Abstract
The present study aims at characterization of Jatropha species occurring in India using nuclear and organelle specific primers for supporting interspecific gene transfer. DNA from 34 accessions comprising eight agronomically important species (Jatropha curcas, J. gossypifolia, J. glandulifera, J. integerrima, J. podagrica, J. multifida, J. villosa, J. villosa. var. ramnadensis, J. maheshwarii) and a natural hybrid, J. tanjorensis were subjected to molecular analysis using 200 RAPD, 100 ISSR and 50 organelle specific microsatellite primers from other angiosperms. The nuclear marker systems revealed high interspecific genetic variation (98.5% polymorphism) corroborating with the morphological differentiation of the species used in the study. Ten organelle specific microsatellite primers resulted in single, discrete bands of which three were functional disclosing polymorphism among Jatropha species. The PCR products obtained with organelle specific primers were subjected to sequence analysis. PCR products from two consensus chloroplast microsatellite primer pairs (ccmp6 and 10) revealed variable number of T and A residues in the intergenic regions of ORF 77–ORF 82 and rp12–rps19 regions, respectively in Jatropha. Artificial hybrids were produced between J. curcas and all Jatropha species used in the study with the exception of J. podagrica. Characterization of F1 hybrids using polymorphic primers specific to the respective parental species confirmed the hybridity of the interspecific hybrids. Characterization of both natural and artificially produced hybrids using chloroplast specific markers revealed maternal inheritance of the markers. While the RAPD and ISSR markers confirmed J. tanjorensis as a natural hybrid between J. gossypifolia and J. curcas, the ccmp primers (ccmp6 and 10) unequivocally established J. gossypifolia as the maternal parent. Evaluation of backcross interspecific derivatives of cross involving J. curcas and J. integerrima indicate scope for prebreeding and genetic enhancement of Jatropha curcas through interspecific hybridization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jatropha curcas L. (Family Euphorbiaceae) has assumed paramount importance as a potential biodiesel crop in more than 50 countries. It is a plant with several attributes, multiple uses and considerable potential (Heller 1996; Openshaw 2000). The major limitation with the currently used planting material is the narrow genetic base, low productivity and vulnerability to a wide array of biotic and abiotic stresses. Genetic variability and divergence studies in seed traits and oil content revealed variability for these traits (Kaushik et al. 2007). However, the evaluation was carried out with germplasm collected from trees of different regions, different aged plants (3–20 years) and propagated through seeds or vegetative cuttings. Comparison of yield contributing traits based on such material results in erroneous conclusions about the superiority of the identified clone as it is influenced by the mode of propagation and climatic conditions. Results of provenance trials indicated strong genotype and environment interactions for several quantitative traits (Heller 1996). Genetic variability assessment of J. curcas germplasm using molecular markers indicated modest levels of inter-accessional variability (Basha and Sujatha 2007). Hence, there is an immediate need to widen the genetic base of J. curcas. Among the various crop breeding approaches, interspecific hybridization is an immediate option for genetic enhancement of J. curcas (Sujatha 2006).
The genus Jatropha is morphologically diverse with 160–175 old and new world woody species comprising of trees, shrubs, rhizomatous subshrubs, tuberous perennial herbs, geophytes and facultative annuals which are distributed chiefly in the tropical and sub-tropical regions of America, Africa and India (Dehgan 1984). Dehgan and Webster (1979) recognized two subgenera (Curcas, Jatropha), ten sections and ten subsections. The subgenus Curcas comprises all Mexican, one Costa Rican, two African and one Indian species, while the subgenus Jatropha includes all South American, African (except two), Antillean, all Indian (except one) and two North American species (Dehgan 1984). Of the 175 reported species, only nine species are available in India. J. villosa and its allied species are of Indian origin. With the exception of J. curcas, all other species existing in India belong to the subgenus Jatropha.
Several Jatropha species are cultivated for their ornamental leaves and flowers, while some are grown in the tropics for their economic uses. Variation for fatty acid profiles, photoperiod insensitivity, flowering and fruiting pattern has been reported in different Jatropha species (Banerji et al. 1985; Sujatha 1996). Screening of Jatropha species against foliage feeders, which attack other Euphorbiaceous members revealed varying levels of resistance within the Jatropha species, with J. integerrima conferring maximum resistance in terms of larval mortality, feeding cessation and with or without pupation (Lakshminarayana and Sujatha 2001). Jatrophas are rich sources of hydrocarbons and J. multifida possesses higher oil content (50%) as compared to J. curcas (23–38%) (Banerji et al. 1985; Sujatha 1996). Determination of the energy values of the oils indicated much higher energy content for J. gossypifolia (42.2 MJ/kg), J. glandulifera (47.2 MJ/kg) and J. multifida (57.1 MJ/kg) than for J. curcas (39.8–41.8 MJ/kg) (Banerji et al. 1985; Jones and Miller 1991). Jatropha multifida, J. podagrica, J. integerrima and J. gossypifolia are well known and cultivated throughout the tropics as ornamental plants. J. gossypifolia, a facultative annual, has heavy fruit bearing ability and thrives well on saline soils. The species J. integerrima, J. multifida and J. podagrica are drought hardy and have continuous bearing unlike J. curcas which has two to four flowering flushes depending on the agro-ecological conditions. There is immense scope for transfer of beneficial traits from other Jatropha species to J. curcas such as, heavy bearing, photoperiod insensitivity, improved fuel characteristics, high oil content, desired oil quality, plant architecture, earliness, reduced toxicity of endosperm proteins and wider adaptability (Sujatha 2006).
