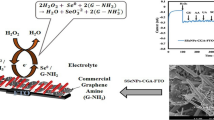
Abstract
In this paper, pure SnO2 and Ce-doped SnO2 nanosheets were synthesized through a facile hydrothermal method. The synthesized materials were characterized by different techniques for their physico-chemical properties. The XRD data indicated the characteristic tetragonal rutile crystal phase for SnO2. Ce doping was ascertained by the presence of the diffraction peaks of CeO2 in all the doped samples of the SnO2 nanosheets. FESEM images revealed highly rough surfaces as well as the agglomeration of a large number of small nanoparticles of multiple shapes to form nanosheets like morphologies for pure SnO2 and Ce-doped SnO2. Electrochemical techniques like cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and chronoamperometry were applied to demonstrate the electrochemical performances of the pure SnO2 and Ce-doped SnO2 nanosheets/Nafion-modified glassy carbon electrode (GCE). The 3% Ce-doped SnO2 nanosheet/Nafion-modified GCE showed a remarkable sensitivity of 0.9986 μA μM−1 cm−2 over a linear dynamic range of 0.5–20.3 µM. The corresponding linear regression equation was Ip (μA) = 0.0709 [2-nitroaniline (μM)] + 0.1385 with R2 = 0.99325. The LOD of the modified sensor was found to be 6.3 ± 0.1 nM at the signal-to-noise ratio of S/N = 3. The newly developed sensor electrode exhibited good selectivity toward 2-nitroaniline in the presence of common interfering species.
Graphical abstract
Fabrication and characterization of highly sensitive and selective 2-nitroaniline chemical sensor based on cerium-doped tin oxide nanosheets/Nafion-modified glassy carbon electrode.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tin dioxide (SnO2) due to its excellent optical and electrical properties has been reported for applications such as supercapacitors [1], gas and chemical sensors [2], lithium-ion batteries [3], dye-sensitized solar cells [4], antimicrobial agents [5], electrochemical biosensors [6], and electrocatalysis [7]. A wide variety of applications associated with SnO2 nanostructures are due to the ease of synthesis of SnO2 nano/microstructures with versatile morphologies, n-type semiconducting nature, a wide-band gap of 3.6 eV, high carrier density, and excellent chemical and thermal stabilities [8]. However, for further improvement for specific applications, many methods have been adopted. Among the various methods adopted, doping is considered as one of the best methods for altering the microstructural, surface morphology, electronic, optical, and bandgap properties of the SnO2 nano/microstructures [9].
Cerium-a lanthanide element has received extensive attention as a dopant material for semiconductor metal oxides due to its mixed valences of +3 and +4, availability of vacant 4f orbitals, redox characteristics (Ce4+ ↔ Ce3+), rapid oxygen ion mobility, and electrocatalytic properties [10,11,12,13]. The reduction of Ce4+ to Ce3+ ion has been found to initiate the generation of lattice mobile oxygen, which in turn results in the formation of surface oxygen vacancy defects. These defects act as hot spots for the synergistic binding of other nanomaterials especially the metal oxides [14, 15]. Ce-doped metal oxides such as Ce-doped ZnO nanoparticles [16, 17], Ce-doped SnO2 thin films [18], Ce-doped NiO nanoparticles [19], Ce-doped In2O3 nanostructure [20], Ce-doped hierarchical flower-like In2O3 microspheres [21], Ce-doped Fe3O4 magnetic particles [22], Ce-doped NiO micro-flowers [23], Ce-doped TiO2 nanoparticles [24], Ce-doped WO3 with raspberry-like architecture [25], Ce-doped CuO nanoparticles [26], and many more have been explored and reported for a variety of applications.
Among the various applications, the electrochemical sensors based on Ce-doped semiconductor metal oxide nanomaterials have extensively been fabricated to detect hazardous and toxic chemicals. One such class of hazardous materials is of nitroanilines which are the derivatives of aniline and exist in different isomeric forms. These chemicals are widely used as raw materials in industries such as dyes, pharmaceuticals, polymers, developing agents, gasoline, insecticides, pesticides, rubber, photo-stabilizers, antioxidants, explosives, anti-aging agents, and paints [27, 28]. Excess release of these chemicals as effluents into the water bodies has a devastating effect not only on the environment but also on human health. In living beings, these chemicals can potentially cause damage to the liver, kidneys, DNA mutations upon inhalation, and even skin diseases. As far as environmental effects are concerned, nitroanilines are non-biodegradable and hence are considered as typical organic pollutants [29]. Many metal oxide semiconductor materials have been explored as transducers for the sensing of nitro-anilines. Bitter gourd–shaped Yb2O3-doped ZnO nanostructures [30], ZnO nanoflower CeO2 nanoparticles [31], ZnO nanorods on fluorine-doped SnO2 [27], Sm2O3-doped ZnO beech fern hierarchical structures [32], novel sphere-like Co2SnO4 [33], K+ intercalated MnO2-rGO composite [34], Yb-doped ZnO nanopencils [35], CdO-ZnO hexagonal nanocones [36], etc. have been reported as electrochemical sensor materials towards nitroaniline isomers. Since Ce-doped SnO2 nanomaterials show improved electric and electronic properties as compared to pure SnO2, they can be potential materials for electrochemical sensing applications. Further, limited studies have been conducted to use Ce-doped SnO2 nanomaterials for 2-nitroaniline detection.
Therefore, the main focus of the present research conducted herein was to synthesize pure SnO2 and Ce-doped SnO2 nanosheets with different Ce- concentrations through a facile hydrothermal method. As-synthesized materials were characterized through different techniques to confirm the composition, morphology, crystallinity, and optical and vibrational properties. The electrochemical sensing behavior of Ce-doped SnO2 nanosheets was compared with that of pure SnO2 nanosheets for 2-nitroaniline. It was found that the 3% Ce-doped SnO2 nanosheets/Nafion-modified GCE exhibited.
