Abstract
Factorial design and Response Surface Methodology (RSM) were used to model the recovery of polyphenols from Schinus molle L. peel (SM) by solvent extraction. Experiments carried out according to a Central Composite Design (CCD) allowed evaluation of the effects of temperature, extraction time, solvent composition and solid to liquid ratio on the yield of polyphenol. Statistical analysis showed that temperature was the most influential factor. A reduced polynomial model was developed by the stepwise regression method. The model was checked by performing validation experiments both inside and outside the factorial region. A very good agreement was observed between experimental and calculated extraction yields, thus supporting the use of the model to quantitatively describe the recovery of polyphenols from SM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The genus Schinus is native to South America. Schinus molle L. (Anacardiaceae) (pepper tree) is native to South Brazil, Uruguay, Bolivia, Perú and Central Argentina and has been cultivated throughout Europe. It was introduced in Tunisia, as an ornamental plant, by French colonizers in the late 1900s. Schinus molle L. is a quick-growing evergreen tree (8–10 m high), with perennial foliage that loses one-third of its leaves per year. The fruit is a greenish drupe that when mature, turns pink or light brown, depending on the area where it grows [1].
As a food ingredient, fruits of Schinus molle have been used in many countries as a substitute for black pepper and to prepare alcoholic drink and beverages. In Peru, molle fruit were soaked in water, sugar added, and then the mixture treated in specific vessels to obtain chicha de molle, which is drunk during certain festivities [2, 3]. Substantial data have shown that Schinus molle possesses anti-bacterial, anti-viral, antifungal, anti-inflammatory, anti-tumoral, anti-spasmodic, analgesic and anti-depressant properties [2, 4]. Other uses were also reported to treat fever, cough, colds, bronchitis, tuberculosis, asthma, conjunctivitis, ophthalmia, stomach pain (gastrointestinal disorders) and hemorrhoids. It has also been used for the treatment of toothache, rheumatism, menstrual disorders, respiratory and urinary tract infection [2, 5, 6]. In addition, Schinus molle L. extracts obtained with various solvents and its essential oil possess anti-bacterial, anti-fungal, antioxidant, insecticidal, analgesic, cytotoxic and trypanocidal properties [6, 7]. The essential oils and extracts obtained from many plants have recently gained popularity and scientific interest because of their use in the food, drug and perfumery industries [2]. These biological activities of Schinus molle were attributed to a wide array of bioactive components such as phenolic acids: gallic, caffeic, syringic, p-coumaric, trans-Ferulic and Quinic, as well as flavonoids including: Rutin, Hyperoside, Luteolin-7-O-glucoside, Quercetrin, Quercetin, Kaempferol, Naringenin, Apigenin [5]. Quantity and quality of plant extracts were dependent on and many factors including type of solvent, temperature, time, solid to liquid ratio and particle size of the solute contribute to the efficacy of the extraction process. In classical optimization experiments only one factor is variable at a time and this method was called as one-factor-at-a-time approach. This technique was tedious, expensive, consuming and often fails to elaborate the interaction effects between variables. Response surface methodology (RSM) is a useful method to evaluate the effects of multiple factors and their interactions on one or more response variables. RSM can effectively be used to find a combination of factor levels that produce an optimum response.
Therefore, the main goal of this research was to optimize a quick and low cost method in order to prepare a phenolic rich extract from Schinus molle L. peel growing in Gafsa (South of Tunisia). In addition, the response surface methodology (RSM) was applied to find the optimal conditions for the extraction with regard to four independent variables including: solvent composition (ethanol–water), temperature, extraction time and solid to liquid ration of dry matter plant.
2 Experimental
2.1 Chemical and Reagents
Folin–Ciocalteu reagent, sodium carbonate anhydrous (Na2CO3), gallic acid and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Fluka (Buchs, Switzerland).
2.2 Plant material
Schinus molle L. peels (500 g) from adult trees was collected randomly on March 2018 from an experimental plantation in Zarroug Province (a city in the south-west of Tunisia, Governorate of Gafsa, 34° 3′8.899″ North, 8°28′26.072″ East). The samples were air-dried at room temperature (20 ± 2 °C) for 1 week. Samples were identified by Dr Abdessatar Ghobtane and according to the morphological description presented in Tunisian flora [8]. Voucher specimens were deposited, under the number SM2018, in the herbarium of the Department of biology of the Faculty of Sciences of Gafsa (Tunisia).
