Abstract
Biochar has been used widely as a soil amendment to improve plant growth, nutrient acquisition, stress tolerance and to improve soil biological, chemical and physical properties. Several studies suggest biochar as a carrier for bacterial inoculants under various climatic and environmental conditions because of the properties that favour microbial life. Biochar is rich in organic carbon, contains nutrients, such as N, P, K, has a high porosity, and high water-holding capacity. In this review, we synthesise results on the effectiveness of biochar as a carrier for inoculum in pot and field conditions. Biochar as a carrier supported a high survival rate of introduced bacteria and significantly increased colonization in the plant rhizosphere. Soil microbes are known to play an essential role in soil biochemical processes, and nutrient cycles; improve plant stress tolerance, and nutrient acquisition through their ability to fix atmospheric nitrogen, solubilize phosphate, or by enhancing decomposition of plant residues. Moreover, biochar-based inoculants increased root and shoot biomass, nodulation and nutrient uptake of plants in pot and field experiments. Biochar-based inoculants were also effective in enhancing plant growth and grain yield in pot and field experiments. These studies demonstrate that biochar can be considered as a suitable carrier or formulation of bacterial inoculants even in hostile environments and might contribute to replace other commercially used materials successfully. Biochar-based rhizobial inoculants can significantly improve the symbiotic performance of legumes with rhizobia which may reduce N fertilizer demand and thus promote the sustainability of crop production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacterial fertilizers containing effective microorganisms are considered as an attractive and environmentally friendly technology to improve plant growth and health under various climatic conditions (Fravel et al. 2003; Berg 2009; Mendes et al. 2013; Hashem et al. 2016). The bacteria used for inoculants may belong to various genera such as Azotobacter, Azospirillum, Bradyrhizobium, Bacillus, Enterobacter, Mesorhizobium, Pseudomonas, Stenotrophomonas among others (Berg 2009; Egamberdiyeva 2005, 2007; Aung et al. 2015; Shahzad et al. 2016). The bacterial inoculant can potentially increase plant nutrient acquisition through fixing atmospheric nitrogen, solubilizing insoluble soil phosphate, improving root systems by the production of plant growth stimulators, and protecting plants from soil-borne pathogens through antimicrobial activity (Forchetti et al. 2010; Hashem et al. 2016). Their application in crop production could also reduce the use of chemical fertilizers and pesticides (Shahzad et al. 2016; Egamberdieva et al. 2015, 2017a).
Legumes have symbiotic relationships with rhizobia and are known as the most efficient system for biological nitrogen fixation (Reckling et al. 2016). However, several abiotic factors including high or low temperature, salinity, and drought have a substantial adverse effect on bacterial survival and their interaction with plants (Ardakani et al. 2010, Vardharajula et al. 2011; Egamberdieva et al. 2011, 2015, 2017b). The survival of introduced bacterial inoculants in the soil and the rhizosphere is the key to significant benefits of PGPR symbiosis (Egamberdieva and Kucharova 2009). The formulation of microbes with various carrier substrates enables to extend their colonization ability and effectiveness on plant growth and development (Trivedi et al. 2005; Cho et al. 2015; Tamreihao et al. 2016).
Several carrier materials were used for bacterial formulations including peat, alginate beads, methyl ethyl cellulose, corn cobs, rice husk, sodium alginate, vegetable oils, coal, perlite, or clay (Xavier et al. 2004; Trivedi et al. 2005; Stephens and Rask 2000; Abd El-Fattah et al. 2013; Egamberdieva and Adesemoye 2016). The carrier material has to support bacterial survival during storage time and also in the soil (Somasegaran and Halliday 1982). For example, peat, carrier material for rhizobial inoculants, was not effective against water and temperature stresses (Ardakani et al. 2010). Furthermore, compost was considered as suitable carrier material for root associated beneficial bacteria (Muenchang et al. 2006). Gandhi and Sivakumar (2010) used vermicompost as carrier material for formulation of Bacillus megaterium, Pseudomonas fluorescens and Azospirillum lipoferum, and found that vermicompost enhanced the shelf life and survival of bacterial inoculants. Kaljeet et al. (2011) studied peat, kaolin and rice husk on the survival of bacterial inoculants and observed a higher bacterial survival only in peat as compared to other carriers. A similar observation was reported by Albareda et al. (2008), whereas bacterial strains better survived in peat and perlite as compared to compost, amorphous silica or attapulgite and bagasse.
