Abstract
Activated carbon (AC) modified with triphenyl phosphine oxide (TPPO) was tested for thorium(IV) adsorption. Different factors affecting the adsorption process such as pH, contact time, modified AC dose, initial thorium concentration, triphenyl phosphine oxide (TPPO) concentration and temperature were investigated to optimize Th(IV) adsorption operating conditions. Kinetic and equilibrium studies of the adsorption process have been studied. The obtained most favorable conditions for Th(IV) adsorption were: pH 3.6, 40 min contact time, 0.5 g modified AC dose, 0.02 M TPPO in toluene and 400 mg/L of thorium concentration at room temperature. The maximum sorption capacity according to Langmuir isotherm reached 71.94 mg/g for modified AC. Adsorption kinetics obeys pseudo-second order kinetic model. Thorium(IV) was efficiently desorbed from the loaded composite using 0.5 M H2SO4 solution. The optimized factors have been carried out to recover thorium(IV) from Abu Rusheid leach liquor, south Eastern Desert, Egypt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Thorium is widely distributed in trace amounts throughout the earth’s crust, occurring mainly within several minerals, such as monazite sands, thorianite, thorite and thoganite [1]. Thorium is three times more abundant than uranium in the earth’s crust. Direct toxicity with thorium is low due to its stability at ambient temperatures [2, 3]. Thorium reactors produce less waste than conventional uranium based ones. Waste solutions bearing thorium can accidently contaminate underground waters, which is considered as an environmental concern [4, 5]. Thorium was used in a variety of applications during the last century. It has been paid more attention recently as a potential nuclear resource due to the large demand of energy. Thorium is an important alloying agent, as it offers alloys greater strength at high temperatures, making it useful in manufacturing jet engines [6]. Thorium dioxide is added to glass to increase its refractive index, producing thoriated glass for use in high-quality camera lenses [7]. 227Th is an alpha-particle emitting isotope that can be used to create localized tumor-killing cancer treatments [8].
Various methods have been developed for separation and utilization of Th(IV). Such methods include bio-sorption [9], liquid–liquid extraction [10] and solid-phase adsorption [11, 12]. Adsorption based on carbon materials, such as activated carbon were used due to their high adsorption capacity as well as elevated thermal and radiation stability [13].
Adsorption is a low cost uptake alternative, which is simple and effective. It is obvious that utilizing adsorbents in metal recovery offers more advantages over liquid–liquid extraction technique. The simplicity of equipment and overall operation and the possibility of regenerating the solid adsorbent is a striking feature of the process. Cost is an important parameter for comparing the adsorbent materials. However, the expense of utilizing individual adsorbents varies depending on the degree of processing required and their availability. Although, the development of chelating ion exchangers is of considerable interest, there are still many difficulties related to their preparation and cost. Due to such difficulties, the concept of using solvent impregnated sorbents has been widely utilized and developed [14, 15].
Activated charcoals possess high degree of surface reactivity due to the favorable pore size distribution, which makes their surface accessible and enhances their adsorption rate without sacrificing their mechanical strength [16]. Due to such advantages, activated carbon materials have found their way in many industrial separation and purification applications, such as the removal of different contaminants from water supplies [17].
The concern of this investigation is to introduce activated carbon modified with TPPO as an effective adsorbent for thorium ions.
2 Experimental
2.1 Materials
All the chemicals and reagents used in this work are of the analytical grade. Thorium stock solution was prepared by dissolving 2.38 g of Th (NO3)4·4H2O in 1000 mL of double-distilled water to prepare 1000 mg/L stock solution, that was diluted into other desired concentrations throughout the study. Thorium concentration was determined photometrically using Arsenazo III complex, [18].
2.2 Apparatus
Thorium, silica, alumina, TiO2, and P2O5 were measured spectrophotometrically using Metertech Inc, model SP-8001, UV–Visible spectrophotometer. Sodium and potassium were determined by a Sherwood flame photometer model 410 (England), using a series of chemical solutions, while CaO, MgO and Fe2O T3 were determined by titration [19]. Trace elements were analyzed using ICP-OES.
The Fourier Transform Infrared Spectrometer (FT-IR) (Thermo Scientific - NICOLET IS10 USA) spectrometer has been used to characterize the major functional groups of AC modified with TPPO before and after adsorption of thorium. Scanning electron microscope (SEM) was used for the specification of the modified AC before and after adsorption of thorium, as well as the final obtained thorium oxide precipitate. The modified activated carbon was identified at laboratories of the Atomic Energy Authority, Anshas, Egypt using X-ray fluorescence (XRF) “JSX-3222 Element Analyzer” with automatic sample changer (JEOL) PW 1510, connected to a computer system using X-40 program for spectroscopy with a detection limit of 5.0 mg/L.
