Abstract
Peat moss has been a standard carrier of inoculum for experimentation and in agriculture. Peat moss is, however, a non-renewable resource. Alternatively, biochar could serve as an inoculum carrier. Here, we tested the effect of biochar-based seed coatings as a carrier for the phosphorous-solubilizing Pseudomonas libanensis inoculum, on corn growth after soluble and insoluble P addition. The survival of P. libanensis was determined based on the measure of colony-forming units from samples of four inoculated guar gum-based biochar coatings and was compared to peat. Storage experiments were performed on inoculated biochars for 22 weeks at 25 °C and on coated corn seeds for 16 weeks at 4 °C. Seed coatings were prepared with inoculated and uninoculated biochars (100 seeds treatment−1), and effects of these treatments are reported on indices of seed germination after 7 days. A greenhouse experiment investigated the effects of the inoculated and uninoculated biochar seed coating on corn plants. The parameters measured from the greenhouse-grown corn plants were germination, fresh weight, dry weight, height, root length, basal stem diameter, leaf area, chlorophyll content, and tissue phosphorous. Our results show that corn plants grown from seeds coated with a biochar from hardwood feedstock are 2 to 10 g heavier than controls and that controls are 4 to 26 % shorter than the plants grown from biochar-coated seeds, where soluble phosphorous is applied. Moreover, corn seeds that were coated with a biochar produced from softwood feedstock germinated more quickly, based on the speed of germination index. Overall, we show that a biochar-based seed coating can benefit sustainable agriculture by carrying P. libanensis and enhancing the growth of corn, but according to parametric statistical tests, it does so without increasing the phosphorous content of the plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The appropriate water holding capacity, consistency, and high porosity of peat moss are qualities that have contributed to its worldwide use as an ingredient of growing substrates and as a carrier for commercial bacterial inoculants (Eudoxie and Alexender 2011). Rapidly decreasing reserves of exploitable non-renewable peat moss have led to price increases which will ultimately limit its use (Tariq et al. 2012).
The chemical and physical characteristics of biochar, a product of biomass pyrolysis, such as high porosity, water holding capacity, and elevated concentrations of organic carbon and nutrients, have had positive effects on soil quality and plant growth (Xu et al. 2012; Graber et al. 2010; Major et al. 2010). Postma et al. (2013) showed that animal bone charcoal can be colonized by bacteria to mobilize phosphate and control plant pathogens.
Pseudomonas libanensis solubilizes inorganic phosphorous. Phosphorous (P) is one of the essential macronutrients for plant growth and development (Hameedaa et al. 2008). The population of P-solubilizing bacteria is usually not large enough to compete with other bacteria that are established in the rhizosphere, and the amount of P liberated by P-solubilizing bacteria is generally not sufficient for a significant increase in plant growth. As a consequence of this, higher concentrations of P-solubilizing bacteria that are normally found in soil are required for the improvement of plant growth and yield (Rodriguez and Fraga 1999).
Corn was selected for this study as an experimental plant because of its importance as a crop and its high P demand. Zea is a genus of the family Gramineae (Poaceae), commonly known as the grass family. Corn is a tall, monoecious annual C4 grass. It is used mainly for human consumption and animal feed, but also for biofuel production. Corn is presently the foremost of all crops in terms of total mass and grain production: global corn production increased from 265 million tonnes (Mt) in 1970 to 844 Mt in 2010 (FAO 2010).
The experimental objectives were to determine whether or not biochar can serve as a suitable carrier for P. libanensis, to create a seed coating system with biochar and P. libanensis that could potentially be used in the agriculture industry, to evaluate the potential of P. libanensis to solubilize inorganic P, and to evaluate the effect of the inoculum and seed treatment on corn growth. It was hypothesized that P. libanensis would mobilize insoluble forms of P and make it available for plant uptake, but the experimental data did not support this hypothesis.