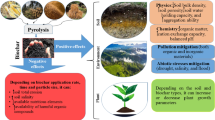
Recently, several reports indicated biochar as a carrier for bacterial inoculants under various climatic conditions (Tamreihao et al. 2016; Egamberdieva et al. 2017c). Biochar is rich in nutrients and may provide a favourable habitat for bacterial proliferation and survival (Pietikainen et al. 2000; Głodowska et al. 2017). This review summarizes the state of the art of microbial inoculants based on biochar and their effect on plant growth and nutrient uptake.
Biochar as a carrier for bacterial inoculants
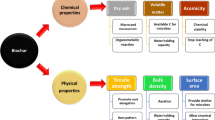
Biochar is produced by pyrolysis or by heating organic biomass in an environment under low or in the complete absence of oxygen and has been used worldwide as a soil amendment, or an approach for carbon sequestration (Lehmann et al. 2011; Ippolito et al. 2012; Biederman and Harpole 2013). The soil amendment with biochar resulted in plant growth promotion, disease resistance and improved soil biological and physical properties (Islami et al. 2011; Graber et al. 2014; Soudek et al. 2016). Also, it has been reported that biochar application improves stress tolerance of plants to salinity, drought, metal toxicity and high temperature (Kammann et al. 2015; Schmidt et al. 2015).
Biochar is characterised by high porosity on the surface area which may provide additional pore space for water and microbes for proliferation (Saranya et al. 2011; Głodowska et al. 2017). Microbes living inside pores may get better protected from external factors such as desiccation, adverse pH, or toxic substances in soil (Chen et al. 2013). It has been reported that population of rhizobia associated with chickpea was higher in a soil-charcoal mixture indicating the suitability of this material as an inoculant carrier (Beck 1991). Pseudomonas libanensis was able to survive in Dynamotive, Pyrovac and Basque biochars and suggested as a suitable carrier for bacterial formulations (Głodowska et al. 2016). Glodowska et al. (2017) reported a significant effect of physical properties of biochars on the survival of Bradyrhizobium japonicum. In this study, Dynamotive-DM and Pyrovac-PR biochars produced from hardwood feedstock were found as suitable carriers for bacterial inoculants, since bacteria survived for 9 months. Furthermore, the survival of Bradyrhizobium sp. in hydrochar and biochar produced from maize silage was investigated by Egamberdieva et al. (2016) revealing that hydrochar sustained a higher log CFU in comparison with biochar from wood. Correspondingly, survival of B. japonicum in biochar pores for more than 6 months was observed by Khavazi et al. (2007). Hale et al. (2015) studied the effect of biochar on the survival of plant growth promoting Enterobacter cloacae UW5 and found that biochar produced from pinewood at the highest treatment temperature of 600 °C, sustained higher population densities of bacteria as compared to vermiculite. In another study, two different biochars from acacia wood and coconut shell were used for the bacterial formulation of Azospirillum lipoferum, and showed a higher survival rate of bacteria for the coconut shell-based biochar (Saranya et al. 2011). Saranya et al. (2011) also found that applying biochar with Azospirillum inoculants significantly increased the rhizosphere population of Azospirillum and other diazotrophic microorganisms. Biochar produced by slow pyrolysis of agricultural wastes at 600 °C significantly increased the survival of Burkholderia sp. and Bacillus sp. Additionally, formulation with biochar stimulated seed germination, plant growth and yield of tomato, as well as soil biological activity (Tripti et al. 2017). Characteristics of biochar such as source material, pyrolysis temperature and granulate size can further influence the interaction among biochar and microbial colonization.