2 Experimental details
2.1 Materials
To synthesize Ce-doped SnO2 nanosheets, all chemicals of AR grade were procured from Sigma-Aldrich and used as obtained without any further purification. For the synthesis, various chemicals, i.e., stannous chloride dihydrate [SnCl2·2H2O], cerium(III) chloride heptahydrate [CeCl3·7H2O], hexamethylenetetramine [HMTA; C6H12N4,], and ammonium hydroxide [NH4OH], were used. For the sensing purpose, 2-nitroaniline (2-NA) was also procured from Sigma-Aldrich and used as obtained. To prepare all solutions for the synthesis and sensing applications, deionized (DI) water was used.
2.2 Synthesis of pure SnO2 and Ce-doped SnO2 nanosheets
A facile hydrothermal process was used to synthesize pure and Ce-doped SnO2 nanosheets. For the synthesis of SnO2 nanosheets, 100 mM equimolar aqueous solutions of SnCl2·2H2O and HMTA (40 mL each) were mixed well under stirring for 30 min. Consequently, several drops of NH4OH were added, to maintain the solution pH = 11, into the resultant mixture and stirred again for further 30 min. After vigorous stirring, the obtained solution was transferred to a Teflon-lined stainless steel autoclave which was sealed and heated at 160 °C for 8 h. After the desired reaction time, the autoclave was cooled to room temperature and the obtained precipitate was centrifuged and washed several times with DI water and ethanol. Finally, the obtained white precipitate was dried at room temperature and annealed at 450 °C for 3 h.
Ce-doped SnO2 nanosheets were prepared following the same synthetic procedure of SnO2 nanosheets; however, for Ce doping, different concentrations of CeCl3·7H2O (1 mM for 1%, 3 mM for 3% and 5 mM for 5%), made in 20 mL DI water, were added in the mixture of SnCl2·2H2O and HMTA, then completed the same hydrothermal procedure of SnO2 nanosheets as described above.
2.3 Characterizations
The synthesized pure SnO2 and Ce-doped SnO2 materials were analyzed by several techniques to examine the morphologies, crystal structures and phases, composition, and optical and vibrational properties. Morphology, composition, purity, and elemental distribution in the material matrix were analyzed through field-emission scanning electron microscopy (FESEM: JEOL-JSM-7600F, Hitachi, Japan) attached with EDS for elemental mapping and composition. The crystallinity, crystal size, and crystal phases were determined by X-ray diffractometer (XRD; PANalyticalX’Pert PRO; Cu-Kα radiations source with λ = 0.1542 nm) in the range of 20–80° with a scan speed of 2°/min. Bandgap energies were determined by UV–vis spectroscopy (Perkin Elmer-UV/VIS, Lambda 950). Vibrational properties were examined by FTIR spectroscopy (Perkin Elmer-FTIR Spectrum-100). FTIR analysis of the synthesized nanomaterials was carried out by initially mixing the product homogeneously with powdered KBr (1% by weight of the sample) followed by palletization at high pressure. The scattering properties of the synthesized nanomaterials were investigated by Raman scattering (Perkin Elmer-Raman Station-400 series) at room temperature. Raman analysis was carried out by directly placing the dry powders of the synthesized nanomaterials below the probe.
2.4 Fabrication of 2-nitroaniline chemical sensors
Initially, the glassy carbon electrode (GCE) with apparent surface area 0.071 cm2 (BAS Inc. Japan) was thoroughly polished with a 1-μm polishing diamond followed by polishing with 0.05 μm alumina slurry. After that, the polished electrode was washed several times with distilled water and dried in an air oven at 100 °C for 1 h. The surface of the GCE was coated with a thin layer of hydrothermally synthesized sensor materials. A homogeneous suspension from the sensor materials was made using Nafion solution as a conducting binder to provide stability and firmness to the coated material on the surface of the electrode. Typically, 5.0 mg of active material was dispersed in 0.45 mL of propan-2-ol containing 0.05 mL of 5% Nafion, followed by ultra-sonication for 20 min to get a homogeneous suspension. Of this suspension, 1.5 μl was gently coated onto a clean GCE surface and dried at ambient conditions for 5 min, followed by drying at 60 °C for 20 min to get a homogeneous layer of active material on the GCE surface. A three-electrode electrochemical cell connected to an electrochemical workstation (Zahner Zennium, Germany) was used for various electro-analytical measurements. A Pt wire as a counter electrode, modified GCE as a working electrode, and an Ag/AgCl (saturated KCl) electrode as a reference electrode were used. All the electro-analytical observations were recorded in 0.1 M phosphate-buffered solution (PBS) of pH 7.0 at room temperature. The sensitivity of the fabricated sensor was determined from the slope of the calibration curve divided by the active area of the modified electrode.
3 Results and discussion
3.1 Properties of the pure SnO2 and Ce-doped SnO2 nanosheets
The diffraction peaks of the SnO2 lattice planes are shown in the form of an XRD spectrum in Fig. 1. Well-defined diffraction peaks corresponding to (110), (101), (200), (111), (211), (220), (002), (310), (112), (301), (202), and (321) diffraction planes are well-matched with the reported literature [37,38,39] and JCPDS: 41–1445. The XRD data indicated the characteristic tetragonal rutile crystal phase of SnO2. The presence of CeO2 in all the prepared doped samples of the SnO2 nanosheets is confirmed by the presence of a small but well-defined peak corresponding to (111) diffraction plane which indicated the cubic fluorite phase of CeO2(JCPDS card no. 81–0792) [40, 41]. No other peak was detected, except for SnO2 and CeO2 in the XRD spectra of un-doped and doped samples which further confirmed the high purity of the samples. The crystallite size was calculated using the Scherrer equation (Eq. 1) [42, 43].