Mc Vaugh (1945), Wilbur (1954) and Dehgan and Webster (1979) regarded J. curcas as the most primitive member of the genus because of its ability to interbreed with species from both subgenera, palmately lobed leaves, arborecsent growth habit and occasional hermaphrodite flowers. Inter and intra-sectional hybrids can be produced with J. curcas as the maternal parent as the barriers to sexual crossability are weak (Dehgan 1984; Sujatha 1996). Dehgan (1984) attempted interspecific hybridization of 20 species in eight of the ten sections and established the phylogenetic significance of interspecific hybridization in Jatropha. The study was confined to identification of crossability barriers and morphological characterization of the F1 hybrids. The species that could be crossed unilaterally with J. curcas as ovule parent include J. macrorhiza, J. capensis, J. cathartica, J. multifida, J. podagrica, J. cordata, J. cinerea (Dehgan 1984). Reciprocal crosses are possible with J. integerrima and interspecific hybrids have been developed between J. curcas and J. integerrima (Rupert et al. 1970; Dehgan 1984; Sujatha and Prabakaran 2003). One to two backcrosses of the F1 hybrids to J. curcas resulted in transgressive segregants exhibiting variation for fruit and seed characters (Sujatha and Prabakaran 2003).
Determination of genetic relationships among species is critical for the management of genetic resources and success of interspecific hybridization. In Jatropha, taxonomic classification and infrageneric relationships were based on leaf epidermal morphology (Dehgan 1980), petiolar anatomy (Dehgan 1982); crossability relationships (Dehgan 1984) and phenetic and cladistic analysis by analyzing 32 morphological characters in herbarium specimens (Dehgan and Webster 1979; Dehgan and Schutzman 1994). Anatomical features of the petiole are singularly not sufficient in delineating evolutionary phylogenetic sequences but strengthen other anatomical, morphological and experimental approaches in solving taxonomic problems. Likewise, morphological studies of epidermis and other traits are insufficient by themselves as taxonomic evidences. Electrophoretic patterns of seed and leaf proteins of Jatropha species found in India were determined to assess similarity index between the species (Sathaiah and Reddy 1985; Sujatha 1996). Molecular markers reveal more quickly and accurately, genetic differences far exceeding those obtainable using morphological or biochemical methods without the obscurance of environment. Nuclear and plastid DNA analysis represent an important tool for phylogenetic and diversity analysis of plants. RAPD markers cover the entire genome revealing length polymorphisms in coding or non-coding and repeated or single copy sequences while ISSR markers generate polymorphism from sequences between two microsatellite primer sites (Williams et al. 1990; Zietkiewicz et al. 1994). Universal primers targeted to mononucleotide repeats present in chloroplast genomes serve as a valuable tool to study chloroplast variation (Weising and Gardner 1999). Chloroplast specific microsatellites have been used for assessment of maternal versus paternal plastid inheritance (Cato and Richardson 1996), assessment of interspecific polymorphism (Weising and Gardner 1999), the detection of hybridization and introgression (Bucci et al. 1998) and phylogeny of plant populations (Grivet and Petit 2003). Ganesh Ram et al. (2007) assessed genetic diversity of five Jatropha species along with five accessions of J. curcas using just 18 RAPD markers.
The aim of the present study was to assess genetic relationships among Jatropha species from India using molecular markers. Some putative natural hybrids and artificially produced hybrids were also included in the study. Four types of molecular markers were applied: RAPD, ISSR, consensus chloroplast microsatellite primers from angiosperm taxa and organellar specific primers from rice. We also evaluated the nuclear and organelle specific primer polymorphism in confirmation of hybridity and direction of gene flow in natural and synthetic hybrids of Jatropha.
Materials and methods
Plant material
The Jatropha species found in India with the exception of J. heterophylla (J. heynii), an ephemeral species and J. nana, a species with localized distribution in a small pocket in western part of India were used in the study (Table 1). Jatropha species growing wild in non-arable lands or cultivated as ornamentals in horticultural gardens and avenue planting were collected and assembled in the Jatropha species garden at the Directorate of Oilseeds Research, Hyderabad, India. The species J. curcas, J. multifida and J. podagrica were established through seeds while the other species were established from stem cuttings and tuberous root stocks. The characteristics of the species were confirmed with those described by Sujatha and Prabakaran (1997). The species J. villosa var. villosa, J. villosa var. ramnadensis (Ramamurthy 1967) and J. maheshwarii (Subramanyam and Nayar 1964) were collected from one state (Tamil Nadu) and only one accession each was used in the study. Plants resembling J. tanjorensis were collected from Pudukottai and Coimbatore from Tamil Nadu state and Kakinada and Vizianagaram from Andhra Pradesh state and were confirmed according to the description of Ellis and Saroja (1961). For other species, five accessions each were initially subjected to molecular studies using 20 RAPD primers producing robust amplification profiles for assessment of intraspecific variability and for detection of off-types, if any. As occurrence of natural hybrids in genus Jatropha is reported, pollen from all the accessions was studied for fertility and pollen grain polymorphism by staining in a 1:1 mixture of 1.0% acetocarmine and glycerol.
DNA extraction
DNA isolation was performed on five grams of leaf tissue ground in liquid nitrogen. Total genomic DNA was extracted individually from younger leaves of 34 accessions of Jatropha species following the standard CTAB method with minor modifications (Doyle and Doyle 1990). As there was no detectable intra-accessional variation, DNA of species bulk was constituted with DNA from single plants. Based on previous molecular studies (Sujatha et al. 2005; Basha and Sujatha 2007), one each of the toxic Indian and non-toxic Mexican genotypes of J. curcas were included keeping in view the genetic divergence between them. Likewise, both the cultivars of J. villosa (villosa and ramnadensis) were analyzed separately. DNA concentrations were determined electrophoretically versus known amount of λ DNA as standards. For PCR, DNA samples were adjusted to a concentration of 2.5 ng/μl.
