Abstract
Novel polyethylene oxide (PEO)/N,N-dimethylacrylamide (DMA) blend hydrogel (PD hydrogel) has been prepared by applying gamma radiation on aqueous mixture of PEO and DMA. Different concentrations of DMA have been blended with aqueous solution of PEO and then gamma radiation of different doses was applied to form the hydrogels. The synthesized hydrogels were characterized using Fourier transform infrared-attenuated total reflection spectroscopy (FTIR-ATR) and scanning electron microscopy (SEM). The effect of different radiation doses, concentration of monomers on the properties of prepared hydrogel (PD hydrogel), such as gel content, swelling behavior, and mechanical properties (Tensile strength) was investigated thoroughly. Results showed that hydrogels were formed at the experimental conditions without the use of any external cross-linker. All the prepared hydrogels showed a significantly larger swelling ratio and improved mechanical strength.

Novel hydrogels were prepared by applying gamma radiation on a mixture containing commercially available polymer and monomer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydrogels are three-dimensional (3D) network of hydrophilic cross-linked polymer that can absorb water and swell to several times at their initial dry volume. Owing to the large quantities of incorporated water and their elastic properties, hydrogels are considered as excellent biocompatible materials. Physical cross-links (e.g., electrostatic forces, hydrogen bonds, hydrophobic interactions) or chemical cross-links (covalent bonds) are responsible for the hydrogels’ three-dimensional structure and distinctive swelling properties [1,2,3]. Recently, these hydrophilic materials have achieved great attention due to their versatile uses such as heavy metal adsorption [4, 5], wound dressing [6], superabsorbent [7, 8], adsorbent for dye removal [9], and drug-delivery systems [1, 10,11,12]. Several methods to prepare hydrogel are cross-linking (physical and chemical) and copolymerization of one or more monofunctional and one multifunctional monomer simultaneously or cross-linking of a homopolymer or copolymer in solution [13,14,15]. Some “smart” hydrogels show swelling and deswelling behavior reliant on the external environment such as temperature, pH, solvent composition, and salt composition depending on the type of functional groups in the structure [1, 16, 17]. More recently, hydrogels synthesized by radiation polymerization and grafting have been reported [18, 19]. Radiation cross-linking is a widely used technique since it does not incorporate the use of any cross-linker. Also, the modification can be achieved in single step and hence it is a cost-effective process to transform biopolymers having their end-use specifically in biomedical application [19, 20]. The technique mainly relies on producing free radicals in the polymer following the exposure to the high energy source such as UV, gamma ray, x-ray, or electron beam. The action of radiation (direct or indirect) will depend on the polymer environment such as dilute solution, concentrated solution, and solid state.
Polyethylene oxide (PEO) is a flexible chain biodegradable polymer, which is also a semicrystalline, thermoplastic water-soluble polymer; the chains contain oxygen atoms at every third position of the polymer backbone. The O–C–C–O bond sequence acts as a significant electron donor, providing the PEO polymer with hydrophilic properties, such as solubility in water as well as in many non-polar organic solvents [21]. In recent years, PEO has become a focus of scientific interest not only because of its unique properties in solution but also its extensive applications, such as drug delivery, tissue implant, and wound healing [22,23,24]. The PEO hydrogels imbibe a considerable amount of water which affects the mechanical properties and make them too weak to withstand high stress [25]. Much research has been carried out on PEO-based hydrogel by using radiation-induced processes, for instance, radiation grafting on tragacanth, acrylic acid, chitosan, and polyacrylamide onto PEO [26,27,28,29]. There are nearly no studies on PEO’s property modification by the grafting of N,N-dimethylacrylamide (DMA) based on gamma radiation, so far. DMA is a non-ionic, hydrophilic monomer, its homopolymers are soluble in water within the temperature ranges of 0–100 °C and also soluble in less polar organic solvents and used in the fabrication of hydrogels with good mechanical properties [15, 30, 31]. DMA-based homopolymers and copolymers have many practical applications in molecular biology, DNA sequencing [32], medical and pharmaceutical fields including contact lenses and in drug delivery [15, 33, 34], and in dye removal [18]. In the present work, we demonstrate to prepare a series of PEO-DMA-based hydrogels by using gamma radiation, without the use of external cross-linking agents or other additives. The effect of DMA concentration on various properties such as gel fraction, swelling ratio, and mechanical properties of the prepared hydrogel was investigated thoroughly.
