Abstract
Alzheimer's disease (AD) is a progressive, chronic and age-related neurodegenerative disorder that affects millions of people across the world. In pursuit of new anti-AD remedies, 2-[Hydroxy-(4-nitrophenyl)methyl]-cyclopentanone (NMC), a β hydroxyl ketone derivative was studied to explore its neuroprotective potentials against AD. The in-vitro AChE and BuChE enzymes inhibition were evaluated by Ellman protocol and antioxidant potentials of NMC by DPPH free radical scavenging assay. In-vivo behavioral studies were performed in the transgenic 5xFAD mice model of AD using shallow water maze (SWM), Paddling Y-Maze (PYM), elevated plus maze (EPM) and balance beam (BB) tests. Also, the ex-vivo cholinesterase inhibitory effects of NMC and histopathological analysis of amyloid-β plaques were determined in the frontal cortex and hippocampal regions of the mice brain. NMC exhibited significant in vitro anti-cholinesterase enzyme potentials with an IC50 value of 67 μg/ml against AChE and 96 μg/ml against BuChE respectively. Interestingly, the activities of AChE and BuChE enzymes were also significantly lower in the cortex and hippocampus of NMC-treated groups. Also, in the DPPH assessment, NMC displayed substantial antioxidant properties with an IC50 value observed as 171 μg/ml. Moreover, histopathological analysis via thioflavin-s staining displayed significantly lower plaques depositions in the cortex and hippocampus region of NMC-treated mice groups. Furthermore, SWM, PYM, EPM, and BB behavioral analysis indicated that NMC enhanced spatial learning, memory consolidation and improved balance performance. Altogether, to the best of our knowledge, we believe that NMC may serve as a potential and promising anti-cholinesterase, antioxidant and neuroprotective agent against AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD) is a chronic, age-related and progressive neurological disease linked with dementia and memory disturbances [1]. AD affected 26.6 million patients worldwide in the year 2006 and the number of patients increases as the population ages and 1 in 85 persons will be affected by the year 2050 [2]. The formation of Aβ plaques, synaptic loss and Neurofibrillary tangles (NFTs) development is the most important neuropathological hallmarks of AD [3]. AD has multifactorial pathogenesis and mostly occurs due to the accumulation of amyloid-β (Aβ) plaques, deficiencies of neurotransmitters such as acetylcholine, neuro-inflammations, oxidative stress-induced neuronal damage, tau hyperphosphorylation and synaptic loss [4, 5]. The Aβ plaques form due to the successive cleavage of APP protein by γ-secretase and β-secretases enzymes. An increase amassing of Aβ results in the worsening and decline of cholinergic neurons in the brain regions and is thoroughly associated with cognitive dysfunction [6, 7]. Reducing the level and formation of Aβ plaques is an important therapeutic approach toward AD. Acetylcholine has a key part in the cognitive functions and inhibitors of AChE enhance the level of acetylcholine. Therefore inhibitors of AChE are another effective therapeutic strategy against AD [8]. Antioxidants also contribute to a therapeutic strategy that aid in the slowing and reduction of development of AD and other neurological disorders [9, 10].
Two groups of drugs were approved by the Food and Drug Administration (FDA) for the management of AD. One is AChE inhibitors which include well-known drugs; Galanthamine, rivastigmine, donepezil and tacrine which are mostly prescribed in clinical settings for the symptomatic treatment of mild to moderate AD [11]. Another group of drugs is N-methyl D aspartate (NMDA) receptor antagonist which comprise of only memantine, prescribed in the clinical setting for the management of moderate to severe type of AD [12]. Memantine is an uncompetitive NMDA receptor antagonist that reduces neuronal excitotoxicity [13]. These drugs are used for the symptomatic treatment of AD and hence no effective treatments exist to manage AD efficiently [14]. As the available AChE inhibitors display neuroprotective effects against neurodegeneration but several associated adverse effects minimize its usefulness in the clinical settings. Therefore further research is required to developed and improve anti-AD drugs to effectively cure AD [15].
Recently, hydroxyl ketones and their derivatives grabbed the interest of researchers in the field of medicinal research because of their fascinating pharmacological properties [16]. Literature search has shown that Hydroxyl ketones are important and noteworthy functionality in medicines, predominantly used in the production of different drugs particularly Antibiotics like tetracycline, Antifungal agents like amphotericin B and Statins like atorvastatin. Hydroxyl ketones possess important antioxidant properties, anti-inflammatory, and is also notable for the design of anticancer drugs [17]. A β-hydroxyl carbonyl functionality is also very common in several natural products and is the building block of numerous remarkable synthetic compounds including pheromones, antibiotics and many other well-known pharmaceutically active compounds. The hydroxyl ketones were readily proceeded to amino alcohols, syn-diols, anti-diols and to other crucial functional groups in biomedical research [18]. The aldol derivative like atorvastatin abolished the effect of anti-Aβ plaques and anti-inflammatory responses which suggest the potential mechanism involved in demonstrating its anti-inflammatory effects [19].
Keeping in mind the interesting pharmacological properties of hydroxy ketone derivatives with neuroprotective and antioxidant potential, the study was designed to explore the selected synthetic β hydroxyl ketone derivative i.e. NMC for prospective usefulness in the treatment of AD (Fig. 1a).