RAPD analysis
Two hundred RAPD primers (Operon Technologies, Alameda, CA, USA) were used for amplification of DNA according to the method of Williams et al. (1990). PCR amplification was carried out in a 10 μl reaction mixture containing 2.5 ng of genomic DNA, 1× PCR buffer containing 1.5 mM MgCl2, 0.4 μM of RAPD primer, 100 μM of each of the four dNTPs and 0.3 U of Taq DNA polymerase (Bangalore Genei, India). PCR amplification was carried out in Applied Biosystems GeneAmp 9700 thermal cycler with an initial denaturation at 94°C for 3 min followed by 45 cycles of denaturation at 94°C for 45 s, annealing at 36°C for 30 s and extension at 72°C for 2 min with a final extension at 72°C for 7 min. The amplified PCR products were resolved by electrophoresis on 1.5% agarose (Bangalore Genei, India) gel in 1× TAE buffer by electrophoresis at 100 V for 3 h and visualized with ethidium bromide staining. The gel images were recorded using the Alpha Innotech Fluorchem gel documentation system. Every PCR reaction was repeated twice to check reproducibility of bands and a negative control (no DNA) was used in all reactions to avoid erroneous interpretations.
ISSR analysis
A total of 100 ISSR primers (UBC primer set No. 9, University of British Columbia, Canada) were used for the analysis. The PCR amplification was empirically determined by testing different concentrations of genomic DNA and primer. The optimal annealing temperature was found to vary according to the base composition of the primers. A negative control that contained all PCR components except DNA was included in every experiment to test for DNA contamination of the reagents.
PCR amplification was performed in 10 μl reaction mixture containing 2.5 ng of template DNA, 150 μM of each of the four dNTPs, 1× PCR buffer (10 mM Tris pH 9.0, 50 mM KCl, 1.5 mM MgCl2), 0.2 μl of 25 mM MgCl2, 0.4 μM ISSR primer and 0.6 U Taq DNA polymerase (Bangalore Genei, India). PCR amplifications were performed in Applied Biosystems GeneAmp 9700 thermal cycler with initial denaturation at 94°C for 4 min followed by 35 cycles of denaturation at 92°C for 30 s, 1 min at the annealing temperature (Ta), elongation at 72°C for 2 min and final extension at 72°C for 7 min. The amplified products were electrophoretically separated in 1.7% agarose (Bangalore Genei, India) gels buffered with 1× TAE at 100 V. The EcoRI and HindIII double digest DNA ladder was used as molecular weight standard.
Statistical analysis
The banding patterns obtained with PCR amplification were analyzed to assess the genetic relationships among the Jatropha species. Each RAPD and ISSR band was scored for the presence (1) or absence (0) to create a binary matrix. Pairwise similarity of banding was analyzed and simple matching coefficients (SM) were generated in SimQual of NTSYS pc version 2.02i (Applied Biostatistics Inc., Setauket, USA). These similarity coefficients were used to construct dendrograms based on unweighted pair group method with arithmetical averages (UPGMA) according to SAHN method (Sneath and Sokal 1973). The significance of genetic similarity matrix data generated with RAPD and ISSR markers was determined using Mantel test.
Organellar genome analysis
A set of ten consensus chloroplast microsatellite primers (ccmp1 to 10) specific to chloroplast genomes of dicotyledonous angiosperms (Weising and Gardner 1999) and 40 rice mitochondrial and chloroplast specific (RMT1 to 40) primers (Rajendra Kumar et al. 2007) were used for characterization of organellar genome of Jatropha species. The PCR amplifications were carried out in a 10 μl reaction mixture containing 10 ng of genomic DNA, 1× PCR buffer containing 1.5 mM MgCl2, 0.4 μM each of forward and reverse primers, 150 μM of each dNTPs and 0.6 U of Taq DNA polymerase (Bangalore Genei, India). PCR amplifications were performed in GeneAmp 9700 thermal cycler (Perkin Elmer Applied Biosystems) with the following cycling conditions: 94°C for 4 min; 35 cycles of 92°C for 30 s, 55°C for 30 s, and 72°C for 45 s; and a final extension at 72°C for 5 min. The amplified PCR products were resolved by electrophoresis on 4% agarose (Bangalore Genei, India) gel and visualized by ethidium bromide staining. Banding pattern was recorded under ultraviolet light and documented in Alpha Innotech Fluorchem gel documentation system. As the amplification products that resulted from organelle specific markers were run on agarose gels, variation due to single base changes cannot be detected credibly. Hence, all the amplicons were subjected to sequence analysis.
Cloning and sequencing of PCR fragments
PCR products amplified with RMT1 and nine ccmp primer pairs (except ccmp8) were ligated into pTZ57R (Insta) T/A cloning vector using cloning kit (MBI Fermentas, USA). The recombinant plasmids were transformed into competent E. coli cells (DH5α) and the plasmid DNA purified from the white colonies as described by Sambrook et al. (1989). Selected transformed clones were screened by PCR analysis with corresponding ccmp primer pairs and the size of inserts was checked by EcoRI and HindIII restriction digestion. The inserted DNA fragments were sequenced at Bioserve Biotechnologies (Hyderabad, India) using M13 vector specific primers. For each amplicon, two sequences were cloned and subjected to sequence analysis. Sequences were edited and assembled in Chromas 1.45 and multiple sequence alignments were performed using Genetool Lite 1.0 software. Alignments were adjusted manually where necessary and 101 sequences were deposited in NCBI GenBank (accession numbers EF990032 to EF990129 and EU167920 to EU167922).
Characterization of interspecific hybrids and validation of polymorphic nuclear and ccmp primers
Interspecific crosses were effected between J. curcas and other Jatropha species. In Jatropha, female flowers open first and are easily distinguished from male flowers by their oblong shape and presence on the central axes of the inflorescence. At flowering, the female parents were dusted with pollen from the male parent and crosses were made in both direct and reciprocal directions. The F1 seeds were germinated on moist vermiculite under high humidity and later transferred to pots. Observations were made on vegetative characters and pollen fertility of the F1 hybrids. The cross involving J. curcas × J. integerrima was advanced to F2, BC2F1 and BC1F2 generations. The advanced generation interspecific derivatives were characterized for seed weight, seed oil content and fatty acid profiles. For fatty acid analysis, methyl esters of seed oil were prepared by extracting oil with petroleum ether and refluxing with methanolic sodium methoxide as described by Schneider et al. (1968). Gas liquid chromatography was carried out using Nucon D 5700 unit coupled with flame ionization detector and Hewlett Packard 3390 integrator.
