Abstract
The present work aims to highlight the use of polyethylene terephthalate (PET) plastic waste for the conception of a new PET-siliceous sand composite material (WPLA) to be used, after heat treatment, as a light aggregate in various screed mortars. This composite is intended to be employed as a substitute for conventional aggregate at the rates of 0, 25, 50, 75, and 100% by weight. Reinforcement corrosion, caused by the attack of chloride ions, is the main reason for the deterioration of reinforced concrete structures around the world. To determine the effects of waste PET as a lightweight aggregate (WPLA), five WPLAX composite mortar formulations were immersed into a 5% NaCl solution. The mechanical strength, absorption of water by capillary suction, and chlorine ion penetration into mortars were all studied. Additional information on the microstructure of the materials was also collected. The results obtained indicated a decrease in the compressive strength of WPLAX. Moreover, Fick’s second law made it possible to observe a decrease in the penetration of chlorine ions, ranging from 40 to 90% in WPLAX mortars as the replacement ratio increased. Likewise, it was found that the sorptivity coefficients of WPLAX mortars decreased from 43 to 65% as compared to that of reference mortar. These encouraging results open up new prospects for using these composite materials as protective mortars for reinforced concrete structures. At the same time, it is one way of getting rid of these PET plastic wastes which represent a serious pollution form to the environment and a real threat to human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the field of construction, eco-materials based on recycled tailings and plastic waste (2iE 2013) are currently among the best solutions to ecological and technical problems. This must be part of everybody’s sustainable development policy. Unfortunately, these materials are not sufficiently exploited despite their technical and economic benefits, in addition to their ecological assets.
About 320 million tons of plastic were produced worldwide in 2015, which represents nearly 10.1 tons per second, for an approximate consumption of 8% of world oil production (Planetoscope 2012). According to a study conducted at the University of Santa Barbara (California, USA) in 2017 (Planetoscope 2012), 9.1 billion tons of plastic have already been produced to date in the world! More than half of this quantity, i.e., 5.4 billion tons, ended up in the environment. Each year, 4.8–12.7 million tons of plastic waste are dumped into the seas and oceans, which end up in microparticles ingested by marine fauna. It is worth recalling that each gram of plastic contains about 1 g of oil (Planetoscope 2012). China is the largest plastic producer in the world, with a 27% share of the world total. Europe follows it closely, with 20%. In contrast, Africa and the Middle East produce only 7%, and Latin America 5% (Planetoscope 2012).
In addition, 25 million tons of plastic wastes are collected in Europe each year, but only 25% of this amount is recycled (Planetoscope 2012). On the other hand, the recycling and recovery rate of these plastic wastes is almost 100% in Switzerland, where the disposal of plastic waste is prohibited. Plastic waste collection and management have become an environmental, societal, and economic issue that needs to be mastered and resolved. However, these wastes could be exploited as a source of raw materials in the context of sustainable development (circular economy) (Planetoscope 2012).
The use of upgraded plastic waste in the development of eco-materials in the field of construction has grown exponentially over the last 2 decades. This period saw the emergence of composite mortars and concretes, plastic aggregates (Badache et al. 2018; Gu and Ozbakkaloglu 2016; Latroch et al. 2018; Saikia and de Brito 2012; Senthil Kumar and Baskar (2015b); Sharma and Bansal 2016; Sulyman et al. 2016), composite aggregates (Alqahtani et al. 2014, Alqahtani 2017a, b, c; Choi et al. 2005, 2009; Ge et al. 2013, 2014; Gouasmi et al. 2017; Zuccheratte et al. 2017), rubber aggregates (Akinyele et al. 2016; Rashad 2016), and many others (Adedayo Badejo et al. 2017; Debska and Licholai 2017a, b). Eco-materials are environmentally friendly products that meet the requirements of professionals in the construction sector (Building and Public Works), particularly those related to durability and to thermal and acoustic comfort.
