). Ultrasound irradiation breakdown the cell wall, and raise the number of exposed solvents, enhance diffusion, swelling furthermore improve the extraction efficiency. Ethanol and methanol like polar solvents desirable for extraction due to their selectivity towards polyphenols [82]. Along with the benefit that the requirement of shorter extraction time (< 1 h), which is higher than microwave extraction, certain drawbacks were associated with URE. No higher temperature (55 °C) is required, while the significant drawback of this process is the non-uniformity in the strength of ultrasound radiation [83].
4 Tannins
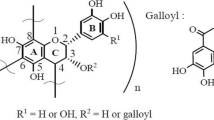
Tannins, as in Fig. 5, are a chemically distinct group of water-soluble phenol that bind proteins to form soluble or insoluble complexes. Depending on the chemical structure tannins are, catalogued into two classes, condensed and hydrolysable tannins or flavonoid.
Proanthocyanidins (condensed tannins) formed by the condensation of flavans, and hydrolysable tannins (decomposable in water) are gallic acid. Decomposition of hydrolysable tannins forms hexahydroxydiphenic acid (gallic acid) are easily decomposed in water, forming gallic acid or hexahydroxydiphenic acid and polyols.
4.1 Tannin gels (TG)
A stepwise reaction conducted in the presence of an acid or base catalyst attained through reaction with formaldehyde results in tannin insolubilization [43, 84]. Tannin resin or gel (insoluble) synthesized when an aldehyde forms a bond with monomers of tannin, serves as a crosslinking agent for the reaction. Thereby an insoluble tannin polymer (non-linear) acknowledged as tannin resin/gel developed. The sodium hydroxide provided eventually results in the dissolution of tannin extract under basic conditions [84] and high temperature (80 °C) [85], by the simultaneous addition of formaldehyde for 8–12 h [43], followed by and, finally, bathing, dehydration, and separating. Excellent gelation can be achieved generally within 1 and 5.7 mmol formaldehyde per gram of tannin extract [10]. While comparing to formaldehyde, acetaldehyde reveals a flimsy polymerization action. TG’s can be concocted without extraction using vegetables (raw peels). Crosslinking between the hydroxyl groups catalyzes spontaneous condensation reaction by blending with sulphuric for immobilization of tannin from astringent permission.
The carboxylic, phenolic, lactonic, and carbonyl groups [84] latter an acidic surface to the TG, where the potential difference between the adsorbent and liquid characterizes the adsorbent at diverse pH range according to its surface charge. The anionic nature of tannin resins can be endorsed at shallow pH values of approximately around 2 [86] by analyzing the isoelectric point [zero points of charge (ZPC)]. The probability of proton discharge by the phenolic group within the tannin accords an anionic feature by setting up a resonance stabilized negative charge. Phenolic groups present shows the possibility to release protons, which accords them an anionic feature by creating a resonance-stabilized negative charge. Adsorption of cations by the electrostatic force of attraction and an ion-exchange mechanism which involves cationic adsorbates and H+ ions that delivers cations to these requisites. A greater electrostatic attraction is shown by cations without any further modification while comparing to anions.
4.2 Tannin foam
Decisively stirring a bulk of extracted condensed (mimosa-60% and pine extract-40%) tannin together with furfuryl alcohol, formaldehyde-water solution, and water. Diethyl ether and toluene-4-sulphonic acid were added after attaining a homogeneous aggregation of the solution and transferred to a case for foaming followed by continuously stirring for about 10 s. The foaming process yields a foam black in colour, having a density of 0.05 and 0.25 g/cm3 upon rising the temperature to about 40 °C within 2 s. The entrapped blowing agents within the pores get eliminated by keeping for 24 h after cutting off the edge skin. Test samples were prepared according to the required dimensions (20 mm) by precisely trimming. The sample of each test should be homogenous, and the prepared foam precisely cut off into 20 mm cubes for testing with 20, 40, and 80 ppm CuSO4 solution and Pb(NO3)2 prepared with 20, 40, and 80 ppm in a 250 mL flask and an analytical balance. A dilute H2SO4 and NaOH were employed to regulate the solution pH at 5.0. A closed container of 150 mL with corresponding metal ion water solution were prepared, and the specimen of 20 mm × 20 mm × 20 mm size immersed forcedly into the container specimen for about 4 and 8 h. After the filtration of solutions, a solid and a liquid phase obtained by squeezing out the foam where the non-adsorbed metals removed by subsequent washing with 10–20 mL deionized water. Replication of the experiment conducted with 60–40% of pine tannin and 80–20% of mimosa tannin sample foams [87]. The optimum experimental conditions for the synthesis of TRF are illustrated in Table 1.
5 Tannin based adsorbents
5.1 Tannic acid (TA) modified polyvinylidene fluoride (PVDF) microfiltration membrane
The commercially available PVDF membrane subjected to ethanol conditioning, followed by soaking for 5 min into an aqueous solution (20 mg/mL) of TA and thereafter soaked in aqueous NaIO4 solution for the same period. In order to eliminate the unstable resins as well as nitrogen gas, the PVDF substrate modified rinsed several times with deionized water after the dipping process. Van der Waals forces were believed to be accountable for the bonding on the PVDF membrane in accordance with hydrogen bonding. The quinone group keeps on degradation on account of the oxidative cleavage with NaIO4, which is an oxidizing agent adequate to produce quinone groups to earn. Moreover, a reliable oxidizing agent like NaIO4 strong enough to create quinone groups via TA oxidation to yield [88]. For an effective of oil-in-water emulsion separation, a straightforward and facile bioinspired methodology performed that transforms the superhydrophobic PVDF membrane into a super hydrophilic PVDF membrane and oleophobicity properties underwater. That involves oxidation of periodate followed, but the coating a TA layer on the surface of the PVDF membrane (Fig. 6a) [89].
The TA coating procedure lasts for only 10 min while maintaining its super hydrophilicity even after the oil-in-water emulsion process safely after 20 cycles. The increase in the number of OC=O groups within the modified PVDF membrane than TA further points out the oxidative cleavage of NaIO4 ensuing deterioration of quinone to carboxyl functionalities, as in Fig. 6b.
5.2 Gelatin/PVA nanofiber band (GPNB) for uranium adsorption from simulated seawater
Meng et al. proposed a novel bayberry tannin (BT) GPNB composite by electrospinning for uranium(VI) adsorption from simulated seawater. Extent of uranium(VI) adsorption by GPNB-BT gauged through a series of adsorption prepared test accordingly through electrospinning technique that escalates the rate of tannin immobilization as it accommodates fibers in nanoscale size (Fig. 7). 50 mL of uranium solution mixed along with 0.02 g of GPNB-BT at pH 5.5 and followed by jolted in an electric shaker. The mixed solution filtered, and adsorption capacity evaluated as
qe is uranium adsorbed per unit gram in GNPB-BT; C0 represents the initial concentration of uranium; Ce represents the equilibrium concentration of uranium; V indicates volume of solution; M indicates adsorbent mass.