Here, λ = wavelength of X-rays (1.542 Å), β = full width half maximum (FWHM), and θ = Bragg angle of diffraction. For analysis, the three most intense peaks corresponding to (110), (101), and (211) diffraction planes were considered. The resulting parameters are tabulated in Table 1. The average crystallite sizes for pure SnO2, 1%, 3%, and 5% Ce-doped SnO2 nanosheets were found to be 31.07, 30.19, 32.10, and 33.86 nm, respectively. Nominal change in the diffraction angles for Ce-doped SnO2 nanosheets as compared to pure SnO2 further confirmed the replacement of the Sn4+ ions by Ce4+ ions from some of the lattice points from the tetragonal rutile crystal phase of SnO2. However, for pure SnO2 and 1% Ce-doped SnO2 nanosheet diffraction angles were almost the same due to the very low concentration of the dopant. With the increase in dopant concentration diffraction angles were found to increase.
The FESEM images of the pure SnO2 and Ce-doped SnO2 nanosheets are shown in Fig. 2a–p. Panoramic images reveal highly rough surfaces and illustrate that the pure, as well as Ce-doped SnO2 nanosheets, are formed by the agglomeration of a large number of small nanoparticles of multiple shapes. High-resolution FESEM images showed that the thickness of the 3% Ce-doped SnO2 nanosheets (Fig. 2j–l) was more as compared to 5% Ce-doped SnO2 nanosheets (Fig. 2n–p). The cross-edge sections FESEM image of pure SnO2 and Ce-doped SnO2 nanosheets displayed a dense distribution and close packing of the nanoparticles to form layered nanosheets. For 3% Ce-doped SnO2 nanosheets, a comb-like morphology was also observed from cross-edged FESEM images. Such small nanoparticle-layered nanosheets of pure SnO2 and Ce-doped SnO2 provide a large surface area for the chemisorption of analytes, required as an important pre-requisite to perform the role of electron mediator, by synthesized nanosheets for efficient and reliable electrochemical sensing.
EDS-selected area and the corresponding EDS spectrum of pure SnO2 are shown in Fig. 3a and (a1), respectively. The EDS spectrum showed peaks for Sn and O only. The EDS spectrum without any other peaks corresponding to any impurities confirms the fact that the synthesized SnO2 nanosheets have appreciable purity. To further confirm the EDS results, the composition and element distribution of the constituent elements of SnO2 nanosheets were studied by the FESEM-elemental mapping technique (Fig. 3 (a2, a3)). The uniform distribution of Sn and O content in the entire structure of the nanosheets lattice can be seen. Similar to pure SnO2 nanosheets, the Ce-doped SnO2 nanosheets were also analyzed by EDS selected area, EDS spectra, and FESEM-elemental mapping technique. The doping of the Ce4+ into the crystal lattices of the SnO2 nanosheets was confirmed for all the doped samples. The EDS spectra for all the doped nanosheets exhibited peaks for Sn, O, and Ce only. The fact was further supported by the FESEM-elemental mapping images as portrayed in Fig. 3 (b2–b4) for 1% Ce-doped, Fig. 3 (c2–c4) for 3% Ce-doped and Fig. 3 (d2–d4) for 5% Ce-doped SnO2 nanosheets. The Sn, Ce, and O contents were uniformly distributed in doped SnO2 nanosheets.
The molar ratio of [Sn2+] and [OH−], pH of the growth solution, and the presence of directing agents like HMTA significantly affect the shape, size, and aspect ratio of the SnO2 nanomaterials [44]. NH4OH present in the reaction mixture provides a sufficient amount of HO– ions which control the nucleation and crystal growth process which then controls the shape, size, and morphology of the SnO2 nanostructures. The presence of the HMTA further adds to the concentration of HO– ions by hydrolysis (Eqs. 2 and 3).
During the hydrothermal growth at 160 °C for 8 h, initially, there is the formation of different hydroxylated species like \({\mathrm{Sn}(\mathrm{OH})}_{3}^{-}\), \({\mathrm{Sn}(\mathrm{OH})}_{4}^{2-}\), and Sn(OH)2 in alkaline medium with pH 11 (Eqs. 4–6) [45, 46].
During the nucleation, these hydroxides are converted to SnO at high growth temperature and pressure conditions in the autoclave. High-temperature annealing in the presence of air at 450 °C for 3 h oxidizes SnO to SnO2 nanoparticles which aggregate together to result in the formation of layered nanosheets like morphologies (Eqs. 7–9) [39].
The presence of CeCl3·7H2O, during the hydrothermal synthesis of Ce-doped SnO2 nanosheets, results in the formation of Ce(OH)3 and \({\mathrm{Ce}(\mathrm{OH})}_{4}^{-}\) in strong alkaline medium (Eqs. 10, 11). Due to electrostatic interactions, Ce(OH)3 and \({\mathrm{Ce}(\mathrm{OH})}_{4}^{-}\) are mixed easily with \({\mathrm{Sn}(\mathrm{OH})}_{3}^{-}\), \({\mathrm{Sn}(\mathrm{OH})}_{4}^{2-}\), and Sn(OH)2 in the alkaline medium during the nucleation and growth process. High-temperature annealing in the presence of air oxidizes Ce(OH)3 and \({\mathrm{Ce}(\mathrm{OH})}_{4}^{-}\) hydroxides into CeO2 (Eqs. 12,13).
Figure 4a represents the FTIR spectra of as-synthesized pure SnO2 and Ce-doped SnO2 nanosheets. The fingerprint region of pure as well as Ce-doped SnO2 nanosheets showed vibrational peaks in the region 604–614 cm−1 which may be assigned to the vibration of constituent ionic species in the crystal lattices and are due to the M − O stretching vibrations [47]. A large intensity band near 3430 cm−1 may be attributed to the stretching vibrational mode of O–H bonds of the H2O molecules adsorbed on the surface of pure SnO2 and Ce-doped SnO2 nanosheets during sample preparation.
