Abstract
Fatliquors are oil-in-water emulsions added during the fatliquoring process of leather manufacture to lubricate and prevent the fibre structure resticking during drying. They also increase softness, flexibility, and tensile strength of fixed leather. In this study, Jatropha curcas oil of no commercial value in Nigeria was sulfonated. The physicochemical properties of both the sulfonated and unsulfonated oils were determined. The sulfonated and unsulfonated oils were also characterized using DSC, FT-IR, 1H NMR, 13C NMR, and 13C NMR DEPT. The prepared sulfonated J. curcas oil was applied onto goatskin and compared with commercial sulphated fatliquor in the processing of shoe upper leather. Physical/mechanical analyses were carried out on fixed leather. Tensile strength, Sudan stain, elongation at break, and double edge tear test results showed notable improvement in the mechanical properties of the leather processed with the sulfonated J. curcas oil. The microscopic analysis also showed fibre structures that were adequately opened up. This study revealed that the sulfonated J. curcas oil can be a good sustainable substitute for commercially available fatliquor as its application in the processing of shoe upper leather shows properties that are comparable with the renowned fatliquors which are normally utilized in leather industries. It also raises the possibility of commercialization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
The need to conserve resources spent on importation of raw materials for industrial use has brought about a quest for cheaper alternatives. Such a search focuses on underutilized seeds for possible development and uses [1, 2]. J. curcas is a drought-resistant shrub that belongs to the family Euphorbiaceae [3]. Although the seeds have an oil content of 40–50%, they are not edible as they contain curcin and phorbol esters which are highly toxic [4].
Jatropha curcas is planted chiefly as a hedge around gardens and in the reclamation of wastelands [5]. Its seed oils have also been studied and used as biofuels [6, 7]. The importance of Jatropha notwithstanding, the seeds are underutilized and neglected in Nigeria; they still constitute a nuisance, other uses had to be researched on, hence this work. This work, therefore, studies its potential as a leather lubricant in the leather industry.
The importance of leather lubrication in the leather industry cannot be overemphasized. Leather manufacture has been with man from pre-historic times. It involves the following processes: soaking, unhairing/liming, deliming/bating, pickling, tanning, neutralization/dyeing, fatliquoring, drying, and finishing. Tanning is one of the most important processes of leather manufacture as it permanently alters the protein structure and converts animal hide (or skin) into a durable non-putrescible substrate [8, 9]. The preliminary stages of leather manufacture such as dehairing, liming, and bating often involve degreasing and most of the natural oils from the skins are removed. Fat removal improves the efficiency of tanning and prevents the formation of undesirable products during leather manufacture [10].
After the tanning process, the leather does not have a sufficient quantity of lubricants within its fibres. When tanned leather which is processed without a leather lubricant (fatliquor) is dried, it results to a hard, intractable material that is difficult to work with; this is due to the collagen fibres sticking together [11]. The last of the wet processing stages of leather manufacture known as the fatliquoring process, therefore, adds oil-in-water emulsion (fatliquor / leather lubricant) into the leather fibre. This lubricates the leather fibres, reduces the frictional forces between them, and enables fatliquored leather to have improved mechanical properties such as tensile strength, tear strength as well as a soft feel [8]. Such polar oils which readily form oil-in-water emulsions are obtained by the chemical interaction of the double bonds in the fatty acid chains of oils with certain polar functionalities or chemical entities.
The traditional raw material used in making fatliquors has for many decades been fish oil which has its disadvantages of non- sustainability and high cost. Subsequently, edible vegetable oils such as flax, soya as well as synthetic oils [12] have been used. The non-sustainability of synthetic oils and the unhealthy competition brought about by the increasing food uses of the edible natural oils have generated a need to research into other substitutes for traditional raw materials used in fatliquor production.
This article reports the use of J. curcas seed oil for the development of sulfonated oil and its sequential use in the processing of a leather shoe upper. This study presents a viable industrial utilization for this readily available, underutilized, non-edible oil with no economic value in Nigeria. Figure 1 gives a schematic diagram of the entire research work done.