The mechanism involves the formation of hydrogen bonds among the phenolic hydroxyl within BT and the peptide chain of gelatin. Upon the inclusion of glutaraldehyde, a new covalent bonding happens by the consolidation of the amine group of peptide chain and PHG of BT; after that, the highly active hydroxyl group of BTs bonded covalently with an aldehyde group from glutaraldehyde. Chelation ability of PHG towards uranyl ions enhances after BT loading, which ensures more PHG group to the system. GNPB-BT achieved paramount adsorption of uranium(VI) and adsorbed 1.4 µ/g of uranium from simulated seawater even at 3 µg/L of initial concentration. At an optimum pH of 5.5, maximum adsorptions of GNPB-BT were found to be higher than that of GPNB by a value 170 mg g−1 at an initial concentration of 80 mg/L with a 0.02 g adsorbent dosage and contact time for 24 h at 25 °C. Uranium adsorption capacity demonstrated [90] by GPNB-BT established by the immobilization of tannin onto nanofiber that, moreover, improves the rate of adsorption through nanorization, fiber porosity, and rate of immobilization possibly incurred due to active sites and surface area [90]. Significant improvement in the adsorption capability of uranium on GPNB-BT crop ups the permeability that furnishes more binding spots for adsorption of Ur(II) ions while providing great mass transfer. The tendency for complexation ability with uranium concluded to be more excellent for BT, which holds phenolic hydroxyl groups while correlating with another hydroxyl (–OH), carboxyl (COOH), and an amine (–NH2) functional groups in gelatin. The active sites within the adjacent phenolic hydroxyl group of tannins where the complexation reactions take place with uranium ions beget the evolution of stable five-membered ring betwixt uranium and phenolic hydroxyl group [91]. The pH dependence of GPNB-BT can be analyzed and shown to increase adsorption as pH increases Fig. 8.
5.3 Tannin immobilized activated clay (TI-AC) spent adsorbent
The highly deleterious Cr(VI) in water can be separated by using spent adsorbents. Li et al. proposed tannin immobilized activated clay (TI-AC) as a spent adsorbent for hexavalent chromium ion removal from effluents. TI-AC prepared by dissolving 0.3 g of AC in 100 mL of tannin solution at pH 3.5 having desired concentration suffered for 2-h agitation in an electric stirrer, thereby acquiring appropriate equilibrium at room temperature [92] typical mechanism of Cr(VI) adsorption by TI-AC in Fig. 9. Adsorption of Cr(VI) strictly follows pseudo-second-order kinetics can be explained through Freundlich isotherm. As shown in Fig. 9, the chromium ions entrapped within the porous spaces of AC and by the esterification of the phenolic group, which attracts anionic forms of chromium. Optimum adsorption pH found to be at pH 2.5 [93], where < 2.5 pH almost all ions get disappeared. H+ concentration patronage adsorption of Cr(VI) ions, whereas higher OH+ concentration inhibits adsorption rate. A series of experiments proposed that TI-AC ascertain splendid adsorption capability in the case of chromium (24.09) at 2.5 pH 1 [92].
5.4 Bayberry tannin immobilized collagen fiber (BTICF)
Collagen fiber (protein fiber), well known for its hydrophilicity [94] mostly extracted from animal skin, preferred to immobilize with tannin covalently by crosslinking with an aldehyde and the BTICF obtained possess exceptional hydrophilic character propitious for fast adsorption rate along with exclusive fibrous structure. Leakage of tannin might occur during the adsorption phenomenon of metals in other procedures is prevented as the tannin, and the collagen fibers are covalently cross-linked. Adsorption behavior of BTICF towards mercury (Hg(II)) had been investigated. BTICF prepared by incorporating 15.0 g of collagen fiber into a previously prepared solution of BT (9.0 g) in 300 mL of deionized water followed by stirring for 24 h at 24.85 °C. After the filtration and washing, 7-ethylic-oxazolidine (2% w/w) 300 mL were mixed and stirred for 1 h at 298 K, continued by stirring for further 4 h at 323 K and subsequent rinsing in water and dried under vacuum for 12 h yields BTICF adsorbent [95].
The capability of BTICF for Hg(II) adsorption has been depicted in Fig. 10 [96], the pyrogallols structure associated with BT with its high electrophilic reaction ability exhibit greater metal ion affinity. The reaction capability of BT over metal ion such as mercury improved due to the attachment galloyl group. Hg adsorption on BTICF takes place by the chelation of BT by adjacent phenolic hydroxyl group within pyrogallol structure on Hg(II) ion.
The Hg(II) adsorption on to of BTICF carried out by a chelation reaction occurs across adjacent phenolic hydroxyl groups within BT and Hg(II) (Fig. 11). Excellent adsorption of Hg(II) (198.49 mg/g where the initial concentration of Hg was 200.00 mg/L) at 303 K temperature and pH 7 befell due to the existence of BTICF in fiber state and position of BT within the collagen fiber, i.e., in the outer space results in faster adsorption [97].
5.5 CNT incorporated tannin formaldehyde resin
The incorporation of carbon nanotubes (CNT) and coconut fibers to mimosa tannin (Acacia mearnsii) and formaldehyde to fabricate tannin–formaldehyde resin (MTCNT-FR) for the adsorption of lead from water (Fig. 12) through a polycondensation reaction where the multiwalled CNT (MWCNT) and coconut fibers were supplied at the gel point at a temperature of 20 °C ± 1 °C.
Luzardo implemented a three levels practice: first as a factorial experiment, second as quadratic factorial and third as incremental addition of CNT to evaluate the difference in the adsorption capacity observe of these systems. Accordingly, the progressive addition of CNT results that with an increase in the nanotube addition, an improvement in the adsorption capacity (79%) of the resin can be observed [98]. Considering the above mentioned three experiments, the resin prepared with an optimum combination range (tannin-59%, HCL-3%, coconut fibre-5%, formaldehyde-29%, and CNT-4) shows the maximum adsorption. Figure 13a, shows a rise in the removal rate of lard in every time, which reaches 90% at the optimum condition. It has been ascribed that the inclusion of CNT and coconut fibers into tannin formaldehyde resin enhances the lead removal rate to a maximum level of 90%, where the pH range of maximum adsorption capacity reached in between 3.5 and 5 (Fig. 13b) during 24 h and Langmuir model reaches an adsorption range more than 13.8 mg g−1 followed by pseudo-second-order kinetics [98].