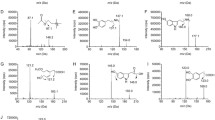
a Chemical structure of the test compound (NMC). b PCR image of Tg APP. The double bands in the figure confirm the presence of transgene at 377 bp and the other 324 bp of internal positive control. While the samples showed a lack of transgene 377 bp band is considered as non-Tg mice. c Schematic representation of experimental design showing the duration of administration of different chemicals/drugs in experimental mice groups and behavioral studies conducted
Material and methods
Reagents/chemicals
Acetylthiocholine Iodide (Sigma Aldrich, UK), Acetylcholinesterase (AChE) from Electrophorus electricus (Sigma Aldrich St, Louis, MO, USA), Ascorbic acid (Sigma-Aldrich, Germany), Butyrylthiocholine iodide (Sigma Aldrich Switzerland), Butyrylcholinesterase (BuChE) derived from equine serum (Sigma Aldrich GmbH, Germany). Galanthamine HBr (Sigma Aldrich, France), 5,5-dithiobis-2-nitrobezoic acid DTNB (Sigma Aldrich, Germany), 2,2-diphenyl-2-picrylhydrazyl DPPH (Sigma Aldrich St, Louis, MO, USA). Dimethyl sulfoxide (99.5% DMSO UniChem Greenville, US). Ethanol (Merck, Germany), Tween 80 (Scharlu, Barcelona, Spain). Thioflavin S (Sigma Aldrich St, Louis, MO, USA). Potassium phosphate monobasic KH2PO4 (Sigma-Aldrich, USA), Potassium hydroxide KOH (Sigma-Aldrich, USA), Potassium phosphate dibasic K2HPO4 (Sigma-Aldrich, USA), Xylene (Thermo Fisher Scientific, USA), DNA extraction Kit (Novel genomic DNA mini Kit), Agarose (Invitrogen, US), Boric Acid (Sigma-Aldrich USA), DNA ladder (Serva CAT; 15165, Germany), DNTPs (Promega, US), Ethidium Bromide (Sigma Aldrich St, Louis, MO, USA), MgCl2 (Invitrogen, US), Primers for PCR (Thermo Scientific, US), Thermus aquaticus polymerase (Thermo Scientific, US), Tris (Invitrogen, USA), Distill water of PCR grade (Thermo Scientific, USA,), NaCl (Invitrogen, USA), 2X PCR Master Mix (Fermentas, Lithuania), Tris–EDTA (Sigma-Aldrich USA), ATChl (Sigma-Aldrich, USA), BTChl (Sigma-Aldrich, USA). All chemicals were of analytical grade and obtained from certified suppliers in Pakistan.
Animals
Transgenic 5XFAD (Tg) male mice (6–7 months age) were used (Jackson Lab, Bar-Harbor, US; strain name B6C3Tg (APP Swe, PSEN 1dE9). 5XFAD mutation includes Swedish (APP KM670 / 671NL), Florida (APP 1716 V), London (APP V717I), PSEN1 M146L, PSEN1 L286V that leads to the early formation of Aβ, neuroinflammation and cognitive deficits. The letter of authorization was granted by Jackson laboratory, USA to exploit these Tg mice only for the research purpose. All Tg mice were housed in cages under conditions of 12 h light/dark cycle. The animals analyzed for genotyping, and the animals which lack transgene were considered as wild type and were utilized as normal mice. All Tg mice were provided with water and food ad libitum. The behavioral studies were performed in a separate experimental room. Both rooms were maintained on a standard laboratory condition. All experiments were performed according to the Animals Scientific Procedure Act UK 1986 and according to ethics and rules of the institutional Ethical Committee granted with vide reference number 25/EC-18/Pharm, dated. 16/10/2018.
Genotyping of 5XFAD transgenic (Tg) mice
Genotyping of all animals was done to confirm the generic APP transgene existence by following the previous protocol [20]. The mice which lack transgene were considered as wild and used as normal mice (Fig. 1b).
Animals grouping and drug administration protocol
Animals were separated into six groups with each group comprised of six mice. Group one is a non-Tg wild type (WT) mice receiving normal saline intraperitoneally. Group two consists of Tg mice receiving normal saline intraperitoneally. Group three comprised of Tg mice receiving standard Galanthamine at a dose of 8 mg/kg intraperitoneally. Group four consists of Tg mice receiving test compound NMC at a dose of 15 mg/kg intraperitoneally. Group five consists of Tg mice receiving test compound NMC at a dose of 30 mg/kg intraperitoneally. Group six also consists of Tg mice receiving intraperitoneally test compound NMC at a dose of 45 mg/kg. NMC was dissolved in a vehicle comprised of DMSO, Tween 80 and Normal saline in a ratio of 5:1:94. All drugs solutions were freshly prepared before drug administration. Drugs were administered intraperitoneally, once daily, for a total of 4 weeks to the respective groups (Fig. 1c).
in-vitro cholinesterase inhibition assay
For cholinesterase inhibition activity, AChE and BuChE assays were performed for the assessment of in-vitro inhibition possibility of NMC using classical Ellman’s protocol [21]. Briefly, the standard drug Galanthamine and test compound (NMC) solutions were made in methanol in different concentrations (62.25 to 1000 μg/ml). The enzymatic solutions of AChE (518 U/mg) and BuChE (7–16 U/mg) were made in phosphate buffer and diluted with a final concentration of 0.03 U/ml and 0.01 U/ml respectively. The DTNB, ATchI and BTchI solutions were made in distilled water. 5 ml from each enzyme solution was added with successive adding of test samples and DTNB reagent. Then the solutions were placed at 30 °C for 15 min and at last substrates solutions were added. The absorbance was measured at 412 nm with UV visible spectrophotometer (Lambda 25, PerkinElmer, USA). Each sample reading was taken as triplicate under identical conditions. Percent enzyme activity and inhibition was calculated by the following formula;
In-vitro antioxidant DPPH radical scavenging assay
The 2,2-diphenyl-2-picrylhydrazyl (DPPH) free radicals scavenging potential of the test compound NMC were determined by the following reported procedure [22]. Briefly, the DPPH solutions were prepared in the methanol and kept in dark. The stock solutions of the test compound and standard having concentrations of 1 mg/ml were made in methanol and then diluted to concentrations of 62.25 μg/ml, 125 μg/ml, 250 μg/ml, 500 μg/ml and 1000 μg/ml. The diluted solutions from each sample were and then added to the DPPH solutions prepared in the methanol. Incubate the solutions for 30 min time and after incubation record the absorbance of the samples at 517 nm with a UV visible spectrophotometer. For comparison, ascorbic acid was used as standard and the solutions were prepared in identical concentrations as that of the sample. Each sample reading was taken as triplicate under identical conditions. The Percent radical scavenging which was calculated by the following mathematical formula;
Xo = Absorbance of Control while X1 = Absorbance of Sample.
The inhibition curves were made through GraphPad Prism program (GraphPad Prism, San Diego, California USA) and median inhibitory concentrations (IC50) values were determined.
Ex-vivo evaluation of cholinesterase inhibition in frontal cortex and hippocampus
After behavioral studies, all experimental mice were sacrificed after ether anesthesia. The frontal cortex and hippocampus were separated from the brain in an ice-cold phosphate buffer saline (0.1 M). The hippocampal and frontal cortex tissue was then homogenized by using homogenizer in phosphate buffer saline. The tissues were then centrifuge 1000×g for 10 min at 4 °C. The supernatant was used for the cholinesterase enzyme following an Ellman protocol. Acetylcholinesterase activities were assessed in the frontal cortex and hippocampus of mice brain homogenates which were standardized for the protein contents (5 mg/ml) [23].