2 Experimental
2.1 Materials
Polyethylene oxide with average molecular weight (Mv) of 400,000 was purchased from Sigma-Aldrich Co., USA. N,N-dimethylacrylamide was obtained from Fluka Chemika, Switzerland. All other reagents were of analytical grade and were used as received.
2.2 Preparation of PEO/DMA hydrogels
Radiation-cross-link technique was employed for the preparation of proposed hydrogel. Firstly, for the synthesis of pure PEO gel, 5% aqueous solution of PEO was prepared by dissolving 5 g of PEO in a beaker (250 ml) with 100 ml distilled water and using a heated sonicator bath, the solution was mixed to have a homogeneous solution. To prepare PEO/DMA hydrogels (PD hydrogels), the PEO concentration was kept constant (5%) and DMA was added with different concentrations (1.25–3.75%, w/w) at a ratio 2:0.5, 2:1, and 2:1.5. The solutions were stirred by a glass rod continuously to make a homogenous solution. The bubbles are generated after mixing and then the solutions left for 1 h in a vacuum oven for outgassing. The mixtures were poured into polyethylene plastic bags (15 cm × 2.5 cm), sealed, placed in glass tubes, and then irradiated by gamma rays from 60Co source (dose rate 5.92 kGy/h). The samples were irradiated at radiation doses from 5 to 30 kGy [26].
2.3 FTIR-ATR spectral analysis
Fourier transform infrared (FTIR) spectra of PEO and PD hydrogels were obtained by PerkinElmer Spectrum 2 FTIR spectrometer (Paragon 500, PerkinElmer, UK). This method is very easy and convenient in comparison to conventional KBr pellet technique. The dried sample pressed against a high-refractive-index prism and measured the infrared spectrum using infrared light that is totally internally reflected in the prism. The spectra were recorded in the range from 4000 to 400 cm−1.
2.4 SEM of freeze-dried hydrogels
The morphology of PEO and PEO-DMA hydrogels were examined under scanning electron microscopy (SEM). Firstly, samples were swollen up to equilibrium in distilled water, then frozen in liquid nitrogen, and then immediately freeze dried in Freeze Drier, FRD-mini (IWAKI, Asahi Techno Glass, Japan) in vacuo at − 50 °C for 2 days to completely remove water. After that, the freeze-dried hydrogels were fractured carefully, and the cross section or interior morphology of the hydrogels was studied with SEM (JCM-5700, JEOL, Japan). Before SEM observation, specimens of the hydrogels were fixed onto a stage and given a coat with osmium at a thickness of 20 nm in osmium plasma coater OPC60 N (Filgen Inc., Japan) [1].
2.5 Gel fraction of prepared hydrogel
The hydrogel samples were dried to constant weight (Wi), immersed in distilled water in beakers at room temperature for 16 h to remove any soluble fraction or unreacted components. The gel samples were taken out from beakers, dried to constant weight (Wd) in a oven at 60 °C temperature. The gel fractions (GF) of the samples were calculated according to the following equation [26]:
where Wd is the weight of dried gel after extraction and Wi is the initial weight of dried gel.
2.6 Swelling ratio of prepared hydrogel
The dried gels (1 cm × 0.5 cm) were immersed in distilled water at room temperature and allowed for swelling. The swollen gels were then taken out periodically and weighed after the excess surface water was removed with a filter paper (Whatman, 125 mm). The procedure was repeated until there was no further weight increase. The swelling ratio (SR) was calculated from the following equation [26]:
where Wd is the weight of dried gel before swelling and Wt is the weight of the swollen gel at time t.
2.7 Measurement of mechanical properties (tensile strength) of prepared hydrogels
Tensile strength (TS) of hydrogel samples (width 15 mm and thickness about 3.23 mm and height 30 mm) was measured with a universal testing machine (Hounsfield Series S, England). The maximum load capacity was 5 N, extension range 75 mm, and the crosshead speed was 10 mm/min. For each sample, a minimum of three measurements was taken, and the average result was calculated.