2 Materials and Methods
Healthy matured seeds of J. curcas were obtained from a farm in Ndufu-Alike town, Ikwo, Ebonyi State, Nigeria. The plant was authenticated by a taxonomist at the International Centre for ethnomedicine and drug development (InterCEDD), Nsukka, Enugu State, Nigeria. Voucher samples were reserved and a voucher number kept for future reference. Wet blue goatskin was obtained from the tannery at the Institute for Creative Leather Technologies (ICLT), The University of Northampton (UoN), Northampton, United Kingdom. Reagents used in the laboratory for extraction, synthesis, and analysis were of analytical grade while those used for leather processing were of commercial/industrial grade.
2.1 Extraction of Jatrophacurcas Seed Oil
Dehusked J. curcas seeds were dried in an oven at 40 °C for 5 h. 100 g of the dehusked seeds were transferred into a thimble and the oil extracted using n-hexane as a solvent with the aid of a soxhlet-apparatus for 4 h. The solvent was recovered in the in-built capacity in the Soxhlet extractor leaving the concentrated oil sample for analysis. The process of extraction was repeated until a sufficient quantity of the oil was obtained.
2.2 Physicochemical Properties Determination on the Extracted Jatrophacurcas Oil
Physicochemical characteristics of J. curcas oil like specific gravity (Ta 1b-64), acid value (Cd 3a-63), iodine value (Cd 1–25), and saponification value (Cd 3–25) were determined using AOCS methods [7].
2.3 Determination of Fatty Acid Composition
The fatty acid composition was investigated as described by a published procedure [13]. In a typical determination, the refluxing of oil at 70 °C for 3 h in 2% sulfuric acid in methanol was carried out for the preparation of the fatty acid methyl ester (FAME). The extraction of the ester into ethyl acetate was achieved and washed clear of acid, then passed over anhydrous sodium sulfate. To determine the fatty acids existing in the oil, gas chromatography-mass spectrometry (GC–MS) of the FAME was used for the determination. Agilent 19091S-433HP-5MS capillary column (30 m × 250 µm × 0.25 µm) was used to conduct the GC–MS analysis. A pressure program of 11.6 psi, an average velocity of 44.3 cm/s, and a temperature of 35 °C to 325 °C were used. The carrier gas is helium gas. 0.2 µL, 300 °C, and 1.5 ml min−1 were used as the injection volume, temperature, and column flow. The oven program was 35 °C for 3 min and finally increased to 280 °C at 5 °C min−1.
2.4 Sulfonation Process
The reaction of the fatty acids and sulfuric acid for the formation of sulfonated oil was carried out as described by a published procedure [14]. In a conventional reaction, concentrated sulfuric acid (45 ml) was transferred dropwise into a beaker containing 150 g of J. curcas oil (with continuous stirring at 20 °C for 2 h). The crude mass was dissolved in 450 ml of ethanol and neutralized utilizing 15% NaOH (dissolved in methanol). The salts were filtered off under vacuum. The solvent was removed and recovered using a rotary evaporator. The resulting sulfonated product was set for use as a leather fatliquor (Scheme 1).
The side reaction can be found below (Scheme 2).
2.5 Characterisation of the Sulfonated Oil
The functional groups of the studied samples were characterized by FT-IR measurement (600–4000 cm−1), normal resolution of 4 cm−1 using a Shimadzu 8400S FT-IR instrument (Shimadzu, Milton Keynes, UK). 1H nuclear magnetic resonance (NMR), 13C NMR and 13C NMR Distortionless Enhancement by Polarization Transfer (DEPT) were obtained on a Bruker Biospin® AV500—5 mm BBO probe with Z-axis gradient, TOPSPIN v 2.1, 1H = 500.13 MHz, 13C = 125.76 MHz (Brucker, Coventry, UK) and used in the examination of the sulfonation process.