6 Removal of heavy metals and toxic metalloids
Cr3+ [99, 100], Pb2+ [101], Cu2+ [101, 102], Zn2+ [43, 101], and Ni2+ [103] like heavy metals ions under aqueous conditions were sophisticatedly attracted by TG. To evade the precipitation of metals at the basic condition to handle effluents in electroplating enterprise, the adsorption of the adsorbates is ordinarily estimated at acidic pH where these metals exist as positively charged ions particularly as double-charged metal (M2+). In an account of heavy metal ions, dismissal from wastewater observed that the tannin resins could do an adsorption capacity of 0.4–1.0 mmol g from various vegetable sources. Evidence states shreds of evidence that ion exchange and complexation are the methodologies commonly employed for the uptake of metals. Catechol is commonly established metal complexes with two adjacent hydroxyl groups, but enhanced stability attained in pyrogallols (with a one more hydroxyl group) [104]. The higher electrostatic attraction between the negative bisorbent surface and positive adsorbate and greater complexation ability enhance with pH as a result of dissociation of the surface-active groups. The maximum removal of metals such as Ni(II), Pb(II), Zn(II), and Cu(II), witnessed in acidic zones with an increase in pH of metals at a pH around 4 or 5 [86]. It is evident from the competition adsorption studies that the higher affinity was disclosed by valonia tannin resin for lead than other metal ions like solutions of Pb(II), Zn(II), and Cu(II). The affinity is as follows (Pb2+ > Cu2+ > Zn2+) [101].Chromium exists in two oxidation states in aqueous solutions Cr(VI) and Cr(III), where high toxicity [101] of hexavalent forms ascribed to their carcinogenicity and mutagenicity to living organisms have got attention over time. The adsorption characteristics of Cr(III) species in the at acidic pH solutions like CrOH2, Cr(OH) 5+4 , Cr3+, so they adsorb metal ions like lead, zinc, copper, and nickel. Huang et al. [105] reported that the Cr(III) eradication increased with an increased pH of 2.5–5.5 utilizing tannin immobilized silica beads(mesoporous structure) where the Cr(III) chelating interaction increases with an increase in pH above the rate as the pHZPC of the bisorbent was reported as 4.01. Cr(VI) exists as CrO4, or (CrO4)2 species in aqueous solutions, having pH nearly neutral so that electrostatic attraction of anionic family towards the surface not happened in the case where the surface pHZPC of tannin (nearly pH 2) is below the pH conditions. However, Cr(VI) displays a perfect adsorption rate at a very low pH (pH 1–2). A very high Cr(VI) adsorption interpreted by Rodrigues et al. [84] at pH 1as per the electrostatic attraction on TBA surface (positively charged) and HCrO4 (negatively charged) ion, and while pH increases the adsorption of Cr(VI) seems to be decreased (Fig. 14a). Stunning adsorption of metals has been observed from different tannin resources (5.5–9.4 mmol). The Cr(VI) uptake by mimosa TG (Acacia mollissima) [106, 107] (illustrated in Fig. 14b): (a) chromate esterification reaction with tannin, (b) redox reaction: Cr6+ gets reduced to Cr3+ followed by the oxidation of tannin molecules forming carboxyl group along with (c) Columbic attraction of Cr3+. The oxidation proficiency of chromate was founded at high Cr3+ (1 g L−1) and a very low pH 1. The study of adsorption on Persimmon TG used a low acidic environment of pH 3 and less concentration of chromium of about approximately 10 mg L−1, 0.2 mmol L−1 [108]. The endorsed results with the preceding findings having high efficiency but evidences for Cr3+ adsorbed on the gel was not reported. The aptitude of biosorption of chromium(VI) attained as a part of acid-catalyzed polymerization as a result of selective adsorption test [99] by the aid of a waste persimmon gel. The chromium adsorption was found to be optimum at pH 3 though the chromium selectivity against rest of metals will be greater than Pb2+, Cd2+, Fe3+, and Zn2+ at pH 1.
Cu(II), Cd(II), and Zn(II) adsorption towards tannin-immobilized hydrotalcite (TI-HTC) have been examined through batch experiments along with the effect of pH on adsorption over a pH range of 2–9. The removal efficiency was improved to about 99.2% from 68.3% within 2–3 pH, which remains constant at pH 5 while a decrement can be observed at pH 9 (alkaline range) [109]. The metal ions get adsorbed to TBA by releasing proton (cationic exchange mechanism) from the hydroxyl group present. An improvement in the adsorption is likely due to the exchange of cations (hydrolysis) where the hydroxyl groups get adsorbed than un-complexed cations [110]. The significant mechanism underlying in the removal of metal ions at higher pH range were the formation of aqueous metal hydroxide and ion exchange. Thus, an optimum pH ~ 6 (initial) was chosen to correlate the ion exchange along with the removal rate while conducting experiments.
The stock solution for the extraction of metals from aqueous solution are made of different concentrations. In one study, the solution was prepared by the dissolution of corresponding metal ions in water or HCL having a suitable grade. Furthermore, these solutions were diluted into specified concentrations using HCL solution. In other studies, Tondi et al. have simply prepared 20, 40 and 80 ppm of copper sulfate solution and 20, 40, and 80 ppm lead nitrate solution in a 250 mL container into which the prepared foam later immersed for adsorption. The pH of the solution (pH = 5) was regulated by using diluted H2SO4 and NaOH solution [48]. Nakano et al. [111], in his study, demonstrated Cr(VI) adsorption by TG in a 40 mL laboratory prepared acidic solution containing Cr(VI) shaked (per minute 90 shakes) with TG particles kept over 1 week at 303 K. In another study, TA coated the PVDF membrane tested for their efficiency in carrying metal ions by immersing them into the FeCl3 solution (5 min) and in the AgNO3 solution. Uranium adsorption capability of GPNB-BT demonstrated by Meng et al. [91] by the addition of prepared 50 mL uranium solution having pH 5.5 with an initial concentration of 80 mg/L uranium were thermostatically mixed using a mechanical shaker (rpm-180) followed by filtration. Formulation of seawater, according to Gunathilake et al., with dissolving chemicals like uranyl nitrate (17 mg), sodium bicarbonate (193 mg), and sodium chloride (25.6 g) in ultrapure water (1 L). Dissolving the solution to get a different uranium concentration (3 μg/L, 5 μg/L, 20 μg/L, 50 μg/L, 100 μg/L). the adsorption efficiency of TI-AC towards chromium was established by Li et al. [92], during his study, a precise amount of K2Cr2O7 dissolved in deionized water used as the stock solution. Further dilution with water gives various concentrations, and the pH of the solution was controlled by the addition of NaOH or HCL (0.1 M). The potential use of collagen fiber immobilized tannin for Hg(II) adsorption performed with a stock solution (1000 mb/L) containing Hg(II) prepared by dissolving Hg(NO3)2·(1/2) H2O in deionized water upon dilution gives required concentration [97]. Dissolving 500 mL of lead nitrate solution was prepared for the analysis of lead adsorption property shown by MWCNT-TF resin. A solution having a different concentration of lead was prepared by the dilution of the stock solution having standard Pb(II) concentration (1 g/L). Literature studies with natural waters are not available; however, the scientific community and researchers have mostly explored with the simulated water samples with the required metal ion (Table 2).