3 Results and discussion
3.1 Optimization of absorbed dose
To optimize the radiation dose for our experiments, PD hydrogel with a PEO (5 g) to DMA (2.5 g) ratio 1:0.5 was synthesized using different radiation doses (5–30 kGy). Gel fraction and swelling ratio were then measured. The results are shown in Fig. 1. As is observed from Fig. 1, the gel content increased up to 10 kGy and then it was almost constant indicating a leveling off tendency at doses from 15 to 30 kGy (Fig. 1a).
We assumed that this might be due to oxidative degradation of main chain (PEO) from –CH2–O– linkages by forming hydrogen peroxide during the radiolysis of water [35, 36]. The results of swelling ratio experiments are shown in Fig. 1b. In this figure too, the maximum swelling ratio was observed at 10 kGy after which swelling ratio decreased. Clearly, it is the highest gel formation with high water absorbing ability obtained at 10 kGy and is thus chosen as optimized dose for our further research.
3.2 Synthesis of PD hydrogels at optimized dose (10 kGy)
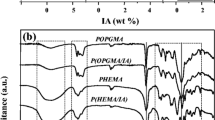
After optimizing the radiation dose which is 10 kGy, a series of hydrogels with different composition of DMA by mixing aqueous solution of PEO and DMA (Fig. 2a, b) have been prepared. Table 1 lists the feed composition of the prepared hydrogels. The possible scheme for the preparation of hydrogels is shown in Fig. 2a. It is reported that, in aqueous solutions, after irradiation, the radical processes are initiated mainly by the reactive intermediates (•OH, •H, e•aq, H2O2, H2, and H+) formed in the radiolysis of water as a result of direct action of radiation [19, 37]. They may attack the reactive molecules such as PEO and DMA to generate free radicals of PEO and DMA. Generally, network structure of this type of hydrogel is formed as a consequence of graft homopolymerization, copolymerization, and cross-linking of monomers and polymers used [19]. A typical image of the prepared hydrogels is shown in Fig. 2b. The presence of functional groups in the prepared hydrogel samples was evaluated via FTIR spectra as shown in Fig. 3. For pure PEO and PD2.50 gel, the broad absorption band at 3469 cm−1 and 3442 cm−1 respectively is ascribed to the existence of –OH (stretch). The band observed at 2888 and 2895 cm−1 can be ascribed to aliphatic stretching of –CH2. The presence of DMA in PD2.50 gel is displayed by a strong absorption band at 1619 cm−1 attributed to free C=O group of amide. As for the pure PEO gel, the absorption band at 1652 cm−1 indicates bound water solvent as in crystal form, which did not appear in spectra of PD2.50 gel. On comparing PD2.50 hydrogel with pure PEO hydrogel, –CH2 scissoring vibration of the –CH2OH group of PEO shifts higher wave number (1467 to 1469 cm−1) with higher intensity. This peak shifting indicates interaction between –CH2 groups of PEO and DMA [18, 29, 38,39,40].
3.3 Morphologies of freeze-dried hydrogels
The cross-sectional morphologies of freeze-dried hydrogels are studied by SEM and represented in Fig. 4. The SEM micrographs of the hydrogels revealed a porous internal structure that confirmed the three-dimensional structure of the hydrogels and controlled the swelling and deswelling process of hydrogel. It is evident from the microphotographs that PD hydrogels exhibit a highly interconnected porous structure with smaller and thicker pore distribution in the presence of DMA. As expected, the pores inside the PD gels become denser with increasing composition of DMA. Hence, this result indicates that pore size and swelling properties of the obtained hydrogels could be tailored by changing the composition of DMA in PEO/DMA ratio.
3.4 Gel fraction
Gel is an insoluble fraction of a cross-link polymer network and measured after removing soluble fraction (sol part) in a suitable solvent. The progressive formation of gel in polymer system depends on various parameters such as weight-average functionality, molecular weights of monomers, and the reaction conditions (e.g., concentration of monomers) [41]. Figure 5 shows the effect of DMA concentration on gel fraction of PD hydrogel, it is found that the gel fraction of hydrogels increased with increased concentration of DMA. For instance, gel fraction of PD hydrogel varies from 84.78 to 91.48% for increased concentration of DMA (1.25–3.75%), whereas, the gel fraction of P5 gel is 83.3% as PEO is a radiation degradable polymer [35, 36]. When PEO and DMA irradiated in aqueous solution, water absorbed radiation to form reactive hydroxyl radicals. The hydroxyl radicals generated PEO and DMA radicals (macro-radicals) by abstracting hydrogen atoms and random reactions of these radicals result in formation of cross-link that path produces gel [19]. In these circumstances, both the concentration of free radical in monomer/polymer system and the distance between the neighboring radicals are favorable for the cross-link formation. Hence, the addition of DMA increases the radical formation and reduces the distance between two macro-radicals leading to increased gel fraction [42].