Figure 4b shows the Raman spectra of the pure SnO2 and Ce-doped SnO2 nanosheets. The rutile phase crystal structure of SnO2 consists of two SnO2 formula units per unit cell. In a single unit cell, each Sn atom is surrounded by six O-atoms whereas each O-atom is surrounded by three Sn-atoms. Symmetry point group and space group of the rutile phase of SnO2 are D4h and p42/mnm, respectively, which have 15 optical phonons with symmetry symbols A1g + A2g + A2u + B1g + B2g + 2B1u + Eg + 3Eu. Among these, only the A1g, B1g, B2g, and Eg optical phonons are Raman active, and the remaining are Raman inactive [48]. The Raman spectra for the pure, as well as Ce-doped SnO2 nanosheets, show three fundamental Raman-active peaks situated at 464, 630, and 766 cm−1.
The peak at 464 is assigned to the vibration of oxygen and corresponds to Eg Raman active mode. Peaks centered at 630 and 766 cm−1 are assigned to the expansion and contraction vibrational modes of Sn–O bonds and correspond to the A1g and B2g optical Raman active modes, respectively [49, 50]. Since the main Raman active peak corresponding to the F2g mode of CeO2 cubic structure is ~ 463 cm−1 which is close to the Eg Raman active mode of the SnO2, no separate Raman active peak for CeO2 in Ce-doped SnO2 nanosheets was observed.
3.2 2-Nitroaniline chemical sensor applications of pure SnO2 and Ce-doped SnO2 nanosheets
Cyclic voltammograms (CV) were recorded and compared to analyze the electro-catalytic performance of bare GCE and modified GCE by pure SnO2 and Ce-doped SnO2 nanosheets/Nafion towards 10 μM 2-nitroaniline within the potential range + 0.5 to + 1.5 V at a scan rate of 50 mV/s in PBS at 7.0 pH vs. Ag/AgCl (saturated KCl) (Fig. 5a). As compared to bare GCE, the pure SnO2 and Ce-doped SnO2 nanosheets/Nafion modified GCE showed better electrocatalytic performances as shown by distinct anodic peaks in different voltammograms. The well-shaped reversible 2-nitroaniline oxidation peak appeared on bare as well as different modified GCEs. For bare GCE, pure SnO2/GCE, 1% Ce-doped SnO2/GCE, 3% Ce-doped SnO2/GCE, and 5% Ce-doped SnO2/GCE oxidation peak currents of 0.6165, 1.646, 1.704, 3.057, and 2.779 μA were observed at potentials 1.291, 1.496, 1.335, 1.316, and 1.336 V, respectively for 10.0 µM 2-nitroaniline at 50 mV/s in PBS. The Ce-doping into the crystal lattice of the SnO2 nanosheets affects the microstructures, optical band gap energies, electrochemical, and redox properties of the Ce-doped SnO2 nanosheets. Further, the unique two-dimensional sheet-like morphology, comprising very small nanoparticles, of the synthesized Ce-doped SnO2 nanomaterials provides a large surface to volume ratio for the greater extent of adsorption of analyte species onto the surface.
(a) CV representing the electro-catalytic performance of bare GCE and modified GCE/Nafion electrodes based on pure SnO2 and Ce-doped SnO2 nanosheets towards 10 μM 2-nitroaniline within the potential range + 0.5 to + 1.5 V and at a scan rate of 50 mV/s in PBS with pH 7.0 and (b) CV for 3% Ce-doped SnO2 nanosheets in the absence and presence of 10 μM 2-nitroaniline
Among all the modified GCEs, the electrode comprising 3% Ce-doped SnO2 nanosheets exhibited maximum electrocatalytic activity for 10 μM 2-nitroaniline. This can be attributed to the presence of comb-like nanoparticles in case of 3% Ce-doped SnO2 nanosheets. Therefore, in the subsequent electrochemical analysis, only 3% Ce-doped SnO2 nanosheet/Nafion-modified GCE was studied under different conditions. This 3% Ce-doped SnO2 nanosheet/Nafion-modified GCE showed no distinct anodic peaks in the absence of 2-nitroaniline, whereas, in the presence of 10 μM 2-nitroaniline, amplified anodic current was observed at the modified active GCE at a scan rate of 50 mV/s in PBS (Fig. 5b). Thus, as fabricated 3% Ce-doped SnO2 nanosheet/Nafion-modified GCE can potentially be used for electrocatalytic oxidation/reduction and hence as electrochemical sensor towards 2-nitroaniline even at very low concentration of 10 μM.
The electro-catalytic sensing characterizations of the 3% Ce-doped SnO2 nanosheet/Nafion-modified GCE were analyzed as a function of scan rate through CV within the potential range + 0.5 to + 1.5 V. Figure 6a represents the effect of change of scan rates from 10 to 60 mV/s on the electrochemical behavior toward 5.0 μM 2-nitroaniline. With the increase in the scan rate, a continuous increase in anodic peak current was observed. A high determinant coefficient (R2) value of 0.9643 was observed from a linearized variation of anodic peak currents with scan rates which further indicate the diffusion-controlled electrochemical oxidation of 2-nitroaniline [51] (Fig. 6b). The corresponding calibration plot for the anodic current responses for 5.0 μM 2-nitroaniline vs. (scan rate)1/2 with the determinant coefficient R2 = 0.99344 (Fig. 6c) and the plot representing the Ep vs. log scan rate with the determinant coefficient R2 = 0.99801 (Fig. 6d) for 3% Ce-doped SnO2 nanosheets/Nafion modified GCEs were also linear confirming the pure diffusion-controlled process.