7 Stability of TBA
The values at various pH were analyzed to find out if there is any leakage from TBA after several cycles of adsorption. Secondary contamination can be caused by leaching from adsorbent to water by repeated exposure to acidic solvents and temperature. Tannin demonstrates no leakage within 3–8 pH while in higher pH (8.5–10), a negligible amount (5–11%) of tannin can leach out of adsorbent. The small amount of lixiviation may not create any problem since the extraction of metal ions can be possible at pH below 7 (optimum pH) due to its acidic nature. TI-HTC does not display any significant shreds of leaching (up to 11%) after 4 cycles of the adsorption process [109]. Tannic acid upon oxidation produces toxic quinones [112] further contaminates the water and consumption leads to severe side effects. However, no literature is available on leaching of toxic quinones from TBA to water.
8 Effect of interfering ions
The existence of two or more metal ions in an aqueous medium may affect the adsorption rate of the corresponding ion on TBA. Nevertheless, the existence of other metal ions did not affect the adsorption of Hg(II). The adsorption capacity of Hg(II) in BTICF observed to be the same in the presence of co-existing ions like Al(II), Cu(II), Pb(II), Cd(II) and Zn(II). Even after adsorbing 20 mg/g of copper from the aqueous solution, BTICF exhibits no significant change in the adsorption of Hg(II). The adsorption studies revealed that the selective adsorption capability of BTICF towards Hg(II) in aqueous solution would be the same. Results were different in terms of chloride ion, which forms charged (ZnCl+, CuCl+, and CdCl+) and uncharged (ZnCl2, CuCl2, and CdCl2) gets adsorbed (weakly) on TBA surfaces because of lower affinity [109]. Therefore, cation adsorption from mixtures having chloride ions hinders the adsorption of cations on TBA.
9 Desorption and reusability
Desorption studies performed under the batch experimental condition and the efficiency of desorption were compared with an initial value to explore the probability of adsorbent regeneration for reuse. During desorption, bonded metal ions leave the surface of the adsorbent and escape into the surrounding. Several eluents (HCL, HNO3, deionized water) can be employed for the desorption of metal ion entrapped within the surface of TBA, where the attachment between ions loaded in the TBA surface gets weakened. Neutral pH water can desorb the Cu(II) and Ni(II) by reducing the bond strength with the adsorbate [113]. TG, along with ions agitated with neutral water, removes 53.85% of Cu(II) and 51.85% of Ni(II) after 2 h [113]. It is found that lactic acid serves as an effective solvent in removing the bonded Hg(II) from BTICF. They observed that the desorbed Hg(II) concentration was 10 times than comparing the original solution. The desorbed BTICF is able to adsorb Hg(II) 4 times with the same efficiency while recycled without impacting the adsorption rate discloses potential application as reusable and inexpensive adsorbent material for Hg(II) expulsion from aqueous solutions [97]. Subsequent washing of tannin resin with HNO3, H2SO4 removes adsorbed Ag+ ions [114]. The adsorbents after desorption of metal ions hired for further adsorption cycles. The adsorption efficiency of TBA remains unaltered for repeated 4 cycles of adsorption experiments indicated the absence of irreversible sites on the TBA surface [97, 114]. Although tannin shows effective desorption of metal ions studies with an adsorption column before implementing an industrial application (large scale) is recommended.
10 Conclusion and future perspectives
In this review, tannin adsorbents adequacy in removing toxic heavy metal ions and dyes from wastewater have been reviewed. Natural polyphenols, along with their structures, classification, extraction from plants, are also addressed. Among the various extraction methods, microwave-assisted extraction was more selective to extract polyphenols and tannins and shown to produce the best polyphenols (condensed or hydrolysable tannins) using methanol as the solvent. For the cleaning-up of several wastewater and effluents, TRF represents a new adsorbent material. Since their preparation, not ultimately mandated under a strict protocol. TRF can anionic take off anionic detergents, dyes, and several toxic metal ions. TRF can adsorb copper (12.5%) and lead (20.1%) ions. The overall anionic nature of tannin resin revealed its stunning capacity to attract cationic breeds such as heavy metal ions. Although tannin alone exhibit adsorption property while tannin implanted with other materials has displayed higher efficiency than simple tannin. CNT can be quickly immobilized into mimosa tannin formaldehyde resin confessed maximum adsorption of greater than 13.8 mg g−1 around 3.5–5.5 pH followed by a pseudo-second-order mechanism. The chelation process stumbles between adjacent phenolic hydroxyl members therein BT and Hg(II) culpable for adsorption of Hg(II). BTICF parades a wide pH adsorption range of 4.0–9.0 owing to a maximum adsorption capacity for Hg(II) 198.49 mg g−1 estimated through a pseudo-first-order equation. Cr(VI) a deleterious metal ion can be detached from water effectively by freezing AC on to tannin as spent adsorbent with 24.09 mg g−1 of adsorption accompanied by pseudo second-order mechanism. The kinetics of adsorption—pseudo first order and pseudo-second-order, adsorption isotherms—Langmuir and Freundlich were also analyzed for adsorption of metals ions and dyes in this survey along with the thermodynamics for the feasibility studies. The adsorption efficiency of TBA remains unaltered for repeated 4 cycles of adsorption without leaching. From this review, it is evident that the tannin, being water-soluble phenol, has the prodigious ability to detach toxic heavy metals and dyes dexterously, thereby make a promising system for wastewater purification. The future of tannins as adsorbents relies on the progressive complexation ability with polymers and fibrous materials for enhanced absorptivity. More research could be done on the adsorption kinetics and thermodynamics of tannin biosorbent as well as the desorption of metals from adsorbents in the case of precious metals. Literature studies stated that no studies were carried out using natural water; experiment results were based on the simulated stock solution. There is still space for research including natural water testing with TBA, quinone and organic carbon leaching, and competing ion effects that have not yet been reported. Tannin owing to its low cost and better efficiency in the removal of contaminants from aqueous solutions used as a novel adsorbent material. Nevertheless, its application was not used on a wide scale; however, the introduction of TBA’s in many effluent treatment plants would be a viable approach. Our studies focus on reusing of adsorbent material without affecting the adsorption rate even after multiple cycles of the adsorption process will also be an enticing area of research.