Ex-vivo DPPH free radical’s scavenging assay
The Dpph scavenging assay of the frontal cortex and hippocampus brain homogenates were performed for the evaluation of the antioxidant activity. Briefly, the brain tissue homogenates of the hippocampus and frontal cortex of all mice groups were homogenized in one ml methanol through continual addition of 0.4 ml Dpph solution (0.1 mM). The solutions mixtures were then incubated at 37 °C for 30 min and the absorbance was taken with a UV visible spectrophotometer at 517 nm [24].
Shallow water maze behavioral tests
The shallow water maze (SWM) apparatus (MK2/Octagonal) is a paddling pool consists of eight exits. Among these 8 exits, one is the true exit and the other seven being false exits which are closed by plugs made up of plastic materials. The one exit of the apparatus was opened into a plastic pipe which is detached so that animal can be carefully shifted to their corresponding cages. There was a shallow water level (2 cm) in the tub and maintained at 20–25 °C to offer an escape stimulus to the animals. The apparatus was placed in the experimental room and many visual cues like colorful charts or pictures were placed exterior to the maze that could be used for spatial orientation. The place of the visual cues were maintained at the same place during whole experiments. The assignment and placement of mice were semi-random and the training and test trials were performed in triplicates during the daytime (9:0 am–4:0 pm). Mice were placed at the center of the apparatus in one of four positions on the perimeter (if the escape exit was on 6 o'clock, then 9, 12 or 3 o’clock positions were used). All experimental mice were trained in the shallow water maze apparatus for five consecutive days, three trials per day with 1 h interval. Similarly, test trials were performed in triplicates for five consecutive days after one hour of intraperitoneally drug administration to respective groups by allowing the animal in the tub for 60 s to find an escape platform. An escape latency time was noted for all groups of mice [20, 25, 26].
Y-maze behavioral test
The paddling Y-maze test is another experiment performed for evaluating learning and memory abilities in animals. The Y-maze apparatus was made up of Polystyrene having three arms of the apparatus with dimensions of 30 × 8 × 20cm. There are three exits among which one is a true exit which is linked with a detachable pipe through which the animals were safely transferred to their corresponding cages while the other two are false exits. There is a clean low water level about 2 cm in the maze that offers an escape stimulus to the animals towards the exit arm. The animals were trained for 5 consecutive days with three trials per day and 1 h apart from each other. After the training, test sessions were performed in triplicates for five consecutive days. Both training and test sessions were carried out during day time (9:0 am to 4:0 pm). In the test session, the mice were placed in one of the closed arms facing away from the center to move towards the safe exit of the y-maze apparatus. The escape latency time was noted. For spontaneous alternation behavior tests, the animals were kept in Y- maze to explore freely the apparatus for 8 min. The sequence and number of arm entries by animals were noted. Spontaneous alternations performance = (Successive triplet sets/Total number of arm entries − 2) × 100.
Elevated plus maze behavioral test
The elevated plus-maze test is a behavioral test used to assess learning and memory in mice. EPM consists of two open arms and two closed arms. Briefly, the mice were positioned at open arm end and the transfer latency time was noted. The mice were then allowed for 2 min to explore the apparatus. The retention trials were done for the learned task and determined after 24 h of day first trials [27].
Balance beam behavioral test
The balance beam apparatus consists of large wood or tubes. The start site of the beam will be lit while the other side keeps it dark, the soft foam will be placed under the beam so that if the animals fell, to minimize the injury. Each mouse was trained on the balance beam, to diminish neophobia. After drug administration, the animals were subjected to test sessions. The narrow beam crossing time of animals was noted [20].
Histopathological analysis (thioflavin-S staining)
After behavioral experiments, Tg mice were killed after ether anesthesia and carefully the intact brain was removed on ice plate, washed with normal saline, and fixed in neutrally buffered 4% paraformaldehyde solution. The standard routine processes were done for fixation, dehydration, paraffin embedding and cutting as previously described [28]. Briefly, the brain tissue was dehydrated through a series of ethanol solutions (50, 70, 80, 90 and 100%), followed by xylene (100%) and then impregnated with xylene paraffin overnight, afterward embedded in melted paraffin wax. The paraffin blocks were then sectioned coronally with a rotary microtome (SLEE Mainz CUT 5062 Germany) at 30 µm thickness. The brain sections were deparaffinized with xylene and then rehydrated with a graded series of ethanol before staining. Thioflavin S staining was performed by incubating deparaffinized and rehydrated sections with thioflavin S solutions for 15 min and then the slides were washed with ethanol (80% and 70% each for 1 min) and finally rinsed with distilled water twice. The slides were then mounted with mounting medium, sealed with a coverslip and kept in dark for 2 h. Finally, the slides were observed under a fluorescence microscope (10X objective). The thioflavin S stained plaques were determined using an image J software [29].
Statistical analysis
The data of all behavioral tests and the biochemical tests were calculated as Mean ± SEM. Data analyses were done by ANOVA followed by suitable post hoc tests and p < 0.05 was considered as significant. All statistical analysis was done with Graph-Pad Prism Version-5 software (GraphPad Software Inc San Diego, CA, US). For the shallow water maze and paddling Y-maze test the results were analyzed with two-way repeated measure ANOVA followed by post hoc Bonferroni analysis. Balance beam test, elevated plus maze test, Y-maze spontaneous alternations test and for all biochemical tests one-way ANOVA followed by post hoc Tukey’s test.
Results
NMC inhibits acetylcholinesterase and butyrylcholinesterase in-vitro
First of all, we examined the inhibitory effects of NMC on the AChE and BuChE which play an important role in the hydrolysis of acetylcholine and are associated with neurodegenerative disorders such as AD [30]. For this, we performed the in-vitro inhibition assay of AChE and BuChE (Table 1). The test compound NMC showed an upsurge in the percent inhibition of AChE and BuChE in a concentration-dependent manner. The inhibition of AChE by NMC observed at 62.25 μg/ml and 1000 μg/ml was 49% and 69% respectively. Galanthamine, used as a positive control, also showed concentration-dependent inhibition of AChE. The inhibition of AChE observed for Galanthamine at 62.25 μg/ml and 1000 μg/ml was 63% and 85% respectively. Similarly, NMC displayed an increase in the percent inhibition of BuChE in a concentration-dependent manner. The percent inhibition of BuChE at concentrations 62.25 μg/ml and 1000 μg/ml was 46% and 66%. The IC50 value of NMC for AChE inhibition was 67 μg/ml while that was 96 μg/ml for the inhibition of BuChE. Similarly, the IC50 value of standard Galanthamine for AChE inhibition was 21 μg/ml and 46 μg/ml for inhibition of BuChE. These results indicated that NMC has significant potential to inhibit AChE and BuChE.