Mettler DSC 2 Star System in a temperature range of − 80 to 180 °C, using an identical program given was used to determine the thermal behaviour of the unsulfonated and sulfonated oils. The purge gas (nitrogen) had a flow rate of ~ 60 ml/min. Samples of oils, of between 5 and 7 mg, were weighed into low-pressure aluminum crucibles and sealed hermetically. The sealed crucibles were pierced before analysis. An empty, hermetically sealed aluminum crucible with a pinhole was used as a reference. A temperature profile of -80 to 180 °C was run using the following temperature program: − 80 °C isotherm for 3 min; dynamic ramp at − 80 °C to 180 °C (at 10 °C min−1), isotherm at 180 °C for 3 min; isotherm at 30 °C for 2 min. The resulting DSC data was analyzed for peak temperature, onset temperature, and melting temperature for comparison. All DSC experiments were carried out in triplicate and average values were reported.
2.6 Physicochemical Properties Determination on the Sulfonated Oil
The pH, specific gravity, total organic SO3, percentage ash, and stability of the emulsion were examined according to the standard methods prescribed by the Society of Leather Chemists and Technologists [15].
2.7 Sampling of Animal skin
Putting into consideration the variability and anisotropic nature of the animal skin or hide, the butt region is taken to be the official sampling position (OSP). The butt has a tight fibre structure, which makes the skin relatively firm and stiff when compared to other skin parts. The OSP accommodates the physiological functions of leather and makes the best comparisons of leather properties. Also, the anisotropic nature of leather brought about the official two-way sampling of leather for physical testing: parallel to the backbone and perpendicular to the backbone. Sampling is also done on the skin of one particular animal as the properties of animal skins vary with age, sex, breed, diet, husbandry, history, storage, curing [16]. Selected wet blue goatskin, (without defects) was shaved to get a consistent thickness in the butt area (1.0–1.1 mm). The butt was subdivided into four quarters such that the sampling location was evenly represented in all the four quarters.
Three of the four quarters labeled NC, (Negative control—no fatliquor); PC, Positive control- reference commercial sulfated fatliquor—TRUPON DXV (TrumplerGmbh, Worms, Germany) and SJCO—sulfonated J. curcas oil were used for proper comparison as shown in Fig. 2 and used for the processing of leather.
2.8 Fatliquoring Process
A conventional shoe upper manufacturing process, Table 1 [17] was carried out on the wet blue goatskin (400 g each) (simultaneously with the aid of three separate tanning drums). Leather dyeing was omitted to enable the Sudan IV stain test (for fatty substances) to be carried out effectively after the leather manufacture.
2.9 Mechanical Properties of Leather
Each of the leather samples was conditioned according to standards [18], before staking twice employing a Cartigliano PAL 160 leather staking machine (Cartigliano) and consequent mechanical examination.
The mechanical characteristics of leather samples were determined using: tensile strength [19] elongation at break and tear strength of leather [20], and grain strength [21] test standards. These mechanical tests were carried out using four measurements per test. For each test, two leather test pieces were cut parallel to the backbone while another two test pieces were cut perpendicular to the backbone. The data obtained for each physical testing are the average of four measurements for each sample. The softness test was carried out using the ST300 leather softness tester according to British softness standards [22]. The softness gauge was opened and an aperture of 25 mm was inserted. The metal plate was placed over the aperture and the gauge closed by pressing down the lever at the front of the machine. The gauge dial was zeroed with a metal plate still in place. The gauge was opened and metal plate removed. Each of the leather samples was tested over aperture, gauge closed for the reading to be stabilized. Reading was taken randomly to the nearest 0.1 mm in five different portions within the butt area and the average value noted. Thin divisions (50 μm) of the leather samples were made with a Leica 1850 cryostat microtome (Leica, Wetzler, Germany) (set at − 20 °C) and used in Sudan (IV) stain test for the measurement of the degree of permeation of the fatliquor within the leather fibrils. The morphologies of the samples were investigated for variations in fibre structure using Hitachi S-3000 N scanning electron microscope, SEM (Hitachi, Maidenhead, UK).
3 Results and Discussions
3.1 Fatty Acid Composition
The types of long-chain fatty acid and percentage compositions of the studied J. curcas seed oil are shown in Table 2. The results show that the oil contained a higher percentage of unsaturated fatty acids (74.53%) when compared to the saturated fatty acids (25.47%). The observed ratio of the unsaturated fatty acid vs saturated fatty acid in the J. curcas oil is consistent with results established by the previous studies [23]. This observed property favours sulfonation of the studied oil due to the presence of a large number of double bonds [24].