2 Materials and methods
2.1 Biochar source, characterization, and carrier preparation
Four biochars were selected: two from hardwood feedstocks (Dynamotive, West Lorne, Ontario, Canada, and Basque, Rimouski, Québec) and two from softwood feedstocks (BlueLeaf, Drummondville, Québec, and Pyrovac, Saguenay, Québec). Peat moss (PRO-MOSS TBK, Rivière-du-Loup, Québec) was used as a standard inoculant carrier and, in this context, a control. Water holding capacity and pH of each were determined in house, porosity by the Micromeritics Analytical Services Lab (Norcross, Georgia), and elemental analysis, particle size, and composition by the Soil Control Laboratory (Watsonville, California) (Table 1). Two hundred grammes of each material was oven-dried at 75 °C for 3 days, powdered, sieved (500 μm), bagged in polypropylene, and autoclaved for 3 days at 121 °C for 20 min.
2.2 P. libanensis: culture and phosphate solubilization
The pH of King’s B medium (20 g peptone, 1.5 g MgSO4, 1.5 g K2HPO, 10 mL glycerol L−1 distilled water) was adjusted to 7.0, and the medium was sterilized for 20 min at 121 °C, cooled, and inoculated with a phosphate-solubilizing strain of P. libanensis isolated from a soybean rhizosphere. The culture was incubated in a gyratory shaker for 7 days at 25 °C in the darkness. A log phase culture was plated onto Petri plates, and the number of viable cells (colony-forming units (CFU)) was determined.
The capacity of the P. libanensis strain to solubilize inorganic phosphate in a solid medium was determined in a Petri plate assay according to the method of Nautiyal (1999). Estimation of phosphate solubilization in broth was carried out according to Nautiyal (1999). The experiment was performed in triplicate. Phosphate in culture supernatant was estimated using the Murphy and Riley (1962) method with ascorbic acid and ammonium molybdate. Samples were analysed by a spectrophotometer (UV/Visible Spectrophotometer, Ultraspec 4300pro) at 870 nm.
2.3 Response of P. libanensis to biochar carrier
To evaluate the carriers (BlueLeaf, Pyrovac, Dynamotive, Basque, or peat moss), a storage experiment was conducted. P. libanensis culture (1.2 mL) in late log phase (4.9 × 109 CFU mL−1) was aseptically injected with 11 mL of sterile water (40 % moisture of the carrier) into sterilized bags with 30 g of carrier, manually mixed, and sealed with clips. Every treatment was replicated three times at room temperature (21 °C) in a completely randomized design.
Preliminary experiments were conducted to determine the most suitable adhesive for the seed coating (Table 1). For the seed coating, the prepared biochars and peat moss were mixed (under a laminar hood) with suitable amounts of sterile water, liquid culture of P. libanensis, and guar gum (Table 1). Corn seeds were sterilized in 70 % alcohol, washed twice in sterile water, dried (2 h), dipped in the prepared formulation, placed in a sterile Petri plate (20 seeds plate−1), and left to dry (2 h). Each treatment was replicated five times. Sealed Petri plates were subjected to 4 and 21 °C.
2.4 Seed coating storage time experiment
The survival of bacteria (CFU mL−1) as a function of time was determined for each biochar type and peat moss at the beginning of the experiment (day 0), 1 week later, and at biweekly intervals for 16 weeks. The following formula determined the number of CFU mL−1: \( \mathrm{C}\mathrm{F}\mathrm{U}\ {\mathrm{mL}}^{-1}=\left(\frac{{\displaystyle \sum }N}{P\times \left(1/A\right)\times \left(1/D\right)}\right) \), where N is the number of colonies per plate, P is the number of plates, A is the amount (in mL) of the aliquot, and 1/D is the decimal dilution.