2.3 Plant Extraction
2.3.1 Maceration
For the extraction process, 10 g of plant material were placed into glass bottle containing 100 mL of extraction solvents under continuous agitation. The extraction temperature varied between 40 and 60 ± 1 °C, time extraction ranged from 40 to 120 min, solvent proportion (ethanol–water 40 to 60% (v/v)) and solid to liquid ratio (5 to 7.5 g/mL) were predetermined using the experimental design summarized in Table 1 [9]. Some research conducted the choice of parameters for example the research conducted by Azrie et al. [10] showed that water due to a higher polarity factor elutes more phenolic compounds from the solid than ethanol. Ethanol was chosen as solvent due to its different molecular structure, is more effective in eluting such compounds as chlorogenic acid and flavonoids [11]. The mixture of water and ethanol provides higher polyphenol extraction efficiency than the use of these solvents separately. Galván d’Alessandro et al. [12] and Virot et al. [13] studying the solubility of polyphenols in various mixtures of water and ethanol achieved the highest solubility of these compounds in a 50% ethanol solution.
2.3.2 Determination of Total Phenolics
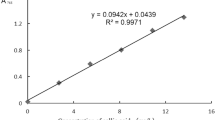
Total Phenol content (TPC) was determined according to the Folin–Ciocalteu method as described before by Yahyaoui et al. [14] with some modification. Briefly, 300 μL sample of extract diluted in methanol (2 mg/5 mL) were added to 1.5 mL of Folin–Ciocalteu reagent (10/100). After 1 min, 1.2 mL of aqueous sodium carbonate (7.5%) was added. The mixture was vortexed and allowed to stand at room temperature with exclusion of light for 120 min. The absorbance was read 760 nm, using a UV–Visible spectrometer (BECKMAN DU 800). The total phenolic content in the extract was calculated from the calibration curve, using gallic acid as a standard, and the results were expressed as mg of gallic acid equivalents (mg GAE) per g of plant dry matter (DM). Three determinations were performed on each sample. For gallic acid, the curve of absorbance A versus concentration C of gallic acid as mg/mL is described by the equation Eq. (1):
2.3.3 Experimental design for the Response Surface Methodology
The optimization of phenolics compounds extraction was carried out using RSM. The three level four-factor rotatable central composite design (CCD) consisting of 28 experimental runs with three replicates at the center point. The independent variables were the temperature (X1, °C), time (X2, min), ethanol proportion (X3, %, v/v ethanol/water) and solid to liquid ratio (X4, g/mL). As displayed in the Table 1, three levels of values for each independent parameter were presented in their original and coded forms. Yield of phenolic coupounds extraction was chosen as the response of the design experiments Table 2.
A second-degree polynomial equation from RSM was used and given below Eq. (2).
where Y is the response (dependent variables); b0, b1, b2, b3, b11, b22, b33, b44, b12, b13 and b14 are the regression coefficients for intercept, linear, quadratic and interaction terms; X1, X2, X3 and X4 were the non-coded values for temperature, time, solvent extraction and solid to liquid ratio, respectively. Based on the variance analysis, the regression coefficients of individual linear, quadratic and interaction terms were calculated.
2.4 Antiradical Activity
Antiradical activity was evaluated using 1, 1-diphenyl-2-picrylhydrazyl free radical (DPPH) with some modifications [14, 15]. Aliquots (1.5 mL) of various dilutions of the plant extracts were mixed with 1.5 mL of methanolic DPPH solution (0.2 mM). The mixtures were incubated for 30 min at 25 °C, then the absorbance at 520 nm was measured using a UV–Visible spectrometer (BECKMAN DU 800). The absorbance in the presence of plant extract was recorded as Asample while the absorbance of the control reaction was recorded as Ablank. The free radical-scavenging activity of each solution was then calculated as inhibition percentage as follows Eq. (3):
Antiradical activity was expressed as IC50 (mg/mL), defined as the concentration of the extract required to cause a 50% decrease in initial DPPH concentration. Ascorbic acid was used as positive control. All measurements were performed in triplicate.
2.5 Statistical Analysis
All results were expressed as means ± standard deviations and all experiments were done in triplicate. The experimental data was analyzed by Nemrodw software.