3.5 Swelling ratio
Swelling in water is one of the most important and promising properties of hydrogel polymers. Most industrial applications of hydrogel in biomedical, environment, and agriculture field are associated with their ability to swell in water. When a dry hydrogel sample is brought in contact with water, water diffuses in the hydrogel and the hydrogel swells. Figure 6 presents the swelling behavior of prepared hydrogels containing different concentration of DMA. The equilibrium-swelling ratio as a function of time for PD hydrogels with varying DMA concentration is illustrated in Fig. 6a. As it is observed from Fig. 6a, all the hydrogels have much larger swelling ratio (e.g., 2730, 2595, and 1948% for PD1.25, PD2.50, and PD3.75 respectively). This might be explained by the fact that PEO shows a high interaction with water because of ether oxygen in the polymer backbone and there is a very sensitive balance between hydrophilic –CONH– and hydrophobic (–CH3)2 groups in DMA, which leads to absorption of water by forming a hydrogen bond [25, 31, 43]. It was also observed that the swelling ratio was increased with time and reached equilibrium after about 48 h. On comparing among the prepared hydrogels, it was evident that the swelling ratio was decreased with increased DMA concentration. We think that this might be due to the formation of more compact structure with the addition of DMA in PEO/DMA mixture. The compact hydrogel structure hindered the network expansion during the swelling. The DMA in PEO/DMA gel reduced the flexibility of PEO network; thus, expansion ability became lesser, and this results in smaller pore structure (Fig. 4). This can explain the decrease in swelling ratio with the increased amount of DMA. Figure 6b shows the photographs of initial state, before and after swelling state of PD2.50 hydrogel. The swollen transparent hydrogel held its shape after 3 weeks swelling. Hydrogels based on PEO and other polymers (e.g., PVA) have been reported with low swelling ratio in comparison with prepared hydrogels (PD gel). For example, Haryanto et al. [44] found maximum 870% SR for PEO/poly(ethylene glycol) dicarboxylate (PEGDC) hydrogel; likely, PEO/poly (ethylene glycol) dimethacrylate (PEGDMA) and poly(vinyl alcohol) (PVA)/kappa-carrageenan (KC) hydrogels showed 300 and 410% swelling ratio respectively [45, 46].
3.6 Mechanical properties (tensile strength)
Generally, pure PEO gels have poor mechanical characteristics, i.e., gels are weak, brittle, and the gel cannot withstand large deformation [47]. It is reported that majority of the gels are weak in nature [48, 49], which confine their applications. Scientists have queries to improve mechanical strength of hydrogel. Several approaches have been made to overcome this limitation in the past [50, 51]. Mechanical properties of hydrogel can be controlled by various routes, for example, changing the co-monomer structure, increasing or diminishing the cross-linking density [22, 52]. One of the most effective methods to improve PEO gel properties is the combination of PEO with other polymers or monomer(s). The gels formed by the combination of two or more polymers usually result in adjusted physical and mechanical properties compared to the gels made of the homopolymers [52]. It is reported that increasing the physically stronger components will lead to an escalation in the mechanical strength of the ultimate product [52]. Figure 7 shows the typical stress-strain curve for hydrogels. The tensile stress at break is plotted as a function of DMA concentration. It is apparent from Fig. 7 that tensile strength value of prepared hydrogels was significantly increased with the addition of DMA. For example, the TS value of sample P5 was only 0.0015 MPa, whereas that of PD1.25, PD2.50, and PD3.75 hydrogels was 0.0025, 0.0031, and 0.0043 MPa respectively. We consider that this marked change in TS with increased DMA concentration might be because of the formation of high cross-linking network between polymer chains. Cross-linking hinders the flexibility of the polymer chain which forms a rigid structure; hence, mechanical strength of hydrogel is increased. Most notably, the improvement in mechanical properties of hydrogel is reliable with their pore size. As mentioned earlier, PD hydrogels with different DMA compositions exhibited a well-formed porous structure with the smallest pores, this compact structure contributed to give out stress throughout the hydrogel surface more evenly [53]. Thus, the TS of PD 3.75 was the highest among all the samples, conversely, P5 was the lowest. This is also in accordance with the morphology of the hydrogels (Fig. 4). As the network becomes more compact with increasing DMA, the mechanical strength also increased.
