Abstract
Sclerotium rolfsii Sacc. is one of the important soil borne pathogen causing stem rot of groundnut prevalent in all growing area worldwide. The present study aimed on the identification of native Trichoderma isolates, and its efficacy against the stem rot pathogen in groundnut at field level. Thirty-five isolates of Trichoderma spp. isolated from the groundnut rhizosphere were comparatively evaluated for their biocontrol potential against S. rolfsii Sacc. and growth promoting traits in groundnut. The morphological studies of the 35 isolates were supported molecularly by amplifying of ITS region and classified into four species namely, T. asperellum, T. citrinoviride, T. longibrachiatum and T. harzianum which were further subjected to biocontrol efficacy tests. The highly efficient representative isolates namely, T. harzianum Thar23, T. asperellum Tasp49, T. longibrachiatum Tlongi5 and T. citrinoviride Tcitri2 were evaluated to produce lytic enzymes and growth promoting traits. The comparative study of these isolates revealed that, T. harzianum Thar23 produced significant (P < 0.05) amount of lytic enzymes viz., chitinase (31.36 U/ml), β 1, 3 glucanase (4.1 U/ml) and protease (2.76 U/ml). T. harzianum Thar23 promotes plant growth traits namely germination efficacy (31.48%), increase in the shoot length (42%) and root length (42.43%), improved vigor index, and increased relative water content (25.56%). Soil application, seed treatment and drenching with the powder formulation of Thar23 in field for the years 2019 and 2020 significantly (P < 0.05) reduced stem rot disease incidence to 59.45% and 53.79% and increased pod yield to 2.85 t/ha and 2.68 t/ha respectively. T. harzianum isolate Thar23 will help the groundnut growers for eco-friendly management of stem rot disease and increased yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundnut (Arachis hypogaea L.) is an important food and oil seed crop due to its high protein and oil content. Several biotic and abiotic factors are responsible for dismal productivity. Diseases like stem rot, collar rot, root rot, leaf spot, bud necrosis, etc., are critical. Stem rot is also known as sclerotium blight caused by soil borne fungi S. rolfsii causes yield loss over 20–25 percent (Annual Report 2015–16). Under warm and high moisture conditions, white mycelium spread over the plant debris, soil and infect the host. The dark brown sclerotia of the pathogen are hard, spherical and 0.5–1.5 mm in size often found in the infected are of host and soil (Aycock 1966). Though fungicides are effective against pathogens, but they cause adverse effect on the environment thus can be replaced by biocontrol agents.
Trichoderma spp. (Teleomorph: Hypocrea) is an omnipresent ascomycetous fungus known for its biocontrol and industrial properties. This fungi were named Trichoderma in 1794 (Persoon 1794) and years later in 1865, the sexual stage Hypocrea species was suggested (Tulasne and Tulasne 1865). Diverse species of Trichoderma namely, T. harzianum, T. asperellum, T. viride, T. virens, T. hamatum and T. atroviride have been reported as biocontrol agents. T. reesei, T. parareesei and T. longibracheatum are known for industrial enzyme production. Trichoderma species are widely present in the soil rhizosphere and documented for symbiotic relationship with the host roots. Due to the importance of the application of Trichoderma spp. as biocontrol agent in field condition, it is necessary to explore its biogeography. There are different studies conducted by the researchers to decipher the diversity of the native Trichoderma spp. and its application against major plant pathogens at national (Kumar et al. 2012; Agrawal and Kotasthane 2012; Devi et al. 2021; Manzar et al. 2021; Jambhulkar et al. 2022) and global (Li et al. 2016; Boat et al. 2020; Ma et al. 2020; Nofal et al. 2021). However, there is a need to explore the diversity of the native species at groundnut growing area of Jaipur, a semi-arid eastern plain zone of Rajasthan (Agro-climatic Zone- III-A), India.
There are various biocontrol mechanisms viz., mycoparasitism, antibiosis, induced systemic resistance in Trichoderma spp. and also known for production of many lytic enzymes viz., chitinases, glucanases, xylanase and proteases etc., as their primary weapons against the fungal pathogens (Sharma et al. 2014) and induce the systemic defence response by activating defence enzymes like peroxidases (PODs), polyphenol oxidases (PPO) and phenylalanine ammonia lyase (PAL) (Malolepsza et al. 2017). Plant growth promotion is crucial component of Trichoderma spp. which helps in improvement of plant growth in terms of increased plant biomass, root and shoot length and grain yield. Trichoderma colonizes fully on root tissues and triggers various mechanisms which induce plant growth promotion, facilitate nutrient uptake, induce plant defence mechanisms, helps in rhizosphere construction, increase carbohydrate metabolism, induce of phytohormones, root exudates and photosynthesis in host (Sallam et al. 2019). Among the genus of Trichoderma spp., T. harzianum is the most researched biocontrol species followed by others such as T. viride, T. asperellum, T. hamatum, T. virens and T. koningii (Keswani et al. 2014). Species like T. longibrachiatum and T. citrinoviride needs to be studied for its biocontrol and plant growth promoting capabilities. Therefore, the comparative evaluation of biocontrol efficacy and plant growth promoting traits of native isolates of Trichoderma spp. will be helpful in the characterization of biocontrol control agents and potential strains can be utilized at field conditions.