In-vitro free radical scavenging potentials of NMC
Next, to evaluate the antioxidant effects of NMC, we performed in-vitro DPPH free radical scavenging assay. We used ascorbic acid as a standard antioxidant and free radical scavenger in this case (Table 2). The test compound NMC showed an upsurge in the % inhibitions of free radicals in a concentration-dependent manner. The % inhibition of free radicals noticed at a concentration of 62.25 μg/ml was 37% and at 1000 μg/ml was 67%. Similarly, the IC50 value of NMC for the inhibition of free radicals was 171 μg/ml. The ascorbic acid, used as a positive control, also displayed an upsurge in the % inhibitions of free radicals in a concentration-dependent manner. The % inhibition of radical scavenging observed at a concentration of 62.25 μg/ml was 56% and at 1000 μg/ml was 86%. Similarly, the IC50 value ascorbic acid for the inhibition of free radicals was 38 μg/ml. These findings support the notion that NMC exhibits remarkable free radical scavenging and antioxidant properties.
NMC reduced the AChE and BuChE enzyme activity in frontal cortex and hippocampus tissues ex-vivo
To support our in-vitro outcomes, we performed ex-vivo cholinesterase assays in the frontal cortex and hippocampus of the Tg mice model of AD. The NMC produced noteworthy changes in the AChE and BuChE enzyme inhibition in the frontal cortex and hippocampus regions of the brain (Fig. 2a–d). The Tg-saline group displayed a significant increase (p ≤ 0.001) in the AChE activity in the frontal cortex and (p ≤ 0.01) hippocampus regions while revealed a significant increase in BuChE activity (p ≤ 0.001) in both cortical and hippocampus of the brain tissue lysates. The Galanthamine-treated group showed a significant decline in the AChE (p ≤ 0.01) and BuChE (p ≤ 0.01) enzyme activity in the tissue lysates. Although, the NMC-treated Tg-group at a dose of 15 mg/kg exhibited less inhibition of the frontal cortex and hippocampal AChE and BuChE enzyme activities. On the other hand, NMC at doses of 30 and 45 exerted significant reduction of AChE activity in the frontal cortex (p ≤ 0.05) and at 45 mg/kg in the hippocampus (p ≤ 0.05). However, NMC only at a dose of 45 mg/kg significantly (p ≤ 0.05) reduced the BuChE enzyme activity in the frontal cortex and hippocampus tissue lysates. These results supported the in-vitro outcomes of the NMC effects on AChE and BuChE enzymes suggesting that NMC might be a potential cholinesterase inhibitor.
Protective effects of NMC on the AChE\ and BuChE enzymes and free radical scavenger in the frontal cortex and hippocampal tissue lysates of mice. a Percentage of AChE inhibition in the frontal cortex. b Percentage of AChE inhibition in the Hippocampus. c Percentage of BuChE inhibition in the frontal cortex. d Percentage of BuChE inhibition in the Hippocampus. e Percentage DPPH scavenging of free radicals in the frontal cortex. f Percentage DPPH scavenging of free radicals in the Hippocampus. The results are expressed as Mean ± SEM and were analyzed by one-way ANOVA followed by post hoc Tukey’s analysis. The p ≤ 0.05 value was considered statistically significant. The symbols *p ≤ 0.05 and **p ≤ 0.01 as compared to the Tg-saline group while ###p ≤ 0.001 as compared to WT-saline administered group
NMC mitigated the free radical scavengers activity ex-vivo
Similarly, to confirm the in-vitro outcomes of NMC on free radical scavengers, we performed ex-vivo DPPH assay (Fig. 2e, f). The % DPPH level was significantly elevated (p ≤ 0.001) in the frontal cortex and hippocampus of the Tg saline-treated group as compared to the WT-saline group. However, treatment with standard Galanthamine reduced the DPPH radicals level significantly (p ≤ 0.001). On the contrary, the NMC-treated Tg group at 30 and 45 mg/kg exerted a substantial reduction in free radicals in the frontal cortex (p ≤ 0.05 & p ≤ 0.01) and hippocampus (p ≤ 0.05) respectively. From these findings, we concluded that NMC might ameliorate the oxidative brain damage as reflected by its potentials in reducing the levels of free radical scavengers.
Effects of NMC on frontal cortex and hippocampus Aβ plaques
We further proceeded to evaluate the level of Aβ plaques which is a typical pathological hallmark of AD. As expected, the results of the histopathological analysis of the WT-saline group showed no Aβ plaques deposition in both the frontal cortex and hippocampus regions of the brain (Fig. 3). However, the Tg-saline administered group showed robust and clear (p ≤ 0.001) Aβ plaques in both the frontal cortex and hippocampus as compared to the WT-saline group. On the other hand, the Tg-Galanthamine groups displayed a significant decrease (p ≤ 0.05) in the Aβ plaques load in the frontal cortex and hippocampus regions compared to the Tg-saline group. The NMC-treated group at 30 mg/kg dose exhibited a little reduction in the Aβ plaques load. However, NMC at 45 mg/kg dose displayed a significant decline (p ≤ 0.05) in Aβ plaques formation in the frontal cortex while showed a decrease (but not significant) in the hippocampal Aβ plaques load as compared to the Tg-saline group.
