Abstract
Background
Previous studies show evidence that brain aging is mainly due to oxidative stress, mitochondrial damage, and apoptosis. In this study, we have selected a natural compound to know the protective effect against neurodegeneration. Both in -vitro and in-vivo studies were performed to understand the potency of the Kaempferide.
Methodology
In our research, in-vitro studies were performed by using PC-12 and SHSY5Y cell lines. Neuroprotective, anti-apoptotic, scavenging activity, and caspase enzyme activity were evaluated to know the effect of kaempferide. Results from in-vitro encourage to performance of in-vivo studies. D-galactose (200 mg/kg body wt.) for 45 days induces brain aging. Donepezil is used as a standard drug at a dose of 3 mg/kg body wt. Kaempferide at two different doses—5 mg/kg and 10 mg/kg body wt were given daily. Behavioral studies like the Morris Water Maze test, Rotarod test, and Randell sellito test were performed during the study. Estimation of tissue parameters like Lipid peroxidation, Anti-oxidant activity enzymes, Acetylcholinesterase Enzyme, Amyloid, and Tau protein and histopathological studies.
Results
In-vitro results revealed that Kaempferide exerts neuroprotective activity by inhibiting apoptosis, caspase 3/7 enzyme, and free-radical generation. In-vivo studies report that, KPD at 10 mg/kg bw. exerts more potent neuroprotective activity than Donepezil which was clearly indicated in the results. Western blot report clearly indicates that Kaempferide exerts a protective effect on Amyloid B and Tau protein in brain tissue.
Conclusion
The scope of in-vitro data encouraged us to conduct in-vivo studies. Kaempferide had a defensive impact on D-galactose-induced brain aging by spatial learning and memory enhancement. Therefore, kaempferide improves D-galactose-induced neurodegeneration through anti-oxidant and neuroprotective effects. It has an anti-aging effect on the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Neurodegeneration mainly leads to dementia and cognitive decline [1, 2]. Pathological changes in the brain are primarily due to excess production of free radicals, mitochondrial damage, amyloid-β, and tau protein aggregation [3]. According to recent demographic data, nearly 48 million people over the age of 55 are affected [4]. Nowadays, stress and a lack of nutritional supplements are the main causes of oxidative stress. Medicinal plants have powerful anti-oxidant and protective properties [5]. The onset of neurodegeneration was delayed and avoided by the use of natural compounds with anti-oxidant and neuroprotective properties [6].
The D-galactose-induced model is a popular model for inducing brain aging because the symptoms mimic natural aging. D-galactose is a monosaccharide molecule that mainly causes oxidative stress by Advanced glycation end products (AGE), Lipid peroxidation, and Mitochondrial damage [7]. D-galactose converts into galacitol by aldol reductase, which increases osmotic stress and finally leads to mitochondrial dysfunction and oxidative damage. The primary cause of D-galactose toxicity is mitochondrial dysfunction, which is a major cause of brain aging [8, 9].
Dietary flavonoids and micronutrients are natural antioxidants found in many fruits and vegetables. Recent studies have primarily focused on the development of natural immunity and antioxidant activity, both of which can aid in the prevention of oxidative stress [10]. The natural products holistic approach since it is safe, potent, less toxic, widely available, and reasonably priced. Antioxidants’ ability to scavenge free radicals reduces oxidative damage and slows aging. There is no standard drug to cure brain aging at all stages [11]. So, by identifying this gap in research, we can try to find a natural molecule that will protect the brain from aging.
Future therapy will necessitate the identification and preparation of medicinal plants. The primary function of natural products is to reduce oxidative stress in living cells through antioxidant activity. Because oxidative stress and amyloid beta generation are thought to be the primary causes of neurodegeneration [12], we speculate on the function of selected phytocandidates for neuroprotection. PC-12 and SHSY5Y human neuroblastoma cell lines were used in in-vitro studies to assess the antioxidant and neuroprotective activity of selected phytocandidates [13].
Kaempferide is an O-methyl derivative of kaempferol. It is a natural compound found in Prunus domatica, Alpinia conchigera, Chrmolaena odorata, Syzygium aromaticum, Citrus X paradisi, and several herbs and spices [14]. KPD has anti-oxidant, anti-hypertensive, and anti-cancer drugs. According to recent research, it is effective against SARS-Cov-2 Nucleocapsid Phosphoprotein [15]. Norbergenin is an O-methyl derivative of bergenin. It is a natural compound found in Ardisia sanguinolenta, Ficus recemosa, Mallotus japonicus, and in several fruits. NRG exerts anti-oxidant activity, acts against lung carcinoma and inhibition of adrenal tyrosine hydroxylase in in-vitro studies.