References
Lea A (2008) Analysis of polyphenol antioxidants in fortified foods and supplements. In: Ottaway PB (ed) Food fortification and supplementation. Elsevier, Amsterdam, pp 175–194. https://doi.org/10.1533/9781845694265.2.175
Quideau S, Deffieux D, Douat-Casassus C, Pouységu L (2011) Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed 50:586–621. https://doi.org/10.1002/anie.201000044
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2:270–278. https://doi.org/10.4161/oxim.2.5.9498
Haslam E, Cai Y (1994) Plant polyphenols (vegetable tannins): gallic acid metabolism. Nat Prod Rep 11:41–66. https://doi.org/10.1039/NP9941100041
Abbas M, Saeed F, Anjum FM, Afzaal M, Tufail T, Bashir MS, Ishtiaq A, Hussain S, Suleria HAR (2017) Natural polyphenols: an overview. Int J Food Prop 20:1689–1699. https://doi.org/10.1080/10942912.2016.1220393
Rice-Evans C (2012) Flavonoid antioxidants. Curr Med Chem 8:797–807. https://doi.org/10.2174/0929867013373011
Agarwal AK (2007) Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog Energy Combust Sci 33:233–271. https://doi.org/10.1016/j.pecs.2006.08.003
Sánchez-Martín J, Beltrán-Heredia J, Delgado-Regaña A, Rodríguez-González MA, Rubio-Alonso F (2013) Adsorbent tannin foams: new and complementary applications in wastewater treatment. Chem Eng J 228:575–582. https://doi.org/10.1016/j.cej.2013.05.009
Gonte R, Balasubramanian K (2016) Heavy and toxic metal uptake by mesoporous hypercrosslinked SMA beads: isotherms and kinetics. J Saudi Chem Soc 20:S579–S590. https://doi.org/10.1016/j.jscs.2013.04.003
Gurung M, Adhikari BB, Morisada S, Kawakita H, Ohto K, Inoue K, Alam S (2013) N-Aminoguanidine modified persimmon tannin: a new sustainable material for selective adsorption, preconcentration and recovery of precious metals from acidic chloride solution. Bioresour Technol 129:108–117. https://doi.org/10.1016/j.biortech.2012.11.012
Gonte RR, Balasubramanian K, Mumbrekar JD (2013) Porous and cross-linked cellulose beads for toxic metal ion removal: Ur(II) ions. J Polym 2013:1–9. https://doi.org/10.1155/2013/309136
Ayalew A, Gonte RR, Balasubramanian K (2012) Development of polymer composite beads for dye adsorption. Int J Green Nanotechnol 4:440–454. https://doi.org/10.1080/19430892.2012.739480
Gonte RR, Shelar G, Balasubramanian K (2014) Polymer–agro-waste composites for removal of Congo red dye from wastewater: adsorption isotherms and kinetics. Desalin Water Treat 52:7797–7811. https://doi.org/10.1080/19443994.2013.833876
Gonte RR, Balasubramanian K (2012) Chemically modified polymer beads for sorption of gold from waste gold solution. J Hazard Mater 217–218:447–451. https://doi.org/10.1016/j.jhazmat.2012.03.020
Gonte R, Balasubramanian K, Deb PC, Singh P (2012) Synthesis and characterization of mesoporous hypercrosslinked poly(styrene co-maleic anhydride) microspheres. Int J Polym Mater Polym Biomater 61:919–930. https://doi.org/10.1080/00914037.2011.610057
Raj RBA, Gonte RR, Balasubramanian K (2017) Dual functional styrene–maleic acid copolymer beads: toxic metals adsorbent and hydrogen storage. In: Khalid S, Shahid M, Niazi NK, Rafiq M, Bakhat HF, Imran M, Abbas T, Bibi I, Dumat C (eds) Enhancing cleanup of environmental pollutants. Springer International Publishing, Cham, pp 255–295
Gore PM, Khurana L, Dixit R, Balasubramanian K (2017) Keratin-Nylon 6 engineered microbeads for adsorption of Th(IV) ions from liquid effluents. J Environ Chem Eng 5:5655–5667. https://doi.org/10.1016/j.jece.2017.10.048
Khurana L, Balasubramanian K (2016) Adsorption potency of imprinted starch/PVA polymers confined ionic liquid with molecular simulation framework. J Environ Chem Eng 4:2147–2154. https://doi.org/10.1016/j.jece.2016.03.032
Gore PM, Khurana L, Siddique S, Panicker A, Kandasubramanian B (2018) Ion-imprinted electrospun nanofibers of chitosan/1-butyl-3-methylimidazolium tetrafluoroborate for the dynamic expulsion of thorium(IV) ions from mimicked effluents. Environ Sci Pollut Res 25:3320–3334. https://doi.org/10.1007/s11356-017-0618-6
Gautam A, Gore PM, Kandasubramanian B (2020) Nanocluster materials in photosynthetic machines. Chem Eng J 385:123951. https://doi.org/10.1016/j.cej.2019.123951
Gore PM, Purushothaman A, Naebe M, Wang X, Kandasubramanian B (2019) Nanotechnology for oil–water separation. In: Prasad R, Karchiyappan T (eds) Advanced research in nanosciences for water technology. Springer, Cham, pp 299–339. https://doi.org/10.1007/978-3-030-02381-2_14
Rajhans A, Gore PM, Siddique SK, Kandasubramanian B (2019) Ion-imprinted nanofibers of PVDF/1-butyl-3-methylimidazolium tetrafluoroborate for dynamic recovery of europium(III) ions from mimicked effluent. J Environ Chem Eng 7:103068. https://doi.org/10.1016/j.jece.2019.103068
Gore PM, Dhanshetty M, Balasubramaniam K (2016) Bionic creation of nano-engineered Janus fabric for selective oil/organic solvent absorption. RSC Adv 6:111250–111260. https://doi.org/10.1039/C6RA24106A
Gore P, Khraisheh M, Kandasubramanian B (2018) Nanofibers of resorcinol–formaldehyde for effective adsorption of As(III) ions from mimicked effluents. Environ Sci Pollut Res 25:11729–11745. https://doi.org/10.1007/s11356-018-1304-z
Gore PM, Zachariah S, Gupta P, Balasubramanian K (2016) Multifunctional nano-engineered and bio-mimicking smart superhydrophobic reticulated ABS/fumed silica composite thin films with heat-sinking applications. RSC Adv 6:105180–105191. https://doi.org/10.1039/c6ra16781k
Gore PM, Kandasubramanian B (2018) Heterogeneous wettable cotton based superhydrophobic Janus biofabric engineered with PLA/functionalized-organoclay microfibers for efficient oil–water separation. J Mater Chem A 6:7457–7479. https://doi.org/10.1039/C7TA11260B
Gore PM, Naebe M, Wang X, Kandasubramanian B (2019) Silk fibres exhibiting biodegradability and superhydrophobicity for recovery of petroleum oils from oily wastewater. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121823