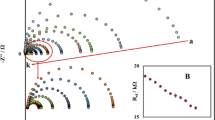
To further investigate the electrochemical properties of the pure SnO2 and Ce-doped SnO2 nanosheets/Nafion-modified GCE, a comparative study of EIS was also carried out. The EIS data was collected in 1.0 mM K3Fe(CN)6/K4Fe(CN)6/0.1 M KCl solution at + 0.6 V. The AC signal amplitude was 10 mV and the frequency range was 0.1 Hz to 100 kHz. The corresponding Nyquist plots drawn between real variables (ZReal) and the negative value of the imaginary impedance variable (ZImag) are shown in Fig. 7. The semicircle shape of the Nyquist plots for the modified electrodes represents the electron transfer resistance, which resulted due to the electron transfer by the redox probe [Fe(CN)6]3−/4−. The diameter of the semicircle plot is correlated to the charge-transfer resistance (Rct) which controls the rate of electron transfer kinetics of the [Fe(CN)6]3−/4− probe at the interface of the electrode. As revealed, the lowest semicircle’s diameter was obtained for the 3% Ce-doped SnO2-modified GCE, indicating a decreased Rct value for this active electrode than other modified working electrodes including pure SnO2/GCE, 1% Ce-doped SnO2/GCE, or 5% Ce-doped SnO2/GCE. This again confirmed an enhanced electron-transfer capability for the 3% Ce-doped SnO2 modified GCE which is advantageous for the electrochemical sensing performance toward 2-nitroaniline, consistent with the above CV results of Fig. 5a.
The amperometric technique was also explored under constant stirring conditions in PBS at an applied potential of 1.3 V against Ag/AgCl (saturated KCl). Figure 8a displays the current real-time response of 3% Ce-doped SnO2 nanosheets/Nafion-modified GCE in the presence of different 2-nitroaniline concentrations ranging from 0.5 to 38.0 µM. In the absence of 2-nitroaniline, a stable background response was observed by the modified GCE. Amperometric current responses increased as soon as 2-nitroaniline with different concentrations was added to the PBS.
(a) The amperometric response curve of 3% Ce-doped SnO2 nanosheets/Nafion-modified GCE in the presence of different 2-nitroaniline concentrations ranging from 0.5 to 38.0 µM at an applied potential of 1.3 V in PBS and (b) corresponding calibration plot of [2-nitroaniline] vs. amperometric current responses
To determine the sensitivity, LOD, and LDR, a calibration plot of the amperometric response current vs. [2-nitroaniline] was plotted (Fig. 8b). The 3% Ce-doped SnO2 nanosheet-modified/Nafion GCE showed a linear dynamic range of 0.5–20.3 µM. The corresponding linear regression equation was Ip (μA) = 0.0709[2-nitroaniline (μM)] + 0.1385 with R2 = 0.99325. The LOD of the modified sensor was found to be 6.3 ± 0.1 nM at the signal-to-noise ratio S/N = 3. The sensitivity of the sensor is estimated to be 0.9986 μA μM−1 cm−2.
The effect of some interfering electro-active chemical species on the selectivity of the 3% Ce-doped SnO2 nanosheet/Nafion-modified GCE towards 2-nitroaniline was also examined. Figure 9 represents the variations of the amperometric current responses of modified GCE during the successive addition of 2-nitroaniline (5.0 μM), 4-nitroaniline (50 μM), 4-nitrophenol (50 μM), NaCl (50 μM), and CaCl2 (50 μM) after certain time intervals and at an applied potential of 1.3 V. Significant increase in current response upon the addition of even 5.0 μM 2-nitroaniline as compared to negligible current changes on the additions of 4-nitroaniline, 4-nitrophenol, NaCl, and CaCl2, is undoubtedly confirming the excellent selectivity of the fabricated 3% Ce-doped SnO2 nanosheet based sensor for 2-nitroaniline.
3.3 Sensing mechanism
Figure 10 illustrates the proposed mechanism for the better electrochemical sensing of the 2-nitroaniline by Ce-doped SnO2 nanosheets. The presence of Ce4+ ions into the SnO2 crystal lattice promotes the charge transfer from the conduction band of SnO2 nanosheets to the surface-adsorbed O2 molecules which were present in the PBS. Additionally, Ce-doping also increases the density of oxygen vacancies and roughness on the surface of the nanosheets required to generate sufficiently more adsorption sites for O2 molecules. Conduction band electrons reduce the adsorbed O2 molecules to different oxygenated species like \({O}^{-}\), \({O}^{2-}\), and \({O}_{2}^{-}\) [30]. Further, the rates of intermolecular charge transfer process and adsorption of the 2-nitroaniline are facilitated on the surface of the Ce-doped SnO2 nanosheets due to the simultaneous presence of electron-rich amino (–NH2) group and an electron-deficient nitro (–NO2) group [52, 53]. Different reactive oxygenated species on the surface of the nanosheets initially reduce 2-nitroaniline into 2-hydroxylamine aniline. The reduction process is followed by a fast reversible oxidation process which oxidizes the 2-hydroxylamine aniline into 2-nitrosoaniline. The electrons released during the oxidation process are transferred back to the conduction band of Ce-doped SnO2 nanosheets which increases the current response and electrical conductivity [54].
Furthermore, there is significant potential for improving the sensitivity of this chemical sensor by applying external photons, which could result in improving its behavior toward 2-nitroaniline [55,56,57].
4 Conclusions
Herein, a novel Ce-doped SnO2 nanosheet/Nafion-modified GCE has been prepared to quantify the 2-nitroaniline in PBS at pH 7.0. Among the various modified electrodes, the 3% Ce-doped SnO2 nanosheets exhibited better electrochemical sensing behavior as compared to others. As-fabricated 3% Ce-doped SnO2 nanosheets/Nafion-modified GCE showed a linear dynamic range of 0.5–20.3 µM, LOD 6.3 ± 0.1 nM at the signal-to-noise ratio S/N = 3 and a remarkable sensitivity of 0.9986 μA μM−1 cm−2. High sensitivity, very low detection limit, wide dynamic range, quick response time, and excellent selectivity of the fabricated sensor may be attributed to high surface area and easy diffusion of the 2-nitroaniline into the sensor matrix. Hence, the hydrothermal synthesis of Ce-doped SnO2 nanosheets has great sensor potential and can be explored for future sensing applications towards toxic and harmful chemicals.