Representative images showing the protective effects of NMC on Aβ plaques in the frontal cortex and hippocampus of Tg mice model (n = 6 mice per group). a Frontal cortex region of the WT-saline group. b Frontal cortex region of Tg-saline group. c Frontal cortex region of Tg-Galanthamine treated group. d–f Frontal cortex region of Tg-NMC 15 mg/kg, 30 mg/kg & 45 mg/kg dose treatment groups respectively. g Hippocampus region of the WT-saline group. h Hippocampus region of Tg-saline group. i Hippocampus region of Tg-Galanthamine treatment group respectively. j–l Hippocampus region of Tg-NMC 15 mg/kg, 30 & 45 mg/kg dose treated groups. m Shows integrated density of Aβ plaques in the frontal cortex & hippocampus regions of the brain in different experimental groups. The results are expressed as Mean ± SEM and were analyzed by one-way ANOVA followed by post hoc Tukey’s analysis. The p ≤ 0.05 value was considered statistically significant. The symbols *p ≤ 0.05 as compared to the Tg-saline group while ###p ≤ 0.001 as compared to WT-saline administered group. (Scale bar 50 µm)
Effects of NMC on learning and memory formation of Tg mice model
To evaluate the effects of NMC on learning and memory formation, we performed SWM behavioral analysis. In the SWM test an escape latency time was noted for all mice groups. The SWM results revealed that the saline-treated Tg group displayed a significant (p ≤ 0.001) increase in escape latency time from day1 to day 5 as compared to the WT saline-treated group. The Galanthamine (standard)-treated group displayed noteworthy diminution (p ≤ 0.001) in escape latency time from day 1 to day 5 compared to the Tg saline-treated group. The NMC-treated group at a dose of 15 mg/kg resulted in a significant (p ≤ 0.05) reduction of escape latency from day 1 to day 5 in comparison with the Tg saline-treated group. The test compound NMC at a dose of 30 mg/kg exhibited a substantial decrease ( p ≤ 0.05) in the escape latency time on day 1 to day 5 in comparison with the Tg saline group. Similarly, NMC at a dose of 45 mg/kg has shown a significant decrease (p ≤ 0.01) in escape latency time from day 1 to day 5 in comparison with the Tg saline group (Fig. 4a).
Effects of NMC on learning, memory and sensorimotor behaviors. a NMC significantly attenuated learning and memory consolidation during the SWM task in different experimental mice groups. b Histogram representing the effects of NMC on escape latency in Paddling Y-maze. The data are presented as the mean ± SEM of 6 mice per group and were analyzed by two-way ANOVA followed by post hoc Bonferroni analysis. c Effects of NMC on the spontaneous alternation behavior percentage in Y-maze. d and e represents initial and final latency respectively. f The effects of NMC in the BB test in different experimental groups. Data are presented as the mean ± SEM (n = 6 mice/group) and were analyzed by one-way ANOVA followed by post hoc Turkey analysis. The p ≤ 0.05 was statistically considered significant.. #p ≤ 0.05, ##p ≤ 0.01 and ###p ≤ 0.001 in comparison with WT saline-administered group, and *p ≤ 0.05, **p ≤ 0.01 & ***p ≤ 0.001 in comparison with Tg saline-administered group
NMC enhanced spatial learning and memory formation in Tg mice
Next, to check the effects of NMC on Spatial Learning and Memory formation, paddling Y-maze analysis was performed. The effects of NMC in paddling Y-maze showed that the saline-treated Tg group (p ≤ 0.001) presented elevated latency time on day 1 to day 5 in comparison with WT saline-treated group. The Galanthamine-treated group displayed a significant decrease (p ≤ 0.01) on day 1 while noteworthy diminution (p ≤ 0.001) from day 2 to day 5 in the escape latency time as compared to the saline-treated Tg group. The test compound NMC at a dose of 15 mg/kg exhibited significant reduction (p ≤ 0.05) at day 2 to day 5 in the escape latency time in comparison to the Tg saline-treated group. Similarly, NMC at a dose of 30 mg/kg showed a significant reduction (p ≤ 0.05) at day 1 to day 5 in the escape latency time in comparison to the Tg saline group. At a dose of 45 mg/kg, NMC showed a significant reduction (p ≤ 0.05) at day 1 while (p ≤ 0.01) at day 2 to day 5 in escape latency time in comparison to the Tg saline group (Fig. 4b). Furthermore, spontaneous alternation behaviors (SAB) percentage in Y-maze was assessed. The Tg saline group displayed a notable (p ≤ 0.001) decrease in the SAB% in comparison to the WT saline-treated mice group. The WT group exhibited an upsurge in the SAB% performance. The Galanthamine-treated group revealed noteworthy (p ≤ 0.01) improvements in the spontaneous alternation behavior. Interestingly, the NMC-treated group at various doses displayed a significant increase in SAB% as compared to the Tg saline-treated group (Fig. 4c). The effects of NMC in paddling Y-maze suggests that NMC has significant potential to improve spatial learning memory and exploratory behaviors.
NMC ameliorated learning & memory deficits-related behaviors of Tg mice model
We performed EPM for the assessment of NMC effects on learning and memory and the response of mice to a novel approach/avoidance situation by measuring their relative exploration of two distinct environments. The initial and final transfer latency times were noted for all the experimental groups. The Tg saline-administered group spent significantly (p ≤ 0.05) more time in initial transfer latency as compared to WT saline-administered group. The Galanthamine-treated group has revealed a significant (p ≤ 0.05) reduction in the initial transfer latency time as compared to the Tg group. Interestingly, the NMC-treated group at a dose of 45 mg/kg showed a marked reduction (p ≤ 0.05) in the initial transfer latency time as compared to the saline-treated Tg mice group (Fig. 4d). After twenty-four hours, the final transfer latency time was noted for all groups. The saline-administered Tg group again exhibited significantly (p ≤ 0.01) more time in retention transfer latency as compared to the WT group. The Galanthamine-treated group revealed a substantial (p ≤ 0.01) decrease in the retention transfer latency time as compared to the Tg saline-administered group. Again, the NMC-treated group at a dose of 15 and 30 mg/kg showed less retention time while at a dose of 45 mg/kg showed significant (p ≤ 0.05) decrease in the retention transfer latency time (Fig. 4e).
NMC improved motor coordination of Tg mice
Motor Coordination of the mice was evaluated by the BB test. In the BB test, the results indicated that there were no major significant differences observed between different groups for motor coordination, although the Tg-Galanthamine group, in comparison with the Tg-saline group, crossed the beam swiftly. The NMC treated group at a dose of 30 mg/kg and 45 mg/kg also showed a reduction in the crossing time but is non-significant as compared to Tg saline-administered group (Fig. 4f). The results also confirmed that the observed memory impairments in the mice model were not attributed to the differences in their motor and coordination.