4 Conclusion
A novel class of hydrogel (PEO-DMA) was successfully synthesized by the application of gamma radiation without any conventional cross-linker or additives. Due to the simplicity of the preparation method, i.e., only by mixing the aqueous polymer and monomer, the hydrogel properties can be tuned or adjusted very easily. There are remarkable impacts of DMA concentrations on the properties (e.g., swelling behavior, gel fraction, and tensile strength) of hydrogels. High swelling ratios were obtained for all the prepared hydrogels. For example, swelling ratio of PD1.25, PD2.50, and PD3.75 hydrogels are approximately 2730, 2595, and 1948% respectively. In addition, gel fraction was increased from 84 to 91% for 1.25–3.75% of DMA. Incorporation of DMA increased the mechanical strength of hydrogels significantly. Mechanical properties of pure PEO gel at 10 kGy was 0.0015 MPa, on the other hand, for PD gels, it was 0.0025, 0.0031, and 0.0043 MPa for 1.25, 2.50, and 3.75 wt% of DMA respectively. While high mechanical strength ascribed to the dense cross-linking formation in the prepared hydrogels, there was a slight lowering in swelling ratio of hydrogels. From the prior discussion, it may be concluded that PD hydrogel with 5:2.50 wt% can be suitable for many applications as well as with specific physical properties: gel fraction (88%), swelling ratio 2595%, and tensile strength 0.0031 MPa.
References
Alam MA, Takafuji M, Ihara H (2013) Thermosensitive hybrid hydrogels with silica nanoparticle-cross-linked polymer networks. J Colloid Interface Sci 405:109–117
Garcia DM, Escobar JL, Noa Y, Bada N, Hernaez E, Katime (2004) Timolol maleate release from pH-sensible poly(2- hydroxyethyl methacrylate-co-methacrylic acid) hydrogels. Eur Polym J 40:1683–1690
Hoffman AS (2002) Hydrogels for biomedical applications. Adv Drug Deliv Rev 54:3–12
Hui B, Ye L (2016) Structure of polyvinyl alcohol-g-acrylic acid-2-acrylamido-2-methyl-1-propanesulfonic acid hydrogel and adsorption mechanism for advanced Pb(II) removal. J Ind Eng Chem 35:309–317
Li Z, Wang Y, Wu N, Chen Q, Wu K (2013) Removal of heavy metal ions from wastewater by a novel HEA/AMPS copolymer hydrogel: preparation, characterization, and mechanism. Environ Sci Pollut Res 20:1511–1525
Kanarat N, Nantarat S, Chinanat W, Robert M (2007) Design and preparation of amps-based hydrogels for biomedical use as wound dressings. Chiang Mai J Sci 34:183–189
Hosseinzadeh H (2013) Synthesis and swelling properties of a poly (vinyl alcohol)-based superabsorbing hydrogel. Curr Chem Lett 2:153–158
Thakur S, Pandey S, Arotiba OA (2016) Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr Polym 153:34–46
Fang R, He W, Xue H, Chen W (2016) Synthesis and characterization of a high-capacity cationic hydrogel adsorbent and its application in the removal of acid black 1 from aqueous solution. React Funct Polym 102:1–10
Alam MA, Takafuji M, Ihara H (2014) Silica nanoparticle-crosslinked thermosensitive hybrid hydrogels as potential drug-release carriers. Polym J 46:293–300
Atta S, Khaliq S, Islam A, Javeria I, Jamil T, Athar MM, Shafiq MI, Ghaffar A (2015) Injectable biopolymer based hydrogels for drug delivery applications. Int J Biol Macromol 80:240–245
Hong KH, Jeon Y, Chung DJ, Kim J (2010) Drug release characteristics of modified phema hydrogel containing thermo-responsive pluronic copolymer. Macromol Res 18:204–207
Mohan YM, Murthy PSK, Raju KM (2005) Synthesis, characterization and effect of reaction parameters on swelling properties of acrylamide–sodium methacrylate superabsorbent copolymers. React Funct Polym 63:11–26