Applications of biochar-based microbial inoculants
Biochar-based inoculants were reported to promote plant growth and nutrient uptake (Egamberdieva et al. 2017c; Tripti et al. 2017). Some examples of biochar based bacterial inoculants and their effect on plant growth are given in Table 1.
Sun et al. (2016) evaluated the survival and root colonization potential of Pseudomonas putida in pinewood biochar that was produced by pyrolysis at 600 °C. They found that biochar was effective as an inoculum carrier and supported a high population of bacteria, and the strain was able to effectively colonize the rhizosphere of plants which resulted in increased plant biomass. The pinewood based inocula of E. cloacae UW5 significantly increased root and shoot biomass and root system development of cucumber (Hale et al. 2015). Similar observations were reported by Glodowska et al. (2017), where biochar-formulated B. japonicum improved the symbiotic performance of soybean, plant growth and physiological properties under greenhouse conditions. The biochar-based seed coating with P. libanensis also improved germination, root and shoot growth, root system, chlorophyll content, and phosphorous uptake of plants (Głodowska et al. 2016). Lupine seeds coated with hydrochar based inoculant of Bradyrhizobium sp. showed an increased nodule number, root and shoot growth and nitrogen, phosphorus and magnesium uptake compared to plants that were inoculated only with Bradyhribium inoculant. Also, the nodule number of lupine plants where seeds were treated with biochar based B. japonicum was increased by up to 146% under drought stress conditions compared to plants inoculated with B. japonicum alone. These results suggest a more profound effect of biochar-based inocula under drought conditions as compared to irrigated conditions (Egamberdieva et al. 2016). The survival of inoculated bacteria in soil and the rhizosphere is crucial for their beneficial effect on plant development (Cho et al. 2015; Egamberdieva and Kucharova 2009), however abiotic stress factors negatively affect bacterial proliferation in soils (Van Dyke and Prosser 2000; Romdhanea et al. 2009). However, previous work showed that the colonization ability of a Bradyrhizobium strain formulated with biochar was not severely affected by drought stress. The pores of biochar support bacterial proliferation through improved aeration and favourable nutrient supply for growth, as well as protection against various environmental stresses such as desiccation, adverse pH, high salinity, and drought (Sangeetha 2012). Steiner et al. (2004) observed that biochar stimulated soil microbial populations and activity also improving plant–microbe interactions. Saranya et al. (2011) observed an increase of plant growth, uptake of N, P, and K and yield of maize by addition of biochar and Azospirillum inoculants. Ghazi (2017) evaluated biochar produced from rice straw as carrier material for rhizobia and found evidence for improved colonisation and survival of bacterial inoculants. The biochar-based inocula increased root and shoot biomass, nodulation and nutrient uptake of kidney bean.
A study on the efficiency of biochar-based Bradyrhizobium sp. (HTC-BR) on lupin plant growth and yield under field conditions showed that HTC-BR inoculant was effective in lupin growth promotion, pod formation and yield under both irrigated and rainfed conditions in comparison to the uninoculated control. There was an increase of 22 and 29% in dry weight of plants when seeds were treated with HTC-BR (Fig. 1). However, pod numbers were not increased by HTC-BR treatments under irrigated conditions, whereas under rainfed conditions, the HTC-BR treated seeds of lupin plants had 39% more pods compared to the uninoculated control. Grain yields were significantly influenced by drought while 51% higher yields were achieved under the irrigated treatment. The yield of inoculated lupin with HTC-BR was 11 and 31% higher compared to the un-inoculated plants under irrigation and rainfed conditions, respectively. Grain yields were significantly influenced by irrigation but not by inoculation. A cause for this finding could be the particular dry conditions in the year of the study where water was the primary limiting factor causing severe yield losses in both, the irrigated and rainfed conditions. Irrigation resulted in significant increase in pod numbers, plant biomass and seed weight. Only two main factors, i.e. irrigation and biochar-based inoculation, showed an enhancement of number of pods (Table 2). In another study, the root and shoot biomass, the number of pods and seed weight of common bean (Phaseolus vulgaris) were increased by biochar and Bacillus sp. treatment (Saxena et al. 2013). In addition, biochar application combined with bacterial inoculants stimulated the population of phosphate solubilizing bacteria in the rhizosphere of plants as well as the P content in plant tissues.