The interspecific hybrids were characterized using RAPD and ISSR primers disclosing polymorphism between the parental species for confirmation of hybridity. For verification of the polymorphism detected with ccmp primers, both natural and artificially produced hybrids were amplified with primer pairs, ccmp6 and 10. J. tanjorensis along with two more naturally occurring hybrids resembling it in morphological characters were tested with all the Jatropha species. Three sets of artificially produced hybrids derived from J. gossypifolia × J. curcas, J. curcas × J. integerrima and J. maheshwarii × J. curcas were amplified with ccmp primers along with their respective parental species.
Results
All the species were characterized morphologically. There was high morphological diversity among the species used in the study. The pollen grains were fertile in all the species and the size varied between 50 and 85 μm. All the four accessions resembling J. tanjorensis exhibited high pollen polymorphism with pollen grain size ranging from 15 to 100 μm and with sterility of more than 95%.
Out of the 200 RAPD primers tested, 168 primers gave amplification products. The number of amplicons per primer varied from 5 (OPF 11) to 30 (OPI 7) and the amplicon size varied from 200 bp to 3.8 kb. The total number of bands generated was 2,678 of which 2,634 revealed polymorphism (98.4%) between the species with an average of 15.7 polymorphic bands per primer (Fig. 1). The extent of polymorphism ranged from 91.7% (OPB 4) and 100% (OPI 3). Genetic similarity based on simple matching coefficients derived from RAPD markers was maximum (0.83) between J. tanjorensis and J. gossypifolia while the minimum (0.59) was recorded between J. curcas and J. multifida.
Of the 100 ISSR primers tested, 52 primers resulted in 856 amplicons with an average of 16.5 bands per primer. Of these, 844 were polymorphic resulting in a polymorphism of 98.6% (Fig. 2). The polymorphism was maximum (100%) with the tetranucleotide primer, UBC 873 and was low (84%) with UBC 867. The number of amplicons per primer ranged from 7 (UBC 867) to 32 (UBC 873) and the size varied from 100 bp to 4.0 kb. Averaged over different types of ISSR primers, maximum number of bands (19.5) was generated by pentanucleotide primers while maximum polymorphism (99.1%) was generated by dinucleotide primers. The nine mononucleotide primers from the UBC set failed to give amplification products. Genetic similarity based on ISSR markers was maximum (0.88) between J. tanjorensis and J. gossypifolia while it was low (0.54) between J. tanjorensis and J. maheshwarii. The correlation between the matrices generated with RAPD and ISSR markers was highly significant (r = 0.953928, P < 0.001) indicating goodness of fit between the two molecular marker systems.
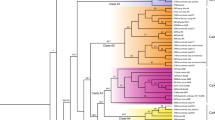
The dendrogram constructed based on RAPD and ISSR marker data resolved six clusters at 72% similarity (Fig. 3). Cluster I consisted of J. curcas. Cluster II comprised of J. tanjorensis and J. gossypifolia. Clusters III and IV included J. glandulifera and J. integerrima, respectively. Cluster V included J. villosa and its allied members, J. villosa var. ramnadensis and J. maheshwarii. The species of section peltatae viz., J. multifida and J. podagrica grouped together in cluster VI. Based on combined marker data analysis, maximum similarity index (0.85) was recorded for J. tanjorensis and J. gossypifolia while the minimum (0.59) was observed for J. curcas and J. multifida.
All the ccmp primers except ccmp8 yielded a single, discrete PCR product (Fig. 4). There was no stuttering in the amplification reactions with ccmp primers. With the exception of ccmp1, all other primers gave amplification product, which was different from that of tobacco (Table 2). The regions ORF77–ORF82 and rp12–rps19 amplified by ccmp6 and ccmp10 primers, respectively exhibited maximum variability. Both these primers differentiated the chloroplast genome of the Jatropha species both in terms of the amplicon size and repeat length. Each species was characterized by a unique haplotype except for J. tanjorensis, which was identical to J. gossypifolia.
Forty primers specific to rice mitochondrial and chloroplast specific genomes gave amplification products in Jatropha species with 22 primers of which four revealed polymorphism among species (data not presented). However, three primers gave multiple bands while the primer RMT1 resulted in a single polymorphic band (Fig. 5). The amplicon sizes with RMT1 varied between 333 and 388 bp in Jatropha species while it was 216 bp in rice (Table 2).
Sequence length, composition of each region sequenced and the repeat region are summarized in Table 2. The sequences obtained with two primer pairs viz., ccmp6 and ccmp10 were aligned with the corresponding tobacco sequences derived from the database (Figs. 6, 7). Sequence alignments demonstrated that variable number of mononucleotide repeats is the cause of polymorphism generated by the ccmp primers. These two primers generated seven variants each both in terms of allele size and microsatellite repeat motifs. Most of the mononucleotide repeats were T mononucleotides with the exception of J. multifida in which the amplicon with ccmp6 resulted in repeat region with mononucleotides of A and exhibited a complex pattern (Fig. 6). In J. integerrima, the sequence amplified with ccmp10 had an additional repeat region of T mononucleotides (Fig. 7). The repeat region in amplicons of J. tanjorensis and J. gossypifolia derived with ccmp10 primers was not distinct. None of the ccmp primers differentiated J. tanjorensis from J. gossypifolia either in terms of repeat length or repeat composition. Unlike the repeat region polymorphism detected with ccmp primers, there was no microsatellite variation within Jatropha with RMT1 and the fragment size variation was found only in the adjacent regions. The repeat motif in rice was [(gtag)4] while it was shorter and identical in all the Jatropha species [(gtag)2] regardless of the variation in amplicon size.