2.3 Preparation of activated carbon
Commercial grade activated carbon was calcinated at 500 °C for 4 h. The powder was stirred with 10% hydrochloric acid solution for 4 h in order to remove any adsorbed species. The residue was filtered off and washed with double distilled water to retain neutral pH. The purified activated carbon was stirred with 10% mixture of [Fe2(SO4)3·5H2O and FeSO4·7H2O] for 3 h and solid/liquid (S/L) ratio 1/5 followed by a rewashing step. The oxidized product was dried in a drying oven at 110 °C for 4 h to enhance the hydroxyl capacity of AC, and (HO-AC) was obtained [20].
2.4 Preparation of modified activated carbon
A series of different concentrations (0.005–0.03 M) of triphenyl phosphine oxide (TPPO) were used to modify activated carbon by dry technique; 0.5 g of activated carbon was stirred with different TPPO in toluene concentrations with S/L ratio (1/10) for 40 min at room temperature till the toluene was evaporated. The modified AC was dried at room temperature.
2.5 Preparation of Abu-Rusheid sample
The studied sample from Abu Rusheid area (south Eastern desert, Egypt) was leached using mixture of acids HF, HNO3, HClO4 and HCl, to determine the percent of major oxides (Table 1). Trace elements (mg/kg) were determined using ICP-OES technique as shown in (Table 1).
2.6 Preparation of Abu-Rusheid leach liquor
The studied sample was subjected to thorium leaching using optimum conditions that verify the best thorium leaching efficiency (90%), while ensuring the minimal dissolution of unwanted impurities. The optimum conditions are − 200 mesh size ground sample, 3 M H2SO4 as a leaching agent, 1/5 solid/liquid ratio, 200 rpm stirring speed for 0.5 h at room temperature. Thorium was measured in filtered leach liquor and found to be 1800 mg/L.
2.7 Adsorption procedures
Batch adsorption studies were conducted by stirring the solution bearing thorium with a certain amount of modified activated carbon for a period of time at constant temperature till equilibrium was achieved. In order to predict the most favorable conditions, such procedures were carried out at different pH values ranging from 1 to 7, different thorium concentrations varying from 50 to 1000 mg/L, different time intervals ranging from 10 to 90 min., different temperatures and different adsorbent doses while keeping the agitation speed constant at 200 rpm. The adsorbent was separated thorium was measured in the filtrate. The thorium uptake capacity (qe, mg/g), adsorption efficiency (E %) and distribution coefficient (Kd) were calculated from the following equations:
where Co and Ce are the initial and equilibrium thorium (IV) concentrations (mg/L), respectively, V is the volume of the aqueous solution (L) and m is the dry resin weight (g).
3 Results and discussions
3.1 Characterization of the synthesized modified activated carbon
3.1.1 Fourier-transform infrared spectrometer characterization
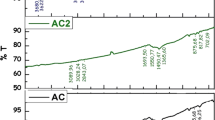
FTIR is a useful tool to identify molecular functional groups [21]. FTIR model Thermo Scientific Nicolet IS10 instrument via the KBR pressed disc method was used in a range starting from 400 to 4000 cm−1 wave numbers. The (FTIR) results of AC modified with TPPO before and after adsorption of thorium are given in (Fig. 1A, B). The obtained (FTIR) results of prepared AC modified with TPPO showed peaks at 3852.96, 3728.69, 3703.62, 3625.37, 3601.97 and 3352.23 cm−1 corresponding to AC-OH stretching. The peaks at 1735.42 to 1654.30 cm−1 corresponding to mono substituted aromatic. The bands 1576.70 and 1503.74 cm−1 are related to C=C ring stretching. While, the bands 1483.25 and 1413.39 cm−1 are attributed to asymmetric CH3 stretching. Two bands 1357.12, and 1332.67 cm−1 are related to (Ar)3–P=O stretching. One peak at 1167.16 cm−1 is related to P=O aromatic stretching. The peaks at 799.93, 720.53, 681.02, 668.88, 657.67 and 617.95 cm−1 are corresponding to out of plane C–H aromatic bending. Finally, peaks at 539.64, 511.30, 473.13, and 463.45 cm−1 are attributed to hydrogen bond out of plane OH (Fig. 1A). While, after adsorption of thorium the peaks at 1413.39, 1357.12, 720.53 and 617.95 cm−1 disappeared and new peak appeared at 1384.10 cm−1. Most bands are shifted which indicated the adsorption of thorium onto AC modified with TPPO (Fig. 1B).
3.1.2 Scanning electron microscope (SEM)
SEM and EDX of the AC modified with TPPO before and after adsorption of thorium were shown in (Fig. 2a, b). Results show the presence of thorium after adsorption onto the modified AC surface.