In the present study, we have isolated and characterized native isolates of Trichoderma from groundnut rhizosphere and potent isolates were comparatively evaluated to assess biocontrol and plant growth promoting potential against groundnut stem rot pathogen S. rolfsii under field conditions.
Materials and methods
Collection and isolation of Trichoderma isolates
The 60 rhizospheric soil samples were collected from groundnut growing areas of Jaipur (Agro-climatic Zone- III-A), a semi-arid eastern plain zone of Rajasthan, India. The longitude and latitude of collection locations were recorded and are given Table 1. For the isolation of Trichoderma spp., the rhizospheric soil samples were serially diluted on Trichoderma selective medium (TSM) (Elad et al. 1981) and incubated at 28 °C ± 1 °C for 4 days. The newly emerging mycelia of fungal colonies were subcultured to fresh potato dextrose agar (PDA) plates and incubated at 28 °C ± 1 °C for 7 days and maintained in potato dextrose agar (PDA) slants at 4 °C for further use in experiment. Culture of S. rolfsii was available at Department of Plant Pathology, SKN Agriculture University, Jobner- Jaipur.
Identification of Trichoderma isolates
Morphological identification
The purified 35 isolates were identified based on the different morphological characters viz., cultural characters like colour, growth and texture, microscopic features branching of conidiophores, phialides disposition, size and shape of conidia were identified based on the Rifai (1969), Bissett (1984) and Samuels et al. (1999).
Molecular identification
Actively growing Trichoderma isolates (5 mm disc) were inoculated into 50 mL potato dextrose broth (PDB) (HIMEDIA Labs, India) and incubated at 28 ± 2 °C for 5–6 days at 180 rpm. The fungal mycelia were harvested using Whatman No. 1 filter paper and washed three times with sterile distilled water. Collected mycelium was grounded finely with liquid nitrogen and stored at − 80 °C till further use. Cetyltrimethyl ammonium bromide (CTAB) method was followed for total fungal DNA extraction (Culling 1992). The internal transcribed spacer (ITS) region was amplified by using universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′)/ITS4 (5′-TCCTCCGCTTATTGATATGC3′) (White et al. 1990).
PCR reaction mixture was prepared in the final volume of 25 µl containing 2.5 µl of 10X PCR buffer with MgCl2, 1 µl of forward and reverse primer each (10 pM), 0.5 µl of 10 mM dNTP’s, 0.5 µl of DyNAzyme II DNA Polymerase (2 U/µl), 2 µl of genomic DNA (50 ng/µl) and 17.5 µl of Molecular biology grade water and amplified by following protocol: initial denaturation for 1 min at 95 °C, 30 cycles of denaturation for 30 s at 95 °C, primer annealing for 1 min at 60 °C, extension at 72 °C for 1 min and a final extension period for 7 min at 72 °C. The amplified PCR products were electrophoretically separated using 1.2% agarose gel in 1X TAE buffer at 80 V for 1 h. Amplified PCR fragments were visualized in UV light and gel documented. The desired amplified products were gel eluted (GeneJET Gel Extraction Kit, Thermo Scientific™, USA) and sequenced through the Sanger sequencing method (Eurofins Pvt. Ltd). The sequence contig was prepared using CAP3 sequence assembly program and aligned sequence were confirmed with nBLAST (www.ncbi.nlm.nih.gov/BLAST) and submitted to NCBI (http://www.ncbi.nlm.nih.gov/).
Phylogenetic analysis
The phylogenetic tree was constructed by aligning the generated sequences using ClustalW multiple sequence alignment program (Thompson et al. 1994) and MEGA7 software program (Kumar et al. 2016). Maximum Composite Likelihood (MCL) method was used to estimate the pairwise distances and bootstrap method was used to study the nodal robustness with a replication of 1000. The Kimura 2-parameter distance model (Kimura 1980) was used for the construction of maximum-likelihood (ML) tree (Kumar et al. 2016).
Screening for antagonistic activity of Trichoderma isolates
Antagonistic activity of different 35Trichoderma isolates against groundnut stem rot pathogen S. rolfsii was done by dual culture plate method (Dennis and Webster 1971). Seven days old actively growing mycelial disc (5 mm) of Trichoderma isolates and S. rolfsii were placed on PDA plates opposite from the periphery and plates without Trichoderma served as a control and plates were incubated at 28 ± 2 °C for 5–7 days. The percentage of inhibition was calculated by following formula
where ‘C’ is the radial growth of pathogen in the control PDA plate in cm and ‘T’ is the radial growth pathogen in test plate in cm.
The antagonism level of these isolates was evaluated according to Bell et al. (1982). Trichoderma isolates with significant antagonistic potential against S. rolfsii were evaluated for production of lytic enzymes and plant growth promoting traits in groundnut.
Lytic enzymes assay of selected isolates of four Trichoderma spp.