References
Xu CH, Chen JZ (2016) Atmospheric-pressure plasma jet processed SnO2/CNT nanocomposite for supercapacitor application. Ceram Int 42:14287–14291. https://doi.org/10.1016/j.ceramint.2016.06.023
Padvi MN, Moholkar AV, Prasad SR, Prasad NR (2021) A critical review on design and development of gas sensing materials. Engineered Science. 15. https://doi.org/10.30919/es8d431.
Li Y, Wang X, Wang Z, Chen L (2019) Facile synthesis of SnO2 nanorods for Na-ion batteries, ES Energy & Environment 3 (2019) 55–59. https://doi.org/10.30919/esee8c198.
Dimarco BN, Sampaio RN, James EM, Barr TJ, Bennett MT, Meyer GJ (2020) Efficiency Considerations for SnO2-Based Dye-Sensitized Solar Cells. ACS Appl Mater Interfaces 12:23923–23930. https://doi.org/10.1021/acsami.0c04117
Sumathi P (2020) Synthesis, Characterization and Antimicrobial Studies of SnO2 Nanoparticles, Int. J. Chem Tech Res. 13: 203–209. https://doi.org/10.20902/ijctr.2019.130317.
Yang L, Zhou Q, Wang G, Yang Y (2013) Acetylcholinesterase biosensor based on SnO2 nanoparticles-carboxylic graphene-nafion modified electrode for detection of pesticides. Biosens Bioelectron 49:25–31. https://doi.org/10.1016/j.bios.2013.04.037
Li X, Yan J, Zhu K (2021) Fabrication and characterization of Pt doped Ti/Sb-SnO2 electrode and its efficient electro-catalytic activity towards phenol. Engineered Science. 15 (2021). https://doi.org/10.30919/es8d432.
J. Divya, A. Pramothkumar, S. Joshua Gnanamuthu, D.C. Bernice Victoria, P.C. Jobe prabakar, Structural, optical, electrical and magnetic properties of Cu and Ni doped SnO2 nanoparticles prepared via Co-precipitation approach, Phys. B Condens. Matter. 588 (2020) 412169. https://doi.org/10.1016/j.physb.2020.412169.
Chattopadhyay S, Kumawat A, Misra KP, Halder N, Bandyopadhyay A, Antony A, Rao A, Poornesh P, Jedryka J, Ozga K, Kucharska B (2021) Micro-strain administered SHG intensity enhancement by heavy Ce doping in co-precipitated ZnO nanoparticles, Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 266:115041. https://doi.org/10.1016/j.mseb.2021.115041.
Chen K, Zhang H, Tong H, Wang L, Tao L, Wang K, Zhang Y, Zhou X (2021) Down-conversion Ce-doped TiO2 nanorod arrays and commercial available carbon based perovskite solar cells: Improved performance and UV photostability. Int J Hydrogen Energy 46:5677–5688. https://doi.org/10.1016/j.ijhydene.2020.11.074
Islam MR, Saiduzzaman M, Nishat SS, Kabir A, Farhad SFU (2021) Synthesis, characterization and visible light-responsive photocatalysis properties of Ce doped CuO nanoparticles: A combined experimental and DFT+U study Colloids Surfaces A Physicochem Eng Asp 617:126386 https://doi.org/10.1016/j.colsurfa.2021.126386
Kumawat A, Sharma A, Chattopadhyay S, Misra KP (2021) Temperature dependent photoluminescence in Sol-gel derived Ce doped ZnO nanoparticles. Mater Today Proc 43:2965–2969. https://doi.org/10.1016/j.matpr.2021.01.322
Zhang Y, Wang C, Zhao L, Liu F, Sun X, Hu X, Lu G (2021) Preparation of Ce-doped SnO2 cuboids with enhanced 2-butanone sensing performance, Sensors Actuators B Chem. 130039. https://doi.org/10.1016/j.snb.2021.130039.
Sawant JP, Shaikh SF, Kale RB, Pathan HM (2020)Pathan, Chemical bath deposition of CuInS2 thin films and synthesis of CuInS2 nanocrystals: A review. Engineered Science 12:1–12. https://doi.org/10.30919/es8d1142.
Kumar M, Chauhan MS, Akhtar MS, Umar A (2021) Effect of cerium ions in Ce-Doped ZnO nanostructures on their photocatalytic and picric acid chemical sensing. Ceram Int 47:3089–3098. https://doi.org/10.1016/j.ceramint.2020.09.145
Kumar R, Umar A, Kumar G, Akhtar MS, Wang Y, Kim SH (2015) Ce-doped ZnO nanoparticles for efficient photocatalytic degradation of direct red-23 dye. Ceram Int 41:7773–7782. https://doi.org/10.1016/j.ceramint.2015.02.110
Karunakaran C, Gomathisankar P, Manikandan G (2010) Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater Chem Phys 123:585–594. https://doi.org/10.1016/j.matchemphys.2010.05.019
Liang YC, Lee CM, Lo YJ (2017) Reducing gas-sensing performance of Ce-doped SnO2 thin films through a cosputtering method. RSC Adv 7:4724–4734. https://doi.org/10.1039/c6ra25853k
Gawali SR, Patil VL, Deonikar VG, Patil SS, Patil DR, Patil PS, Pant J (2018) Ce doped NiO nanoparticles as selective NO2 gas sensor. J Phys Chem Solids 114:28–35. https://doi.org/10.1016/j.jpcs.2017.11.005
Liu X, Jiang L, Jiang X, Tian X, Sun X, Wang Y, He W, Hou P, Deng X, Xu X (2018) Synthesis of Ce-doped In2O3 nanostructure for gas sensor applications. Appl Surf Sci 428:478–484. https://doi.org/10.1016/j.apsusc.2017.09.177
Wei D, Huang Z, Wang L, Chuai X, Zhang S, Lu G (2018) Hydrothermal synthesis of Ce-doped hierarchical flower-like In2O3 microspheres and their excellent gas-sensing properties. Sensors Actuators, B Chem 255:1211–1219. https://doi.org/10.1016/j.snb.2017.07.162
Qi Z, Joshi TP, Liu R, Liu H, Qu J (2017) Synthesis of Ce(III)-doped Fe3O4 magnetic particles for efficient removal of antimony from aqueous solution. J Hazard Mater 329:193–204. https://doi.org/10.1016/j.jhazmat.2017.01.007
Zhu L, Zeng W, Yang J, Li Y (2019) Unique hierarchical Ce-doped NiO microflowers with enhanced gas sensing performance. Mater Lett 251:61–64. https://doi.org/10.1016/j.matlet.2019.05.055
Dil EA, Ghaedi M, Asfaram A, Mehrabi F, Bazrafshan AA, Tayebi L (2019) Synthesis and application of Ce-doped TiO2 nanoparticles loaded on activated carbon for ultrasound-assisted adsorption of Basic Red 46 dye, Ultrason. Sonochem. 58:104702. https://doi.org/10.1016/j.ultsonch.2019.104702.