Alignment of DNA sequences at the consensus chloroplast microsatellite primers ccmp6 locus from various Jatropha species and comparison with the corresponding sequences of Nicotiana tabacum (ORF77-ORF82 intergenic region). Only unique sequences are shown for each species. Microsatellites are shown in bold type with repeat originally targeted in the tobacco alignment
Alignment of DNA sequences at the consensus chloroplast microsatellite primers ccmp10 locus from various Jatropha species and comparison with the corresponding sequences of Nicotiana tabacum (rp12–rps19 intergenic region). Only unique sequences are shown for each species. Microsatellites are shown in bold type with repeat originally targeted in the tobacco alignment
Large differences were observed in allele sizes of different species with ccmp6, and ccmp10. The polymorphism obtained with these primers was validated on natural and artificially produced hybrids. Use of ccmp6 and 10 primer sets on J. tanjorensis and two more accessions resembling it clearly showed that all three natural hybrids are genetically similar (Fig. 8a, b). With primer pair ccmp6, the hybrids had an amplicon of size 83 bp similar to that of J. gossypifolia while, the other putative parent J. curcas had an amplicon of 81 bp. Likewise with ccmp10, the natural hybrids and J. gossypifolia resulted in an amplicon of 69 bp while J. curcas amplified a band of 118 bp. Further, to confirm the maternal inheritance of plastid genes, the primers were tested on three artificially produced hybrids viz., J. gossypifolia × J. curcas, J. curcas × J. integerrima and J. maheshwarii × J. curcas. Analysis with ccmp6 primers clearly showed the banding pattern of hybrids identical to their maternal parent (Fig. 9).
PCR amplification profile of the natural hybrids resembling J. tanjorensis along with putative parental species and other Jatropha species for confirmation of the maternal parent. a Generated by ccmp6 primers; b generated by ccmp10 primers. M molecular marker (50 bp DNA ladder), Nc negative (no DNA) control and the arrows indicate the size of the band in hybrids and putative parental species
Interspecific hybrids could be produced between J. curcas and all other species used in the study with the exception of J. podagrica. All the F1s showed morphological intermediacy in terms of leaf pigmentation, number of leaf lobes and flower color. The hybrid between J. multifida and J. curcas had thin hard stem typical of the maternal parent (Fig. 10a). The hybrids of J. maheshwarii × J. curcas were early and flowered within 58–65 days of sowing (Fig. 10b). Flower color was green in hybrids of J. curcas with J. maheshwarii, J. glandulifera, J. villosa and J. villosa var. ramnadensis, while it was pinkish green in case of hybrid with J. multifida and purplish green in hybrid with J. gossypifolia. Flower color of hybrids involving J. curcas and J. integerrima was not representative of the parental flower colors and has been reported earlier (Sujatha and Prabakaran 2003). Pollen staining revealed pollen heteromorphism in hybrids of all the interspecific crosses with pollen fertility ranging from 42 to 69% in different hybrids. Presence of RAPD markers specific to the parental species confirmed the hybridity of different interspecific hybrids (Fig. 11).
Interspecific derivatives of J. curcas crossed with different Jatropha species. a F1 hybrid of J. multifida × J. curcas; b F1 hybrid of J. maheshwarii × J. curcas with the parents; c BC1F1 progenies of J. curcas × J. integerrima showing distinct variations in vegetative characters; d BC1F1 of J. curcas × J. integerrima with increased fruit size and good bearing ability
The interspecific hybrids of J. curcas × J. integerrima were advanced to F2, BC1F1, BC1F2 and BC2F2 generations. The interspecific derivatives derived through one to two backcrosses of the cross involving J. curcas × J. integerrima resulted in lines exhibiting wide variability in qualitative and seed characters (Fig. 10c; Table 3). Pollen fertility improved from 64% (associated with pollen polymorphism) in F1s to 85.4% in BC1F1 (no pollen polymorphism) to >95.0% in BC2F1 generations. The fruits of F1s were small with poor filling but one backcrossing to J. curcas resulted in improved fruit set and increased fruit size (Fig. 10d). BC2F1 and BC2F2 plants with high seed yield and high oleic acid have been selected for further breeding.
Discussion
Polymorphism based on RAPD and ISSR markers was high (98.5%) and sufficient in distinguishing each of the Jatropha species. The two nuclear marker systems were equally effective and produced similar levels of polymorphism in Jatropha. Sathaiah and Reddy (1985) used isozymes for characterization of four Jatropha species. The study revealed distinct protein profile for each of the four Jatropha species used in their study. High polymorphism between species indicates variation in genic, intergenic and repeated sequences regions in Jatropha. Both the RAPD and ISSR primers generated several unique species specific markers. Sequencing of such unique amplicons would be useful in phylogenetic studies and establishment of gene introgressions between different taxa.
Clustering based on molecular markers in the present investigation was in agreement with the previously reported taxonomic classification founded on morphological characteristics (Dehgan 1982, 1984; Dehgan and Webster 1979). J. curcas, a perennial shrub and J. gossypifolia, a facultative annual were the closest relatives and clustered together. This grouping could also be due to the inclusion of J. tanjorensis, a natural hybrid between the two species. However, exclusion of J. tanjorensis from the cluster analysis still revealed the closeness of the two species. Studies of Sathaiah and Reddy (1985) based on seed protein profiles revealed maximum similarity (45.0%) between J. curcas and J. gossypifolia. The species, J. maheshwarii and J. villosa var. ramnadensis are allied to J. villosa, but differ in plants being glabrous, leaves oblong-ovate, entire, acute to acuminate at apex and petals united only at base (Subramanyam and Nayar 1964; Ramamurthy 1967). Molecular analysis was consistent with morphologically based grouping of these accessions. The species J. integerrima of section polymorphae formed a separate cluster. The species J. multifida and J. podagrica belonging to the section peltatae clustered together. Leaf protein profiles of total protein (native PAGE), protein subunits (SDS–PAGE) and esterase isozymes showed maximum similarity (50.9%) between J. multifida and J. podagrica (Sujatha 1996). The section peltatae is considered to be the most advanced section based on petiolar anatomy (Dehgan 1982), crossability relationships (Dehgan 1984) and morphological differentiation (Dehgan and Webster 1979). Molecular profiling of Jatropha species also revealed maximum genetic distance between J. curcas and members of the section peltatae. Morphologically, J. glandulifera has closer resemblances to J. gossypifolia and is often confused with the latter. However, it is distinguished from the latter by complete green leaves devoid of anthocyanin pigmentation, the serrate gland-tipped leaves, stout branches, long branched gland tipped stipules and greenish yellow flowers (Anonymous 1959). The RAPD and ISSR markers separated these two species into different clusters and the ccmp6 primer distinguished both the species based on the variability in ORF 77-ORF 82 intergenic region.