Preparation of cell-free culture filtrate
The cell-free culture filtrate from selected isolates T. harzianum Thar23, T. asperellum Tasp49, T. longibrachiatum Tlongi5 and T. citrinoviride Tcitri2were prepared using freeze-dried mycelia of S. rolfsii as a sole carbon source. Actively grown mycelial mat of S. rolfsii was harvested from 7 days old PDB broth and homogenized by using liquid nitrogen. The freeze dried pathogen mycelial powder was stored at − 20 °C. A 5 mm mycelial disc of actively growing selected Trichoderma isolates was inoculated in autoclaved 250 ml of minimal synthetic broth (MSB) containing (g/l) FeSO4-0.01, MnSO4-0.01, ZnSO4- 0.01, KCl-0.5, MgSO4-0.5, K2HPO4-1.0, NaNO3-3.0; pH 5.5 amended with 1% freeze dried mycelia of S. rolfsii and flasks were kept at 28 ± 2 °C at 180 rpm and filtered through Whatman no. 1 filter paper at different time interval from day 1 to 10.
Estimation of chitinase (EC 3.2.1.14)
Dinitrosalicylic acid (DNSA) method was used to estimate the chitinase production from Trichoderma isolates. One millilitre of culture filtrate with 0.5 ml of colloidal chitin and 0.5 ml of 1 M sodium acetate buffer was mixed and incubated at 40 °C for 6 h and centrifuged at 12,000 rpm for 5 min at 4 °C. One millilitre of supernatant was mixed with 0.5 ml of DNSA in 1 M NaOH and 0.1 ml of 10 M NaOH and kept at 100 °C for 5 min. The assay mixture was recorded spectrophotometrically at 582 nm and N-acetylglucosamine (GlcNAc) was used as standard. Specific chitinolytic activity was defined as unit of GlcNAc released by 1 ml of enzyme solution under assay conditions.
Estimation of β-1,3-glucanase (EC 3.2.1.39)
β-1,3-Glucanase activity was determined using laminarin as a substrate. The assay mixture contains 0.5 ml of culture filtrate with 1 ml of laminarin in 50 mM acetate buffer (pH 4.8) and was incubated at 50 °C for 10 min. One ml of dinitrosalicylic acid was added to the reaction mixture and kept at 95 °C for 5 min and total amount of reducing sugar was recorded at 540 nm. One unit of β-1,3-glucanase activity was defined as the amount of enzyme required to release one µmol of reducing sugar per minute.
Estimation of protease (EC 3.4.21.4)
Protease activity was determined using 1% casein as substrate in 50 mM phosphate buffer (pH 7.0) was denatured at 100 °C for 15 min in the water bath. The reaction mixture containing 1 ml of casein substrate was added with 3 ml of 10% tricholoroacetic acid (TCA) and kept at 4 °C for 1 h. This mixture was centrifuged at 8000 rpm for 15 min at 4 °C, and supernatant was recorded at 280 nm. One unit of protease activity was defined as the amount of enzyme solution equivalent to release 1 µmol of tyrosine under assay conditions.
Plant growth promoting traits of selected isolates of four Trichoderma spp. in groundnut
The plant growth promoting ability of the selected Trichoderma isolates in groundnut (RG-510 Spreading variety) was studied under pot conditions. Groundnut seeds were treated with spore suspensions of each selected Trichoderma isolates containing 2 × 108spores ml−1and were soaked for one hour. Spore suspensions from selected isolates were prepared from PDA plates containing 7 days old cultures of Trichoderma by scraping gently on the surface of the plates with sterile distilled water containing 0.01% Tween 20 and filtered through two layers of sterile muslin cloth. The spore concentration was adjusted with the aid of haemocytometer.
Efficacy on seed germination, root, and shoot length and relative water content (RWC)
The germination efficacy of selected Trichoderma isolates in groundnut seeds was studied by treating with Trichoderma spore suspension (2 × 108 spores ml−1) and transferred to respective pots containing sterile soil along with farm yard manure (FYM) in 10:1 ratio. Seeds treated with sterile water served as control. After 10 days, the number of germinated seedlings in each replication was counted and the germination was calculated and expressed by using the following formula
Groundnut plants (30 days old) were harvested from each treatment and washed three times with sterile distilled water. The root and shoot length were observed and based on the root and shoot length with germination percentage, the vigour index was calculated by using formula given by Abdul Baki and Anderson (1973).
To determine relative water content, the harvested plants were air dried and weighed (fresh weight). For dry weight, the plants were kept in hot air oven at 100 °C for 20 min, and then kept at 80 °C for 24 h at oven then weighed and recorded (Tian et al. 2015). Each control and treatment were repeated three times. The following formula was used to determine RWC of shoots and roots.
where, RWC is relative water content, FW: fresh weight, and DW: dry weight.
Groundnut stem rot management by application of selected isolates of four Trichoderma spp. under field conditions
The field experiment was conducted in the randomized block design with three replications in the kharif season of 2019 and 2020 at Agronomy Farm, S.K.N. College of Agriculture, Jobner situated 260 05′ N-latitude and 750 28′ E-longitudes and at an altitude of 427 m above mean sea level in Jaipur district of Rajasthan. The region falls in agroclimatic zone III-a (semi-arid eastern plain), and variety RG 510 was used for both experimental years. The seeds were treated with talc-based bioformulation of different Trichoderma isolates at 8 g/kg. The spore concentration of the bioformulation was maintained 2 × 108 CFU/g. The treatment schedule is as follows.