Discussion
Keeping in mind the important role of β hydroxyl ketone in medicinal research, in this study, we aimed to evaluate the potential neuroprotective effects of synthetic β hydroxyl ketone derivative i.e. 2-[hydroxy-(4-nitrophenyl)methyl]-cyclopentanone (NMC). We performed in vitro and ex-vivo AChE and BuChE inhibition assays, and free radical DPPH scavenging assay to explore its cholinesterase inhibitory and antioxidant effects. Then, we assessed the effects of NMC on Aβ plaques which are the most important pathological hallmarks of AD. Finally, we performed the behavioral analysis to examine the NMC effects on cognitive deficits and motor coordination.
AD involves the dysfunctions of several neurotransmitters, predominantly in cholinergic pathways of the brain. The cholinergic hypothesis explains the key role of acetylcholine neurotransmitter in cholinergic pathways and the deficiency of acetylcholine play a key role in the pathophysiology of AD. Therefore prolonging the neurotransmitter acetylcholine functions by inhibiting the cholinesterase enzymes is one of the effective therapeutic approaches towards AD [31]. Similarly, BuChE is responsible for the hydrolysis of the acetylcholine neurotransmitter. Therefore, the inhibition of BuChE is also considered a useful therapeutic target in AD therapeutics. The inhibition of these cholinesterases also aids in slowing the development of amyloidogenic products providing important disease-modifying mechanisms and improved clinical outcomes [32]. It has also been reported that the deterioration of cholinergic neurons located in the basal forebrain is linked with AD and dementia [33]. Furthermore, the management of AD involves drugs which are AChE inhibitors. However, due to adverse effects and high cost, there is a dire need for other cost-effective and safest drugs [34]. Also, AChE inhibitors are effective in protecting the cortical neurons from glutamate-induced neuronal toxicity [35]. In this study, we examined the in vitro capability of the anticholinesterases mechanism of NMC. Interestingly, NMC exhibited 69% of AChE inhibition at high concentration (1000 μg/ml) with an IC50 of 67 μg/ml. Also, NMC revealed 66% of BuChE inhibition at the highest concentration (1000 μg/ml) with an IC50 of 96 μg/ml. These results were comparable with the standard anticholinesterase drug Galantamine which showed an IC50 value of 21 μg/ml and 46 μg/ml for AChE and BuChE enzymes. These results suggest that NMC has significant anticholinesterase properties which might be a potential neuroprotective agent. To support our in-vitro findings and further elucidate the cholinesterase inhibitory effects of NMC, we performed ex-vivo studies on AChE and BuChE enzyme inhibition in the frontal cortex and hippocampal area of the Tg-mice brain. Remarkably, the results suggest that NMC displayed significant inhibition of both AChE and BuChE in the frontal cortex and hippocampal areas of the Tg mice as compared to the Tg-saline treated group.
Oxidative stress is also a major and leading player in the pathogenesis of cancer, neuropathy, cardiovascular diseases, AD, Parkinson disease, Rheumatoid arthritis, Atherosclerosis and other neurological disorders [36]. Overproduction of ROS results in oxidative stress, which adversely affects and causes damage to the cell structures, including membranes, lipids, DNA and proteins [37,38,39]. Reducing oxidative stress by antioxidant mechanisms is one of the important and effective therapeutic approaches to neurological disorders such as Alzheimer’s and Parkinson's disease [40]. Furthermore, it is established that chronic oxidative stress will cause tau protein phosphorylation’s which further leads towards the formation of neurofibrillary tangles which is one of the major pathological hallmarks of AD [41, 42]. We examined the antioxidant activity of NMC by DPPH free radical scavenging assay. The antioxidant activity of the test compound NMC revealed an IC50 value of 171 μg/ml in-vitro, while displayed antiradical scavenging properties compared to the Tg-saline treated group. These results indicate that NMC is a potent antioxidant compound and may contribute to a potential therapeutic strategy that will aid in the reduction and prevention of oxidative stress-mediated neuronal toxicity and the pathogenesis of AD and other related neuropathological hallmarks.
The important pathophysiological changes that occur in the brain of AD patients include Aβ plaques formation and neurofibrillary tangles development [43]. Elevated buildup and accumulation of Aβ oligomers and fibrils leads towards the formation of senile plaques in the cortical and hippocampal areas of the brain and is considered as the major hallmark of AD [44]. The increased level of Aβ in the brain regions is correlated with oxidative brain damage, neuroinflammation and cognitive decline [45, 46]. The reduction of cortical cholinergic neurons is also closely linked and interrelated with the Aβ plaques formation [47]. Therefore, we further examined the effects of NMC on Aβ plaques by histological analysis of brain regions hippocampus and frontal cortex which are associated with the memory and cognitive function in the Tg mice model of AD. Thioflavin-S staining results demonstrates that the frontal cortex and hippocampal areas of NMC treated groups markedly reduced the Aβ as compare to the Tg-saline treated group. Henceforth, these outcomes suggest that NMC exhibits disease-modifying and neuroprotective properties against the neurotoxic Aβ plaques and therefore will be a beneficial agent for the treatment of AD.
Another main hallmark of AD is cognitive deficits, which arise due to oxidative stress and other related detrimental factors [36, 48]. Consistent with previous reports, oxidative stress and Aβ plaques lead to synaptic dysfunction and behavioral impairments [36]. Learning and memory deficits, behavioral disturbances are the major symptoms of AD, for that purpose we performed SWM, Y-Maze, EPM and BB tests. Morris water maze was used to measure spatial learning and memory in experimental animals [49]. Well-defined regions of the brain particularly the hippocampus, striatum and cortical parts are involved. MWM test is comprised of a water tub containing water with a platform for the escape of animals. It is a well-known test in behavioral neuroscience to examine memory deficits in different experimental models [49]. Our results of the SWM test demonstrated an improvement in the spatial learning and memory of the NMC treated group. While Y-Maze test noticeably indicated an improvement in learning and memory by reducing the latency time and also enhancing the spontaneous alternation behavior which suggests that NMC possesses cognitive enhancing properties. In the EPM test, our results show that NMC reduced the retention transfer latency time also indicating improvement of memory performance. Furthermore, AD is linked with impairments in sensorimotor processing, and due to which falls are very common in aged patients of AD [50]. For this, we performed the BB test to evaluate sensorimotor skills. Our results on BB performance revealed that NMC promoted motor coordination by reducing the time to crossing the beam. Overall, these results suggest that NMC possesses cognitive enhancing properties which may possibly be due to the inhibition of cholinesterases, reduction in the Aβ level and/or potential antioxidant properties.