3 Results and Discussion
3.1 Optimisation of TPC Extraction Yield
Fitting the model. The phenolic compounds extraction of S. molle extracts was further improved through RSM approach. The Experimental data and responses were reported in Table 2. The independent factors used in the design were extraction temperature (X1), extraction time (X2), ethanol proportion (X3) and solid to liquid ratio (X4). The extraction yield of TPC was the responses. The model provided a good fit to the data, with coefficient of determination (R2), adjusted-R2 and prediction-R2 equal to 0.982, 0.962 and 0.859, respectively. In this investigation, a greater model fit was obtained. The determination coefficient of responses is near to 0.9, proving a good representation of the variability of the factors by the models. It is important to note that the model was considered accurate and reliable as the R2 is superior to 0.75 [16]. A comparison between experimental and calculated extraction yields is shown in Fig. 1.
Analysis of residuals indicated no apparent departures from basic ANOVA assumptions, i.e., normally distributed errors with constant variance and independent of one another. Furthermore, the lack of fit was not significant (p = 0.181, see Table 3), which further supports the model adequacy to describe the experimental data.
3.1.1 Response Surface Analysis for Total Phenolics Content (TPC)
The results obtained during the optimization of the process showed that the amount of TPC varied considerably between 65.51 ± 0.67 and 332.55 ± 3.59 mg GAE/g DM (Table 4). The highest amount was obtained with experiment number 5 while the lowest was obtained with experiment number 26 (50% of ethanol proportion with 7.5 mg/mL solid to liquid ratio, 50° C and 90 min) and 5 (40% of ethanol proportion with 7.5 mg/mL as a solid to liquid ratio, 50° C and 90 min). The linear effect of extraction temperature (ρ < 0.001) and solid to liquid ratio (ρ < 0.001) was found statistically significant. Different empirical models (linear, two-factor interaction, quadratic and cubic) were tested for their ability to fit the experimental data listed in Table 5. For all independent variables tested, the quadratic effect of extraction temperature, solid to liquid ratio and solvent was statistically significant (ρ < 0.001).
Therefore, the polynomial equation for TPC response came as Eq. (4):
From the estimated regression coefficients, we could conclude that:
-
The main factors temperature (T), solid to liquid ratio (p) and solvent composition (S) were all significant and have effect on polyphenols recovery. The ethanol proportion had a positive linear effect, which means that the use of higher ethanol proportion give higher TPC in the extract. In contrast, extraction temperature had a negative effect. These results could be explained as reported by Silva and co-workers [17]. They mentioned that some flavonoid compounds were thermo-sensitive, that high temperature leads to the flavonoid compounds decomposition, mainly flavan-3-ol and derivatives as well as anthocyanin, which required keeping the temperature extraction below at a certain limit. To conclude, it is likely that our extract contained thermo-sensitive flavonoids as phenolic compounds. On the other hand, our results were not in accordance with those presented by Liu et al. [18] who cited that extraction of phenolic compounds from plant material could be carried out at high temperatures more than 60 °C.
As reported previously by Kasmi et al. [19], the phenolic compounds content of the various S. molle fruits extracts, this research team noted that ethyl acetate extract was the richest (123.7 ± 1.7 mg GAE/g DW), followed by methanol (86.2 ± 2.3 mg GAE/g DW) and ethanol extracts (82.5 ± 1.6 mg GAE/g DW). The lowest value was 9.8 ± 0.4 mg GAE/g DW in hexane extract. They also mentioned that flavonoids were detected in schinus molle fruit extracts. The results showed a strong variation of composition with the extraction solvent. The ethanol extract being the richest (74.6 ± 2.54 mg QE/g DW). Methanol and ethyl acetate extracts showed the lowest value with 4.3 ± 0.15 and 1.7 ± 0.04 mg QE/g DW, respectively. Hexane extract contained no flavonoids. The amount of total phenolics extracted with aqueous solutions from schinus molle fruits was 7.6 mg of GAE/g DW [20]. In other investigations on schinus molle, Yueqin et al. [21] and Ono et al. [22] had identified the biflavonoids chamaejasmin, agathisflavone and tetrahydroamentoflavone as well as the dihydroflavonol engeletin and the flavonol quercetin-3-rhamnoside.
Little is known about the polyphenol content of the schinus molle peel extracts, therefore in this study we found that for them:
-
Temperature (T) and solid to liquid ratio (p) affected the response through both a linear and a quadratic term.