The durability of a cementitious material is measured by its ability to withstand the attack and penetration of chloride ions that cause the corrosion of reinforcements in ferroconcrete structures (Adam Neville 2000). Corrosion is one of the main causes of deterioration of reinforced concrete constructions. Its process usually begins with the dissolution and decomposition of the metal, followed by the formation of ferrous ions. The hydrolysis of these ions leads to the acidification of the material and the formation of insoluble ferric hydroxides which give rust over time (Raharinaivo et al. 1998).
The diffusion of chloride ions into concrete is the result of complex mechanisms. Actually, the ion exchange between the aggressive diffusing solution and the interstitial solution in concrete plays an essential role (Stanish et al. 1997). The binding capacity of chloride ions depends on several factors, including the nature of cement, C3A aluminate content, compactness of concrete, and water-to-cement (W/C) ratio which decreases the porosity of the cementitious paste and thus makes ion penetration slower. Other species, such as mineral and polymeric additions, can affect the chemical fixation of chlorides.
Kou et al. (2009) found out that the partial substitution of natural aggregates by PVC waste materials allows increasing the resistance to chloride ion penetration. The penetration depth decreases linearly with the substitution rates, i.e., 5, 15, 30, and 45% PVC, by volume. Fraj et al. (2010) reported that the addition of dry aggregates of polyurethane foam in a concrete mixture gives a chloride penetration coefficient below the reference value. On the other hand, the addition of pre-wet aggregates of polyurethane foam leads to significant increases in the same coefficient. Benosman et al. (2011) observed that replacing cement with PET waste particles helps to reduce the rapid penetration of chlorine ions (RCPT). On the other hand, Ghernouti and Rabehi (2012) noted that the substitution of crushed sand by plastic bag waste, which is a low-density polyethylene (LDPE) material, decreases the penetration depth of chlorine ions. Similarly, Onuaguluchi and Panesar (2014) indicated that the rapid chloride permeability (RCPT) of concrete decreases by partially replacing 5, 10, and 15% by volume of natural fine aggregates with crumb rubber. Senhadji et al. (2015) found out that the partial substitution (30, 50, and 70% v) of natural aggregates (fine sand and aggregates of class 3/8) by PVC granules increases the resistance to penetration of chlorine ions. Omrane et al. (2016) made the same observation by reporting that PET-based mortar–polymer composites, when cement is replaced by PET granules at ratios equal to 2, 4, and 6% w, have a chloride ion penetration depth much lower than that of the reference mortar. Shanmugapriya and Helen Santhi (2017) indicated that the obtained rapid chloride permeability values decrease with the incorporation of high-density polyethylene (HDPE) waste in concrete, either as fine or coarse aggregates.
The present study focuses first on the evolution of the mechanical strength, capillary water absorption coefficient (sorptivity) and resistance to the penetration of chloride ions in composite mortars designed by substitution of the conventional aggregates, such as calcareous sand, by the composite material PET-siliceous sand composite, also called WPLA. It also tries to make a comparison with the unmodified mortar for the purpose of designing an industrial composite screed mortar resistant to the penetration of aggressive agents in a saline environment (5% NaCl). This is interesting for the protection of the environment, which fits in the framework of sustainable development. Some key substitution proportions are also investigated to determine the feasibility limits, which has not been done before, in the previous research (Alqahtani et al. 2014, 2017a; Choi et al. 2005, 2009; Debska and Licholai 2017b; Gouasmi et al. 2017; Zuccheratte et al. 2017). In addition, further information on the microstructure of the material was obtained using the XRD and SEM techniques.
Materials used
Cement
The cement used is type CPJ 42.5 CEM II/A, from the LCO plant of the HOLCIM-LAFARGE group, located in OGGAZ (Northwestern Algeria). This cement has a fineness of 4500 cm2/g, an absolute density equal to 3.09 g/cm3 and average compressive strengths of 22 MPa at 2 days and 48 MPa at 28 days. The chemical and mineralogical compositions of cement and its clinker are given in Tables 1 and 2, respectively.