Conclusion
In conclusion, the findings of our study have revealed that NMC exhibits potential cholinesterases inhibitory, antioxidant and neuroprotective effects. However, the potency of NMC is less than that of the Galanthamine, a specific anticholinesterase drug. Furthermore, NMC has significantly reduced the Aβ load and enhanced learning, memory, and motor coordination (Supplementary Fig.). Though, the effects of NMC on other pathological hallmarks of AD such as synaptic dysfunctions, neuroinflammation, and neurofibrillary tangles remain unclear. Therefore, more detailed studies are required to evaluate the mechanistic role of NMC in neurodegenerative disorders.
Abbreviations
- AD:
-
Alzheimer disease
- NMC:
-
2-[Hydroxy-(4-nitrophenyl)methyl]-cyclopentanone
- SWM:
-
Shallow water maze
- PYM:
-
Paddling Y-maze
- AChE:
-
Acetylcholinesterase enzyme
- BuChE:
-
Butrylcholinesterase enzyme
- CNS:
-
Central nervous system
- DTNB:
-
2,2 Dithiobisnitrobenzoic acid
References
Cummings JL, Cole G (2002) Alzheimer disease. JAMA 287(18):2335–2338. https://doi.org/10.1001/jama.287.18.2335
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimer’s Dementia 3(3):186–191. https://doi.org/10.1016/j.jalz.2007.04.381
LaFerla FM, Oddo S (2005) Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol Med 11(4):170–176. https://doi.org/10.1016/j.molmed.2005.02.009
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16(6):358. https://doi.org/10.1038/nrn3880
Hamos JE, DeGennaro LJ, Drachman DA (1989) Synaptic loss in Alzheimer’s disease and other dementias. Neurology 39(3):355–355. https://doi.org/10.1212/wnl.39.3.355
Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C (2017) Molecular pathogenesis of Alzheimer’s disease: an update. Ann Neurosci 24(1):46–54. https://doi.org/10.1159/000464422
Kumar A, Singh A (2015) A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol Rep 67(2):195–203. https://doi.org/10.1016/j.pharep.2014.09.004
Holzgrabe U, Kapková P, Alptüzün V, Scheiber J, Kugelmann E (2007) Targeting acetylcholinesterase to treat neurodegeneration. Expert Opin Ther Targets 11(2):161–179. https://doi.org/10.1517/14728222.11.2.161
Muhammad T, Ali T, Ikram M, Khan A, Alam SI, Kim MO (2019) Melatonin rescue oxidative stress-mediated neuroinflammation/neurodegeneration and memory impairment in scopolamine-induced amnesia mice model. J Neuroimmune Pharmacol 14(2):278–294. https://doi.org/10.1007/s11481-018-9824-3
Khan A, Ikram M, Muhammad T, Park J, Kim MO (2019) Caffeine modulates cadmium-induced oxidative stress, neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J Clin Med 8(5):680. https://doi.org/10.3390/jcm8050680
Schelterns P, Feldman H (2003) Treatment of Alzheimer’s disease; current status and new perspectives. Lancet Neurol 2(9):539–547. https://doi.org/10.1016/s1474-4422(03)00502-7
Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ (2003) Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 348(14):1333–1341. https://doi.org/10.1056/NEJMoa013128
Danysz W, Parsons CG (2003) The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int J Geriatric Psychiatry 18(S1):S23–S32. https://doi.org/10.1002/gps.938
Huang Y, Mucke L (2012) Alzheimer mechanisms and therapeutic strategies. Cell 148(6):1204–1222. https://doi.org/10.1016/j.cell.2012.02.040
Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M (2010) Alzheimer’s disease: clinical trials and drug development. Lancet Neurol 9(7):702–716. https://doi.org/10.1016/S1474-4422(10)70119-8
Trost BM, Brindle CS (2010) The direct catalytic asymmetric aldol reaction. Chem Soc Rev 39(5):1600–1632. https://doi.org/10.1039/B923537J
Padrón JM, Miranda PO, Padrón JI, Martín VS (2006) β′-Hydroxy-α, β-unsaturated ketones: a new pharmacophore for the design of anticancer drugs. Bioorg Med Chem Lett 16(8):2266–2269. https://doi.org/10.1021/jo048410j
Mandal S, Mandal S, Ghosh SK, Ghosh A, Saha R, Banerjee S, Saha B (2016) Review of the aldol reaction. Synth Commun 46(16):1327–1342. https://doi.org/10.1080/00397911.2016.1206938
Kurata T, Miyazaki K, Kozuki M, Morimoto N, Ohta Y, Ikeda Y, Abe K (2012) Atorvastatin and pitavastatin reduce senile plaques and inflammatory responses in a mouse model of Alzheimer’s disease. Neurol Res 34(6):601–610. https://doi.org/10.1179/1743132812Y.0000000054
Ayaz M, Junaid M, Ullah F, Subhan F, Sadiq A, Ali G, Ovais M, Shahid M, Ahmad A, Wadood A (2017) Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front Pharmacol 8:697. https://doi.org/10.3389/fphar.2017.00697
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Kamal Z, Ullah F, Ayaz M, Sadiq A, Ahmad S, Zeb A, Hussain A, Imran M (2015) Anticholinesterse and antioxidant investigations of crude extracts, subsequent fractions, saponins and flavonoids of Atriplex laciniata L.: potential effectiveness in Alzheimer’s and other neurological disorders. Biol Res 48(1):21. https://doi.org/10.1186/s40659-015-0011-1
Brasford M (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Braca A, De Tommasi N, Di Bari L, Pizza C, Politi M, Morelli I (2001) Antioxidant principles from bauhinia t arapotensis. J Nat Prod 64(7):892–895. https://doi.org/10.1021/np0100845
Petrasek T, Prokopova I, Bahnik S, Schonig K, Berger S, Vales K, Tews B, Schwab ME, Bartsch D, Stuchlik A (2014) Nogo-A downregulation impairs place avoidance in the Carousel maze but not spatial memory in the Morris water maze. Neurobiol Learn Mem 107:42–49. https://doi.org/10.1016/j.nlm.2013.10.015
Deacon RM (2013) Shallow water (paddling) variants of water maze tests in mice. J Vis Exp 76:e2608. https://doi.org/10.3791/2608
Kulkarni PD, Ghaisas MM, Chivate ND, Sankpal PS (2011) Memory enhancing activity of Cissampelos pariera in mice. Int J Pharm Pharm Sci 3(2):206–211
Shahid M, Subhan F, Ali G, Ullah I, Alam J, Ullah S, Rauf K (2017) Neuroprotective effect of Bacopa monnieri against morphine-induced histopathological changes in the cerebellum of rats. Pakistan J Pharm Sci 30(6):2067–2074