Wistar rats were used in the current study to induce brain aging with D-galactose, and KPD and NRG were used to treat brain aging by demonstrating their effect on improving cognitive function.
2 Methodology
2.1 In Vitro Studies
Isolated compounds procured from—Prunetin (No. 29432) were procured from cayman chemicals, Michigan 48,108 USA. Kaempferide and Piceatannol were purchased from sigma Aldrich, USA and Norbergenin was obtained from chemscene chemicals, NJ, USA, and Isookanin from Merck chemicals, Thermoscientifics, USA.
2.1.1 Cell Culture
Cell culture experiments were carried out using PC-12 and SHSY5Y neuroblastoma cell lines obtained from Sigma Aldrich, United States. The PC-12 cell lines were grown in RPMI1640 medium, while the SHSY5Y cells were grown in DMEM (Dulbecco's modified eagle medium), both were supplemented with 10% Foetal Bovine Serum, 100 µg/mL streptomycin, and 100 IU/mL penicillin. Cells were kept at 37 °C in a humidified atmosphere of 5% CO2. PC12 cells were cultured for an additional 5 days after seeding before being treated with mouse nerve growth factor (mNGF) and medium (1:100) to induce neuronal differentiation. Every other day, the culture medium was changed. Routine enzymatic digestion and passage were performed on the cells. A hemocytometer was then used to count the cultured cells.
2.1.2 Cell Viability Assessment
PC12 and SHSY5Y cells were treated with concentrations ranging from 5 to 100 M for 72 h and subjected to the MTT assay to investigate the cytotoxicity effects of phytocandidates (Kaempferide, and Norbergenin). In 96-well culture plates, cells were plated at densities of 3500 and 5000 cells per well. The medium was replaced with fresh media after overnight incubation, and the compounds were added to achieve the desired concentrations. After a 24-h incubation period, all groups were tested for cell viability using the MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). MTT (5 mg/mL) was added to both cells at a rate of 20 g per well and incubated for 4 h. The culture plates growth medium was then removed, and the formazan crystals were dissolved in dimethyl sulfoxide. For quantitative analysis, a Multiwell Microtitre Spectrophotometer was used. The percentages of viable cells are calculated using absorbance intensities and based on the survival of the control group. Each experiment was carried out three times.
2.1.3 Neuroprotective Effects of Phytocandidates
To test the neuroprotective effects of the flavonoids kaempferide and norbergenin on SHS5YS cells, the cells were treated with a range of 0 to 50 µM for 24 h before being treated with 2 M β-amyloid (Aβ) for 48 h before the MTT assays. The PC-12 cells were pre-treated with test samples for 24 h before being exposed to 6- hydroxydopamine (6- OHDA) for an additional 48 h, and the cells were analyzed using the MTT assay. Following treatment, the cells were exposed to 5 mg ml-1 MTT for 4 h at 37 °C. After carefully removing the media, 100 µL DMSO (99%) was added to dissolve the formazan crystals formed. At 570 nm, the absorbance was measured. Cells were treated with 0.15% DMSO (control), 2 µM Aβ without prior treatment with test compounds (A control), and 10 mM 6-OHDA without prior treatment with test compounds (Aβ control) (OHDA control). To determine the neuroprotective effects of flavonoids kaempferide and norbergenin, against SHS5YS cells, the cells were treated in the range of 0 to 50 μM for 24 h, and were further subjected to treatment with 2 µM β-amyloid (Aβ) for 48 h before the MTT assays. To analyze the neuroprotective effect, the PC-12 cells were pre-treated with test samples for 24 h, followed by exposure to 6-hydroxydopamine (6-OHDA) for an additional 48 h, and the cells were analyzed using MTT assay. After treatment, the cells were treated with 5 mg ml−1 MTT for 4 h at 37 °C. After the media were carefully removed, 100 μL DMSO (≥ 99%) was added to dissolve the formazan crystals formed. The absorbance was measured at 570 nm using a microplate reader (TECAN, Switzerland). Controls consisted of cells treated with 0.15% DMSO (vehicle control, VC) and cells treated with 2 µM Aβ without prior treatment with test compounds (Aβ control), and cells treated with 10 mM 6-OHDA without prior treatment with test compounds (OHDA control).