Elaboration of the composite material waste PET lightweight aggregate (WPLA)
A new eco-friendly composite material is developed by mixing a siliceous sand-based composite material and waste PET bottles. The upgrading and recycling of such waste material turns out to be an interesting initiative for the production of a lightweight composite material which has the characteristics and properties that are required in the field of construction. This composite material should have 35% of its weight in recycled PET. On the other hand, this operation aims at protecting the global environment from plastic wastes.
A 1.5-l bottle of mineral water contains an average of 3.5 g of PET; this means that making 1 kg of composite material allows getting rid of about 100 used mineral water bottles. The experimental work consisted of conducting physico-mechanical and physico-thermal tests, and also assessing the durability of the prepared materials (Gouasmi 2012). These experiments required the use of more than 80 kg of WPLA composite aggregate. For economic reasons, siliceous sand was chosen, because it is a natural material found in abundance. Its ease of extraction and treatment and its mode of use make its cost per ton relatively low compared to the calcareous sand which is composed of treated silico-calcareous rocks, in addition to all the constraints of extraction, crushing, grinding, selection, and sieving.
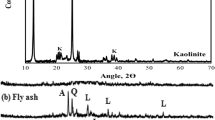
The development of the composite aggregate (WPLA) involves a semi-industrial process in a ready-to-use mortar production unit. Slabs with hardness close to that of natural rock were made. These slabs were prepared with siliceous sand (Ss), brought from the sand quarry of Sfisef, a small town in the Province (Wilaya) of Sidi Bel Abbès, along with used PET bottles of mineral water and soft drinks, after being washed and cut into small squares with side not exceeding 2.5 cm. The chemical composition of the siliceous sand used is given in Table 1. Figure 1 illustrates the microstructural XRD analysis results of siliceous sand, PET scrap, and WPLA composite aggregate. It should be noted that no chemical interaction was observed between the mineral compounds of sand (Ss) and the polymeric molecules (PET), which means that there is total absence of new compounds. These findings are in agreement with those reported by Zuccheratte et al. (2017).
The components of the mixture (siliceous sand and PET) were subjected to a heat treatment at a temperature not exceeding 290 °C, accompanied by a thorough mixing, at 50–60 revolutions per minute. At the end, the mixture undergoes a long stepwise cooling process; this should subsequently affect the grindability and hardness of the prepared slabs (Fig. 2a). It is useful to remember that this hardness is close to that of natural rock. These slabs then go through a semi-industrial grinding and sieving process to finally give several grain size classes, ranging from 0 to 2.7 mm. The material thus obtained is then called composite aggregate WPLA. The particle-size class 0/0.3 mm of the waste PET lightweight aggregate (WPLA) was used throughout this study for the fabrication of the composite mortars WPLAX. The main aggregate physical properties used in this study are presented in Table 3. The morphologies of PET and composite aggregate WPLA are illustrated in Figs. 2c and 2d. Figure 2d, representing the composite aggregate WPLA, shows that there is a perfect microstructural arrangement of the organic phase (PET) and mineral phase (siliceous sand). This allows the composite mortars WPLAX to develop interesting durability properties against chemical attack (Gouasmi et al. 2015), and particularly against the penetration of the material by chloride ions.
Calcareous sand (C S)
The calcareous sand (CS) used, which is replaced by the composite aggregate WPLA throughout this study, is a silico-calcareous sand from quarries belonging to the HASNAOUI group of companies, located in the region of Sidi Ali Benyoub, in the Wilaya of Sidi bel abbes. The chemical composition of calcareous sand (CS), its XRD spectrum, and physical properties are shown in Tables 1, 3 and Fig. 1, respectively.
The particle-size distributions of calcareous sand and composite aggregate WPLA were determined by laser by means of the MASTER SIZER 2000 type MALVERN. The materials used were analyzed by the XRD technique using a diffractometer powder (Bruker D8 Advance) and utilizing Cu Kα radiation. Moreover, the PET waste, WPLA composite aggregate, and WPLAX composite mortars were analyzed using a HITACHI TM-1000 SEM (Table Top Microscope).