Conversely, use of few RAPD primers (18) resulted in several ambiguities in establishment of genetic relationships among Jatropha species (Ganesh Ram et al. 2007). J. villosa var. Ramnadensis and J. villosa var. Villosa are varieties of the species, J. villosa (Ramamurthy 1967). While the cv. Ramnadensis clustered with J. gossypifolia, the cv. Villosa formed a separate group with J. tanjorensis. Similarly, J. tanjorensis, a spontaneous hybrid between J. curcas and J. gossypifolia (Prabakaran and Sujatha 1999) failed to show its genetic closeness with either of its parental species. Likewise, J. gossypifolia of section jatropha grouped together with J. integerrima of section polymorphae in principal component analysis. These uncertainities in the study of Ganesh Ram et al. (2007) could probably be due to use of few data points (112 polymorphic bands generated with 18 primers) unlike in the present investigation where a total of 3,478 polymorphic marker data generated with 220 functional nuclear markers were used for establishment of genetic relationships among Jatropha species. Careful understanding of the material and use of adequate number of molecular markers are essential prerequisites for drawing valid inferences about the genetic affinities.
Chloroplast genome evolves slowly and many primers for PCR amplification and analysis of chloroplast sequences can be used across a wide array of genera. Weising and Gardner (1999) designed and developed a set of conserved PCR primers for the analysis of simple mononucleotide polymorphism in chloroplast genomes of dicotyledonous angiosperms. The ten ccmp primers with the exception of ccmp8 gave amplification products in Jatropha. DNA template set comprising six genera of the Euphorbiaceae also failed to amplify with ccmp8 (Vogel et al. 2003). Likewise, ccmp8 failed to produce amplification products with templates from Solanaceae, Actinidia, Cruciferae and monocotyledonous species (Weising and Gardner 1999).
The ccmp primers were able to distinguish all the Jatropha species with a unique set of alleles and also aided in unraveling the direction of the interspecific crosses. Maximum variability among the Jatropha species was disclosed with ccmp primers in the ORF 77–ORF 82 region amplified by ccmp6 primer and rp12–rps19 region amplified by ccmp10. These two primers showed polymorphism in Macaranga species as well (Vogel et al. 2003). The primer ccmp6 failed to reveal variability in Lycopersicon and Actinidia species (Weising and Gardner 1999). Conversely, this primer pair revealed maximum variability among the Jatropha species both in terms of repeat unit size and composition. The rp12–rps19 intergenic region exhibited largest size spectrum with poly(A) tracts of variable size in Actinidia, Cordyline and Solanaceae (Goulding et al. 1996; Weising and Gardner 1999). However, in case of Jatropha, variation was in the T mononucleotides and the species J. integerrima of the section polymorphae had an additional repeat region of T mononucleotides. In chloroplast genomes, gene order is highly conserved but small insertions/deletions (indels) are relatively frequent resulting in intra-species variation (Turkec et al. 2006). The possible explanations for existence of mutational hotspots in noncoding cpDNA or due to small insertion and deletion mutations in the intergenic cpDNA regions has been adequately discussed (Johnson and Hattori 1996; van Ham et al. 1994). Regardless of the causes of genetic variation, characterization of chloroplast genome of Jatropha species using universal primers targeted to mononucleotide repeat regions indicates that ccmp primers 6 and 10 serve as a useful tool for assessment of interspecific genetic variation.
In silico sequence analysis of amplicons obtained using organelle specific primers was done. The ccmp1 primer sequence flanking trnK intron region revealed 100% homology with chloroplast mat K gene for maturase in trnK intron. Sequences derived with ccmp2, ccmp5 and ccmp7 showed high degree of similarity to the chloroplast genome sequences of other euphorbiaceous members indicating that these regions are highly conserved. The ccmp2 sequence of Jatropha possessed 79% homology to Psb1 gene of Macaranga sps, trnS region of Euphorbia millii, and trnS-trnQ intergenic spacer region of Acalypha. The sequence derived with ccmp5 primers had 100% similarity to the 3′ rps2 chloroplast sequence of euphorbiaceae genera, Mallotus, Euphorbia, Acalypha, Mercurialis, Codiaeum variegatum and Macaranga. Likewise, ccmp7 is also highly conserved in the Euphorbiaceae and the amplicons of Jatropha showed 100% similarity to atpB gene in Macaranga, Codiaeum and Euphorbia. The sequences obtained with ccmp9 derived amplicons showed 100% homology to the chloroplast genome of members of Solanaceae.
The consensus chloroplast microsatellite primers developed for dicotyledonous species worked with monocotyledons (Weising and Gardner 1999). The property of maternal inheritance of organellar genomes and its low nucleotide substitution rate has encouraged us to use organelle specific primers from heterologous systems. Based on the personal communication (Sundaram) microsatellite primers specific to organellar genome of rice were used for assessment of genetic relationships among Jatropha species. Of the 40 tested primers, one primer (RMT1) was found functional in giving single, discrete, polymorphic band in Jatropha species. Sequence similarity analysis using the sequence obtained from rice showed 100% homology with genomic DNA of chromosomes 1, 3, 6 and 10 of Japonica cultivar group, chloroplast atpB and atpE genes, mitochondrion and chloroplast genes of zea mays, mitochondrial subunits of beta and epsilon subunits of atpB and atpE genes. However, amplicons derived from Jatropha with RMT1 primers possessed only 19% homology with that of rice. Interestingly, analysis of the sequences derived from Jatropha showed 100% sequence similarity to the chloroplastic atpE gene. The atpE gene from both chloroplast and mitochondria hold high sequence homology. The forward primer of RMT1 (ttc ata cgg cgg gag tc) along with the reverse primer (agc tct cag acg agc tg) amplified an amplicon of size 208–216 bp with the repeat motif (gtag)4 in the mitochondrial genome of rice. The same forward primer with a different reverse primer sequence (gat acg agt cga ggc tg) amplified chloroplast DNA of rice with an amplicon size of 198 bp but with the same repeat motif of (gtag)4. Presumably in Jatropha, the RMT1 primer pair would have amplified the chloroplast region and could be successfully used for characterization of organellar genome variation.