3.1.3 X-ray fluorescence (XRF)
Both the synthesized and loaded modified AC were identified by X-ray fluorescence (XRF) as shown in (Fig. 3a, b). The results show that thorium was successfully adsorbed onto the surface of the modified AC.
3.1.4 Effect of pH
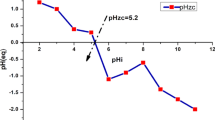
Proton availability is important for metal adsorption nearly by all adsorbents. The pH directly influences the aqueous chemistry of thorium and the properties of the sorbent’s active sites [22]. The retention of thorium(IV) on the prepared modified AC was studied in the pH range from 1 to 7 using 100 mL thorium solution assaying 400 mg/L, 0.5 g modified AC and 200 rpm stirring speed for 40 min at room temp. The obtained results indicated that thorium(IV) adsorption efficiency increases with increasing the pH from 1 to 3.6, where it reaches a maximum of 89% at pH 3.6 then adsorption efficiency decreases with increasing pH up to 7 (Fig. 4). This is due to electrostatic repulsion of the protonated active sites with the positively charged of thorium ion species.
From the obtained results, it is clear that the efficiency of thorium adsorption depends on pH dependent and that maximum adsorption occurs at pH 3.6 due to surface properties of the modified AC as well as the variation of thorium complex species distribution at different pH values [23]. The dominant thorium species at pH 3.6 are Th(OH) 2+2 or Th(OH)3+. The adsorption percent was low at pH values below 3, due to the protonation of residual oxygen-containing groups on the modified AC as well as the competition between H+ and Th4+ ions for the same adsorption sites [24]. However, on increasing the pH beyond 4, hydrolytic precipitation of thorium ions results in declining the adsorption capacity. At pH > 4, thorium precipitates as Th (OH)4. At higher pH values, monomer and polynuclear species of thorium may include Th(OH)3+, Th(OH) +3 , Th(OH) 2+2 , Th2 (OH) 6+2 , Th2 (OH) 4+4 , Th4(OH) 8+8 , and Th6 (OH) 8+14 [25].
3.1.5 Effect of contact time
The effect of contact time was studied while keeping other parameters affecting the adsorption constant. Time was varied from 10 to 90 min. It was found that thorium adsorption efficiency increased with increasing the contact time (Fig. 5). The adsorption efficiency of thorium increased from 55% at 10 min to 89% at 40 min. Sorption reached apparent equilibrium after about 40 min. No significant change in thorium adsorption efficiency was noticed beyond that time, revealing that a state of equilibrium was reached. This indicates that adsorption occurred as monolayer on the surface of the adsorbent. When all the adsorption sites were saturated, the increase in metal ions uptake was seized [26]. Further increase in time encountered a decrease in metal uptake due to intra-particle diffusion processes dominating over adsorption [27].
3.1.6 Effect of triphenyl phosphine oxide (TPPO) concentration
A series of experiments were performed using 0.5 g of activated carbon stirred for 40 min with different concentrations of (0.005 to 0.03 M) TPPO in toluene with S/L ratio (1/10) at room temperature (Fig. 6). The obtained results indicate that thorium adsorption efficiency increased with increasing the concentration of (TPPO) until reaching maximum value at 0.02 M TPPO.
3.1.7 Effect of initial thorium concentration
A series of prepared 100 mL solutions of different initial thorium concentrations ranging from 50 to 1000 mg/L were mixed with 0.5 g of modified AC at pH 3.6 and 200 rpm stirring speed for 40 min at room temperature. The effect of initial thorium concentration is expressed in terms of uptake capacity (qe) (Fig. 7). Accordingly, 400 mg/L of thorium was found optimum to yield maximum uptake capacity, (qe = 71.2 mg/g for AC modified with TPPO).
Increasing thorium concentration results in higher molecular competition on the available surface active sites on the surface of the adsorbent, thus, leading to a decrease in the overall adsorption efficiency [28].
3.1.8 Effect of temperature
Several experiments were performed at temperatures ranging from 25 to 70 °C. Other parameters were fixed at 400 mg/L thorium, pH 3.6 and 200 rpm stirring speed for 40 min. The maximum adsorption efficiency was 89% at 25 °C and dropped down to 50% at 70 °C, (Fig. 8). Such results show that room temperature is the most favorable temperature for thorium adsorption by the modified AC.
3.1.9 Effect of modified AC dose
Different amounts of modified AC ranging from 0.1 to 1 g were mixed with 100 mL of thorium solution at optimum conditions. Thorium adsorption efficiency increased from 55% with 0.1 g to reach 89% with 0.5 g, (Fig. 9). Furthermore, the adsorption efficiency decreased by increasing the adsorbent dose. At low adsorbent doses, all the active sites are available for thorium adsorption. On the other hand, at higher adsorbent amounts, the crowdedness of thorium ions will lead to difficulty to fill the remaining sites due to electrostatic repulsion between thorium ions [29, 30].