Plant biomass (a), pod number (b), and seed weight (c) of lupins when their seeds were inoculated with biochar-based Bradyrhizobium sp. compared to uninoculated seeds. The plants were grown in the field under irrigated and rainfed conditions. The columns marked with an asterisk differed significantly from uninoculated plants at P < 0.05
Conclusion and future prospects
The studies on the possible use of biochar as carrier material for microbial inoculants showed that biochar might provide microbes shelter and nutrients for their proliferation, protect them from various external stress factors such as desiccation, high temperature and toxic elements. The biochar-based inoculants not only showed convincing results on plant growth promotion and yield in pot experiments (Fig. 2), but also in field trials under various climatic and environmental conditions. These results demonstrate that biochar can be considered as a suitable carrier or formulation of bacterial inoculants even in hostile environments and might contribute to replace other commercially used materials successfully. Biochar-based rhizobial inoculants can significantly improve the symbiotic performance of legumes with rhizobia which may reduce N fertilizer demand and thus promote the sustainability of crop production and of agroeco-systems. However, reports suggest that types of biochar should previously be tested for their effect on bacterial survival ahead of any application in crop production.
References
Abd El-Fattah DA, Eweda WE, Zayed MS, Hassanein MK (2013) Effect of carrier materials, sterilization method, and storage temperature on survival and biological activities of Azotobacter chroococcum inoculant. Ann Agric Sci 58:111–118
Albareda M, Dulce N, Navarro R, Camacho M, Tenprano FJ (2008) Alternatives to peat as carrier for rhizobia inoculants, solid and liquid formulations. Soil Biol Biochem 40:2771–2779
Ardakani SS, Hedari A, Tayebi L, Mohammadi M (2010) Promotion of cotton seedlings growth characteristics by development and use of new bioformulations. Intern J Bot 6:95–100
Aung HP, Djedidi S, Oo AZ, Yokoyama T, Suzuki S, Sekimoto H, Bellingrath-Kimura SD (2015) Growth and 137Cs uptake of four Brassica species influenced by inoculation with a plant growth-promoting rhizobacterium Bacillus pumilus in three contaminated farmlands in Fukushima prefecture, Japan. Sci Total Environ 521(52):261–269
Beck DP (1991) Suitability of charcoal-amended mineral soil as carrier for rhizobium inoculants. Soil Biol Biochem 23:41–44
Berg G (2009) Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotech 84(1):11–18
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214
Chen J, Liu X, Zheng J, Zhang B, Lu H, Chi Z, Pan G, Li L, Zhen J, Zhang X, Wang J, Yu X (2013) Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl Soil Ecol 71:33–44
Cho ST, Chang HH, Egamberdieva D, Kamilova F, Lugtenberg B, Kuo CH (2015) Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS One. https://doi.org/10.1371/journal.pone.0140231
Egamberdieva D, Adesemoye T (2016) Improvement of crop protection and yield in hostile agroecological conditions with PGPR-based biofertilizer formulations. In: Arora NK et al (eds) Bioformulations: for sustainable agriculture. Springer, India, pp 199–212
Egamberdieva D, Kucharova Z (2009) Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol Fertil Soils 45:561–573
Egamberdieva D, Kucharova Z, Davranov K, Berg G, Makarova N, Azarova T, Chebotar V, Tikhonovich I, Kamilova F, Validov S, Lugtenberg B (2011) Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol Fertil Soils 47:197–205
Egamberdieva D, Li L, Lindström K, Räsänen L (2015) A synergistic interaction between salt tolerant Pseudomonas and Mezorhizobium strains improves growth and symbiotic performance of liquorice (Glycyrrhiza uralensis Fish.) under salt stress. Appl Microbiol Biotech 100(6):2829–2841