T1—Soil application with T. asperellum Tasp49 enriched FYM (10: 200) + seed treatment with T. asperellum Tasp49 at 8 g/kg seeds + drenching with T. asperellum Tasp49 at 8 ml/l at 40 days after sowing.
T2—Soil application with T. harzianum Thar23 enriched FYM (10: 200) + seed treatment with T. harzianum Thar23 at 8 g/kg seeds + drenching with T. harzianum Thar23 at 8 ml/l at 40 days after sowing.
T3—Soil application with T. longibrachiatum Tlongi5 enriched FYM (10: 200) + seed treatment with T. longibrachiatum Tlongi5 at 8 g/kg seeds + drenching with T. longibrachiatum Tlongi5 at 8 ml/l at 40 days after sowing.
T4—Soil application with T. citrinoviride Tcitri2 enriched FYM (10: 200) + seed treatment with T. citrinoviride Tcitri2 at 8 g/kg seeds + drenching with T. citrinoviride Tcitri2 at 8 ml/l at 40 days after sowing.
T5—Untreated control.
Disease incidence was monitored on a weekly basis by observation of symptoms and was calculated by the following formula
Shelling of the well dried 100 g pods from each treatment was done and recorded weight of kernels and the shelling percentage was calculated by following formula
The pod yield was calculated from each treatment separately after threshing, winnowing, and cleaning the produce was weighed and converted in terms of Tones/ha.
Statistical analysis
The normality of the data was checked and found that data are treatment-wise normally distributed. All the treatments replicated thrice in a completely randomized design and the descriptive statistics of the data are presented as mean value ± SD. Significance of mycelial growth inhibition, enzyme production and growth promotion were tested by a one-way analysis of variance (ANOVA). The data were analysed by ANOVA using R-programming language and treatment means were compared using Fisher’s Protected LSD test at p = 0.05 (Gomez and Gomez 1984).
Results
Morphological characteristics of Trichoderma isolates
Thirty-five isolates of Trichoderma spp. were collected from the rhizospheric soil of groundnut growing area of Jaipur District (26.9706° N, 75.3791° E) of Rajasthan, India, which were further morphologically characterized through microscopic studies. Based on morphological features the isolates were classified into four groups I T. asperellum, group II T. harzianum, group III T. longibrachiatum and group IV T. citrinoviride (Table 1).The group I consisted of 12 isolates of T. asperellum showed dark green and compact colonies on PDA medium with the typically paired and regularly branched conidiophores(Table 1). The conidia were globose to sub-globose in shape with the size of 2.5–3 µm (Fig. 1). A total of 11 isolates of T. harzianum in grouped exhibited whitish green to pale green on PDA surface with short branched and irregular conidiophores at right angle. The shape of conidia was globose to sub-globose to short obovoid with size of 1.5–2 µm (Fig. 1). The 6 isolates of group III were yellowish green or dark olive green on PDA plates with short, branched conidiophores, laginiform or bottle shaped conidia on long main branches with the size of 2–2.5 µm were classified as T. longibrachiatum. The group IV was classified as T. citrinoviride consisted of 6 isolates which showed dusky yellowish green colony on PDA with less ellipsoidal, broadly rounded apex conidia with size of 2–2.5 µm, with relatively straight long branched conidiophores, relatively elongate, lageniform or narrowly shaped phialides (Fig. 1).
PDA culture plates showing 7 days old representative isolates of Trichoderma spp. a, e, i Showing the growth on PDA, branching pattern of phialides and conidia of T. asperellum Tasp49. b, f, j Showing the growth on PDA, branching pattern of phialides and conidia of T. harzianum Thar23. c, f, k Showing growth on PDA, branching pattern of phialides and conidia of T. longibrachiatum Tlongi5. d, h, l Showing growth on PDA, branching pattern of phialides and conidia of T. citrinoviride Tcitri2 (scale bar 10 µm)
Molecular characterization of Trichoderma isolates and phylogenetic analysis
A single amplified product around 550-650 bp of all 35 isolates of Trichoderma spp. were sequenced and confirmed with BLAST search tool and submitted to NCBI (Table 1). The BLAST analysis was differentiating at species level with homology percentage of 95–99%, and results obtained from phylogenetic analysis of ITS sequences showed that the 35 Trichoderma isolates can be separated into four different species with three distinct clades of Trichoderma namely (Fig. 2), the Pachybasium A clade (T. asperellum), the Harzianum clade (T. harzianum), and the Longibrachiatum clade (T. longibrachiatum and T. citrinoviride). The Pachybasium A clade consists of 12 isolates of T. asperellum with a bootstrap value of 98%, the clade Harzianum consisting of 11 isolates of T. harzianum supported by bootstrap value of 81%. The closely associated species both T. longibrachiatum (6 isolates) and T. citrinoviride (6 isolates), fall in the section Longibrachiatum clade with 96% bootstrap value indicating the close relationship of both species (Fig. 2).