2.1.4 Anti-apoptotic Studies
To understand the anti-apoptotic effect of selected phytocandidates, the cells (2 mL) at a density of 5 × 104 were grown in 40-mm Petri dishes and allowed to attach for 24 h. For SHSY5Y cells, after which cells were treated with the EC100 of KPD and NRG for 24 h and were further subjected to treatment with 2 µM β-amyloid (Aβ) for 48 h before the apoptotic analysis. To analyze the anti-apoptotic effect, the PC-12 cells were treated with KPD and NRG at EC100 for 24 h, followed by exposure to 6-hydroxydopamine (6-OHDA) for an additional 48 h, and the cells were analyzed using MTT assay. After the various treatment periods, cells were harvested and centrifuged at 1000 g for 10 min. The supernatants were washed in 1% PBS and resuspended in Annexin V binding buffer. The cells were centrifuged at 1000 g for 10 min and supernatants were discarded. The cell extracts were suspended in 100 μL Annexin V binding buffer and 5 μL Annexin V Alexa Fluor 488 was added and allowed to incubate in the dark for 15 min. PI (4 μL) diluted in 1 × Annexin V binding buffer (1: 10) was added and allowed to incubate for 15 min in the dark at room temperature. Annexin V binding buffer (500 μL) was added to wash the Annexin/PI stained cells. Annexin/PI was evaluated according to the previously described method on a Becton Dickinson FACScan instrument (BD Biosciences Pharmingen, San Diego, CA, USA) fitted with a 488 nm argon laser. A minimum of 10,000 cells per sample were acquired and analyzed using CellQuest Pro software.
2.1.5 Cellular Reactive Oxygen Species (ROS) Activity
To study the effect of KPD and NRG on the production of cellular reactive oxygen species induced by Aβ in SH-SY5Y cells and 6-OHDA in PC-12 cells, were pre-treated with KPD and NRG at EC100 for 24 h before the addition of 2 µM Aβ in SHSY5Y cells and 10 mM of 6-OHDA in PC-12 cells for another 48 h. The effect of KPD or NRG on Aβ/6-OHDA induced production of reactive oxygen species was assessed using the Dichlorodihydroflourescein diacetate (DCFDA)-cellular reactive oxygen species detection assay kit. The treated cells were stained with DCFDA solution for 45 min at 37 °C. The fluorescence at Ex/Em = 485/535 nm was measured using a microplate reader.
2.2 In-Vivo Studies
2.2.1 Animals and Treatments
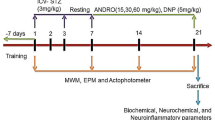
Fourty two (42) adult Wistar male and female rats weighing 120–180 g were used in all experiments of this study. Rats were purchased from the animal house of the National Institute of Nutrition, Hyderabad, India, and were housed in the animal house of Maharajah’s Pharmacy College, Vizianagaram under standard housing conditions (Room temperature 24–27 °C and 60% humidity) with 12-h light and dark cycles. Animals were fed standard laboratory pellets (20% proteins, 5% fats, and 1% multivitamins) with water ad libitum. All animal procedures were performed in accordance with the guidelines of the Institutional animal ethics committee (IAEC) for animal handling at GITAM School of Pharmacy, Visakhapatnam (Approval No. IAEC/GIP-1287/GSS-F/APPROVED/4/2019–2020). Animals were randomly divided into 7 experimental groups of six animals each (n = 6 in each experimental group).
The D-gal Study group is divided as follows:
-
1.
Group 1 (Normal): Rats received normal Phosphate buffer saline every day for 45 days.
-
2.
Group 2 (Diseased group): Administered with D-galactose at 200 mg/kg body wt, orally once daily for 45 days.
-
3.
Group 3 (Standard group): Donepezil (3 mg/kg body wt.) orally once daily 30 min before the administration of D-galactose.
-
4.
Group 4 (Test group 1): Kaempferide (5 mg/kg body weight) was administered orally for a week prior to D-galactose being administered for 45 days thereafter.
-
5.
Group 5 (Test group 2): Kaempferide (10 mg/kg body weight) was administered orally for a week prior to D-galactose being administered for 45 days thereafter.
-
6.