3.1.10 Desorption and regeneration of modified activated carbon
Desorption enhances the economic value of the overall adsorption process [31]. Three mineral acids (H2SO4, HNO3, HCl) having the same concentration (1 M) were examined using 100 mL of diluted acid solution to elute thorium loaded on to 0.5 g modified AC for 30 min at room temperature. From (Table 2), sulfuric acid was found the most suitable acid to elute loaded thorium.
In order to determine the optimum H2SO4 concentration, a series of 100 mL of different acid concentration solutions ranging from 0.1 to 1.0 M were mixed with 0.5 g of modified AC at room temperature for 40 min. Table 2 shows that Th(IV) elution efficiency increases from 42% with 0.1 M H2SO4 till 93% at 0.5 M. Further increase in sulfuric acid concentration shows no enhancement to the elution efficiency. The optimum sulfuric acid concentration sufficient to elute Th(IV) efficiently was 0.5 M H2SO4.
Regeneration is an important feature of the studied adsorbent in order to ensure its economic applicability and reuse. The adsorption–desorption cycle was repeated five times with the same loaded adsorbent using 0.5 M H2SO4. Table 3 shows that the adsorption capacity of Th(IV) decreased slightly after 5 regeneration cycles from 71.2 to 68 mg/g, while the adsorption efficiency decreased from 89 to 85%. After the fifth cycle, the adsorption efficiency decreased sharply to 50%. Thus, the studied modified AC possesses excellent reusability features.
3.1.11 Adsorption equilibrium studies
Adsorption isotherms can clearly characterize the interaction pathway of Th(IV) with the modified AC adsorbent. Equilibrium studies are generally used for both the design of sorption process and understanding the sorption mechanism. Thorium ions can be adsorbed onto the surface of a solid support by several mechanisms. The operative mechanism depends on the nature of sorption sites, surface properties, affinities of the sorbent, the type of the sorbate and the bulk properties of the aqueous solution [32].
In the current study, both Langmuir and Freundlich isotherm models were used to correlate experimental data at the determined optimum conditions. Sorption experiments were conducted by mixing 10 mL of solution of an initial thorium concentration 400 mg/L with 0.5 g of the modified AC at pH 3.6 and 200 rpm agitation speed for 40 min at room temperature.
Langmuir adsorption model assumes that adsorption occurs mainly as a saturated monolayer of adsorbate on the surface of the adsorbent. This model assumes too that the energy of adsorption is constant and there is no transmigration of the adsorbed molecules on the adsorbent surface [33]. Langmuir isotherm model can be expressed in the following form:
where Ce is concentration of thorium in solution at equilibrium (mg/L), qe is the amount of thorium adsorbed per weight unit of adsorbent at equilibrium (mg/g), qmax is the saturated monolayer maximum adsorption capacity (mg/g) and b is Langmuir constant (L/mg). The linear plots of Ce/qe versus Ce are shown in (Fig. 10). The maximum calculated adsorption capacity (qmax) was close to experimental data and the correlation coefficient was 0.9977 at room temperature, (Table 4). The value of saturation capacity determined from Langmuir isotherm model was 71.94 mg/g at room temperature which is near to the experimental value of 71.2 mg/g. The tested modified AC showed high sorption capacity compared to other adsorbents [34,35,36,37,38,39,40]. From Langmuir assumptions, thorium uptake has occurred on a homogeneous surface as a monolayer and all the metal binding sites are energetically the same [41].
The dimensionless constant called separation factor; RL, can be used to predict whether the sorption system is favorable or unfavorable. This parameter can be calculated from the following equation [42]:
where b is the Langmuir constant and Co is the initial Th(IV) concentration (mg/L). The value of RL indicates the nature of the isotherm; if (RL = 0) then irreversible, if (0 < RL < 1) then favorable and if (RL > 1) then unfavorable. The obtained data shows that the values of RL are > 1, indicating a favorable sorption process under the conditions applied.
On the other hand, The Freundlich isotherm model assumes that the adsorption process is carried out on a surface of adsorbent with a heterogeneous energetic distribution of active sites, accompanied by interactions between adsorbed molecules [43]. The Freundlich isotherm model is represented by the equation:
where Ce is the equilibrium concentration of thorium in the solution (mg/L), qe is the amount of thorium adsorbed per weight unit of modified AC at equilibrium (mg/g), Kf is the adsorption capacity (mg/g) and 1/n is the Freundlich constant related to the intensity of adsorption (L/mg).