Diao Q, Yin Y, Jia W, Xu X, Ding Y, Zhang X, Cao J, Yang K, Jiao M (2020) Highly sensitive ethanol sensor based on Ce-doped WO3with raspberry-like architecture Mater Res Express 7:115012 https://doi.org/10.1088/2053-1591/abcabf
Ponnar M, Thangamani C, Monisha P, Gomathi SS, Pushpanathan K (2018) Influence of Ce doping on CuO nanoparticles synthesized by microwave irradiation method. Appl Surf Sci 449:132–143. https://doi.org/10.1016/j.apsusc.2018.01.126
Aggrwal G, Salunke-Gawali S, Gejji SP, Nikalje M, Chakravarty D, Verma PL, Gosavi-Mirkute P, Harihar S, Jadhav M, Puranik VG (2021) Puranik. Reactions of 2,3-Dibromonaphthalene-1,4-Dione and Pyridyl Amines: X-ray Structures, DFT Investigations, and Selective Detection of the Hg2+ and Ni2+ Ions. Engineered Science, 14:78–93. https://doi.org/10.30919/es8d427.
Chen S, Chen X, Xia T, Ma Q (2016) A novel electrochemiluminescence sensor for the detection of nitroaniline based on the nitrogen-doped graphene quantum dots. Biosens Bioelectron 85:903–908. https://doi.org/10.1016/j.bios.2016.06.010
Nie Y, Liu Y, Su X, Ma Q (2019) Nitrogen-rich quantum dots-based fluorescence molecularly imprinted paper strip for p-nitroaniline detection. Microchem J 148:162–168. https://doi.org/10.1016/j.microc.2019.04.080
Umar A, Ibrahim AA, Kumar R, Almas T, Al-Assiri MS, Baskoutas S (2019) Nitroaniline chemi-sensor based on bitter gourd shaped ytterbium oxide (Yb2O3) doped zinc oxide (ZnO) nanostructures. Ceram Int 45:13825–13831. https://doi.org/10.1016/j.ceramint.2019.04.079
Sayyed SA, Beedri NI, Bhujbal PK, Shaikh SF, Pathan HM (2020) Pathan, Eosin Eosin-Y Sensitized Bi-layered ZnO Nanoflower-CeO2 Photoanode for Dye-Sensitized Solar Cells Application. ES Materials & Manufacturing 10:45–51. https://doi.org/10.30919/esmm5f939.
Ibrahim AA, Umar A, Kumar R, Kim SH, Bumajdad A, Baskoutas S (2016) Sm2O3-doped ZnO beech fern hierarchical structures for nitroaniline chemical sensor. Ceram Int 42:16505–16511. https://doi.org/10.1016/j.ceramint.2016.07.061
Palpandi K, Raman N (2020) Electrochemical detection of 2-nitroaniline at a novel sphere-like Co2SnO4 modified glassy carbon electrode. New J Chem 44:8454–8462. https://doi.org/10.1039/d0nj01098g
Yamuna A, Jiang TY, Chen SM (2021) Preparation of K+ intercalated MnO2-rGO composite for the electrochemical detection of nitroaniline in industrial wastewater J Hazard Mater 411:125054 https://doi.org/10.1016/j.jhazmat.2021.125054
Ahmed AI, Ahmad U, Baskoutas S (2017) Ytterbium doped zinc oxide nanopencils for chemical sensor application. J Nanosci Nanotechnol 17:9157–9162. https://doi.org/10.1166/jnn.2017.14702
Umar A, Akhtar MS, Al-Assiri MS, Al-Salami AE, Kim SH (2018) Composite CdO-ZnO hexagonal nanocones: Efficient materials for photovoltaic and sensing applications. Ceram Int 44:5017–5024. https://doi.org/10.1016/j.ceramint.2017.12.098
Umar A, Ammar HY, Kumar R, Almas T, Ibrahim AA, AlAssiri MS, Abaker M, Baskoutas S (2020) Efficient H2 gas sensor based on 2D SnO2 disks: Experimental and theoretical studies. Int J Hydrogen Energy 45:26388–26401. https://doi.org/10.1016/j.ijhydene.2019.04.269
Kumar R, Umar A, Kumar R, Chauhan MS, Al-Hadeethi Y (2021) ZnO–SnO2 nanocubes for fluorescence sensing and dye degradation applications. Ceram Int 47:6201–6210. https://doi.org/10.1016/j.ceramint.2020.10.198
Umar A, Ammar HY, Kumar R, Ibrahim AA, Al-Assiri MS (2019) Square disks-based crossed architectures of SnO2 for ethanol gas sensing applications—An experimental and theoretical investigation Sensors Actuators, B Chem 304:127352 https://doi.org/10.1016/j.snb.2019.127352
Umar A, Kumar R, Akhtar MS, Kumar G, Kim SH (2015) Growth and properties of well-crystalline cerium oxide (CeO2) nanoflakes for environmental and sensor applications. J Colloid Interface Sci 454:61–68. https://doi.org/10.1016/j.jcis.2015.04.055
Umar A, Almas T, Ibrahim AA, Kumar R, AlAssiri MS, Baskoutas S, Akhtar MS (2020) An efficient chemical sensor based on CeO2 nanoparticles for the detection of acetylacetone chemical J Electroanal Chem 864:114089 https://doi.org/10.1016/j.jelechem.2020.114089
Al-Hadeethi Y, Umar A, Ibrahim AA, Al-Heniti SH, Kumar R, Baskoutas S, Raffah BM (2017) Synthesis, characterization and acetone gas sensing applications of Ag-doped ZnO nanoneedles, Ceram. Int. 43:6765–6770. https://doi.org/10.1016/j.ceramint.2017.02.088.