Group 6 (Test group 3): Norbergenin (5 mg/kg body weight) was administered orally for a week prior to D-galactose being administered for 45 days thereafter.
-
7.
Group 7 (Test group 4): Norbergenin (10 mg/kg body weight) was administered orally for a week prior to D-galactose being administered for 45 days thereafter.
2.2.2 Behavioral Examination
In this study, all behavioral examinations were performed from the 46th day. All behavioral tests were conducted at the same time of the day (9:00 am to 6:00 pm) for 7 days.
-
1.
Morris water maze (MWM) test
MWM is carried out as previously described and was used to assess rats learning and memory abilities Sun et al. [16]. The experimental apparatus, a circular water tank (120 cm in diameter, 50 cm in height), was filled to a depth of 30 cm with water (22° C). The water was made opaque by using nontoxic black carbon ink. The water maze was divided into four equal quadrants conceptually (east, south, west, and north). A black platform (10 cm in diameter and 29 cm in height) was submerged 1 cm below the water's surface and placed in the east quadrant's midpoint. The rats were placed in the water with their backs to the pool wall at one of three starting quadrant points (south, west, and north). They were allowed to swim for 60 s to find the hidden platform and rest for 15 s on it. Rats who did not reach the platform in the allotted time were gently guided there and allowed to rest for 15 s. The time it took to reach the platform was measured. The time spent in the target quadrant, distance moved in the target quadrant, platform crossing number, and time of first crossing the platform were all recorded.
-
2.
Randall–Selitto paw pressure test
This method was used to assess the mechanical nociceptive threshold, an index of mechanic hyperalgesia [17]. The Randall-Selitto paw pressure device was used to measure the nociceptive flexion reflex. The nociceptive threshold was determined by calculating the time to withdraw the hind paw.
2.2.3 Brain Tissue Sampling and Preparation
Thiopental was used to anaesthetize the rats before they were sacrificed via cervical dislocation. Each rat's entire brain was quickly placed on ice and then dissected from the olfactory bulb to the cerebellum. After that, it was rinsed with isotonic saline and dried on filter paper. Each brain was sagittally divided into two sections. The first part of the brain (right hemisphere) was kept at – 80 °C for biochemical analysis. The second section was fixed in 10% formalin for histopathological studies (left hemibrain).
2.2.4 Estimation of Total Protein Content
The carbonyl group of protein molecules reacts with copper and potassium reagents and forms a blue-coloured, potassium-biuret complex. This complex together with tyrosine and phenolic compounds present in the protein reduce the phospho-molybdate of the folin reagent to intensify the color of the solution (Lowry method).
To precipitate the protein, 100 mg of brain tissue homogenate (wet weight) was mixed with 10% TCA (Trichloroacetic acid). For 5 min, the sample was centrifuged at 3000 rpm. The supernatant was thrown away. In 1N NaOH, the precipitate was redissolved. 5 ml of reagent C was added to this and thoroughly mixed before being left undisturbed for 10 min. To this, 0.5 ml of Folin phenol reagent was added, thoroughly mixed, and left for 30 min. As standard and blank, 1% BSA and 1N NaOH were used. The developed blue color was measured at 650 nm using a UV–visible double beam bio spectrophotometer (Elico), an autoanalyzer that automatically quantifies the protein content in the sample Tables 1, 2 and 3.
2.2.5 Activities of SOD, GSH-Px, MDA, CAT, T-AOC Levels in the Brain
To determine whether KPD and NRG attenuated oxidative damage in the brain tissue of D- galactose-treated rats. We measured the activities of SOD, GSH-Px, MDA, and CAT along with T- AOC levels in the brain tissue (shown in Table 4). SOD, GSH-Px, CAT as well as T-AOC were significantly lower in positive control rats than in-vehicle control rats. D-galactose treatment reduced the level of MDA. Supplementation with Prunetin effectively treated the brain damage caused by D-galactose. The acid reduction method developed by Green et al. was used to determine the amount of nitric oxide (NO) present in the brain (1982). In this method, the brain homogenate was immersed in an acidic medium until nitrous acid diazotized sulphanilamide coupled with N-(1-naphthyl) ethylenediamine was formed when nitrite was added. The resultant azo dye with its bright reddish-purple colour could be measured at 540 nm.