2.5 Evaluation of seed coating and inoculated biochar on plant growth
2.5.1 Germination experiment
The two biochars (Pyrovac and Dynamotive) that showed the highest colony count over time were used to determine the influence of seed coating on the germination process. Seeds were arranged into two layers of a P8 filter paper (Fisherbrand, Mississauga, Canada) in Petri plates (150 mm in diam., 10 seeds plate−1), soaked with 5 mL of sterile distilled water, and kept at 25 °C, with 60–80 % relative humidity, in the darkness for 7 days. The completed germination events were counted daily. Root and shoot length and seedling dry weight were measured. Germination indices were determined (Zeng and Zhang 2010): germination percentage, GP = (G a /G n ) × 100 %, where G n is the total number of experimental seeds and G a is the number of normal germinating seeds on day 7; germination index, GI = ∑G t /D t , where G t is the number of normal germinating seeds and D t is the number of germination days; seedling vigour index, VI = GP × RL, where GP is the germination percentage and RL is the seedling root length (mm); and the speed of germination, \( \mathrm{S}\mathrm{G}=\frac{n_1}{d_1}+\frac{n_2-{n}_1}{d_2}+\dots \frac{n_n-{n}_{n-1}}{d_n} \), where n is the number of seeds germinated on day d, the serial number of days.
2.6 Greenhouse experiment
For this experiment, only the Dynamotive biochar was used. Plants were grown in sand and Turface (2:1) in plastic pots (50 cm in diam.) (Fig. 1). The treatments tested were control (uncoated uninoculated), uncoated seeds inoculated with P. libanensis, Dynamotive biochar, and Dynamotive biochar inoculated with P. libanensis at a ratio of 5 t ha−1. Pots were watered with either Hoagland’s solution with soluble P (Hoagland’s No. 2 Basal Salt Mixture, Sigma-Aldrich) or an alternative solution with tricalcium phosphate instead of potassium phosphate. All treatments were replicated five times in a completely randomized design (45 pots total, 5 seeds pot−1). Plants were grown in a greenhouse for 16 h at 25 °C during the day or for 8 h at 20 °C during the night, with 60–80 % relative humidity. After 1 week, seedlings were thinned to 3 pot−1 (15 plants treatment−1). Germination percentage, aboveground fresh weight, dry weight, plant height, main root length, basal stem diameter, and leaf area were measured. Chlorophyll content was measured using a chlorophyll meter (SPAD-502, Konica Minolta). The concentration of P in the plant tissue was determined using a colorimetric method (Murphy and Riley 1962). The concentration was read at 660 nm with a spectrophotometer (UV/Visible Spectrophotometer, Ultraspec 4300pro).
The formulation of biochar-based seed coating as an alternative to peat for inoculation. Guar gum was the least expensive of the best sticking agents. Experimentation assessed the survival of a phosphorous-solubilizing P. libanensis in the coating and the effect of seed coating on seed germination. Subsequent greenhouse experiments assessed the effect of the experimental seed coating on corn growth and development
3 Statistical analysis
SAS System software (SAS 9.3, SAS Institute, Inc., 1999, Cary, NC, USA) was used for statistical analyses. The data to describe each of the quantified biochar characteristics was limited to a mean value for each variable for each char type. Therefore, Kendall’s correlation coefficient (τ) was used (SAS PROC CORR KENDALL) to quantify the relationships between the biochar physical and chemical properties.
The bacterial growth data was sorted by temperature. Generalized linear mixed models were fit to the bacterial growth data using SAS PROC GLIMMIX and the DIST = GAMMA option in the MODEL statement. The dependant variable was the log10 CFU (+1). The structure of the covariance matrix was based on time (TYPE = SP(POW)(Week)). The estimates were compared to the peat moss control using Dunnett’s test. Generalized linear mixed models were also fit to the final and repeated measurements of the plant growth variables. Percentages were expressed as continuous values between 0 and 1, and therefore, the Beta Distribution, DIST = BETA, was specified. To make inferences, the LSMEANS statement and Bonferroni adjustment were used. SAS PROC SGPLOT was used with the LOESS statement to produce the scatter-diagram smoothing.