Gore PM, Naebe M, Wang X, Kandasubramanian B (2019) Progress in silk materials for integrated water treatments: fabrication, modification and applications. Chem Eng J 374:437–470. https://doi.org/10.1016/j.cej.2019.05.163
Gupta P, Lapalikar V, Kundu R, Balasubramanian K (2016) Recent advances in membrane based waste water treatment technology: a review. Energy Environ Focus 5:241–267. https://doi.org/10.1166/eef.2016.1227
Gupta P, Kandasubramanian B (2017) Directional fluid gating by Janus membranes with heterogeneous wetting properties for selective oil–water separation. ACS Appl Mater Interfaces 9:19102–19113. https://doi.org/10.1021/acsami.7b03313
Rastogi S, Kandasubramanian B (2019) Progressive trends in heavy metal ions and dyes adsorption using silk fibroin composites. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-07280-7
Thakur K, Kandasubramanian B (2019) Graphene and graphene oxide-based composites for removal of organic pollutants: a review. J Chem Eng Data 64:833–867. https://doi.org/10.1021/acs.jced.8b01057
Sharma G, Kandasubramanian B (2020) Molecularly imprinted polymers for selective recognition and extraction of heavy metal ions and toxic dyes. J Chem Eng Data 65:396–418. https://doi.org/10.1021/acs.jced.9b00953
Rastogi S, Sharma G, Kandasubramanian B (2020) Nanomaterials and the environment. In: Hussain CM (ed) The ELSI handbook of nanotechnology, 1st edn. Scrivener Publishing LLC, pp 1–23. https://doi.org/10.1002/9781119592990.ch1
Rastogi S, Kandasubramanian B (2020) Processing trends of silk fibers: silk degumming, regeneration and physical functionalization. J Text Inst. https://doi.org/10.1080/00405000.2020.1727269
Bhalara PD, Punetha D, Balasubramanian K (2014) A review of potential remediation techniques for uranium(VI) ion retrieval from contaminated aqueous environment. J Environ Chem Eng 2:1621–1634. https://doi.org/10.1016/j.jece.2014.06.007
Bhalara PD, Punetha D, Balasubramanian K (2015) Kinetic and isotherm analysis for selective thorium(IV) retrieval from aqueous environment using eco-friendly cellulose composite. Int J Environ Sci Technol 12:3095–3106. https://doi.org/10.1007/s13762-014-0682-0
Bhalara PD, Balasubramanian K, Banerjee BS (2015) Spider-web textured electrospun composite of graphene for sorption of Hg(II) ions. Mater Focus 4:154–163. https://doi.org/10.1166/mat.2015.1232
Mishra P, Balasubramanian K (2014) Nanostructured microporous polymer composite imprinted with superhydrophobic camphor soot, for emphatic oil-water separation. RSC Adv 4:53291–53296. https://doi.org/10.1039/c4ra07410f
Balasubramanian K, Sharma S, Badwe S, Banerjee B (2015) Tailored non-woven electrospun mesh of poly-ethyleneoxide-keratin for radioactive metal ion sorption. J Green Sci Technol 2:10–19. https://doi.org/10.1166/jgst.2015.1032
Rule P, Balasubramanian K, Gonte RR (2014) Uranium(VI) remediation from aqueous environment using impregnated cellulose beads. J Environ Radioact 136:22–29. https://doi.org/10.1016/j.jenvrad.2014.05.004
Valko M, Morris H, Cronin M (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208. https://doi.org/10.2174/0929867053764635
Sánchez-Martín J, Beltrán-Heredia J, Gibello-Pérez P (2011) Adsorbent biopolymers from tannin extracts for water treatment. Chem Eng J 168:1241–1247. https://doi.org/10.1016/j.cej.2011.02.022
Santos SCR, Bacelo HAM, Boaventura RAR, Botelho CMS (2019) Tannin-adsorbents for water decontamination and for the recovery of critical metals: current state and future perspectives. Biotechnol J 14:1900060. https://doi.org/10.1002/biot.201900060
Lacoste C, Basso MC, Pizzi A, Laborie MP, Celzard A, Fierro V (2013) Pine tannin-based rigid foams: mechanical and thermal properties. Ind Crops Prod 43:245–250. https://doi.org/10.1016/j.indcrop.2012.07.039
Lee CS, Robinson J, Chong MF (2014) A review on application of flocculants in wastewater treatment. Process Saf Environ Prot 92:489–508. https://doi.org/10.1016/j.psep.2014.04.010
Aguilar-Miranda ED, Lopez MG, Escamilla-Santana C, Barba de la Rosa AP (2002) Characteristics of maize flour tortilla supplemented with ground Tenebrio molitor larvae. J Agric Food Chem 50:192–195. https://doi.org/10.1021/jf010691y
Tondi G, Oo CW, Pizzi A, Trosa A, Thevenon MF (2009) Metal adsorption of tannin based rigid foams. Ind Crops Prod 29:336–340. https://doi.org/10.1016/j.indcrop.2008.06.006
Job N, Sabatier F, Pirard JP, Crine M, Léonard A (2006) Towards the production of carbon xerogel monoliths by optimizing convective drying conditions. Carbon N Y 44:2534–2542. https://doi.org/10.1016/j.carbon.2006.04.031
Tondi G, Fierro V, Pizzi A, Celzard A (2009) Tannin-based carbon foams. Carbon N Y 47:1480–1492. https://doi.org/10.1016/j.carbon.2009.01.041
Martinez VD, Vucic EA, Becker-Santos DD, Gil L, Lam WL (2011) Arsenic exposure and the induction of human cancers. J Toxicol 2011:1–13. https://doi.org/10.1155/2011/431287
Ratnaike RN (2003) Acute and chronic arsenic toxicity. Postgrad Med J 79:391–396. https://doi.org/10.1136/pmj.79.933.391
Mudgal V, Madaan N, Mudgal A, Singh RB, Mishra S (2010) Effect of toxic metals on human health. Open Nutraceuticals J 3:94–99. https://doi.org/10.2174/18763960010030100094
Nordberg GF, Nordberg M (1988) Biological monitoring of cadmium. In: Clarkson TW, Friberg L, Nordberg GF, Sager PR (eds) Biological monitoring of toxic metals. Springer US, Boston, pp 151–168
Chatterjee S (2015) Chromium toxicity and its health hazards. Int J Adv Res 3:167–172
Zahir F, Rizwi SJ, Haq SK, Khan RH (2005) Low dose mercury toxicity and human health. Environ Toxicol Pharmacol 20:351–360. https://doi.org/10.1016/j.etap.2005.03.007
Berlin M, Ullberg S (1963) Accumulation and retention of mercury in the mouse. Arch Environ Health Int J 6:602–609. https://doi.org/10.1080/00039896.1963.10663448