The organelle specific markers were validated on both naturally obtained and artificially produced hybrids. In the genus Jatropha, existence of natural hybrid complexes is reported such as, J. curcas-canascens complex in Mexico (Dehgan and Webster 1978), J. integerrima-hastata complex in Cuba and West Indian islands (Pax 1910) and J. curcas-gossypifolia (J. tanjorensis) in India (Prabakaran and Sujatha 1999). J. curcas crosses readily with all the species. Jatropha accessions similar in appearance to J. tanjorensis were identified at several locations. Careful examination of the population structure revealed that the most predominant species in the vicinity were J. curcas and J. gossypifolia. The species J. curcas and J. gossypifolia are sympatric and in India, the ranges of the two species overlap and natural spontaneous hybrids occur between the two species. Studies of Prabakaran and Sujatha (1999) based on morphological, cytological and biochemical characteristics confirmed J. tanjorensis as a natural hybrid between J. curcas and J. gossypifolia and not a distinct species as described by Ellis and Saroja (1961). In the present investigation, RAPD and ISSR markers confirmed the hybridity of J. tanjorensis between J. curcas and J. gossypifolia. The organelle specific primers explicitly established that J. gossypifolia is the maternal parent for the hybrid. Molecular profile of two more natural hybrids resembling J. tanjorensis with ccmp6 and 10 primers indicated unidirectional introgression of J. curcas nuclear genome into that of J. gossypifolia. Thus, the study also demonstrated the usefulness of ccmp primer pairs 6 and 10 in confirmation of hybridity in interspecific crosses and detection of parental species involved in putative hybrids.
RFLP studies indicated paternal mode of inheritance of chloroplast DNA in interspecific crosses within the genus Actinidia (Cipriani et al. 1995). However, characterization of both the natural and artificial hybrids of Jatropha displayed chloroplast genome amplification profiles identical to that of the maternal progenitor, thereby suggesting that the chloroplast genome is maternally inherited in Jatropha, as in the case of most other angiosperms (Mogensen 1996).
This study was undertaken to corroborate morphological variation with genetic variation. We have not made any accurate comparison with the morphology-based taxonomy as the species used in both the studies were not the same. Few of the species used in the present study viz., J. glandulifera, J. villosa and J. maheshwarii were not included in the studies of Dehgan (1980, 1982, 1984), Dehgan and Webster (1979) and Dehgan and Schutzman (1994). Nevertheless, grouping together of species of section peltatae (J. podagrica and J. multifida) and likewise the J. villosa complex based on both morphological and molecular studies indicate that molecular studies will unequivocally help in establishment of genetic relationships between different taxa. Inclusion of both the new and old world species and addition of genomic regions of suitable variability will increase resolution and will help in confirming the systematic position of different Jatropha species and in a better understanding of the genetic relationships and evolution of the genus Jatropha.
Interspecific hybridization revealed the possibility of obtaining hybrids of J. curcas with other Jatropha species. Studies of Dehgan (1984) showed high degree of unilateral compatibility and crosses of J. curcas were successful only when it is used as the female parent with the exception of the cross with J. integerrima where reciprocal hybrids are possible. In this investigation, we obtained seed in crosses of J. curcas as pollen parent with J. multifida, J. maheshwarii, J. gossypifolia and J. villosa as ovule parents. Studies of Dehgan (1984) were confined to development and characterization of interspecific hybrids in Jatropha. Characterization of advanced generation interspecific derivatives of J. curcas and J. integerrima cross carried out in the present investigation indicate ample scope for genetic enhancement of J. curcas through interspecific gene transfer.
References
Anonymous (1959) The wealth of India. Raw materials, vol V. CSIR, New Delhi, pp 293–297
Banerji R, Chowdhury AR, Misra G, Sudarsanam G, Verma SC, Srivastava GS (1985) Jatropha seed oils for energy. Biomass 8:277–282
Basha SD, Sujatha M (2007) Inter and intra-population variability of Jatropha curcas (L.) characterized by RAPD and ISSR markers and development of population-specific SCAR markers. Euphytica 156:375–386
Bucci G, Anzidei M, Madaghiele A, Vendramin GG (1998) Detection of haplotypic variation and natural hybridization in halepensis-complex pine species using chloroplast simple sequence repeat (SSR) markers. Mol Ecol 7:1633–1643
Cato SA, Richardson TE (1996) Inter- and intraspecific polymorphism at chloroplast SSR loci and the inheritance of plastids in Pinus radiata D. Don. Theor Appl Genet 93:587–592
Cipriani G, Testolin R, Morgante M (1995) Paternal inheritance of plastids in interspecific hybrids of the genus Actinidia revealed by PCR amplification of chloroplast DNA fragments. Mol Gen Genet 247:693–697
Dehgan B (1980) Application of epidermal morphology to taxonomic delimitations in the genus Jatropha L. (Euphorbiaceae). Bot J Linn Soc 80:257–278
Dehgan B (1982) Comparative anatomy of the petiole and infrageneric relationships in Jatropha (Euphorbiaceae). Am J Bot 69:1283–1295
Dehgan B (1984) Phylogenetic significance of interspecific hybridization in Jatropha (Euphorbiaceae). Syst Bot 9:467–478
Dehgan B, Schutzman B (1994) Contributions toward a monograph of neotropical Jatropha: phenetic and phylogenetic analyses. Ann Mo Bot Gard 81:349–367
Dehgan B, Webster GL (1978) Three new species of Jatropha (Euphorbiaceae) from western Mexico. Madrono 25:30–39