-
There was a positive interaction between (T) and (p), suggesting that temperature had less pronounced effect on polyphenols recovery at higher solid to liquid ratio; but the interaction were negative between temperature (T) and time of extraction (t) also between (T) and (S) suggesting that temperature had less effect on polyphenols recovery when time of extraction and composition of solvent with ethanol were pronounced.
-
The interaction were negative between composition of solvent (S) and (t) also between (S) and (p) suggesting that composition of solvent had less effect on polyphenols recovery when time of extraction and solid to liquid ratio were pronounced.
-
binary interaction coefficient between (t) and (p) was not significant, indicating that each of them exerted its effect independently of the other.
Figures 2, 3, 4 showed the response surface plots resulting from Eq. (4). The response variable was plotted as a function of two factors varying in the factorial part of the design (–1 ≤ xi ≤ + 1) while setting the others to their center-point values. From Fig. 2, the strong effect of solvent composition and the positive, though less pronounced, effect of temperature on polyphenol recovery was evident. These effects could be explained by considering that the extraction kinetics was negatively affected by temperature. Examination of Fig. 3 confirmed a slight negative effect of temperature and extraction time was not significant on polyphenol recovery. Finally, from Fig. 4, we noted that the solid-to-liquid ratio (p) has a negative effect on polyphenol extraction. This was a consequence of the enhancement of mass transfer of released polyphenols into the solvent caused by less solid-to-liquids ratios.
3.1.2 Optimization and Validation of the Optimal Conditions
Response surface methodology was enabled to evaluate the effects of variables process (temperature, time, solvent composition and solid to liquid ratio) and their interaction on response of TPC extraction yield. In addition, it has been found that RSM was a powerful tool for optimizing experimental conditions to maximize the response variables [23]. Extraction of schinus molle peel part was optimized in order to determine the maximum of TPC extraction yield. Optimum extraction conditions were established according to the desirability function. To conclude, the model showed that the optimum extraction condition for TPC extraction yield response came as: duration of extraction of 60 min, an extraction temperature of 40° C, an ethanol proportion of 60% and a solid to liquid ratio of 5 g/mL. Under these conditions, TPC value was 332.55 mg GAE/g DM.
3.2 DPPH Radical Scavenging Activity
Antiradical activity of schinus molle extracts was proved by the extract concentration providing 50% scavenging (IC50) that listed in Table 4. Overall all the schinus molle peel extracts showed antiradical activity ranging from 56 to 81.7 mg/mL. Schinus molle extracts obtained with run 5 showed the best radical scavenging activity with the smallest IC50 value (56 mg/mL). While, run 26 showed a weaker free radical scavenging activity (IC50 value 81.7 mg/mL). The mean IC50 value of all the prepared extracts was 73.83 mg/mL. A good correlation between total phenolic contents and the IC50 value was found with a R2 value of 0.935 (Fig. 5). Generally extract samples that contained more phenolic compounds contents showed better radical scavenging activity (less IC50). The synthetic antioxidant, ascorbic showed good radical scavenging activity with IC50 values of 5.5 ± 0.26 mg/mL. The results of the present study indicated that phenolic compounds were powerful scavengers of free radicals as demonstrated by a good correlation between TPC and DPPH radical scavenging activity (Fig. 5). Some reports in the literature depicted the strong correlation between phenolic compounds contents and antiradical activity. The antiradical activity of essential oils and extracts was attributed to the presence of phenolic compounds. [19, 24]
4 Conclusion
The response surface methodology was successfully employed to optimize the extraction of phenolic compounds from schinus molle peel. The second-order polynomial model gave a satisfactory description of the experimental data. An optimized condition for maximum extraction of polyphenol extracts was determined. Solvent ratio, solid to liquid ratio and temperature were the most important factors affecting extraction of phenolic compounds. Results of the present study indicate a good correlation between total phenolic contents and DPPH radical scavenging activity. This study can be useful in the development of industrial extraction processes, including further studies concerning the optimal number of sequential steps to enhance the efficacy of a large-scale extraction system. These results demonstrate that the schinus molle peel parts were rich in natural phenolic compounds that could replace synthetic compound and used as a source of natural product.