Test methods
It was decided to make composite screed mortars from Mâtine cement (CPJ CEM II/A), and four mixtures by partially replacing calcareous sand, by weight, in the proportions 0, 25, 50, 75, and 100%, with the WPLA composite aggregate (Table 4). For each preparation, the mixtures were prepared in accordance with the recommendations of the standard ASTM C109 (2011). The workability of each type of mortar prepared was measured using the spreading table, in accordance with the standard ASTM C 1437 (2001).
The molds containing the samples were covered with a plastic film and stored in the laboratory’s maturation cabinet. After 24 h, the samples are demolded and then immersed in lime saturated water (ASTM C511 2006) at the temperature of 20 ± 2 °C, until the test age.
Mechanical strengths and density
Mechanical strength tests were performed, in accordance with the standard ASTM C109 (2011), to characterize the composite mortars (WPLAX) at different time intervals, i.e., 2, 7, and 28 days. The density of WPLAX composites was measured, in accordance with Gouasmi (2012) and Gouasmi et al. (2017).
The water capillary sorption test (sorptivity)
This test was performed according to Choi et al. (2009) and the AFPC-AFREM (1997) procedure. It allows measuring the rate of water absorption by capillary suction of unsaturated mortars placed in contact with water without any hydraulic pressure.
Before starting to measure the sorptivity, the specimens were pre-conditioned in the oven, at a temperature of approximately 105 °C, until reaching a constant mass. The water capillary sorption test was used to determine the absorption rate by capillary rise of a cubic specimen, of dimensions 50 × 50 × 50 mm3, placed on small supports in a tank containing water (Fig. 3), so that water could reach only 5 mm of the height of the test piece; the lateral faces of the specimen had previously been impregnated with an epoxy resin (Fig. 4). The increase in mass of the test piece was then measured as a function of time, i.e., at 1, 4, 9, 16, 25, 36, 49, and 64 min.
The capillary water absorption coefficient was then calculated using the following formula (Eq. 1):
where Q is the amount of water absorbed (cm3), A is the surface of the specimen in contact with water (cm2), t is the time expressed in seconds, and S is the sorptivity coefficient of the specimen (cm/s1/2).
Diffusion of chloride ions
The test samples used are cubic specimens (50 × 50 × 50 mm3), prepared at the TEKNACHEM physico-mechanical test laboratory, which located in the industrial zone of Sidi Bel Abbes (Algeria). The mortars were made in accordance with the recommendations of Standard EN 196-1 (2005).
The WPLAX mortar specimens underwent a 28-day cure, and then, the side faces were covered with epoxy resin to make them impermeable. Therefore, chloride ions’ penetration can occur only along one direction, through the bottom and top sides (Fig. 4). The samples were then introduced into a 5% concentrated NaCl solution.
To evaluate the durability of composite mortars against the penetration of chloride ions and to highlight the effect of the composite aggregate WPLA on the resistance to this penetration, it was decided to follow the evolution of the penetration depth of chloride ions at 7, 28, and 91 days. The solutions were changed every 7 days until the age of 28 days, then every 28 days until the age of 91 days.
For each test, the specimen was cut in half, through the two permeable faces, along the diffusion direction of chloride ions (Fig. 5). Then, a solution of silver nitrate AgNO3 was poured on each section, according to the standard UNI 7928 (1978). The permeable faces of the specimen then changed color; the white color represents the penetration depth of chloride ions. This depth was measured using a caliper, at several positions (Fig. 5). The penetration depth considered (X) is the average of all readings.
Results and discussion
Mechanical properties and density of WPLAX composite mortars
Table 5 shows that the compressive strength decreases with the increase in the substitution rate by the composite aggregate PET-siliceous sand (WPLA), especially at 28 days. On the other hand, at the young age (2 and 7 days), the compressive strength values are seen to evolve positively with the maturity and rate of substitution of sand with WPLA. It is clearly noted that:
-
At 2 days, the compressive strength values develop positively as compared to the unmodified mortar, for each proportion of incorporated WPLA (Table 5).