3.2 Physicochemical Properties
The physicochemical characteristics of JCO and SJCO are given in Table 3. The high percentage of JCO signifies that it has a substantial volume of oil which could be harnessed for industrial/ commercial purposes. JCO has a high iodine value of 104. This is suggestive of a relatively high unsaturation and confirms the presence of C=C double bonds. This characteristic is beneficial for the sulfonation reaction of the oil [25]. The reduction in iodine value to 25 in SJCO signifies the conversion of the olefinic double bond in the fatty acid. SJCO was free from rancid/foul smell and the 10% solution exhibited a mild colour which is very unlikely to influence the colour of the finished product.
It was also seen that SJCO readily formed emulsion in both hot and cold water. Its 10% emulsion stayed stable for > 24 h without separation or creaming; having the pH of the 10% emulsion below 8.0, according to standard specifications [26]. The concept of having a precise pH value for specific sulfonated oils is not significant except for quality control schemes [27].
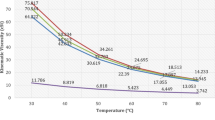
The thermal behaviour of the oils is displayed in Table 4 and Fig. 3. This diversity in melting point range emanated from the combined impacts of the polymorphism, composition of fatty acids in the triglyceride, and history of the thermal behaviours [28, 29].
As a consequence of the numerous fatty acids present in the oil, the end-set temperature is often taken as the melting point [30, 31]. The DSC results showed a substantial rise in the melting point of the oil from 3.18 °C to 14.59 °C on sulfonation. Since the melting point of unsaturated fatty acids is lower than saturated fatty acids [14], it, therefore, means that the substantial rise in melting point revealed by the sulfonated oil is an indication that most of the unsaturated fatty acids in the sulfonated oil were used up during the sulfonation reaction leaving behind saturated fatty acids. The DSC results revealed also that the studied SJCO is thermally stable over a wide temperature range and can be easily used in fatliquoring leather products applicable in diverse climates.
3.3 Structural Characterizations
Figure 4 showed the FT–IR results. It illustrated the functional groups of JCO and SJCO with different intensity levels. The emergence of S=O stretching of the sulfate and sulfonate groups in the SJCO may be attributed to the appearance of the peak at 1198 cm−1. This is an indication that the sulfonation reaction took place in the SJCO. This was not the case for the JCO. The investigated peaks in the studied oils and functional groups are displayed in Table 5.
The 1H NMR of JCO and SJCO are depicted in Fig. 5. The 1H NMR spectra show about nine to ten signals of vital strength before and after sulfonation. A chemical shift (δ) of about 0.85 ppm is ascribed to the terminal methyl group. The emergence of methylene proton signals at various positions of the acyl chain in the triglycerol structure could be attributed to the chemical shifts between 1.0 and 2.2 ppm. The protons of glyceride moiety in the studied oils are ascribed to the peaks at δ 4.11–4.32 ppm, while the protons of the–CH=CH– moiety are due to the peaks at δ 5.26–5.35 ppm. Comparable assignments have been published [32]. Since the protons of the –CH=CH– moiety are sp2 hybridized, their NMR signals are deshielded by the controlling power of the diamagnetic anisotropy of the π system. Saturation of the double bond is normally caused by sulfation or sulfonation. The formed sp3 hybridized protons are therefore presumed to be shielded corresponding to the sp2 olefinic protons. The recently produced protons (H-C-S or H-C-O) in the sulfonated oil recorded signals at δ 3.65 and 3.73 ppm. The oil without sulfonation did not show these proton signals at δ 3.65 and 3.73 ppm.
Figure 6a, b shows the 13C NMR spectra of the JCO and SJCO. A signal at around 14.1 ppm was observed by the methyl groups at the end of the acyl chains in glyceride moiety. It is well separated from other signals and hence easily recognized. The signals linked with the olefinic carbons seem extremely deshielded at δ 127–131 ppm owing to the diamagnetic anisotropic influence of the π system. The detected signals faded completely upon sulfonation because of the loss of the double bonds (Fig. 6b). There is an appearance of sp3 hybridized carbons (C–S and C–O) at 51 and 72 ppm after the completion of sulfonation reactions.