Eggleston DW, Nylander M (1987) Correlation of dental amalgam with mercury in brain tissue. J Prosthet Dent 58:704–707. https://doi.org/10.1016/0022-3913(87)90424-0
Crespo-López ME, Macêdo GL, Pereira SID, Arrifano GPF, Picanço-Diniz DLW, do Nascimento JLM, Herculano AM (2009) Mercury and human genotoxicity: critical considerations and possible molecular mechanisms. Pharmacol Res 60:212–220. https://doi.org/10.1016/j.phrs.2009.02.011
Salonen JT, Seppänen K, Nyyssönen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R (1995) Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 91:645–655. https://doi.org/10.1161/01.CIR.91.3.645
Siblerud RL, Motl J, Kienholz E (1994) Psychometric evidence that mercury from silver dental fillings may be an etiological factor in depression, excessive anger, and anxiety. Psychol Rep 74:67–80. https://doi.org/10.2466/pr0.1994.74.1.67
Kalia K, Flora SJS (2005) Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 47:1–21. https://doi.org/10.1539/joh.47.1
Goyer RA (1993) Lead toxicity: current concerns. Environ Health Perspect 100:177–187. https://doi.org/10.1289/ehp.93100177
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5:47–58. https://doi.org/10.2478/v10102-012-0009-2
Lockitch G (1993) Perspectives on lead toxicity. Clin Biochem 26:371–381. https://doi.org/10.1016/0009-9120(93)90113-K
Renner R (2010) Exposure on tap: drinking water as an overlooked source of lead. Environ Health Perspect. https://doi.org/10.1289/ehp.118-a68
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:317–333. https://doi.org/10.1111/j.1753-4887.1998.tb01670.x
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S. https://doi.org/10.1093/jn/130.8.2073S
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747. https://doi.org/10.1093/ajcn/79.5.727
Belščak-Cvitanović A, Durgo K, Huđek A, Bačun-Družina V, Komes D (2018) Overview of polyphenols and their properties. In: Galanakis CM (ed) Polyphenols: properties, recovery, and applications. Elsevier, Amsterdam, pp 3–44
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306. https://doi.org/10.1080/1040869059096
Spencer JPE, Abd El Mohsen MM, Minihane A-M, Mathers JC (2008) Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr 99:12–22. https://doi.org/10.1017/S0007114507798938
Bouras M, Chadni M, Barba FJ, Grimi N, Bals O, Vorobiev E (2015) Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind Crops Prod 77:590–601. https://doi.org/10.1016/j.indcrop.2015.09.018
Shao P, He J, Sun P, Zhao P (2012) Analysis of conditions for microwave-assisted extraction of total water-soluble flavonoids from Perilla Frutescens leaves. J Food Sci Technol 49:66–73. https://doi.org/10.1007/s13197-011-0265-8
Routray W, Orsat V (2012) Microwave-assisted extraction of flavonoids: a review. Food Bioprocess Technol 5:409–424. https://doi.org/10.1007/s11947-011-0573-z
Fraga-Corral M, García-Oliveira P, Pereira AG, Lourenço-Lopes C, Jimenez-Lopez C, Prieto MA, Simal-Gandara J (2020) Technological application of tannin-based extracts. Molecules 25:1–27. https://doi.org/10.3390/molecules25030614
Li Y, Skouroumounis GK, Elsey GM, Taylor DK (2011) Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem 129:570–576. https://doi.org/10.1016/j.foodchem.2011.04.068
Ngaha Njila MI, Mahdi E, Massoma Lembe D et al (2017) Review on extraction and isolation of plant secondary metabolites. In: May 22–24, 2017 Kuala Lumpur (Malaysia) ICLTET-2017, ACBES-2017. International Institute of Engineers, Kuala Lumpur (Malaysia), pp 67–72. https://doi.org/10.15242/iie.c0517024
Bhadoriya U, Tiwari S, Mourya M, Ghule S (2011) Microwave-assisted extraction of flavonoids from Zanthoxylum budrunga W. Optimization of extraction process introduction. Asian J Pharm Life Sci 1:81–86
Bendicho C, Lavilla I (2018) Ultrasound extractions. In: Reference module in chemistry, molecular sciences and chemical engineering. Elsevier, Amsterdam, pp 1–9. https://doi.org/10.1016/B978-0-12-409547-2.04571-6
Mandal SC, Mandal V, Das AK (2015) Classification of extraction methods. In: Mandal SC, Mandal V, Das AK (eds) Essentials of botanical extraction. Elsevier, Amsterdam, pp 83–136
Dhanani T, Shah S, Gajbhiye NA, Kumar S (2017) Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab J Chem 10:S1193–S1199. https://doi.org/10.1016/j.arabjc.2013.02.015
Khoddami A, Wilkes M, Roberts T (2013) Techniques for analysis of plant phenolic compounds. Molecules 18:2328–2375. https://doi.org/10.3390/molecules18022328
Alvares Rodrigues L, Koibuchi Sakane K, Alves Nunes Simonetti E, Patrocínio Thim G (2015) Cr total removal in aqueous solution by PHENOTAN AP based tannin gel (TFC). J Environ Chem Eng 3:725–733. https://doi.org/10.1016/j.jece.2015.04.006
Fan Z, Liu B, Wang J, Zhang S, Lin Q, Gong P, Ma L, Yang S (2014) A novel wound dressing based on Ag/graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv Funct Mater 24:3933–3943. https://doi.org/10.1002/adfm.201304202
Yurtsever M, Şengil İA (2009) Biosorption of Pb(II) ions by modified quebracho tannin resin. J Hazard Mater 163:58–64. https://doi.org/10.1016/j.jhazmat.2008.06.077
Sánchez-Martín J, Beltrán-Heredia J, Delgado-Regaña A, Rodríguez-González MA, Rubio-Alonso F (2013) Optimization of tannin rigid foam as adsorbents for wastewater treatment. Ind Crops Prod 49:507–514. https://doi.org/10.1016/j.indcrop.2013.05.029
Ong C, Shi Y, Chang J, Alduraiei F, Wehbe N, Ahmed Z, Wang P (2019) Tannin-inspired robust fabrication of superwettability membranes for highly efficient separation of oil-in-water emulsions and immiscible oil/water mixtures. Sep Purif Technol 227:115657. https://doi.org/10.1016/j.seppur.2019.05.099
Xu G, Pranantyo D, Zhang B, Xu L, Neoh K-G, Kang E-T (2016) Tannic acid anchored layer-by-layer covalent deposition of parasin I peptide for antifouling and antimicrobial coatings. RSC Adv 6:14809–14818. https://doi.org/10.1039/C5RA23374G