2.2.6 Acetylcholinesterase Assay
The AChE activity was measured using the Ellman method as shown before using the brain homogenates of the control and treated animals as a source of cholinesterase enzyme. The brain homogenate was prepared from the right cerebral hemisphere cortical and hippocampal areas.
2.2.7 Histological Investigation
The portion of the rats’ brains was used for histopathologic examination, by fixation with 10% buffered-saline formalin. Washing was done in tap water then serial dilutions of alcohol were used to dehydrate. Specimens were cleared in xylene and embedded in paraffin at 56° in a hot air oven for twenty-four hours.
Paraffin beeswax tissue blocks were prepared for sectioning at 4 microns' thickness by slide microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained by cango red stains for examination of histology [18].
2.2.8 Western Blot Analysis
Proteins from brain homogenates were extracted and quantified using the Lowry method. Following Singh et al., Western blotting for proteins Amyloid1-42, phosphorylated Tau, Caspase 3, Bax, Bcl2, CAT, SOD1, SOD2, and the housekeeping protein -actin was performed (2018). The following primary antibodies were used: anti-A1-42 and anti-p-Tau (Beijing Biosynthesis Biotechnology Co., Ltd.), mouse monoclonal caspase-3 p17 (1:1000; Santa Cruz Biotechnology), and anti-Bax (Beijing Biosynthesis Biotechnology Co., Ltd.).(1:400, Cell Signaling Technology, Danvers, MA, USA), anti-Bcl-2 (1:400, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-actin (1:400, Santa Cruz Biotechnology, Santa Cruz, CA, USA) (1:50,000; Millipore). Secondary antibodies were goat anti-rabbit secondary antibody (1:20,000) and horseradish peroxidase-conjugated antimouse antibody (1:20,000). (Both Santa Cruz Biotechnology). The membranes were then washed three times in TBST for 15 min. The protein bands were visualized using a Western blotting technique based on chemiluminescence.
2.2.9 Statistical Analysis
The data were represented as mean standard deviation. One-way analysis of Variance (ANOVA) with Graph Pad instant demo version was used for statistical evaluation, and a P-value of 0.05 was considered statistically significant Fig. 1.
3 Results
3.1 In-Vitro Studies
3.1.1 Cell Culture
3.1.2 Cell Viability Assessment
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) is the gold standard for determining whether or not cells are in a viable environment.Among the five molecules examined, Kaempferide and Norbergenin exhibited the lowest cellular cytotoxicity, i.e., the lowest cell viability in both cells. Furthermore, Piceatannol showed the greatest cytotoxicity against PC-12 and SHSY5Y cells.
Both Prunetin and Isookanin displayed intermediate effects against PC-12 and SHSY5Y cells when compared with other molecules (represented in Table 1).
3.1.3 Neuroprotective Effects of Phytocompounds Against Degenerative SHSY5Y Cells
In the present study, the effect by Aβ treated SHSY5Y cells showed a significant reduction of cell viability with 37.86 ± 2.9% and 6-OHDA treated PC-12 cells showed reduced viability of 43.58 ± 3.9%, whereas DMSO-treated cells showed around 99% viability in both cells, indicating that vehicle had no influence on the growth and metabolism of cells (Table 2 and Fig. 2).
3.1.4 Anti-apoptotic
-
(a)
Anti-apoptotic assay against SHSY5Y cells
The live cell percentage of SHSY5Y cells treated with NRG and KPD increased to 84.1 ± 5.69% and 77.6 ± 4.22% respectively when compared to SHSY5Y cells that had not been treated or normal control cells. According to these results, the total percentage of live cells completely recovered to a level comparable to that of undifferentiated SHSY5Y cells over the treatment of NRG at EC100 (Fig. 3).
-
(b)
Anti-apoptotic assay against PC 12 cells
The live cell percentage of PC-12 cells treated with NRG and KPD increased to 79.1 ± 25.32% and 77.2 ± 9.35% respectively when compared to PC-12 cells that had not been treated or normal control cells. According to these results, the total % of live cells completely recovered to a level comparable to that of undifferentiated PC-12 cells over the treatment of compound NRG at EC100 (Fig. 4).
3.1.5 Cellular Reactive Oxygen Species Activity
Pre-treatment of SHSY5Y cells with KPD or NRG at EC100 decreased ROS production in response to 2 µM Aβ addition to 109.1 ± 8.6% and 111.6 ± 7.6% respectively, demonstrating that KPD is more effective than NRG. While there were no statistically significant differences between the KPD and NRG treatment groups (p > 0.05), a slightly higher NRG concentration was needed to achieve the same level of ROS suppression as in the KPD group (Table 3).