Phylogenetic relationships of Trichoderma isolates inferred by analysis of ITS region and constructed using two parameter model implemented in the MEGA7 inferred by using the Maximum Likelihood method and Tamura-Nei model. Analysis was conducted in MEGA 7 and the percentage of replicate trees in which the associated taxa clustered together in the bootstrap test
Antagonistic activity of Trichoderma isolates against S. rolfsii
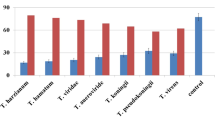
The antagonistic activity of 35 isolates of Trichoderma spp. against S. rolfsii was evaluated by dual culture assay. Two groups of T. asperellum and T. harzianum exhibited higher antagonistic activity with the range of 62% to 81.7% against S. rolfsii. Group III T. longibrachiatum and Group IV T. citrinoviride recorded moderate or lower mycelial inhibition from 51.43 to 67.5% (Fig. 3).The degree of antagonism was measured by the scale described by Bell et al. (1982). Isolates from T. asperellum group namely Tasp1, Tasp3, Tasp6, Tasp46 and Tasp49, from T. harzianum group namely, Thar1, Thar3, Thar4, Thar5, Thar20, Thar21, Thar22, Thar23, Thar24 and Thar25 exhibited class 1 level of antagonism, whereas isolates like Tasp2, Tasp4, Tasp5, Tasp47, Tasp48, Tasp50 and Tasp51 from T. asperellum group, one isolate from T. harzianum Thar2, some of the isolates from T. longibrachiatum group namely Tlongi1, Tlongi2, Tlongi4, Tlongi5and Tcitri2, Tcitri4 and Tcitri6 from T. citrinoviride group expressed the class 2 level of antagonism. Class 3 level of antagonism was observed in Tlongi3 and Tlongi25 from T. longibrachiatum and Tcitri1, Tcitri3 and Tcitri5 from T. citrinoviride against S. rolfsii. Among 35 isolates, the potential isolate from each group namely T. asperellum Tasp49 from group I, T. harzianum Thar23 from group II, T. longibrachiatum Tlongi5 from group III and T. citrinoviride Tcitri2 from group IV were selected for the study of lytic enzyme production and plant growth promoting traits in groundnut.
Lytic enzymes assay of selected isolates of four Trichoderma spp.
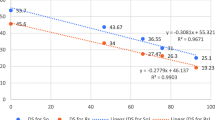
The lytic enzymes like chitinase, β-1,3-glucanase and protease from selected Trichoderma isolates were studied by using freeze dried mycelia of S. rolfsii in MSB as a source of enzyme production. The enzyme activity from the isolates were gradually increased from day 1 to 7 for chitinase and day 1–6 for β-1,3-glucanase and protease and decreased after day 7. Among the selected isolates, T. harzianum Thar23 (31.36 U/ml) significantly produced higher amount of chitinase on day 7 followed by T. asperellum Tasp49 (25.26 U/ml) (Fig. 4). The other selected isolates T. longibrachiatum Tlongi5 (20.1 U/ml) and T. citrinoviride Tcitri2 (17.3 U/ml) exhibited lesser amount of chitinase enzyme activity as compared to other isolates (Fig. 4). Similarly, another lytic enzymes β -1, 3-glucanase and protease are also produced significantly higher 4.1 U/ml and 2.76 U/ml on day 6 by T. harzianum Thar23 followed by T. asperellum Tasp49 (2.6 U/ml and 2.13 U/ml). The other two selected isolates T. longibrachiatum Tlongi5 (1.33 U/ml and 1.16 U/ml) and T. citrinoviride Tcitri2 (0.8 U/ml and 0.83 U/ml) showed lesser production of these enzymes compared to other isolates (Fig. 4).
Plant growth promoting traits of selected isolates of four Trichoderma spp. in groundnut
The selected isolates were comparatively tested for their growth promoting ability in groundnut under greenhouse conditions. The seeds treated with T. harzianum Thar23 and T. asperellumTasp49 significantly increased the germination efficacy to 31.48 and 24.47% and increased the shoot length by 42 and 21.44% and root length by 73.72 and 62.76% compared to control with vigour index of 3598.25 and 3030.65 (Table 2) and increase in plant biomass in terms of the fresh and dry weight of shoot and root. The RWC of shoot and root treated with Thar23 shown higher (81.23%) than control (60.65%) (Table 2). However, the moderate effect on germination efficacy and plant growth promoting traits was observed in seed treatment with T. citrinoviride Tcitri2 as compared to other selected isolates (Table 2).
Groundnut stem rot management by application of selected isolates of four Trichoderma spp. under field conditions
Field experiments were conducted to evaluate the efficacy of native Trichoderma isolates on stem rot disease incidence, shelling percent and pod yield for the year of 2019 and 2020 kharif season. Soil application, seed treatment and drenching with T. harzianum Thar23 and T. asperellum Tasp49 significantly (P < 0.05) reduced stem rot disease incidence up to 59.45%, 52.01% in 2019 and 53.79%, 48.74% for the year of 2020 and increased pod yield in T. harzianum Thar23 (2.85 t/ha and 2.68 t/ha) and T. asperellum Tasp49 (2.62 t/ha and 2.55 t/ha) treated plots with increased shelling per cent (Table 3). However, treatment with T. longibrachiatum Tlongi5 and T. citrinoviride Tcitri2 shows moderate reduction in disease incidence up to 35.11%, 34.16% in 2019 and 34.16%, 33.21% in 2020 with pod yield of 2.13 t/ha, 2.05 t/ha in 2019 and 2.05 t/ha and 1.95 t/ha in 2020 (Table 3). The results obtained from field experiments that indicate the effect of application of T. harzianum Thar23 in improvement of the pod yield up to 51.59% and 38.58% during 2019 and 2020 kharif seasons, respectively.