4 Results and discussion
4.1 Quality of carrier
The biochars utilized were manufactured from various feedstocks and under a range of pyrolysis conditions; thus, physical analysis revealed differences between the carriers and chemical analysis revealed differences between the biochars (Table 1). Unsurprisingly, the average pore diameter was positively correlated with porosity and median pore diameter (area) (both τ = 0.8, p = 0.05) and negatively correlated with bulk density (τ = −0.8, p = 0.05). The data indicated some strong negative relationships (all τ = −1, p = 0.0415): porosity with Cl, porosity with NH4-N, median pore diameter (area) with Co, median pore diameter (area) with P, water holding capacity with Cl, water holding capacity with NH4-N, pH with Co, and pH with P. There were also strong positive relationships (all τ = 1, p = 0.0415): As with Ni, As with B, As with Na, Cd with P, Cr with Zn, Ni with B, Ni with Na, B with Na, Cl with NH4-N, K with NO3-N, and Co with P.
Biochar pH can vary from below 4 to above 12, depending on the feedstock, pyrolysis temperature, and degree of oxidation (Lehmann 2007), which creates extremely different living conditions for bacteria in the pore spaces. Considering the ubiquity and proximity of microorganisms at the biochar surface, biochar pH is considered to have an important effect on total microbial abundance (Lehmann et al. 2011). A neutral pH of 7.0–7.5 is thought to be optimal for the growth of Pseudomonas strains (Thomas et al. 1994). Therefore, it was expected that biochars that were close to neutral pH (Dynamotive, Pyrovac, and Basque) would sustain the number and viability of P. libanensis at higher levels than other carriers, and they did, under controlled 21 °C temperature conditions.
The average pore diameters of the Basque, Dynamotive, and Pyrovac biochars are shown in Table 1. Wood-derived biochar retains the configuration of plant cells, and its pores resemble interconnected chambers, 5–10 μm in diameter, which is an optimum size for the retention and inhabitation of bacteria. On average, bacteria have a diameter range of 0.3–3 μm (Swift et al. 1979) and this range is similar to the average pore diameters of the Basque, Dynamotive, and Pyrovac biochars.
4.2 Storage time of P. libanensis-inoculated biochar and seed coating
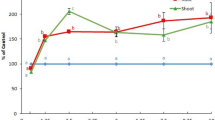
Viable cells were counted from inoculated biochars and peat moss at day 0, after 1 week, and biweekly until week 22. The five carrier materials sustained the survival and viability of P. libanensis to varying degrees. After 22 weeks, the arithmetic mean of viable cells on the log10 scale (log10 CFU) was 5.98 ± 0.14 for the Pyrovac carrier, 5.50 ± 0.10 log10 CFU was present in the Dynamotive biochar carrier, and 5.66 ± 0.18 log10 CFU was present in the Basque biochar carrier. Although these three biochars sustain bacteria populations at similar levels, the 95 % confidence limits indicate statistically significant differences, where the log10 CFU in the Dynamotive biochar was lower than that in the Pyrovac and Basque biochars during the week 10 to week 15 intervals (Fig. 2a). According to the Dunnett-adjusted limits at 95 % confidence, there would be 2 to 26 % more CFUs on the log10 scale with the Basque biochar, as compared to peat moss (p = 0.0134) and 2 to 25 % more log10 CFU with the Pyrovac biochar (p = 0.0182). Surprisingly, peat moss and BlueLeaf biochar were found to sustain bacterial viability for only a relatively short period of time. After 2 weeks of incubation, no viable cells were found in these carriers.
Nonparametric regression plots showing the log10 CFU mL−1 of P. libanensis in a powdered biochar stored at 21 °C (a), biochar seed coating stored at 21 °C (b), and 4 °C (c) as a function of time. Seed coating carriers: PM peat moss, BL BlueLeaf, PV Pyrovac, DM Dynamotive, BA Basque. Solid line indicates predicted value; dotted line indicates 95 % confidence limits
Two sets of biochar-coated corn seeds inoculated with P. libanensis were prepared and stored at two different temperatures (4 and 21 °C) to determine the survival of bacteria within the seed coating material and to determine the influence of temperature on the bacterial population over time. Counts of viable cells were performed for each treatment at day 0, 1 week, and at 1-week intervals until 16 weeks. As with the previous experiment, BlueLeaf, Pyrovac, and Basque biochar coatings supported more abundant bacterial populations (Fig. 2b, c). After storage at 21 °C for 16 weeks, the arithmetic means for log10 CFU was 1.64 ± 0.42 for the Pyrovac biochar, 1.06 ± 0.53 for the Basque biochar, and 2.02 ± 0.54 for the BlueLeaf biochar (and 0 for the Dynamotive biochar and peat moss).