PC-12 cells treated with 6-OHD (6OHDA_PC-12 cells; 142.5 ± 5.3%) produced more cellular reactive oxygen species than vehicle control (99.8 ± 4.1%). Pre-treatment of PC-12 cells with KPD or NRG at EC100 decreased ROS production in response to 2 µM Aβ addition to 106.8 ± 8.3% and 109.7 ± 7.4% respectively, demonstrating that KPD is slightly more effective than NRG (represented in Table 3).
3.2 In-Vivo Studies
3.2.1 Behavioural Examination
3.2.1.1 Morris Water Maze Test
These results demonstrated that the effect of KPD and NRG in at higher dose of 10 mg/kg bw showed learning and memory gaining in prophylactic dose which was found to be superior to the effect showed by the standard control i.e. donepezil Figs. 5,6,7,8,9,10 and11; Tables 4, 5, 6, 7 and 8.
All data were expressed in Mean ± SEM, n = 6. ##P < 0.01 versus control, **P < 0.01 versus disease control group.
3.2.1.2 Randall Selitto Test
These results demonstrated that KPD and NRG, at their prescribed doses, significantly improved the management of neuropathic pain of animals with D-Gal-induced neurodegeneration. It was found that in both KPD and NRG groups, neuropathic pain was significantly managed at their higher doses i.e. 10 mg/kg bw when p < 0.05 was considered.
3.3 Biochemical Examination
3.3.1 Total Protein Content
3.3.2 Activities of SOD, GSH-Px, MDA, CAT, T-AOC Levels in the Brain
To determine whether KPD and NRG attenuated oxidative damage in the brain tissue of D- galactose treated rats. We measured the activities of SOD, GSH-Px, MDA, CAT along with T- AOC levels in the brain tissue (shown in Table 4). SOD, GSH-Px, CAT as well as T-AOC were significantly lower in positive control rats than in-vehicle control rats. D-galactose treatment reduced the level of MDA. Supplementation with KPD effectively treated the brain damage caused by D-galactose.
3.3.3 Ach. E activity
3.3.4 Histopathological Examination
3.3.5 Western Blot Analysis
4 Discussion
In this research, we studied the neuroprotective effect of natural molecules—Kaempferide and Norbergenin against D-galactose-induced brain aging in Wistar rats. KPD and NRG supplementation led to reversing the declined learning memory in D-galactose-induced brain aging rats. Our results showed that 45 days of administration of KPD and NRG attenuate the D-galactose-induced cognitive decline in rats.
Oxidative stress is the primary cause of brain aging, which leads to neurodegeneration [19]. Stress increases the number of reactive oxygen species, and increased ROS production damages the brain, particularly the hippocampus, which is responsible for learning and memory. Flavonoids are promising molecules with more beneficial and protective properties [20]. Various researchers reported that natural molecules with fewer side effects attenuated stress-induced, STZ-induced, and copper-induced neurodegeneration [21, 22]. In this study, we choose two phytocandidates to study the protective effect on Wistar rats. Based on the in-vitro results, we chose molecules for the in-vivo study.
Our in-vitro findings showed that Phytocandidates exert a protective effect against neurodegeneration induced by Aβ and 6-OHDA in cell lines. KPD 5 mg/kg and KPD 10 mg/kg exert a protective effect on neurotoxicity-induced PC-12 and SHSY5Y cell lines. Kaempferide 5 mg/kg and Kaempferide 10 mg/kg treatment significantly reduce Amyloid- and Tau protein levels in the brain. Norbergenin at 5 mg/kg and 10 mg/kg doses reduced oxidative damage, whereas Kaempferide 5 mg/kg and 10 mg/kg treatment significantly reduced oxidative stress compared to Norbergenin.
The levels of oxidative damage can be determined by measuring the activity of antioxidant enzymes such as CAT, SOD, and MDA. Antioxidant enzyme activities such as SOD, CAT, GSH-Px, and MDA were reduced. MDA is one of the lipid peroxidation markers. The most important indicator of oxidative damage. In the serum of rats exposed to D-galactose, CAT, SOD, and GSH-Px levels decreased while MDA levels increased.