Discussion
The present study was focused on morphological and molecular characterization, antagonistic ability, and plant growth promoting traits in the potential native Trichoderma isolates tested against stem rot pathogen S. rolfsii of groundnut. The isolates from rhizosphere soil of groundnut were collected and characterized based on the morphological characteristics to identify the species level by using the reference of Rifai (1969), Bissett (1984) and Samuels et al. (1999) and classified in to four groups, namely T. asperellum (12), T. harzianum (11), T. longibrachiatum (6) and T. citrinoviride (6). Morphological characterization of native Trichoderma isolates has earlier been taken up by several researchers (Rifai 1969; Bissett 1984; Pandian et al. 2016; Devi et al. 2021; Jambhulkar et al. 2022). In addition to supporting the reliability of morphological identification, isolates were further characterized molecularly by amplifying ITS region. Kullnig-Gradinger et al. (2002) described the multigene phylogeny approaches for the evolution of Trichoderma spp. by using the ITS1 and ITS2, 28S rDNA, mitSSU, tef 1 and ech42 genes. Indian researchers have widely surveyed in different locations of the country and have reported from the different geographical locations like New Delhi (Muthu and Sharma 2011), South Andaman Island (Kumar et al. 2012), Chhattisgarh (Agrawal and Kotasthane 2012), Manipur (Kamala et al. 2015) and Uttarakhand (Manzar et al. 2021), different states of India (Devi et al. 2021).The present study revealed the presence of diverse Trichoderma spp. in the rhizosphere of groundnut growing area of Jaipur District of Rajasthan. Mainly T. asperellum and T. harzianum were found to be dominant species with greater antagonistic potential against a wide range of phytopathogens. Till now, 375 species of Trichoderma spp. have been identified and their DNA barcoding information was deposited in the International Subcommission on Taxonomy of Trichoderma (ICTT) (http://www.trichoderma.info). The modern Trichoderma taxonomy methods help in the precise identification and reorganization of 50 new species of Trichoderma per year (Cai and Druzhinina 2021). Similar studies by Manzar et al. (2021) highlighted the phylogenetic relationship among the Trichoderma spp. based on the ITS and tef-1α sequences. Out of 20 isolates, nineteen isolates belonged to T. asperellum as compared to T. harzianum (one isolate). With the upcoming trend of development of potential native strains of Trichoderma spp. characterization through molecular and morphological tools have become very important step in research.
To further utilize the native strains for biological control, antagonism tests are required to understand the mechanism under in vitro and in vivo conditions. The antagonistic ability of the Trichoderma isolates was tested against S. rolfsii showed significant reduction in the mycelial growth of pathogen. Significant variation was observed in the isolates from T. asperellum and T. harzianum while T. longibrachiatum and T. citrinoviride exhibited moderate efficacy. The CWDEs are specialized group (glycosyl-hydrolases, oxidoreductases, lyases, and esterases) of enzymes produced by Trichoderma spp. which are key component against wide range of phytopathogens. Recently Kaur et al. 2021 reported purified proteins both endochitinase and β-1,3-glucanase from T. viride isolate T1#3 degrade the hyphae of R. solani causing sheath blight in rice. Several research findings stated that the genus of Trichoderma is known to produce CDWs like chitinase, β-1,3-glucanase and protease are playing key role in the suppression of the growth of major soil borne pathogens (Guigon-Lopez et al. 2015; Li et al. 2016; Elamathi et al. 2018; Boat et al. 2020; Macena et al.2020). In recent years, green synthesis of nanoparticles by these species made an impact in the agricultural and food sector due to the secretion of bioactive enzymes, metabolites and accumulation of metals are responsible for reduction of metal ions and helping in the formation nanoparticles. Raja et al. 2021 reported that biologically synthesized nanoparticles by using cell free culture filtrate of T. harzianum Th3 inhibits the mycelial growth of groundnut root rot complex pathogens by 60–65%. Production of secondary metabolites during mycoparasitism also a pivotal key of Trichoderma spp. in the antagonistic mechanism. For example, secondary metabolites like harzianic acid (HA), 6-pentyl-α-pyrone (6PP), koninginin, harzianopyridone and etc. can be correlated with biocontrol mechanisms (Vinale and Sivasithamparam 2020).