The Freundlich constants Kf and 1/n are calculated from the slope and intercept of the log qe versus log Ce plots (Fig. 11). The adsorption parameters are shown in (Table 4). The value of Kf (mg/g) is lower than the experimental capacity of Th(IV) on modified AC at room temperature. From the obtained data, the calculated by Freundlich isotherm model does not fit with experimental data.
3.1.12 Sorption kinetics studies
Kinetic studies are helpful for the prediction of adsorption rate and gives important information for designing and modeling extraction processes. Two kinetic models including pseudo-first order and pseudo-second order diffusion models were applied to analyze the kinetics of the sorption process. Thorium sorption kinetics was studied at 400 mg/L concentration, pH 3.6 and stirring speed of 200 rpm at room temperature.
The linear form of pseudo-first order kinetic model can be represented by following equation [44]:
where qt and qe are the amounts of thorium ions adsorbed in (mg/g) at time t (min) at equilibrium. The constant k1 is the adsorption rate constant (min−1), which is determined by plotting Log (qe − qt) versus t (Fig. 12).
From the data in (Table 5), the sorption capacity was found 60.64 mg/g, and R2 value was 0.9864. Accordingly, the adsorption processes for the modified AC does not fit a pseudo-first order kinetic model.
Results were also applied to the pseudo-second order kinetic model which is given in the following form [45]:
where k2 is the rate constant (g/mg min). The slope of the straight lines of plots of t/qt versus t stands for 1/qe while the intercept stands for 1/k2q2e (Fig. 13). The calculated value (qe) for modified AC was found to be 77.51 mg/g and the correlation coefficient (R2) = 0.9968, (Table 5). The obtained experimental data fit well with the pseudo-second order kinetic model. Accordingly, the pseudo second order model is the most suitable model to describe the kinetic process of thorium adsorption by the modified AC. This kinetic process is mainly controlled by the chemisorption which involves chemical bonding between thorium ions and the active sites of the modified AC [46, 47].
4 Case study
Obtained sample of Abu Rusheid weighing 1 kg with thorium content 10,000 mg/kg was ground to particle size − 200 mesh and was subjected to acid leaching with 3.0 M H2SO4 with S/L ratio of 1:5 for 30 min at room temperature. The leach liquor was filtered and thorium was measured in solution was found 1800 mg/L; leaching efficiency was 90%. Five liters of leaching solution were stirred with 22.5 g of modified AC at pH 3.6 at 200 rpm stirring speed for 40 min. Thorium(IV) was eluted from the loaded modified AC using 0.5 M H2SO4 acid and was precipitated using sodium hydroxide at pH 1 as thorium hydroxide then dried at 110 °C as thorium oxide powder. The thorium assayed 83% in the product with 94.3% purity. The chemical composition of thorium precipitate was characterized by SEM and EDX analyses, (Fig. 14). Finally, a schematic flow diagram for the process is presented in (Fig. 15). A comparative study [48,49,50,51,52,53,54,55] of thorium uptake capacities (mg/g) of different adsorbents is shown in (Table 6).
5 Conclusion
Activated carbon modified with TPPO was used for thorium(IV) adsorption at optimum conditions: pH 3.6, 200 rpm agitation speed for 40 min at 25 °C. The maximum uptake capacity of the modified AC was 71.94 mg/g. The obtained data obeyed pseudo-second order kinetic model. Langmuir adsorption isotherm model was found to be the most suitable model for explaining the adsorption process. Finally, the optimized factors have been carried out for thorium adsorption and precipitation from the studied leach liquor of a geologic sample. The final thorium precipitate was measured chemically and characterized by SEM and EDX analyses.