Our findings showed that CAT, SOD, and GPx activity was increased while MDA levels were decreased in the serum of rats given kaempferide. Even at doses of 5 mg/kg and 10 mg/kg, kaempferide inhibits the oxidative damage caused by D-galactose.
According to recent research, cholinergic neurodegeneration is one of the most common causes of Alzheimer's disease and other neurodegenerative disorders. Acetylcholinesterase is an enzyme that breaks down acetylcholine into acetate and choline. AchE inhibition was used to treat the neurodegenerative disorder. NRG and KPD inhibit AchE activity while increasing the acetylcholine neurotransmitter, which is important in the treatment of neurodegeneration.
In the behavioral tests—Morris water maze test, Rotarod test, and Randall sellito test—kaempferide protected rats from D-galactose-induced brain aging. KPD improves D-galactose-induced rats' cognitive and memory abilities.
Neurodegeneration due to Aβ and Tau plaque formation in brain tissue is the common cause of Brain aging and Alzheimer's disease. In Western Blot analysis reports, kaempferide exhibits a protective effect in the brain by decreasing the production of Aβ and Tau Proteins (indicated in KPD treated Group). In the western blot image (control group), the thickness of the band indicates the formation of the protein Plaque.
Kaempferide's pharmacological activities include being a potent anti-oxidant, acting against cancer, cardioprotective, and anti-hypertensive [23]. All of these actions are mediated by modulating apoptosis and the inflammatory response, angiogenesis, and free radical production. Previous research has shown that Kaempferide isolated from C.odorata is effective against cervical cancer [24]. NRG has been reported to have antioxidant, anti-inflammatory, anti-viral, anti-microbial, and urinary system effects. In our study, KPD and NRG clearly show a protective effect against D-galactose-induced neurodegeneration.
We discovered that two flavonols reduce oxidative stress and inhibit caspase enzyme activity in-vitro. On the other hand, KPD and NRG exert anti-apoptotic activity, and aggregation of Amyloid-β and Tau proteins was inhibited. In-vivo studies reveal that KPD exerts potent anti-oxidant activity and inhibitory action on Acetylcholinesterase Enzyme. Both in-vitro and in-vivo studies indicated that Kaempferide shows a stronger effect than Norbergenin. KPD, as a natural molecule, has a neuroprotective effect by showing action on amyloid and Tau protein aggregation. This study adds to the neuroprotective research on kaempferide and norbergenin in neurodegenerative models.
5 Conclusions
Finally, present in-vitro and in-vivo findings show that Kaempferide administration significantly reduces oxidative stress, Acetylcholinesterase enzyme, Amyloid-β, and Tau protein levels in the brain. KPD and NRG boost antioxidant enzyme activity and reduce lipid peroxidation. Kaempferide, at a dose of 5 mg/kg, is also protective. Based on present research, by reducing oxidative damage, KPD has shown promising treatment for neurodegenerative conditions like Alzheimer's disease and brain aging. Additionally, molecular docking data encourages further research on the chemical kaempferide.
Abbreviations
- AGE:
-
Advanced glycation end products
- FBS:
-
Foetal bovine serum
- KPD:
-
Kaempferide
- NRG:
-
Norbergenin
References
Betthauser TJ et al (2020) Amyloid and tau imaging biomarkers explain cognitive decline from late middle-age. Brain 143:320–335
Moreno-Jiménez EP et al (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25:554–650
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57(4):1105–1121
Wrigglesworth Jo, Yaacob Nurathifah, Ward Phillip, Woods Robyn L, McNeil John, Storey Elsdon, Egan Gary, Murray Anne, Shah Raj C, Jamadar Sharna D, Trevaks Ruth, Ward Stephanie, Harding Ian H, Ryan Joanne (2022) Brain-predicted age difference is associated with cognitive processing in later-life. Neurobiol Aging 109:195–203. https://doi.org/10.1016/j.neurobiolaging.2021.10.007
Holland TM, Agarwal P, Wang Y, Leurgans SE, Bennett DA, Booth SL, Morris MC (2020) Dietary flavonols and risk of Alzheimer dementia. Neurology 94(16):e1749–e1756