Al-Hadeethi Y, Umar A, Al-Heniti SH, Kumar R, Kim SH, Zhang X, Raffah BM (2017) 2D Sn-doped ZnO ultrathin nanosheet networks for enhanced acetone gas sensing application. Ceram Int 43:2418–2423. https://doi.org/10.1016/j.ceramint.2016.11.031
Lupan O, Chow L, Chai G, Schulte A, Park S, Heinrich H. (2009) A rapid hydrothermal synthesis of rutile SnO2 nanowires, Mater. Sci. Eng. B. 157:101–104. https://doi.org/10.1016/j.mseb.2008.12.035.
Wu W, Zhang S, Zhou J, Xiao X, Ren F, Jiang C (2011) Controlled synthesis of monodisperse sub-100 nm hollow SnO2 nanospheres: A template-and surfactant-free solution-phase route, the growth mechanism, optical properties, and application as a photocatalyst. Chem - A Eur J 17:9708–9719. https://doi.org/10.1002/chem.201100694
Wang B, Sun L, Wang Y (2018) Template-free synthesis of nanosheets-assembled SnO2 hollow spheres for enhanced ethanol gas sensing, Mater. Lett. 218:290–294. https://doi.org/10.1016/j.matlet.2018.02.003.
Ray A, Roy A, Ghosh M, Ramos-Ramón JA, Saha S, Pal U, Bhattacharya SK, Das S (2019) Study on charge storage mechanism in working electrodes fabricated by sol-gel derived spinel NiMn 2 O 4 nanoparticles for supercapacitor application, Appl. Surf. Sci. 463:513–525. https://doi.org/10.1016/j.apsusc.2018.08.259.
Mariammal RN, Ramachandran K, Renganathan B, Sastikumar D (2012) On the enhancement of ethanol sensing by CuO modified SnO 2 nanoparticles using fiber-optic sensor. Sensors Actuators, B Chem 169:199–207. https://doi.org/10.1016/j.snb.2012.04.067
Poloju M, Jayababu N, Manikandan E, Reddy MR (2017) Reddy, Enhancement of the isopropanol gas sensing performance of SnO2/ZnO core/shell nanocomposites, J. Mater. Chem. C. 5:2662–2668. https://doi.org/10.1039/C6TC05095F.
Zargar RA, Bhat MA, Parrey IR, Arora M, Kumar J, Hafiz AK (2016) Optical properties of ZnO/SnO2 composite coated film, Opt. - Int. J. Light Electron Opt. 127:6997–7001. https://doi.org/10.1016/j.ijleo.2016.05.037.
Wahab R, Ahmad N, Alam M, Ahmed J (2019) Nanorods of ZnO: An effective hydrazine sensor and their chemical properties. Vacuum. https://doi.org/10.1016/j.vacuum.2019.04.036
Naito S, Yokoyama S, Asahara H, Nishiwaki N (2017) Synthesis of functionalized 4-nitroanilines by ring transformation of dinitropyridone with enaminones, Tetrahedron Lett. 58:4699–4702. https://doi.org/10.1016/j.tetlet.2017.11.003.
Muniz-Miranda M, Neto N (2004) Surface-enhanced Raman scattering of π-conjugated “push-pull” molecules: Part I. p-Nitroaniline adsorbed on silver nanoparticles, in: Colloids Surfaces A Physicochem. Eng. Asp. 79–84. https://doi.org/10.1016/j.colsurfa.2004.08.070.
Umar A, Akhtar MS, Dar GN, Abaker M, Al-Hajry A, Baskoutas S (2013) Visible-light-driven photocatalytic and chemical sensing properties of SnS2nanoflakes. Talanta 114:183–190. https://doi.org/10.1016/j.talanta.2013.03.050
Algadi H, Mahata C, Kim S, Dalapati GK (2020) Improvement of Photoresponse Properties of Self-Powered ITO/InP Schottky Junction Photodetector by Interfacial ZnO Passivation, J. Electron. Mater. 1–7. https://doi.org/10.1007/s11664-020-08565-1.
Algadi H, Mahata C, Sahoo B, Kim M, Koh WG, Lee T (2020) Facile method for the preparation of high-performance photodetectors with a GQDs/perovskite bilayer heterostructure, Org. Electron. 76. https://doi.org/10.1016/j.orgel.2019.105444.
Algadi H, Mahata C, Woo J, Lee M, Kim M, Lee T (2019) Enhanced photoresponsivity of all-inorganic (Cspbbr3) perovskite nanosheets photodetector with carbon nanodots (CDs), Electron. 8. https://doi.org/10.3390/electronics8060678.
Funding
The Deputy for Research and Innovation—Ministry of Education, Kingdom of Saudi Arabia, supported the research through a grant (NU/IFC/INT/01/004) under the institutional funding committee at Najran University, Kingdom of Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Umar, A., Kumar, R., Algadi, H. et al. Highly sensitive and selective 2-nitroaniline chemical sensor based on Ce-doped SnO2 nanosheets/Nafion-modified glassy carbon electrode. Adv Compos Hybrid Mater 4, 1015–1026 (2021). https://doi.org/10.1007/s42114-021-00283-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-021-00283-4