References
Cheira MF, Orabi AS, Atia BM, Hassanein MA (2018) Solvent extraction and separation of thorium(IV) from chloride media by a Schiff base. J Solut Chem 47(4):611–633. https://doi.org/10.1007/s10953-018-0740-1
Kaygun AK, Akyil S (2007) Study of the behaviour of thorium adsorption on PAN/zeolite composite adsorbent. J Hazard Mater 147:357–362. https://doi.org/10.1016/j.jhazmat.2007.01.020
Talip Z, Eral M, Hicsonmez U (2009) Adsorption of thorium from aqueous solutions by perlite. J Environ Radioact 100:139–143. https://doi.org/10.1016/j.jenvrad.2008.09.004
Sheng G, Hu B (2013) Role of solution chemistry on the trapping of radionuclide Th(IV) using titanate nanotubes as an efficient adsorbent. J Radioanal Nucl Chem 298(1):455–464. https://doi.org/10.1007/s10967-012-2389-3
Kaynar UH, Sabikoglu I, Kaynar SC, Eral M (2016) Modeling of thorium(IV) ions adsorption onto a novel adsorbent material silicon dioxide nano-balls using response surface methodology. Appl Radiat Isot 115:280–288. https://doi.org/10.1016/j.apradiso.2016.06.033
Murty BS, Yeh JW, Ranganathan S, Bhattacharje PP (2019) Physical metallurgy of high-entropy alloys. High Entropy Alloys 3:31–50. https://doi.org/10.1016/B978-0-12-816067-1.00003-5
Schibille N, Gratuze B, Ollivier E, Blondeau E (2019) Chronology of early Islamic glass compositions from Egypt. J Archaeol Sci 104:10–18. https://doi.org/10.1016/j.jas.2019.02.001
Heyerdahl H, Krogh C, Borrebæk J, Larsen A, Dahle J (2011) Treatment of HER2-expressing breast cancer and ovarian cancer cells with alpha particle-emitting 227Th-Trastuzumab. Int J Radiat Oncol Biol Phys 79(2):563–570. https://doi.org/10.1016/j.ijrobp.2010.08.038
Bhainsa KC, D’Souza SF (2009) Thorium biosorption by Aspergillus fumigatus, a fila-mentous fungal biomass. J Hazard Mater 165:670–676. https://doi.org/10.1016/j.jhazmat.2008.10.033
Deng Q, Jin Y, Wang Q, Zhao R, Pan N, Zhai F, Luo M, Xia C (2013) New cyclen derivative ligand for thorium(IV) separation by solvent extraction. J Radioanal Nucl Chem 295:125–133. https://doi.org/10.1007/s10967-012-1879-7
Anirudhan TS, Suchithra PS, Senan P, Tharun AR (2012) Kinetic equilibrium profiles of adsorptive recovery of thorium(IV) from aqueous solutions using poly (methacrylic acid) grafted cellulose/bentonite superabsorbent composite. Ind Eng Chem Res 51:4825–4836. https://doi.org/10.1021/ie202538q
Deb AKS, Mohanty BN, Ilaiyaraja P, Sivasubramanian K, Venkatraman B (2012) Adsorptive removal of thorium from aqueous solution using diglycolamide functionalized multi-walled carbon nanotubes. J Radioanal Nucl Chem 295:1161–1169. https://doi.org/10.1007/s10967-012-1899-3
Pan N, Deng J, Guan D, Jin Y, Xia C (2013) Adsorption characteristics of Th(IV) ions on reduced graphene oxide from aqueous solutions. Appl Surf Sci 287:478–483. https://doi.org/10.1016/j.apsusc.2013.10.004
Cortina JL, Warshawsky A (1997) Developments in solid–liquid extraction by solvent-impregnated resins. Ion Exch Solvent Extr 13:195–293. https://pure.tue.nl/ws/files/3135867/200910950.pdf
Turanov AN, Karandashev VK, Masalov VM, Zhokhov AA, Emelchenko GA (2013) Adsorption of lanthanides(III), uranium(VI) and thorium(IV) from nitric acid solutions by carbon inverse opals modified with tetraphenyl methylene diphosphine dioxide. J Colloid Interface Sci 405:183–188. https://doi.org/10.1016/j.jcis.2013.05.026
Bansal RC, Donnet JB, Stoeckli F (1988) A review of: active carbon. J Dispers Sci Technol 11(3):323–482. https://doi.org/10.1080/01932699008943255
Qandeer R, Hanif J (1995) Adsorption of dysprosium ions on activated charcoal from aqueous solutions. Carbon 33(2):215–220. https://doi.org/10.1016/00086223(94)00135-M
Marczenko Z (1986) Separation and spectrophotometric determination of elements. Horwood, NewYork. ISBN-13: 978-0853129035
Shapiro L, Brannock WW (1962) Rapid analysis of silicates, carbonates and phosphate rocks. US Geol Surv Bull 114:51–53. https://doi.org/10.3133/b1144a
Zhou H, Chen Y (2010) Effect of acidic surface functional groups on Cr(VI) removal by activated carbon from aqueous solution. Rare Met 29:333–338. https://doi.org/10.1007/s12598-010-0059-6
Dong J, Ozaki Y (1997) FTIR and FT-Raman studies of partially miscible poly(methy1 methacrylate)/poly(4-vinylphenol) blends in solid states. Macromolecules 30:286–292. https://doi.org/10.1021/ma9607168