Dehgan B, Webster GL (1979) Morphology and infrageneric relationships of the genus Jatropha (Euphorbiaceae). Univ Calif Publ Bot 74:1–73
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Ellis JL, Saroja TL (1961) A new species of Jatropha from south India. J Bombay Nat Hist Soc 58:834–836
Ganesh Ram S, Parthiban KT, Senthil Kumar R, Thiruvengadam V, Paramathma M (2007) Genetic diversity among Jatropha species as revealed by RAPD markers. Genet Resour Crop Evol 55:803–809. doi:10.1007/s10722-007-9285-7
Goulding SE, Olmstead RG, Mordent CW, Wolfe KH (1996) Ebb and flow of the chloroplast inverted repeat. Mol Gen Genet 252:195–206
Grivet D, Petit RJ (2003) Chloroplast DNA phylogeography of the hornbeam in Europe: evidence for a bottleneck at the outset of postglacial colonization. Conserv Genet 4:47–56
Heller J (1996) Physic nut—Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops. 1. International Plant Genetic Resources Institute, Rome, Italy (http://www.ipgri.cgiar.org/publications/pdf/161.pdf)
Johnson DA, Hattori J (1996) Analysis of a hotspot for deletion formation within the intron of the chloroplast trnL gene. Genome 39:999–1005
Jones N, Miller JH (1991) Jatropha curcas. A multipurpose species for problematic sites. Land Resour Ser 1:1–12
Kaushik N, Kumar K, Kumar S, Kaushik N, Roy S (2007) Genetic variability and divergence studies in seed traits and oil content of Jatropha (Jatropha curcas L.) accessions. Biomass Bioenergy 31:497–502
Lakshminarayana M, Sujatha M (2001) Screening of Jatropha species against major defoliators of castor (Ricinus communis L.). J Oilseeds Res 18:228–230
Mc Vaugh R (1945) The genus Jatropha in America: principal intergeneric groups. Bull Torrey Bot Club 72:271–294
Mogensen HL (1996) The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot 83:383–404
Openshaw K (2000) A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenergy 19:1–15
Pax F (1910) Euphorbiaceae–Jatropheae. In: Das Pflanzenreich. IV,vol 147. Verlag Von Wilhem Engleman, Leipiz, pp 1–148
Prabakaran AJ, Sujatha M (1999) Jatropha tanjorensis Ellis & Saroja, a natural interspecific hybrid occurring in Tamil Nadu, India. Genet Resour Crop Evol 46:213–218
Rajendra Kumar P, Biswal AK, Balachandran SM, Srinivasan K, Sundaram RM (2007) Simple sequence repeats in organellar genomes of rice: frequency and distribution in genic and intergenic regions. Genome anal 23:1–4
Ramamurthy K (1967) A new variety of Jatropha villosa from Madras state. Bull Bot Surv India 9:278–279
Rupert EA, Dehgan B, Webster GL (1970) Experimental studies of relationships in the genus Jatropha. L. J. curcas × J. integerrima. Bull Torrey Bot Club 97:321–325
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sathaiah V, Reddy TP (1985) Seed protein profiles of castor (Ricinus communis L.) and some Jatropha species. Genet Agr 39:35–43
Schneider EL, Loke SP, Hopkins DT (1968) Gas liquid chromatographic analysis of cyclopropenoid fatty acids. J Am Oil Chem Soc 45:585–590
Sneath PHA, Sokal RR (1973) Numerical taxonomy. Freeman, San Francisco
Subramanyam K, Nayar MP (1964) A new species of Jatropha from Madras state. Bull Bot Surv India 6:331–332
Sujatha M (1996) Genetic and tissue culture studies in castor (Ricinus communis L.) and related genera. Ph.D. Dissertation, Osmania University, Hyderabad
Sujatha M (2006) Genetic improvement of Jatropha curcas L.: possibilities and prospects. Indian J Agrofor 8:58–65
Sujatha M, Prabakaran AJ (1997) Characterization and utilization of Indian Jatrophas. Indian J Pl Genet Resour 10:123–128
Sujatha M, Prabakaran AJ (2003) New ornamental Jatropha hybrids through interspecific hybridization. Genet Resour Crop Evol 50:75–82
Sujatha M, Makkar HPS, Becker K (2005) Shoot bud proliferation from axillary nodes and leaf sections of non-toxic Jatropha curcas L. Plant Growth Regul 47:83–90
Turkec A, Sayar M, Heinze B (2006) Identification of sweet cherry cultivars (Prunus avium L.) and analysis of their genetic relationships by chloroplast sequence-characterised amplified regions (cpSCAR). Genet Resour Crop Evol 53:1635–1641
van Ham RCHJ, Hart HT, Mes THM, Sandbrink JM (1994) Molecular evolution of noncoding regions of the chloroplast genome in the Crassulaceae and related species. Curr Genet 25:558–566
Vogel M, Banfer G, Moog U, Weising K (2003) Development and characterization of chloroplast microsatellite markers in Macaranga (Euphorbiaceae). Genome 46:845–857
Weising K, Gardner RC (1999) A set of conserved PCR primers for the analysis of simple sequence repeat polymorphism in chloroplast genomes of dicotyledonous angiosperms. Genome 42:9–19
Wilbur RL (1954) A synopsis of Jatropha, subsection Eucurcas, with the description of two new species from Mexico. J Elisha Mitch Sci Soc 70:92–101
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acid Res 18:6531–6535
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)—anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgments
The authors wish to thank the RSAD Department of the Government of Andhra Pradesh, India for support of the project. The authors also wish to thank Prof. Klaus Becker, University of Hohenheim, Stuttgart, Germany for the non-toxic Mexican genotype and the Project Director, Directorate of Oilseeds Research for extending all the facilities for carrying out the investigation. (Researchers interested in information about the RAPD and ISSR primers can obtain it from the authors).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Basha, S.D., Sujatha, M. Genetic analysis of Jatropha species and interspecific hybrids of Jatropha curcas using nuclear and organelle specific markers. Euphytica 168, 197–214 (2009). https://doi.org/10.1007/s10681-009-9900-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-009-9900-0