Egamberdieva D, Wirth S, Behrendt U, Abd-Allah EF, Berg G (2016) Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front Microbiol 7:209. https://doi.org/10.3389/fmicb.2016.00209
Egamberdieva D, Davranov K, Wirth S, Hashem A, Abd_Allah EF (2017a) Impact of soil salinity on the plant-growth—promoting and biological control abilities of root associated bacteria. Saudi J Biol Sci 24(7):1601–1608
Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Berg G, Liao H (2017b) Coordination between Bradyrhizobium and root colonizing Pseudomonas alleviates salt stress in soybean (Glycine max L.) through altering root system architecture and improving nodulation. J Plant Interactions 12:100–107. https://doi.org/10.1080/17429145.2017.1294212
Egamberdieva D, Reckling M, Wirth S (2017c) Biochar-based inoculum of Bradyrhizobium sp. improves plant growth and yield of lupin (Lupinus albus L.) under drought stress. Eur J Soil Biol 78:38–42. https://doi.org/10.1016/j.ejsobi.2016.11.007
Egamberdiyeva D (2005) Characterisation of Pseudomonas species isolated from the rhizosphere of plants grown in serozem soil, semi arid region of Uzbekistan. Sci World J 5:501–509. https://doi.org/10.1100/tsw.2005.64
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36:184–189. https://doi.org/10.1016/j.apsoil.2007.02.005
Forchetti G, Masciarelli O, Izaguirre MJ, Alemano S, Alvarez D, Abdala G (2010) Endophytic bacteria improve seedling growth of sunflower under water stress, produce salicylic acid, and inhibit growth of pathogenic fungi. Curr Microbiol 61(6):485–493
Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytol 157:493–502
Gandhi A, Sivakumar K (2010) Impact of vermicompost carrier based bioinoculants on the growth, yield and quality of rice (Oryza sativa L.). Ecoscan 4:83–88
Ghazi AA (2017) Potential for biochar as an alternate carrier to peat moss for the preparation of Rhizobia bio inoculum. Microbiol Res J Intern 18(4):1–9
Głodowska M, Husk B, Schwinghamer T, Smith DL (2016) Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron Sustain Dev 36:21
Głodowska M, Schwinghamer T, Husk B, Smith D (2017) Biochar based inoculants improve soybean growth and nodulation. Agric Sci 8:1048–1064
Graber ER, Frenkel O, Jaiswal AK, Elad Y (2014) How may biochar influence severity of diseases caused by soilborne pathogens? Carbon Manag 5:169–183
Hale L, Luth M, Crowley D (2015) Biochar characteristics relate to its utility as an alternative soil inoculum carrier to peat and vermiculite. Soil Biol Biochem 81:228–235
Hashem A, Abd_Allah EF, Alqarawi A, Al-Huqail AA, Wirth S, Egamberdieva D (2016) The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front Plant Sci 7:1089. https://doi.org/10.3389/fmicb.2016.01089
Ippolito JA, Laird DA, Busscher WJ (2012) Environmental benefits of biochar. J Environ Qual 41:967–972
Islami T, Curitno B, Basuki N, Suryanto A (2011) Maize yield and associated soil quality changes in cassava and maize intercropping system after 3 years of biochar application. J Agric Food Technol 1:112–115
Kaljeet S, Keyeo F, Amir HG (2011) Influence of carrier materials and storage temperature on survivability of rhizobial inoculants. Asian J Plant Sci 10:331–337
Kammann CI, Schmidt HP, Messerschmidt N, Linsel S, Steffens D, Müller C, Koyro HW, Conte P, Joseph S (2015) Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci Rep. https://doi.org/10.1038/srep11080
Khavazi K, Rejali F, Seguin P, Miransari M (2007) Effects of carrier, sterilization method, and incubation on survival of Bradyrhizobium japonicum in soybean (Glycine max L.) inoculants. Enzyme Microbial Techn 41:780–784
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663