Thakur A, Rk W, Singh P (2011) Structural parameters and swelling behavior of ph sensitive poly(acrylamide-coacrylicacid) hydrogels. Chem Biochem Eng Q 25:181–194
Gundogan N, Okay O, Oppermann W (2004) Swelling, elasticity and spatial inhomogeneity of poly(N,N-dimethylacrylamide) hydrogels formed at various polymer concentrations. Macromol Chem Phys 205:814–823
Takafuji M, Alam MA, Goto H, Ihara H (2015) Microspherical hydrogel particles based on silica nanoparticle-webbed polymer networks. J Colloid Interface Sci 455:32–38
Ohmine I, Tanaka T (1982) Salt effects on the phase transition of ionic gels. J Chem Phys 77:5725–5729
Ibrahim AG, Hai FA, Wahab HA, Mahmoud H (2016) Synthesis, characterization, swelling studies and dye removal of chemically crosslinked acrylic acid/acrylamide/N,N-dimethyl acrylamide hydrogels. Am J Appl Chem 4:221–234
Bhuiyan MAQ, Rahman MS, Rahaman MS, Shajahan M, Dafader NC (2015) Improvement of swelling behaviour of poly (vinyl pyrrolidone) and acrylic acid blend hydrogel prepared by the application of gamma radiation. Org Chem Curr Res 4:138–145
Lugao AB, Malmonge SM (2001) Use of radiation in the production of hydrogels. Nucl Instrum Methods Phys Res Sect B 185:37–42
Yuan QW (1999) Poly(ethylene oxide). In: Mark JE (ed) Polymer data handbook, 3rd edn. Oxford University Press, New York, pp 542–552
Rahman MA, Khan MA, Tareq SM (2010) Preparation and characterization of polyethylene oxide (peo)/gelatin blend for biomedical application: effect of gamma radiation. J Appl Polym Sci 117:2075–2082
Byun M, Hong SW, Zhu L, Lin Z (2008) Self-assembling semicrystalline polymer into highly ordered, microscopic concentric rings by evaporation. Langmuir 24:3525–3531
Kashmola TO, Kamil ES (2014) Structure rheology of polyethylene oxide solution. Iraqi journal of chemical and petroleum engineering 15:23–32
Husken D, Gaymans RJ (2009) The tensile properties of poly(ethylene oxide)-based segmented block copolymers in the dry and wet state. J Mater Sci 44:2656–2664
Khoylou F, Naimian F (2009) Radiation synthesis of superabsorbent polyethylene oxide/tragacanth hydrogel. Radiat Phys Chem 78:195–198
Ali AEH, Hegazy ESA (2007) Radiation synthesis of poly(ethylene glycol)/acrylic acid hydrogel as carrier for site specific drug delivery. J Biomed Mater Res Part B: Appl Biomater 81:168–174
Erizal WT (2011) Synthesis of polyethylene oxide (peo)–chitosan hydrogel prepared by gamma radiation technique. Indones J Chem 11:16–20
Patel G, Sureshkumar MB, Patel P (2015) Spectroscopic investigation and characterizations of pam/peo blends films. Soft 4:9–24
Guillaumont L, Bokias G, Iliopoulos I (2000) Hydrophobically modified poly(N,N-dimethylacryl-amide): synthesis, aqueous solution behaviour, and rheological properties in aqueous mixtures with hydrophobically modified poly(sodium acrylate). Macromol Chem Phys 201:251–260
Algi MP, Okay O (2014) Highly stretchable self-healing poly(N,N-dimethylacry-lamide) hydrogels. Eur Polym J 59:113–121
Song L, Liang D, Chen Z, Fang D, Chu B (2001) DNA sequencing by capillary electrophoresis using mixtures of polyacrylamide and poly(N,N-dimethylacrylamide). J Chromatogr A 915:231–239
Liu SQ, Tong YW, Yang YY (2005) Incorporation and in vitro release of doxorubicin in thermally sensitive micelles made from poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)-b-poly(D,L-lactide-co-glycolide) with varying compositions. Biomaterials 26:5064–5074
Kataoka K, Miyazaki H, Okano T, Sakurai Y (1994) Sensitive glucoseinduced change of the lower critical solution temperature of poly[N,N-(dimethylacrylamide)-co-3-(acrylamido)-phenylboronic acid] in physiological saline. Macromolecules 27:1061–1062