Plant growth promoting fungi (PGPF) are majorly associated with wide range of hosts and helps in transformation of soil nutrients, alter the niche of rhizosphere, elucidate the systemic resistance, and improve the plant growth. Trichoderma spp. are one of major beneficial fungal community present in the soil environment which directly create an impact on plants such as increased in number lateral roots and length, cumulative root length and root tips, germination efficacy and seeding growth, improved surface area of roots and leaves, wet and dry weight of plant biomass, and positive effect on flowering. And also responsible for elucidation of plant immunity through increasing jasmonic acid (JA), salicylic acid (SA), ethylene (ET), phytoalexin levels and root exudates in plants, soil nutrients solubilization, and nutrient uptake. Some of the Trichoderma spp. are rhizospheric competent in nature that can be able to colonize the plant roots and enter the epidermis and outer cortex of root system (Harman et al. 2004). Recently Nofal et al. (2021) reported that seedling treatment with 10% cell free culture filtrate of T. atroviride from rhizosphere of tomato could improve the plant growth and decreased wilt incidence percentage (8%) caused by Fusarium oxysporum f. sp. lycopersici. The current study also revealed the impact of seed inoculation with selected native Trichoderma isolates which helps in improvement of germination efficacy, root, and shoot length in groundnut. The RWC of the root and shoot in treated plants has been increased which indicates the acceleration in the plant growth. Based on the obtained results, the highly efficient strain T. harzianum Thar23 exhibits excellent mycelial growth inhibition of pathogen, lytic enzymes production and improve the plant growth could be used against biotic and abiotic stress at greenhouse and field level in pest management practices.
Performance of microbial antagonistic under field condition is one of the important key factors in commercialization of the product at market level. The present findings were in accordance with several research findings stated that the importance of performance of Trichoderma spp. against reduction of different pathogen population at field level (Sharma et al. 2012; Jambhulkar et al. 2022). In this present study, there are differences in performance of Trichoderma isolates, however treatment with T. harzianum Thar23 enhanced groundnut growth, reduction in S. rolfsii disease incidence, significant increase in shelling percentage and pod yield among other isolates.
Conclusion
Based on morpho and molecular characterization 35 native Trichoderma isolates were grouped into four different Trichoderma spp. namely, T. asperellum (12), T. harzianum (11), T. Longibrachiatum (6) and T. citrinoviride (6) from rhizosphere of groundnut and screened based on the antagonistic activity against S. rolfsii. The potential isolates from each group viz., T. harzianum Thar23, T. asperellum Tasp49, T. longibrachiatum Tlongi5 and T. citrinoviride Tcitri2 were selected for lytic enzyme production and plant growth promoting studies in groundnut. The highly efficient isolate T. harzianum Thar23 exhibits excellent mycelial growth inhibition of pathogen, lytic enzymes production and improves the plant growth which could be used in biotic and abiotic stress management in groundnut at both green house and field level.
References
Abdul-Baki AA, Anderson JD (1973) Vigour determination in soybean seed by multiple criteria 1. Crop Sci 13(6):630–633
Agrawal T, Kotasthane AS (2012) Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chhattisgarh in Central India. Springerplus 1(1):1–10
Annual Report (2015–16) ICAR-directorate of groundnut research, Junagadh-362001, Gujarat, India
Aycock R (1966) Stem rot and other diseases caused by Sclerotium rolfsii or the status of Rolfs’ fungus after 70 years. Raleigh (NC): North Carolina Agricultural Experiment Station Bulletin, pp 132–202
Bell DK, Wells HD, Markham CR (1982) In vitro antagonism of Trichoderma spp. against six fungal plant pathogens. Phytopathology 72:379–382
Bissett J (1984) A revision of the genus Trichoderma I sect. Longibrachiaticum sect. nov. Can J Bot 2:924–931
Boat MAB, Sameza ML, Iacomi B, Tchameni SN, Boyom FF (2020) Screening, identification and evaluation of Trichoderma spp. for biocontrol potential of common bean damping-off pathogens. Biocontrol Sci Technol 30(3):228–242
Cai F, Druzhinina IS (2021) In honour of John Bissett: authoritative guidelines on molecular identification of Trichoderma. Fungal Divers 107(1):1–69
Culling KW (1992) Design and testing of a plant specific PCR primer for ecological evolutionary studies. Mol Ecol 1:233–240
Dennis L, Webster J (1971) Antagonistic properties of some species of Trichoderma. III. Hyphal production. Trans Br Mycol Soc 57:363–369
Devi P, Prabhakaran N, Kamil D, Choudhary SP (2021) Polyphasic taxonomy of Indian Trichoderma species. Phytotaxa 502(1):1–27
Elad Y, Chet I, Henis Y (1981) A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 9:59–67
Elamathi E, Malathi P, Viswanathan R, Sundar AR (2018) Expression analysis on mycoparasitism related genes during antagonism of Trichoderma with Colletotrichum falcatum causing red rot in sugarcane. J Plant Biochem Biotechnol 27:351–361