Figure 7a, b shows the result of the 13C NMR DEPT experiments of the JCO and SJCO. The terminal CH3 of the studied JCO and SJCO was observed to be positioned downwards at δ 14 ppm as can be seen in Fig. 7a, b. The C–H–O of the glycerol backbone at δ 68 ppm could be observed to be positioned downward. Similarly, the CH2O pair in the glycerol backbone was positioned upward at chemical shifts of δ 64 ppm and 62 ppm. The –(CH2)2 of the fatty acid chains were detected at several positions at δ 20–30 ppm positioned upward. It was also observed that the HC=CH bond previously found phased down in JCO (δ 127 ppm and 130 ppm) were absent in SJCO. This absence was due to the formation of C–O–S and C–S bonds by the reaction of the C=C in the fatty acid with sulfuric acid. The C–O–S bond could be seen phased down at δ 72 ppm in SJCO while the C–S bond also formed in SJCO was similarly detected phased down at δ 51 ppm. Both C–O–S and C–S bonds are absent in JCO.
3.4 Sudan IV Test Results
Sudan stain test on the cross-section of the trial leather samples revealed the appearance of oil (indicated by red stains) within the leather fibres treated with the synthesized SJCO and that treated with the commercial fatliquor in contrast to the leather without fatliquor (Fig. 8). This is evidence of the degree of penetration of the SJCO.
3.5 Mechanical Properties of Fixed Leather Samples
Experimental results (Table 6) showed that the three leather test pieces, NC, PC, and SJCO have average softness values of 24.6 mm, 28.9 mm, and 29.0 mm respectively. The aforementioned values of the PC and SJCO are within the same range unlike the outcome of the negative control, NC. This is in agreement with the outcome of the Sudan stain test. It shows that the infiltration of the sulfonate group into the double bonds of the JCO improved the softness properties of the studied SJCO fatliquored leather, making its softness property very comparable to leather which commercial fatliquor has been used on (Table 6).
Table 7 shows the tensile strength and elongation at break results of the processed leather test pieces. The test piece is extended to its breaking point to examine its structural resistance while the elongation at break describes its flexibility. The tensile behavior of the longitudinal sample (perpendicular to the backbone) is more unstable and breaks more easily than the transverse sample (parallel to the backbone [31]. It was observed that the leather test piece, negative control, NC had the least values for both the average tensile strength and elongation at break results (15.57 N/mm2 and 27.50% respectively). PC and SJCO had higher and similar average tensile strength values (23.79 N/mm2 and 23.93 N/mm2) respectively. They also had higher and comparable values for the percentage elongation at break results (33.52 and 37.94% respectively). These comparable results demonstrate that the commercial sulfated fatliquor and the prepared sulfonated fatliquor had similar effects on fixed leather.
Table 8 shows the analysis of the double edge tear result of the studied leather test pieces. A test piece placed on an Instron tensile testing machine is used to describe the highest force exerted during the double tearing of the test piece [33]. The greatest force obtained when the three-trial shoe upper leather test pieces were subjected to double edge tearing test is 43.9 N, obtained on the testing of leather processed with SJCO. This shows that the application of SJCO in the processing of the shoe upper leather increased the double edge tear strength of the studied leather. This result is intriguing because the improved tear strength induced by SJCO is far higher than that of PC and NC.
Table 9 presents the strength of the grain surface of the trial leather test pieces. The ball burst test adopted in the determination of the distension and strength of the grain of leather also reveals the capacity of the shoe upper leather to resist the increasing force (leading to cracking or bursting) when ball-shaped steel material is pressed against the centre of the test leather piece [21]. Values for load at grain crack are the average of four separate determinations. The result reveals that the highest force of 260 N and 350 N obtained for the strength at grain crack and strength at grain burst of leather samples fatliquored with PC and SJCO were quite analogous. The grain layers were also elastic (had increasing distension values) as evidenced by the expansion of strains/pressures to which it is exposed to during footwear lasting as carried out in the shoemaking process. From the table, it was observed that the strength of the grain of experimental leather is comparable to the strength properties of the positive control leather.