After 16 weeks of storage at 4 °C, the arithmetic mean log10 CFU was 3.91 ± 0.75 for the Pyrovac biochar, 2.39 ± 0.48 for the Dynamotive biochar, 4.24 ± 0.59 for the BlueLeaf biochar, and 1.51 ± 0.50 for the Basque biochar. Peat moss was the least suitable carrier for P. libanensis: when stored at 21 °C, no viable cells were found in the peat moss seed coat 1 week after inoculation. Nevertheless, when seeds were stored at 4 °C, bacteria were present in the peat moss seed coating until week 8 but the population abundance was quite low (0.26 ± 0.26 log10 CFU). The seed coating had a significant effect on the log10 CFU under 4 °C temperature conditions (F 4,241 = 31.39, p < 0.0001). Under 4 °C temperature conditions, according to the Dunnett-adjusted limits at 95 % confidence, there would be 33 to 77 % more log10 CFU when seeds are coated with the BlueLeaf biochar, as compared to peat moss (p < 0.0001) (Fig. 2c). At the same levels of confidence and temperature, there would be 6 to 41 % more log10 CFU when seeds are coated with the Dynamotive biochar as compared to peat moss (p = 0.0033) and there would be 41 to 86 % more log10 CFU when seeds are coated with the Pyrovac biochar (p < 0.0001).
4.3 Phosphate solubilization by P. libanensis
The solubilization of tricalcium phosphate by P. libanensis was indicated by the halo zone around the colony on an agar medium. The mean halo diameter of ten replicates was 25.6 mm, and the halo/colony diameter ratio was 10.45 (ten replicates). P solubilization in a liquid medium was calculated based on the calibration curve. After 24 h of incubation, 17.4 % of tricalcium phosphate was solubilized by the bacteria. The maximum P-solubilizing activity was recorded 1 week after inoculation (98.2 %) compared to the control. After the first week, the amount of tricalcium phosphate solubilized remained stable until the end of the experiment (5 weeks). The spectrophotometric analysis showed that 465.11 mg L−1 of phosphorous was solubilized in a liquid medium containing tricalcium phosphate, 2 weeks after inoculation (three replicates).
4.4 Evaluation of seed coating and biochar inoculant on corn growth
To evaluate corn seedling growth and the influence of seed coating on corn seedling development, a germination test was carried out by Petri plate assay. Following from the results of previous experiments, Pyrovac and Dynamotive biochars were selected as the most suitable carriers for P. libanensis. Generally, Dynamotive biochar-coated seeds and Dynamotive biochar-coated seeds inoculated with P. libanensis performed better than the control and other treatments (Table 2).
The germination experiment showed that seed coating and inoculation did not affect the seedling dry weight nor the germination percentages (Table 2). The treatments did, however, affect the speed of germination (F 4,45 = 4.82, p = 0.0025). While the treatments did not affect the germination index, they had a significant effect on the seed vigour index (F 4,45 = 16.21, p < 0.0001). The treatments also had a significant effect on the seedling root length (F 4,45 = 13.86, p < 0.0001).
A greenhouse experiment was conducted to evaluate the influence of bacterial seed coating as well as inoculated biochar on corn growth characteristics. The Dynamotive biochar, the best performing biochar in the germination assay, was selected for the greenhouse experiment. Corn plant growth indicators are presented in Table 2. For the plants supplied with Hoagland’s solution with tricalcium phosphate, only Dynamotive biochar-coated seeds showed statistically significant higher (p < 0.05) fresh weight, plant height, stem diameter, total leaf area, and dry weight compared to the other treatments. It was expected that corn seeds inoculated with P. libanensis would result in better growth of plants than uninoculated seeds when plants were supplied with tricalcium phosphate; however, no significant differences in growth were recorded. Also, biochar seed coating did not significantly affect the aboveground plant metrics when tricalcium phosphate was applied (Table 2).