Savas H, Olgun G (2002) Gelation, swelling and water vapor permeability behavior of radiation synthesized poly(ethylene oxide) hydrogels. Radiat Phys Chem 64:35–40
Zhao Y, Wanga M, Tang Z, Wu G (2011) Radiation effects of UHMW-PE fibre on gel fraction and mechanical properties. Radiat Phys Chem 80:274–277
Mathesan MS, Mamou A, Silverman J, Rabani J (1973) Reaction of hydroxyl radicals with poly(ethylene oxide) in aqueous solution. J Phys Chem 77:2420–2424
Kiran E, Rodriquez F (1973) Effects of gamma radiation on aqueous polymer solutions–a comparative study. J Macromol Sci Phys part B 7:209–224
Nita LE, Chiriac AP, Nistor MT, Neamtu I (2013) Hydrogel based on poly(N,N-dimethylacrylamide-co-3, 9-divinyl-2, 4, 8, 10-tetraoxaspiro (5.5) undecane) with dual sensitive behavior: synthesis and characterisation. Rev Roum Chim 58:137–143
Baek S, Kima D, Jeon SN, Seo J (2017) Preparation and characterization of pH-responsive poly(N,N-dimethyl acrylamide-co-methacryloyl sulfadimethoxine) hydrogels for application as food freshness indicators. React Funct Polym 120:57–65
Ahmed Z, Stepto RFT (1982) Effect of monomer functionality and concentration on gelation in non-linear random polymerization. Polym J 14:767–772
Fekete T, Borsa J, Takacs E, Wojnarovits L (2017) Synthesis of carboxymethylcellulose/starch superabsorbent hydrogels by gamma-irradiation. Chem Cent J 11:46–55
Haryanto SD, Han SS, Son JH, Kima SC (2015) Poly(ethylene glycol) dicarboxylate/poly(ethylene oxide) hydrogel film co-crosslinked by electron beam irradiation as an anti-adhesion barrier. Mater Sci Eng C 46:195–201
Haryanto, Fani, Mahardian A (2018) Biocompatible hydrogel film of polyethylene oxide-polyethylene glycol dimetacrylate for wound dressing application. IOP Conf Ser Mater Sci Eng 288:012076
Dafader NC, Manir MS, Alam MF, Swapna SP, Akter T, Huq D (2015) Effect of kappa-carrageenan on the properties of poly(vinyl alcohol) hydrogel prepared by the application of gamma radiation. Sop Transactions on Applied Chemistry 2:1–12
Jassal M, Agarwal AK, Save NS (2006) Thermoresponsive smart textile. Indian J Fibre Text Res 31:52–65
Zivanovic S, Li J, Davidson PM, Kit K (2007) Physical, mechanical, and antibacterial properties of chitosan/peo blend films. Biomacromolecules 8:1505–1510
Fu F, Chen Z, Zhao Z, Wang H, Shang L, Gu Z, Zhao Y (2017) Self-healing structural color hydrogel. Proc Natl Acad Sci 114:5900–5905
Haq MA, Su Y, Wang D (2017) Mechanical properties of PNIPAM based hydrogels: a review. Mater Sci Eng C 70:842–855
Okumura Y, Ito K (2001) The polyrotaxane gel: a topological gel by figure-of-eight cross-links. Adv Mater 13:485–487
Gong JP, Katsuyama Y, Kurokawa T, Osada Y (2003) Double-network hydrogels with extremely high mechanical strength. Adv Mater 15:1155–1158
Anseth KS, Bowman CN, Brannon-Peppas L (1996) Mechanical properties of hydrogels and their experimental determination. Biomaterials 17:1647–1657
Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL (2004) Structure and properties of silk hydrogels. Biomacromolecules 5:786–792
Funding
This study was financially supported by the National Science and Technology (NST) Fellowship and Research cell, Noakhali Science and Technology University (NSTU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Afroz, S., Afrose, F., Alam, A.K.M.M. et al. Synthesis and characterization of polyethylene oxide (PEO)—N,N-dimethylacrylamide (DMA) hydrogel by gamma radiation. Adv Compos Hybrid Mater 2, 133–141 (2019). https://doi.org/10.1007/s42114-018-0058-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-018-0058-x