The result shows that the leather samples fatliquored with PC and SJCO had improved mechanical properties and comparable values which depicts good lubrication of the leather fibres [27].
A similar study on another potential fatliquor, sulfonated Afzelia africana aril cap oil, SACO, recommended for use in the tannery has been carried out [14]. Though not all SACO-induced mechanical properties are better than TRUPON DXV-induced mechanical properties, the results obtained are comparable. This trend is similar to what is obtainable in this present study. Although differences exist in the two studies, a direct comparison of the mechanical properties reported in this present work cannot be made with the properties obtained for leather treated with sulfonated A. africana aril cap oil, SACO. This is because both studies were not carried out on the same goatskin. A comparison between any two skins cannot be expected, even if the same area of the skin is sampled. Variation occurs in animals as a result of the breed, sex, age, history, curing, storage, diet, etc. and these factors affect the mechanical properties of the processed leather [16]. Direct comparison is therefore carried out on different fatliquors processed on the same skin.
3.6 Microscopic analysis
Figure 9 shows the morphology of the leather samples using a scanning electron microscope (SEM). The leather samples fatliquored with PC and SJCO each showed structures that were adequately opened up. However, this was not the case for the leather without fatliquor as its structure showed fibres which had a split-up structure that restuck after drying.
4 Conclusion
A suitable range of techniques has been used to characterize the sulfonated Jatropha oil. The as-synthesized sulfonated J. curcas oil which is readily washed out with water without leaving any oily feeling to hand was also free from rancid or putrefactive odour. There was an improved tensile strength, elongation at break, tear load, grain strength, as well as softness in the leather processed with the prepared sulfonated fatliquor in comparison with an imported sulfated equivalent. This reveals that the sulfonated J. curcas fatliquor can compete favourably and can even be used as a substitute fatliquor for the production of leather shoe upper. It is therefore recommended that attention be paid to the cultivation of J. curcas in Nigeria for possible commercialization of the product.
References
Otabor GO, Ifijen IH, Mohammed FU, Aigbodion AI, Ikhuoria EU (2019) Alkyd resin from rubber seed oil/linseed oil blend: a comparative study of the physiochemical properties. Heliyon 5:e01621
Malik M, Ikhuoria EU, Ifijen IH (2020) Extraction and physiochemical characterization of oils obtained from selected under-utilized oil bearing seeds in Nigeria. Publ Chem Soc Nigeria Kano Chapter, Chem Search J 11(1):110–117
Grimm C, Somarriba A (1999) Suitability of physic nut (Jatropha Curcas L.) as single host plant for the leaf-footed bug Leptoglossus Zonatus Dallas (Het., Coreidae). J Appl Entomol 123(6):347–350
Basha SD, Mulpuri S (2019) Genetic analysis of jatropha species and interspecific hybrids of Jatropha Curcas using nuclear and organelle specific markers. Euphytica 168(2):197–214
Francis G, Edinger R, Becker K (2005) A concept for simultaneous wasteland reclamation, fuel production, and socio-economic development in degraded areas in India: need, potential and perspectives of Jatropha plantations. Nat Resour Forum 29(1):12–24
Jain S, Sharma MP (2010) Biodiesel production from Jatropha curcas oil. Renew Sustain Energy Rev 14(9):3140–3147
Brühl L (1996) Official methods and recommended practices of the American Oil Chemist's Society, physical and chemical characteristics of oils, fats and waxes, section I. In: The AOCS methods editor and the AOCS Technical Department. AOCS Press, Champaign
Dettmer A, Ayub MAZ, Gutterres M (2011) Hide unhairing and characterization of commercial enzymes used in leather manufacture. Braz J Chem Eng 28:373–380
Żarłok J, Śmiechowski K, Mucha K, Tęcza A (2014) Research on application of flax and soya oil for leather fatliquoring. J Clean Prod 65:583–589