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Guigon-Lopez C, Vargas-Albores F, Guerrero-Prieto V, Ruocco M, Lorito M (2015) Changes in Trichoderma asperellum enzyme expression during parasitism of the cotton root rot pathogen Phymatotrichopsis omnivora. Fungal Biol 119(4):264–273
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species: opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56
Jambhulkar PP, Raja M, Singh B, Katoch S, Kumar S, Sharma P (2022) Potential native Trichoderma strains against Fusarium verticillioides causing post flowering stalk rot in winter maize. Crop Prot 152:105838
Kamala T, Devi SI, Sharma KC, Kennedy K (2015) Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma biodiversity hot spot region with special reference to Manipur. BioMed Res Int 1–21
Kaur R, Kalia A, Lore JS, Kaur A, Yadav I, Sharma P, Sandhu JS (2021) Trichoderma sp. endochitinase and β-1,3-glucanase impede Rhizoctonia solani growth independently, and their combined use does not enhance impediment. Plant Pathol 70:1388–1396
Keswani C, Mishra S, Sarma BK, Singh SP, Singh HB (2014) Unravelling the efficient applications of secondary metabolites of various Trichoderma spp. Appl Microbiol Biotechnol 98:533–544
Kimura MA (1980) Simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotides sequences. J Mol Evol 2:87–90
Kumar K, Amaresan N, Bhagat S, Madhuri K, Srivastava RC (2012) Isolation and characterization of Trichoderma spp. for antagonistic activity against root rot and foliar pathogens. Indian J Microbiol 52(2):137–144
Kullnig-Gradinger CM, Szakacs G, Kubicek CP (2002) Phylogeny and evolution of the fungal genus Trichoderma—a multigene approach. Mycol Res 106:757–767
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Li Y, Sun R, Yu J, Saravanakumar K, Chen J (2016) Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J Microbiol 56(3):318–327
Ma J, Tsegaye E, Li M, Wu B, Jiang X (2020) Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 10(8):1–13
Macena AMF, Kobori NN, Mascarin GM, Vida JB, Hartman GL (2020) Antagonism of Trichoderma-based biofungicides against Brazilian and North American isolates of Sclerotinia sclerotiorum and growth promotion of soybean. Biocontrol 65:235–246
Malolepsza U, Nawrocka J, Szczech M (2017) Trichoderma virens 106 inoculation stimulates defense enzyme activities and enhances phenolics levels in tomato plants leading to lowered Rhizoctonia solani infection. Biocontrol Sci Technol 27(2):180–199
Manzar N, Singh Y, Kashyap AS, Sahu PK, Rajawat MVS, Bhowmik A, Sharma PK, Saxena AK (2021) Biocontrol potential of native Trichoderma spp. against anthracnose of great millet (Sorghum bicolour L.) from Tarai and hill regions of India. Biol Control 152:104474
Muthu KA, Sharma P (2011) Molecular and morphological characters: an appurtenance for antagonism in Trichoderma spp. Afr J Biotechnol 10(22):4532–4543
Nofal AM, El-Rahman MA, AbdelghanyTM A-M, M, (2021) Mycoparasitic nature of Egyptian Trichoderma isolates and their impact on suppression Fusarium wilt of tomato. Egypt J Biol Pest Control 31:103
Pandian RTP, Raja M, Kumar A, Sharma P (2016) Morphological and molecular characterization of Trichoderma asperellum strain Ta13. Indian Phytopathol 69:297–303
Persoon CH (1794) Dispositamethodicafungorum. RomersneuesMagazin der Botanik 1:81–128
Raja M, Sharma RK, Jambhulkar PP, Sharma KR, Sharma P (2021) Biosynthesis of silver nanoparticles from Trichoderma harzianum Th3 and its efficacy against root rot complex pathogen in groundnut. Mater Today Proc 43(5):3140–3143
Rifai MA (1969) A revision of the genus Trichoderma. Mycol Papers 116:1–56
Sallam N, Eraky AM, Sallam A (2019) Effect of Trichoderma spp. on Fusarium wilt disease of tomato. Mol Biol Rep 46(4):4463–4470
Samuels GJ, Lieckfeldt E, Nirenberg HI (1999) Trichoderma asperellum, a new species with warted conidia, and redescription T. viride. Sydowia 51:71–88
Schuster A, Schmoll M (2010) Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87(3):787–799
Sharma P, Saini MK, Deep S, Kumar V (2012) Biological control of groundnut root rot in farmer’s field. J Agric Sci 4(8):48–59
Sharma P, Sharma M, Raja M, Shanmugam V (2014) Status of Trichoderma research in India: a review. Indian Phytopathol 67(1):1–19
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tian X, He M, Wang Z, Zhang J, Song Y, He Z, Dong Y (2015) Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul 77(3):343–356
Tulasne LR, Tulasne C (1865) Selecta fungorumcarpologia. Jussu, Paris
Vinale F, Sivasithamparam K (2020) Beneficial effects of Trichoderma secondary metabolites on crops. Phytother Res 34(11):2835–2842
White TJ, Bruns T, Lee SJWT, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Acknowledgements
The authors are thankful to Head, Department of Plant Pathology, Sri Karan Narendra Agriculture University, Jobner for proving research facilities. This present study is part of first author’s Ph.D. programme and thankful to Head, Department of Biosciences, Manipal University Jaipur for necessary support during the research work.
Funding
Funding was provided by Indian Council of Agricultural Research (F. No. Agril. Edn. /9/11/2016/ES/HRD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raja, M., Sharma, R.K., Jambhulkar, P.P. et al. Comparative evaluation of native Trichoderma species from groundnut rhizosphere against stem rot caused by Sclerotium rolfsii Sacc.. Indian Phytopathology 76, 459–471 (2023). https://doi.org/10.1007/s42360-023-00610-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-023-00610-3