For the plants supplied with soluble P, root characteristics were homogeneous (Table 2). Where tricalcium phosphate was supplied, however, the treatments affected the fresh weight of roots (F 4,10 = 7.68, p = 0.0043). Surprisingly, where tricalcium phosphate was applied, the fresh weight of roots was affected by the treatment (F 4,10 = 7.68, p = 0.0043). The dry weight of roots was affected by the treatments where tricalcium phosphate was supplied, too (F 4,10 = 19.67, p < 0.0001) (Table 2). No statistically significant differences between the treatments were found for the root length of the plants from the greenhouse experiment.
In the greenhouse experiment, the treatments affected the fresh weight of corn plants when soluble P was used (F 3,40 = 7.72, p = 0.0003). Nevertheless, the treatments did not affect the fresh weight of plants when tricalcium phosphate was used (F 4,50 = 1.03, p = 0.4018). According to the Bonferroni-adjusted limits at 95 % confidence, Dynamotive biochar-coated seeds would produce plants that were 2 to 10 g heavier than the controls, where Hoagland’s solution with soluble P was used (p = 0.0017).
Similarly, the treatment had a significant effect on plant height where soluble P was applied (F 3,40 = 6.10, p = 0.0016). The treatment did not affect the plant height where tricalcium phosphate was applied (F 4,50 = 0.42, p = 0.7911). According to the Bonferroni-adjusted limits at 95 % confidence, uncoated and uninoculated seeds would produce corn plants 26 to 4 % shorter than the plants produced by Dynamotive-coated seeds, where soluble P was applied.
A chemical analysis of the corn tissue revealed some differences between treatments (Table 2). When tricalcium phosphate was applied, the treatments affected corn nitrogen (N) percentages (F 4,25 = 3.38, p = 0.0243). Corn plants for uncoated inoculated seeds had lower N percentages compared to corn plants that were grown from Dynamotive biochar-coated seeds that were inoculated (p = 0.0166). The treatment affected the N:C ratio, where tricalcium phosphate was applied (F 4,25 = 21.20, p < 0.0001) and where soluble P was applied (F 3,19 = 13.81, p < 0.0001) (Table 2).
The chlorophyll data indicated a relatively steep decrease in the indexed chlorophyll content between weeks 3 and 4. The indexed chlorophyll content remained stable thereafter until week 6. The treatment affected the chlorophyll content where soluble P was applied (F 3,16 = 4.87, p = 0.0136). According to the Bonferroni-adjusted limits at 95 % confidence, Dynamotive biochar-coated seeds would produce plants with 1 to 13 % more indexed chlorophyll content than plants grown from uncoated seeds treated with an inoculated biochar.
Surprisingly (unless the nonparametric tests are considered), the treatments did not increase P concentrations in the corn tissue. It was previously demonstrated that inoculation with P-solubilizing bacteria improves plant N uptake. The chemical analysis revealed improved N uptake in corn shoots. On the other hand, de Freitas et al. (1997) showed that none of their P-solubilizing rhizobacteria enhanced the growth and yield of canola. Furthermore, the results of P analysis showed that the plant tissue P concentrations in the controls were not significantly different from those of the tissue of inoculated plants.