Rehman SU, Shah SA, Ali T, Chung JI, Kim MO (2017) Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol Neurobiol 54:255–271
Nam SM, Hwang H, Seo M, Chang BJ, Kim HJ, Choi SH, Rhim H, Kim HC, Cho IH, Nah SY (2018) Gintonin attenuates D-galactose-induced hippocampal senescence by improving long-term hippocampal potentiation, neurogenesis, and cognitive functions. Gerontology 64:562–575
Islam MT (2017) Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol Res 39(1):73–82
Kim AC, Lim S, Kim YK (2018) Metal ion effects on Aβ and tau aggregation. Int J Mol Sci. https://doi.org/10.3390/ijms19010128
Muthumanickam S, Kamaladevi A, Boomi P, Gowrishankar S, Pandian SK (2021) Indian Ethanomedicinal phytochemicals as promising inhibitors of RNA-binding domain of SARS COVI-2 Nucleocapsid Phosphoprotein: An in-Silico study. Front Mol Biosci. https://doi.org/10.3389/fmolb.2021.637329
Spencer JP, Vauzour D, Rendeiro C (2009) Flavonoids and cognition: the molecular mechanisms underlying their behavioural effects. Arch Biochem Biophys 492:1–9. https://doi.org/10.1016/j.abb.2009.10.003
Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ (2013) NIH toolbox for assessment of neurological and behavioral function. Neurology 80(11 Suppl 3):S2–S6. https://doi.org/10.1212/WNL.0b013e3182872e5f
Chao J, Li H, Cheng KW, Yu MS, Chang RC, Wang M (2010) Protective effects of pinostilbene, a resveratrol methylated derivative, against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. J Nutr Biochem 21(6):482–489
Nath LR, Gorantla JN, Joseph SM, Antony J, Thankachan S, Darsan B, Menon DS, Sankar S, Ravi S, Lankalapalli RS (2015) Anto RJ Kaempferide, the most active among the four flavonoids isolated and characterized from Chromolaena odorata, induces apoptosis in cervical cancer cells while being pharmacologically safe. Royal Soc Chem 5:100912
Gowrishankar S, Muthumanickam S, Kamaladevi A, Karthika C, Jothi R, Boomi P et al (2021) Promising phytochemicals of traditional indian herbal steam inhalation therapy to combat COVID-19–An In Silico Study. Food Chem Toxicol 148:111966. https://doi.org/10.1016/j.fct.2020.111966
Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ et al (2007) Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Experim Neurol 204(1):264–272
Anseloni VC, Ennis M, Lidow MS (2003) Optimization of the mechanical nociceptive threshold testing with the Randall-Selitto assay. J Neurosci Methods 131:93–97
Bancroft's Theory and Practise of Histological Techniques (7th edition), October 2013. Publisher: Elsevier. ISBN: 978-0-7020-4226-3
Aherne SA, O’Brien NM (2002) Dietary flavonols: chemistry, food content, and metabolism. Nutrition 18:75–81
Jeong CH, Kwak JH, Kim JH, Choi GN, Kim DO, Heo HJ (2011) Neuronal cell protective and antioxidant effects of phenolics obtained from Zanthoxylum piperitum leaf using in vitro model system. Food Chem 125:417–422
Ning K, Zhao L, Franklin M, Matloff W, Batta I, Arzouni N, Sun F, Toga AW (2020) Parity is associated with cognitive function and brain age in both fe- males and males. Sci Rep 10(1):6100
Puig J, Blasco G, Daunis-i-Estadella J, Moreno M, Molina X, Alberich-Bayarri A, Xifra G, Pedraza S, Ricart W, Fernandez-Aranda F, Fernandez-Real JM (2016) Lower serum osteocalcin concentrations are associated with brain microstruc- tural changes and worse cognitive performance. Clin Endocrinol (Oxf) 84(5):756–763
Hong JT, Yen JH, Wang L, Lo YH, Chen ZT, Wu MJ (2009) Regulation of heme oxygenase-1 expression and MAPK pathways in response to Kaemperol and rhamnocitrin in PC12 cells. Toxicol Appl Pharmacol 237:59–68
Martineti V, Tognarini I, Azzari C et al (2010) Inhibition of in vitro growth and arrest in the G0/G1 phase of HCT8 line human colon cancer cells by kaempferide triglycoside from Dianthus caryophyllus. Phytother Res 24(9):1302–1308
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nalla, S., Ganta, S. Defensive Impact of Kaempferide Against Neurodegenerative Studies: In Vitro and In Vivo Investigations. Chemistry Africa 6, 2483–2493 (2023). https://doi.org/10.1007/s42250-023-00673-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00673-9