Ferrah N, Abderrahim O, Didi MA, Villemin D (2011) Sorption efficiency of a new sorbent towards uranyl: phosphonic acid grafted Merrifield resin. J Radioanal Nucl Chem 289(3):721–730. https://doi.org/10.1007/s10967-011-1172-1
Langmuir D, Herman JS (1980) The mobility of thorium in natural waters at low temperatures. Geochim Cosmochim Acta 44:1753–1766. https://doi.org/10.1021/ja02242a004
Wang H, Yuan X, Wu Y, Huang H, Zeng G, Liu Y, Wang X, Lin NQY (2013) Adsorption characteristics and behaviors of graphene oxide for Zn(II) removal from aqueous solution. Appl Surf Sci 279:432–440. https://doi.org/10.1016/j.apsusc.2013.04.133
Torapava N, Persson I, Eriksson L, Lundberg D (2009) Hydration and hydrolysis of thorium(IV) in aqueous solution and the structures of two crystalline thorium(IV) hydrates. Inorg Chem 48:11712–11723. https://doi.org/10.1021/ic901763s
Mishra SP, Achary PG, Das M (2012) Adsorption of Cu(II) by used aqua guard carbon (UAC). J Chem Pharm Res 4(2):1207–1216. ISSN: 0975-7384
Sharma I, Goyal D (2009) Kinetic modeling: Chromium(III) removal from aqueous solution by microbial waste biomass. J Sci Ind Res 68:640–646. http://hdl.handle.net/123456789/4427
Miraoui A, Didi MA (2015) Thorium(IV) sorption onto sodium bentonite and magnetic bentonite. Eur Chem Bull 4(11):512–521. https://doi.org/10.17628/ECB.2015.4.512
Liao XP, Shi B (2005) Adsorption of fluoride on zirconium(IV)-impregnated collagen fiber. Environ Sci Technol 39:4628–4632. https://doi.org/10.1021/es0479944
Chaudhary N, Balomajumder C (2014) Optimization study of adsorption parameters for removal of phenol on aluminum impregnated fly ash using response surface methodology. J Taiwan Inst Chem Eng 45(3):852–859. https://doi.org/10.1016/j.jtice.2013.08.016
Kannamba B, Reddy KL, AppaRao BV (2010) Removal of Cu(II) from aqueous solutions using chemically modified chitosan. J Hazard Mater 175(1–3):939–948. https://doi.org/10.1016/j.jhazmat.2009.10.098
Chabani M, Amrane A, Bensmaili A (2006) Kinetic modelling of the adsorption of nitrates by ion exchange resin. Chem Eng J 125:111–117. https://doi.org/10.1016/j.cej.2006.08.014
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Sadeek SA, El-Sayed MA, Amine MM, Abd El-Magied MO (2014) Selective solid-phase extraction of U(VI) by amine functionalized glycidyl methacrylate. Environ Chem Eng 2:293–303. https://doi.org/10.1016/j.jece.2013.12.015
Tag El-Din AF, Elshehy EA, Abd El-Magied MO, Atia AA, El-Khouly ME (2018) Decontamination of radioactive cesium ions using ordered mesoporous monetite. RSC Adv 8:19041–19050. https://doi.org/10.3390/colloids1010002
Abd El-Magied MO, Dhmees AS, Abd El-Hamid AM, Eldesouky EM (2018) Uranium extraction by sulfonated mesoporous silica derived from blast furnace slag. J Nucl Mater 509:295–304. https://doi.org/10.1016/j.jnucmat.2018.06.034
Abd El-Magied MO, Mansour A, Alsayed FA, Atrees MS, Abd Eldayem S (2018) Biosorption of beryllium from aqueous solutions onto modified chitosan resin: equilibrium, kinetic and thermodynamic study. Dispers Sci Technol 39:1597–1605. https://doi.org/10.1080/01932691.2018.1452757
Elshehy EA, Shenashen MA, Abd El-Magied MO, El-Nahas AM, Tolan DA, Halada K, Atia AA, El-Safty SA (2017) Selective recovery of silver(I) ions from e-waste using cubically multi-thiolated cage mesoporous monoliths. Eur J Inorg Chem 2017:4823–4833. https://doi.org/10.1002/ejic.201700644
Abd El-Magied MO, Tolba AA, El-Gendy HS, Zaki SA, Ati AA (2017) Studies on the recovery of Th(IV) ions from nitric acid solutions using amino-magnetic glycidyl methacrylate resins and application to granite leach liquors. Hydrometallurgy 169:89–98. https://doi.org/10.1016/j.hydromet.2016.12.011
Abd El-Magied MO, Hassan AM, Gad HMH, Mohammaden TF, Youssef MAM (2017) Removal of nickel(II) ions from aqueous solutions using modified activated carbon: a kinetic and equilibrium study. J Dispers Sci Technol 39:862–873. https://doi.org/10.1080/01932691.2017.1402337
Tunali S, Akar T (2006) Zn (II) biosorption properties of Botrytis cinerea biomass. J Hazard Mater 131(1–3):137–145. https://doi.org/10.1016/j.jhazmat.2005.09.024