Sun X, Huang X, Liao X, Shi B (2010) Adsorptive recovery of UO22+ from aqueous solutions using collagen–tannin resin. J Hazard Mater 179(1–3):295–302. https://doi.org/10.1016/j.jhazmat.2010.03.002
Meng J, Lin X, Zhou J, Zhang R, Chen Y, Long X, Shang R, Luo X (2019) Preparation of tannin-immobilized gelatin/PVA nanofiber band for extraction of uranium(VI) from simulated seawater. Ecotoxicol Environ Saf 170:9–17. https://doi.org/10.1016/j.ecoenv.2018.11.089
Li W, Tang Y, Zeng Y, Tong Z, Liang D, Cui W (2012) Adsorption behavior of Cr(VI) ions on tannin-immobilized activated clay. Chem Eng J 193–194:88–95. https://doi.org/10.1016/j.cej.2012.03.084
Chand R, Narimura K, Kawakita H, Ohto K, Watari T, Inoue K (2009) Grape waste as a biosorbent for removing Cr(VI) from aqueous solution. J Hazard Mater 163:245–250. https://doi.org/10.1016/j.jhazmat.2008.06.084
Ho Y-S (2003) Comment on “Adsorption of fluoride, phosphate, and arsenate ions on a new type of ion exchange fiber”, by R.X. Liu, J.L. Guo, and H.X. Tang. J Colloid Interface Sci 262:307–308. https://doi.org/10.1016/S0021-9797(03)00173-5
Liao X, Zhang M, Shi B (2004) Collagen-fiber-immobilized tannins and their adsorption of Au(III). Ind Eng Chem Res 43:2222–2227. https://doi.org/10.1021/ie0340894
Xu M, Wang H, Lei D, Qu D, Zhai Y, Wang Y (2013) Removal of Pb(II) from aqueous solution by hydrous manganese dioxide: adsorption behavior and mechanism. J Environ Sci 25:479–486. https://doi.org/10.1016/S1001-0742(12)60100-4
Huang X, Liao X, Shi B (2009) Hg(II) removal from aqueous solution by bayberry tannin-immobilized collagen fiber. J Hazard Mater 170:1141–1148. https://doi.org/10.1016/j.jhazmat.2009.05.086
Luzardo FHM, Velasco FG, Correia IKS, Silva PMS, Salay LC (2017) Removal of lead ions from water using a resin of mimosa tannin and carbon nanotubes. Environ Technol Innov 7:219–228. https://doi.org/10.1016/j.eti.2017.03.002
Inoue K, Paudyal H, Nakagawa H, Kawakita H, Ohto K (2010) Selective adsorption of chromium(VI) from zinc(II) and other metal ions using persimmon waste gel. Hydrometallurgy 104:123–128. https://doi.org/10.1016/j.hydromet.2010.05.005
Claxton LD, Houk VS, Hughes TJ (1998) Genotoxicity of industrial wastes and effluents. Mutat Res Mutat Res 410:237–243. https://doi.org/10.1016/S1383-5742(98)00008-8
Şengil İA, Özacar M (2009) Competitive biosorption of Pb2+, Cu2+ and Zn2+ ions from aqueous solutions onto valonia tannin resin. J Hazard Mater 166:1488–1494. https://doi.org/10.1016/j.jhazmat.2008.12.071
Şengil İA, Özacar M (2008) Biosorption of Cu(II) from aqueous solutions by mimosa tannin gel. J Hazard Mater 157:277–285. https://doi.org/10.1016/j.jhazmat.2007.12.115
Meethale Kunnambath P, Thirumalaisamy S (2015) Characterization and utilization of tannin extract for the selective adsorption of Ni(II) ions from water. J Chem 2015:1–9. https://doi.org/10.1155/2015/498359
Oladoja NA, Alliu Y, Ofomaja A, Unuabonah IE (2011) Synchronous attenuation of metal ions and colour in aqua stream using tannin–alum synergy. Desalination 271:34–40. https://doi.org/10.1016/j.desal.2010.12.008
Huang X, Liao X, Shi B (2010) Tannin-immobilized mesoporous silica bead (BT–SiO2) as an effective adsorbent of Cr(III) in aqueous solutions. J Hazard Mater 173:33–39. https://doi.org/10.1016/j.jhazmat.2009.08.003
Ma J, Jiang Z, Cao J, Yu F (2020) Enhanced adsorption for the removal of antibiotics by carbon nanotubes/graphene oxide/sodium alginate triple-network nanocomposite hydrogels in aqueous solutions. Chemosphere 242:125188. https://doi.org/10.1016/j.chemosphere.2019.125188
Nakano Y, Takeshita K, Tsutsumi T (2001) Adsorption mechanism of hexavalent chromium by redox within condensed-tannin gel. Water Res 35:496–500
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dye Pigment 51:25–40. https://doi.org/10.1016/S0143-7208(01)00056-0
Anirudhan TS, Suchithra PS (2008) Synthesis and characterization of tannin-immobilized hydrotalcite as a potential adsorbent of heavy metal ions in effluent treatments. Appl Clay Sci 42:214–223. https://doi.org/10.1016/j.clay.2007.12.002
Das NC, Bandyopadhyay M (1992) Removal of copper(II) using vermiculite. Water Environ Res 64:852–857. https://doi.org/10.2175/WER.64.7.2
Nakano Y, Tanaka M, Nakamura Y, Konno M (2000) Removal and recovery system of hexavalent chromium from waste water by tannin gel particles. J Chem Eng Jpn 33:747–752. https://doi.org/10.1252/jcej.33.747
Grabber JH (2008) Mechanical maceration divergently shifts protein degradability in condensed-tannin versus o-quinone containing conserved forages. Crop Sci 48:804–813. https://doi.org/10.2135/cropsci2007.08.0461
Makeswari M (2013) Tannin gel derived from leaves of Ricinus communis as an adsorbent for the removal of Cu(II) and Ni(II) ions from aqueous solution. IJMER 3:3255–3266
Yurtsever M, İaşengı L (2012) Adsorption and desorption behavior of silver ions onto valonia tannin resin. Trans Nonferr Met Soc China 22:2846–2854. https://doi.org/10.1016/S1003-6326(11)61541-0
Acknowledgements
The authors are thankful to Dr. C. P. Ramanarayanan, Vice-Chancellor of DIAT (DU), Pune for the motivation and support. First author would like to acknowledge Dr. B. Srinivasulu, Principal Director & Head, CIPET: Institute of Plastic Technology (IPT), Kochi, for the support. The authors would like to thank Mr. RaviPrakash Magisetty, Mr. Prakash Gore, and Mr. Swaroop Gharde for their persistent technical support throughout the review writing. The authors are thankful to all anonymous reviewers and the Editor, for improving the quality of the revised manuscript by their valuable suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kavitha, V.U., Kandasubramanian, B. Tannins for wastewater treatment. SN Appl. Sci. 2, 1081 (2020). https://doi.org/10.1007/s42452-020-2879-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2879-9