Abbreviations
- RSM:
-
Response surface methodology
- SM:
-
Schinus molle
- CCD:
-
Central composite design
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- TPC:
-
Polyphenol compounds yield
- GAE:
-
Gallic acid equivalent
- DM:
-
Plant dry matter
- X1:
-
The non-coded values for temperature
- X2:
-
The non-coded values for time
- X3:
-
The non-coded values for solvent extraction
- X4:
-
The non-coded values for solid to liquid ratio
- A:
-
Absorbance
- IC50 :
-
Inhibition concentration at 50%
- (T):
-
Factor temperature
- (p):
-
Factor solid to liquid ratio
- (S):
-
Factor solvent composition
- (t):
-
Factor time of extraction
- DF:
-
The degree of freedom
- SS:
-
The sum of squares
- F:
-
The F-value
- P:
-
The P value
- SE:
-
Standards errors
- t:
-
The T-value
References
Martins MR, Arantes S, Candeias F, Tinoco MT, Cruz-Morais J (2013) J Ethnopharmacol. https://doi.org/10.1016/j.jep.2013.10.063
Giuffrida D, Martínez N, Arrieta-Garay Y, Fariña L, Boido E, Dellacassa E (2020) Food Res Inter 133:109103
Tlili N, Yahia Y, Feriani A, Labidi A, Ghazouani L, Nasri N, Saadaoui E, Khaldi A (2018) Ind Crops Prod 122:559–565
Ribeiro VE, Pereira MP, Mauro de Castro E, Corrêa FF, Cardoso MG, Pereira FJ (2019) Ind Crops Prod 132:92–98
Ennigrou A, Casabianca H, Laarif A, Hanchi B, Hosni K (2017) South Afr J Bot 108:407–415
Bras C, Gumilar F, Gandini N, Minetti A, Ferrero A (2011) J Ethnopharmacol 137:1450–1456
López A, Castro S, Andina MJ, Ures X, Munguía B, Llabot JM, Elder H, Dellacassa E, Palma S, Domínguez L (2014) Ind Crops Prod 53:209–216
Pottier-alapetite G (1981) Impr Offic Rep Tun 655
Zuorro A (2014) Am J Appl Sc. 11:1463–1471
Azrie AM, Chuah AL, Pin KY, Tan HP (2014) J Chem Pharm Res 6:172–176
Wijngaard HH, Brunton N (2010) J Food Eng 96:134–140
Galvan d’Alessandro L, Kriaa K, Nikov I, Dimitrov K (2012) Sep Purif Technol 93:42–47
Virot M, Tomao V, Le Bourvellec C, Renard CM, Chemat F (2010) Ultras Sonochem 17:1066–1074
Yahyaoui A, Arfaoui MO, Rigane G, Hkir A, Amari K, Ben Salem R, Ammari Y (2019) Chem Afric 2:361–365
Ben Hmed M, Rigane G, Ben Salem R, Zouari N, Cherif S (2020) Rev Roum Chim 65(2):173–178
Ghazouani NM, Abderrabba M, Bouajila J (2016) J Anal Bioanal Chem 49:1258–1271
Silva EM, Da-Silva SJ, Pena RS, Rogez H (2011) Food Bioprod Proces 89:39–46
Liu Y, Wang H, Cai X (2015) Food Sci Technol 52:2336–2343
Kasmi A, Hammami M, Abderrabba M, Ayadi S (2016) J Chem Pharma Res 8:93–101
Ranilla LG, Kwon YI, Apostolidis E, Shetty K (2010) Biores Technol 101:4676–4689
Yueqin Z, Recio MC, Manez S, Giner RM, Cerda Nicolas M, Rios JL (2003) Planta Med 69:893–898
Ono M, Yamashita M, Mori K, Masuoka C, Eto M, Kinjo J, Ikeda T, Yoshimitsu HT, Nohara T (2008) Food Sci Technol Res 14:499–508
Piwowarska N (2012) Gonza´lez-Alvarez. J Biom Bioen 43:42–51
Manoharan KP, Kemelo SM, Mosotho JG, Sibusisiwe M (2018) J Med Plants Res 12:369
Acknowledgements
The authors express sincere thanks to the higher Education and Scientific Research Ministry for its financial support and are grateful to Professor Mohamed Rigane for useful discussions about the English.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Khedher, O., Rigane, G., Salem, R.B. et al. Optimization of Polyphenols Recovery from Schinus molle L. Peel Using Response Surface Methodology (RSM). Chemistry Africa 3, 813–820 (2020). https://doi.org/10.1007/s42250-020-00170-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-020-00170-3