-
At 7 days, the compressive strength values experience alternating positive evolutions for WPLA25 and WPLA50 and negative ones for WPLA75 and WPLA100.
-
At 28 days, the compressive strength values evolve negatively for all the composite mortars containing composite aggregate WPLA as compared to the unmodified mortar WPLA0, which contains only natural aggregate (calcareous sand). These results corroborate those found by Choi et al. (2009), Saikia and de Brito (2012), Senthil Kumar and Baskar (2015a), Zuccheratte et al. (2017) and Alqahtani et al. (2017b).
Table 6 exhibits the flexural strength of composite mortars WPLAX. It can easily be seen from Table 6 that the evolution of the flexural strength varies with the kind of composite materials used. It is, also, noted that:
-
At 2 days, the flexural strength decreases with the incorporation of the composite aggregate; while the composite aggregate content increases, the flexural strength decreases.
-
At 28 days, substituting 25% of mortar by composite aggregate gives the highest strength among all mortars made, with a strength value reaching 5.23 MPa. This is followed successively by the strength of WPLA0 with 4.81 Mpa, WPLA50 with 4.52 MPa, WPLA75 with 4.17 Mpa, and, finally, WPLA100 with 3.97 Mpa.
Consequently, it can be stated that the WPLA improves the flexural strengths at rates which are defined for the desired maturities, i.e., at 7 and 28 days, for a proportion of 25%. This statement can be explicated by the elastic nature and the non-brittle characteristic of polyethylene terephthalate plastic particles under loading (Gouasmi et al. 2017).
In addition, it is easy to observe from Table 4 that the densities of composite mortars tend to decrease as the content of the WPLA rises. The larger the WPLA composite aggregate content, the lighter the resulting modified mortar (Alqahtani et al. 2017b, c; Gouasmi et al. 2017).
The water capillary sorption test
A simple and easy way to characterize the absorption kinetics of materials consists of measuring their absorptivity. The higher the sorptivity, the faster the material is likely to be invaded by the liquid in contact. Sorptivity is also a property that characterizes the porous material which absorbs water and allows its transmission by capillarity. Figure 6 shows the relationship between the cumulative absorption of WPLAX composite mortars and the square root of the elapsed time. It is important to note that the greatest water absorption value by capillarity is 0.524 g/cm2; it was observed in the mixture of control mortar. Incorporating the WPLA composite decreases the capillary water absorption, especially for the modified mortars with a 100% substitution rate; in this case, the capillary absorption decreases by 60% compared to that of control mortar.
Figure 7 shows the influence of the composite aggregate PET-silica sand on water absorptivity by composite mortar specimens. It is noted that sorptivity decreases considerably for the first substitution rate of calcareous aggregate by the composite WPLA; there is a 51% reduction for WPLA 25. For the other two composite mortars, i.e., WPLA 50 and WPLA 75, the decline in sorptivity is equal to 43%. Finally, this decrease is 65% for the composite mortar WPLA100 when compared to unmodified mortar (WPLA0). Zuccheratte et al. (2017) reported that water absorption decreases with the increase in the synthetic gravel substitution ratio and the water-to-cement (W/C) ratio. These findings are consistent with those reported by Zuccheratte et al. (2017) and Alqahtani et al. (2017b). On the other hand, Senthil Kumar et al. (2017) reported that water absorption and sorptivity of high impact polystyrene (HIPS) concrete specimens were higher than the control one. The water absorption and sorptivity increased with an increase in HIPS content.
The results mentioned above make it possible to conclude that the substitution of calcareous sand by the lightweight composite aggregate WPLA as well as the hydrophobic effect of the PET, which is inside the WPLA aggregate, both contribute to block the capillary pores by water-repellent effect. In addition, a reduction in porosity was detected near the inter-facial area between WPLA and the cement matrix (Fig. 8). The strong adhesion between the composite aggregate and cement paste contributes significantly to the reduction in sorptivity.