In conclusion, a germination experiment revealed the positive influence of seed coating on corn seedling growth under laboratory conditions. Clearly, the experimental biochars contributed to improved germination characteristics of corn; however, the impact of bacteria and their P-solubilizing activity is questionable. It is hypothesized that the main reason for improved seedling growth is the biochar coating, which retains water around the seed and thus creates favourable conditions for seedling development. Also, due to the high content of macro- and microelements, a biochar might be a direct source of some nutrients. P solubilization is one of many mechanisms by which bacteria might directly or indirectly affect plant growth. The production of gibberellins, cytokinin, 1-aminocyclopropane-1-carboxylate deaminase, and volatile compounds can be considered as other types of these mechanisms (Podile and Kishore 2006); however, they were not characterized in the present study. Therefore, it is also possible that P. libanensis produces plant-related compounds which may stimulate plant growth. In fact, it has been shown that many of the P-solubilizing bacteria synthetize IAA-like hormones which are well known to influence plant growth development. Future work should investigate the alternative products of P. libanensis that promote plant growth, other than P solubilization.
Abbreviations
- CFU:
-
Colony-forming units
References
De Freitas JR, Banerjee MR, Germida JJ (1997) Phosphate-solubilizing rhizobacteria enhance the growth and yield but not phosphorous uptake of canola (Brassica napus L.). Biol Fertil Soils 24:358–364. doi:10.1007/s003740050258
Eudoxie GD, Alexender IA (2011) Spent mushroom substrate as a transplant media replacement for commercial peat in tomato seedling production. J Agric Sci 3:41–49. doi:10.5539/jas.v3n4p41
FAO (Food and Agriculture Organisation of the United Nations) (2000) Fertilizers requirements in 2015 and 2030. FAO, Rome
Graber ER, Harel YM, Kolton M, Cytryn E, Silber A, David DR, Tschansky L, Borensthein M, Elad Y (2010) Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 337:481–496. doi:10.1007/s11104-010-0544-6
Hameedaa B, Harinib G, Rupelab OP, Wanib SP, Gopal R (2008) Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163:234–242. doi:10.1016/j.micres.2006.05.009
Lehmann J (2007) Bio-energy in the black. Front Ecol Environ 5:381–387. doi:10.1890/1540-9295(2007)5[381:BITB]2.0.CO;2
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota: a review. Soil Biol Biochem 43:1812–1836. doi:10.1016/j.soilbio.2011.04.022
Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333:117–128. doi:10.1007/s11104-010-0327-0
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. doi:10.1016/S0003-2670(00)88444-5
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170(1):265–270. doi:10.1111/j.1574-6968.1999.tb13383.x
Podile AR, Kishore GK (2006) Plant growth promoting rhizobacteria. Springer, Netherlands
Postma J, Clematis F, Nijhuis EH, Someus E (2013) Efficacy of four phosphate-mobilizing bacteria applied with an animal bone charcoal formulation in controlling Pythium aphanidermatum and Fusarium oxysporum f. sp. radicis lycopersici in tomato. Biol Control 67(2):284–291. doi:10.1016/j.biocontrol.2013.07.002
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339. doi:10.1016/S0734-9750(99)00014-2
Swift MJ, Heal OW, Anderson JW (1979) Decomposition in terrestrial ecosystems. Blackwell, Oxford
Tariq U, Rehman SU, Khan MA, Yunus A (2012) Agricultural and municipal waste as potting media components for the growth and flowering of Dahlia hortensis Figaro. Turk J Bot 36:378–385
Thomas KL, Lloyd D, Boddy L (1994) Effects of oxygen, pH and nitrate concentration on denitrification by Pseudomonas species. FEMS Microbiol Lett 118(1–2):181–6. doi:10.1111/j.1574-6968.1994.tb06823.x
Xu G, Lv Y, Sun J, Shao H, Wei L (2012) Recent advances in biochar application in agricultural soils: benefits and environmental implications. Clean Soil Water Air 40(10):1093–1098. doi:10.1002/clen.201100738
Zeng DF, Zhang L (2010) A novel environmentally friendly soybean seed-coating agent. Acta Agric Scand Sect B Soil Plant Sci 60:545–551. doi:10.1080/09064710903334256
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Głodowska, M., Husk, B., Schwinghamer, T. et al. Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron. Sustain. Dev. 36, 21 (2016). https://doi.org/10.1007/s13593-016-0356-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s13593-016-0356-z