Muenchang S, Panichsakpatana S, Weaver RW (2006) Tomato growth in soil amended with sugar mill by-products compost. Plant Soil 280:171–176
Pietikainen J, Kiikkila O, Fritze H (2000) Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 89:231–242
Reckling M, Bergkvist G, Watson CA, Stoddard FL, Zander PM, Walker R, Pristeri A, Toncea I, Bachinger J (2016) Trade-offs between economic and environmental impacts of introducing legumes into cropping systems. Front Plant Sci 7:669
Romdhanea SB, Trabelsib M, Aouanic ME, de Lajudied P, Mhamdia R (2009) The diversity of rhizobia nodulating chickpea (Cicer arietinum) under water deficiency as a source of more efficient inoculants. Soil Biol Bioch 41(12):2568–2572
Sangeetha D (2012) Survival of plant growth promoting bacterial inoculants in different carrier materials. Inter J Pharm Biol Arch 3:170–178
Saranya K, Kumutha K, Krishnan PS (2011) Influence of biochar and Azospirillum application on the growth of maize. Madras Agric J 98:158–164
Saxena J, Rana G, Pandey M (2013) Impact of addition of biochar along with Bacillus sp. on growth and yield of French beans. Sci Hortic 162:351–356
Schmidt HP, Pandit BH, Martinsen V, Cornelissen G, Conte P, Kammann CI (2015) Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine enhanced biochar to a fertile tropical soil. Agriculture 5:723–741
Shahzad R, Waqas M, Khan AL, Asaf S, Khan MA, Kang SM, Yun BW, Lee IJ (2016) Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol Biochem 106:236–243
Somasegaran P, Halliday J (1982) Dilution of liquid Rhizobium cultures to increase production capacity of inoculant plants. Appl Env Microb 44:330–333
Soudek P, Rodriguez Valseca IM, Petrova S, Song J, Vanek T (2016) Characteristics of different types of biochar and effects on the toxicity of heavy metals to germinating sorghum seeds. J Geochem Explor 182:157–165
Steiner C, Teixeira WG, Lehmann J, Zech W (2004) Microbial response to charcoal amendments of highly weathered soils and Amazonian dark earths in Central Amazonia preliminary results. In: Glaser B, Woods WI (eds) Amazonian dark earths: explorations in space and time. Springer Verlag, Heidelberg, pp 195–212
Stephens JHG, Rask HM (2000) Inoculant production and formulation. Field Crop Res 65:249–258
Sun D, Hale L, Crowley D (2016) Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol Fert Soils 52(4):515–522
Tamreihao K, Ningthoujam DS, Nimaichand S, Singh ES, Reena P, Singh SH, Nongthomba U (2016) Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol Res 192:260–270
Tripti, Kumar A, Usmani Z, Kumar V (2017) Biochar and flyash inoculated with plant growth promoting Rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J Environ Manag 190:20–27
Trivedi P, Pandey A, Palni LMS (2005) Carrier-based preparations of plant growth-promoting bacterial inoculants suitable for use in cooler regions. World J Microb Biotech 21:941–945
Van Dyke MI, Prosser JI (2000) Enhanced survival of Pseudomonas fluorescens in soil following establishment of inoculum in a sterile soil carrier. Soil Biol Biochem 32:1377–1382
Vardharajula S, Ali SZ, Grover M, Reddy G, Bandi V (2011) Drought-tolerant plant growth promoting Bacillus spp.: effect on growth, osmolytes, and antioxidant status of maize under drought stress. J Plant Inter 6:1–14. https://doi.org/10.1080/17429145.2010.535178
Xavier IJ, Holloway G, Legget M (2004) Development of rhizobial inoculant formulations. Online. Crop Manag. https://doi.org/10.1094/cm-2004-0301-06-rv
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Egamberdieva, D., Hua, M., Reckling, M. et al. Potential effects of biochar-based microbial inoculants in agriculture. Environmental Sustainability 1, 19–24 (2018). https://doi.org/10.1007/s42398-018-0010-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-018-0010-6