Affiang SD, Ggamde G, Okolo VN, Olabode V, Jekkada J (2018) Synthesis of sulphated-fatliquor from Neem (Azadirachtaindica) seed oil for leather tannage. Am J Eng Res 7(4):215–221
Nasr AI (2017) Reusing limed fleshing wastes as a fatliquor in leather processing. Egypt J Chem 60(5):919–928
Tawfig HM, Gasmelseed GA, Mohammed FE (2017) Application of fatliquor prepared from sudanese castor oil in leather fatliquoring process. Int J Eng Sci Res Technol 6(10):248–253
Orsavova J, Misurcova L, Ambrozova JV, Vicha R, Mlcek J (2015) Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci 16(6):12871–12890
Nkwor AN, Ukoha PO (2020) Evaluation of the leather fatliquoring potential of sulphonated Afzelia Africana Aril Cap Oil. Heliyon 6(1):e03009
SLTC (1996) Society for Leather Technologists and Chemists (SLTC) methods. Withernsea
Covington AD (2011) Tanning chemistry: the science of leather. Royal Society of Chemistry Publishing, Cambridge
ICLT SR 15/31 (2015) Leather manufacture: shoe upper-chromium (non-compact). In: Manual for leather processing, Institute for Creative Leather Technologies, Northampton
BS 2419 (2002) Leather, physical and mechanical tests: conditioning. British Standards Institution, London
BS 3376 (2012) Leather, physical and mechanical tests: determination of tensile strength and percentage extension. British Standards Institution, London
BS 3377 (2011) Leather, physical and mechanical tests: determination of tear load. Double edge tear. British Standards Institution, London
BS 3379 (2015) Leather, physical and mechanical tests: determination of distension and strength of surface leather (ball burst method). British Standards Institution, London
BS 1723 (2015) Leather, physical and mechanical tests: determination of softness. British Standards Institution, London
DiNicolantonio JJ, O’Keefe JH (2017) Good fats versus bad fats: a comparison of fatty acids in the promotion of insulin resistance, inflammation, and obesity. Mo Med 114(4):303–307
Nkwor AN, Ukoha PO, Wise WR, Nwaji NN, Flowers K (2019) Fatty acid profile and production of fatliquor from Canarium schweinfurthii Mesocarp Oil. Pertanika J Sci Technol 27(4):2221–2243
Patel VR, Dumancas GG, Kasi Viswanath LC, Maples R, Subong BJJ (2016) Castor Oil: Properties, Uses, and Optimization of Processing Parameters in Commercial Production. Lipid Insights 9:1–12
IS 6357 (2013) Sulphated oil for leather fatliquoring—specification bureau of Indian standards New Delhi, India
Waite T (1999) Oils, fats, waxes and fatliquors. In: Leafe MK (ed) Leather technologists pocket book. Society of leather technologists and chemists, Withernsea, pp 125–148
Lawer-Yolar G, Dawson-Andoh B, Atta-Obeng E (2019) Novel phase change materials for thermal energy storage: evaluation of tropical tree fruit oils. Biotechnol Rep (Amst) 24:e00359
Maszewska M, Florowska A, Dłużewska E, Wroniak M, Marciniak-Lukasiak K, Żbikowska A (2018) Oxidative stability of selected edible oils. Molecules (Basel, Switzerland) 23(7):1746
Nassu RT, Guaraldo Gonçalves LA (1999) Determination of melting point of vegetable oils and fats by differential scanning calorimetry (DSC) technique. Grasas Aceites 50(1):16–21
Li Z, Paudecerf D, Yang J (2009) Mechanical behaviour of natural cow leather in tension. Acta Mech Solida Sin 22(1):36–44
Guillén MD, Ruiz A (2003) Edible oils: discrimination by 1H nuclear magnetic resonance. J Sci Food Agric 83(4):338–346
Chang SY, Li JX, Sun CC (2017) Tensile and shear methods for measuring strength of bilayer tablets. Int J Pharm 523(1):121–126
Acknowledgements
The authors would want to thank the management of the Institute for Creative Leather Technologies (ICLT), University of Northampton, Northampton, The United Kingdom for the bench space offered for this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Nkwor, A.N., Ukoha, P.O., Ifijen, I.H. et al. The Use of Sulfonated Jatropha curcas Oil for the Processing of Mechanically Improved Leather. Chemistry Africa 3, 911–925 (2020). https://doi.org/10.1007/s42250-020-00189-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-020-00189-6