Ly PT, Cai F, Song W (2011) Detection of neuritic plaques in Alzheimer’s disease mouse model. J Vis Exp 53:e2831. https://doi.org/10.3791/2831
Calderon F, Von Bernhardi R, De Ferrari G, Luza S, Aldunate R, Inestrosa N (1998) Toxic effects of acetylcholinesterase on neuronal and glial-like cells in vitro. Mol Psychiatry 3(3):247–255. https://doi.org/10.1038/sj.mp.4000383
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66(2):137–147. https://doi.org/10.1136/jnnp.66.2.137
Ballard C (2002) Advances in the treatment of Alzheimer’s disease: benefits of dual cholinesterase inhibition. Eur Neurol 47(1):64–70. https://doi.org/10.1159/000047952
Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215(4537):1237–1239. https://doi.org/10.1126/science.7058341
Dunn N, Pearce G, Shakir S (2000) Adverse effects associated with the use of donepezil in general practice in England. J Psychopharmacol 14(4):406–408. https://doi.org/10.1177/026988110001400410
Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A (2006) Acetylcholinesterase inhibitors used in treatment of Alzheimer’s disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology 51(3):474–486. https://doi.org/10.1016/j.neuropharm.2006.04.007
Muhammad T, Ikram M, Ullah R, Rehman SU, Kim MO (2019) Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 11(3):648. https://doi.org/10.3390/nu11030648
Khan A, Ali T, Rehman SU, Khan MS, Alam SI, Ikram M, Muhammad T, Saeed K, Badshah H, Kim MO (2018) Neuroprotective effect of quercetin against the detrimental effects of LPS in the adult mouse brain. Front Pharmacol 9:1383. https://doi.org/10.3389/fphar.2018.01383
Ikram M, Saeed K, Khan A, Muhammad T, Khan MS, Jo MG, Rehman SU, Kim MO (2019) Natural dietary supplementation of curcumin protects mice brains against ethanol-induced oxidative stress-mediated neurodegeneration and memory impairment via Nrf2/TLR4/RAGE signaling. Nutrients 11(5):1082. https://doi.org/10.3390/nu11051082
Khan MS, Muhammad T, Ikram M, Kim MO (2019) Dietary supplementation of the antioxidant curcumin halts systemic LPS-induced neuroinflammation-associated neurodegeneration and memory/synaptic impairment via the JNK/NF-kappaB/Akt signaling pathway in adult rats. Oxid Med Cell Longev 2019:7860650. https://doi.org/10.1155/2019/7860650
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol 147:1–19. https://doi.org/10.1016/j.pneurobio.2016.07.005
Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G (2000) Oxidative stress in Alzheimer’s disease. Biochimica et Biophysica Acta (BBA) 1502(1):139–144. https://doi.org/10.1016/s0925-4439(00)00040-5
Su B, Wang X, Lee H-g, Tabaton M, Perry G, Smith MA, Zhu X (2010) Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neurosci Lett 468(3):267–271. https://doi.org/10.1016/j.neulet.2009.11.010
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356. https://doi.org/10.1126/science.1072994
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054):184–186. https://doi.org/10.1126/science.1566067
Näslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD (2000) Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA 283(12):1571–1577. https://doi.org/10.1001/jama.283.12.1571
Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB (2000) A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer’s disease. Nature 408(6815):975. https://doi.org/10.1023/A:1023255106106
Arendt T, Bigl V, Tennstedt A, Arendt A (1985) Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer’s disease. Neuroscience 14(1):1–14. https://doi.org/10.1016/0306-4522(85)90160-5
Khan M, Ullah R, Rehman SU, Shah SA, Saeed K, Muhammad T, Park HY, Jo MH, Choe K, Rutten BPF, Kim MO (2019) 17beta-estradiol modulates SIRT1 and halts oxidative stress-mediated cognitive impairment in a male aging mouse model. Cells 8(8):928. https://doi.org/10.3390/cells8080928
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Rev 36(1):60–90. https://doi.org/10.1016/s0165-0173(01)00067-4
Morris JC, Rubin EH, Morris EJ, Mandel SA (1987) Senile dementia of the Alzheimer’s type: an important risk factor for serious falls. J Gerontol 42(4):412–417. https://doi.org/10.1093/geronj/42.4.412
Funding
We acknowledge the funding (National research program for universities; NRPU 6671/KP/NRPU/R&D/HEC/2016) from Higher education commission of Pakistan for the completion of necessary part of the project. The funding authorities did not contribute to the plan, design or any other part of the research work.
Author information
Authors and Affiliations
Contributions
SIA performed and carried out all experimental work, data collection and evaluation, and wrote a preliminary draft of the manuscript. GA conceived, designed and supervised the study. Also, he edited and reviewed the final version of the manuscript. RU helped in performing genotyping studies. TM helped in data analysis and drafted the final version of the manuscript. NU synthesized and structurally confirmed the NMC compound (chemistry data published ‘Picolylamine as an Organocatalyst Template for highly Diastero-And Enantioselective Aqueous Aldol Reactions’. ANH helped us in the behavioral studies and edited and reviewed the final draft of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This research was carried out under the project title as Novel targeted heterocyclic compounds, a potential candidate for Alzheimer's disease’ approved by the Research Ethical Committee of Pharmacy Department, University of Peshawar, Pakistan has been approved all experimental procedures on animals vide reference number 25/EC-18/Pharm, dated. 16/10/2018.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmad, S.I., Ali, G., Muhammad, T. et al. Synthetic β-hydroxy ketone derivative inhibits cholinesterases, rescues oxidative stress and ameliorates cognitive deficits in 5XFAD mice model of AD. Mol Biol Rep 47, 9553–9566 (2020). https://doi.org/10.1007/s11033-020-05997-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05997-0