Humelnicu D, Drochioiu G, Sturza MI, Cecal A, Popa K (2006) Kinetic and thermodynamic aspects of U(VI) and Th(IV) sorption on a zeolitic volcanic tuff. J Radioanal Nucl Chem 270(3):637–640. https://doi.org/10.1007/s10967-006-0473-2
Freundlich HMF (1906) Over the adsorption in solution. Phys Chem 57:385–470
Lagergren S (1898) About the theory of so called adsorption of soluble substance. Kungliga Svenska Vetenskapsakademiens, Handlinger 24:1–39
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P (2012) Removal of uranium(VI) from aqueous solutions by magnetic schiff base: kinetic and thermodynamic investigation. Chem Eng J 198:412–419. https://doi.org/10.1016/j.cej.2012.05.090
Wu FC, Tseng RL, Juang RS (2001) Enhanced abilities of highly swollen chitosan beads for color removal and tyrosinase immobilization. J Hazard Mater 81:167–177. https://doi.org/10.1016/S0304-3894(00)00340-X
Ding L, Deng HP, Wu C, Han X (2012) Affecting factors, equilibrium, kinetics and thermodynamics of bromide removal from aqueous solutions by MIEX resin. Chem Eng J 181:360–370. https://doi.org/10.1016/j.cej.2011.11.096
Mahmoud AAA, Fatih C, Sabriye Y, Ceren KA (2017) Assessment of the adsorption of thorium onto styrene divinylbenzene-based resin: optimization using central composite design and thermodynamic parameters. Process Saf Environ Prot 109:192–202. https://doi.org/10.1016/j.psep.2017.02.019
Annam S, Brahmmananda CVS, Sivaraman N, Sivaramakrishna A, Vijayakrishna K (2018) Carbamoylmethylphosphine oxide functionalised porous crosslinked polymers towards sequential separation of uranium(VI) and thorium(IV). React Funct Polym 131:203–210. https://doi.org/10.1016/j.reactfunctpolym.2018.07.026
Ansari SA, Mohapatra PK, Manchanda VK (2009) A novel malonamide grafted polystyrene-divinyl benzene resin for extraction, pre-concentration and separation of actinides. J Hazard Mater 161(2–3):1323–1329. https://doi.org/10.1016/j.jhazmat.2008.04.093
Hritcu D, Humelnicu D, Dodi G, Popa MI (2012) Magnetic chitosan composite particles: evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydr Polym 87:1185–1191. https://doi.org/10.1016/j.carbpol.2011.08.095
Ashraf A, Hany AA, Shawky S, Kandil AT (2014) Separation of thorium from aqueous solution by non living water hyacinth roots. Tech J Eng Appl Sci 4(1):1–10. ISSN 2051-0853 ©2014 TJEAS
Savva I, Efstathiou M, Krasia-Christoforou T, Pashalidis I (2013) Adsorptive removal of U(VI) and Th(IV) from aqueous solutions using polymer-based electrospun PEO/PLLA fibrous membranes. J Radioanal Nucl Chem 298(3):1991–1997. https://doi.org/10.1007/s10967-013-2657-x
Anirudhan TS, Rijith S, Tharun AR (2010) Adsorptive removal of thorium(IV) from aqueous solutions using poly(methacrylic acid)-grafted chitosan/bentonite composite matrix: process design and equilibrium studies. Colloids Surf A 368:13–22. https://doi.org/10.1016/j.colsurfa.2010.07.0055
Hosseini-Bandegharaei A, Allahabadi A, Rahmani-Sani A, Rastegar A, Khamirchi R, Mehrpouyan M, Hekmat-Shoar R, Pajohankia Z (2016) Thorium removal from weakly acidic solutions using titan yellow-impregnated XAD-7 resin beads kinetics, equilibrium and thermodynamic studies. J Radioanal Nucl Chem 309(2):761–776. https://doi.org/10.1007/s10967-015-4689-x
Acknowledgements
This research paper was carried out as a part of the research activities of the authors. This article was reviewed and approved for publishing by the Nuclear Materials Authority. Despite that all the laboratories, instrumentations and chemicals were provided by the Nuclear Materials Authority; the authors still maintain the full right to publish the manuscript wherever they decide without any conflict of interest with the Nuclear Materials Authority.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclosures and disclaimer
This work was funded by the Nuclear Materials Authority as a part of its research activities. This article was reviewed and approved for publishing by the Nuclear Materials Authority with no obligation on the authors’ part to revise the manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yousef, L.A., Bakry, A.R. & Ahmad, A.A. Thorium ions recovery using activated carbon modified with triphenyl phosphine oxide: kinetic and equilibrium studies. SN Appl. Sci. 1, 974 (2019). https://doi.org/10.1007/s42452-019-1006-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1006-2