Therefore, it can be deduced that, for WPLA 25, two effects act simultaneously, namely the hydrophobicity of PET and the effect of the fillers (calcareous sand) that fill up the pores, since the particle-size distribution of calcareous sand is more continuous than that of the composite WPLA (Table 7). It is worth mentioning that the water-repellent effect of WPLA particles, containing PET plastic waste, is greater than that of pore filling by calcareous sand particles.
Finally, the composite aggregate (WPLA) contributes significantly to the impermeability of WPLAX composite mortars. This indicates that the WPLA aggregate provides improved durability to the screed mortars.
The results on the use of light aggregates based on plastic waste are consistent with those previously obtained by different research teams (Alqahtani et al. 2017b; Benazzouk et al. 2004; Benosman et al. 2017; Marzouk et al. 2007; Zuccheratte et al. 2017).
The relationship between the compressive strength and sorptivity coefficient of composite mortars is shown in Fig. 9. It is possible to maintain a polynomial relationship between sorptivity and the compressive strength with a correlation coefficient of about R2 = 0.7577. Regardless of the composite composition, it can be noted that an increase in the sorptivity coefficient is associated with an increase in the compressive strength. Irrespective of the compressive strength, the highest value of the sorptivity coefficient was measured in the composite WPLA0. The addition of the composite WPLA as a substitute for sand caused an improvement in the sorptivity coefficient of composite mortars. On the other hand, Choi et al. (2009) reported that the sorptivity coefficient of mortar-containing 25% of WPLA was 31% lower than that of the control mortar. However, this coefficient increased by 15% and 52% when levels of 50% and 75% of WPLA were used, respectively, in the mix (Choi et al. 2009).
Diffusion of chloride ions inside WPLAX composite mortars
Figure 10 allows showing that the penetration depth of chloride ions in mortar-containing the composite aggregate WPLA is smaller than that in the WPLA0 mortar, containing only conventional aggregate (calcareous sand); this was obtained for all immersion deadlines. This explains the influence of adding composite aggregate PET-silica sand on the microstructure of cementitious materials as well as on their permeability to chloride ions. The comparison of the results relative to the penetration depth of chloride ions in the different mortars allows saying that those containing the composite aggregate WPLA are more resistant to the penetration of chloride ions than those containing the calcareous aggregate alone. It was found that the 7-day penetration depths decreased by approximately 11.8, 22.3, 34.5, and 40.1%, respectively, for the WPLA25, WPLA50, WPLA75, and WPLA100 composite mortars with respect to the reference mortar WPLA0.
On the other hand, it was observed that, at 28 days, these depths decreased by approximately 14.8, 28.1, 36.5, and 41.9% for WPLA25, WPLA50, WPLA75, and WPLA100, respectively, as compared to WPLA0.
In addition, at 91 days, the penetration depth decreased by about 19.2, 41.9, 51.1, and 58.9% for the composite mortars WPLA25, WPLA50, WPLA75, and WPLA100, respectively, when compared to WPLA0.
In addition, it may be noted that, at 28 days, the penetration of chloride ions for WPLA100 remained unchanged, since the same values were observed at 91 days.
Therefore, one can say that adding the composite aggregate WPLA as a substitute for the calcareous aggregate increases the resistance of the composite mortars (WPLAX) to chloride ion penetration. It is worth noting that the higher the substitutions ratios, the more the composite mortars resist to the penetration of chloride ions. This is due to the presence of PET (hydrophobic, 0% water absorption) which blocks the passage of chloride ions. The findings thus obtained are in good agreement with those obtained by Benosman et al. (2008), Kou et al. (2009), Ghernouti and Rabehi (2012), Senhadji et al. (2015) and Omrane et al. (2016).
From this study, it can be observed that the mortars containing the composite aggregate WPLA develop a better resistance to the penetration of chloride ions (Fig. 10). Therefore, these mortars can be recommended as protective materials against reinforcement corrosion in various building structures.
Apparent chloride ion diffusion coefficients
The penetration depth of chloride ions follows the second law of Fick (Crank 1956), under the conditions of diffusion in a non-stationary regime. The mathematical solution of Fick’s second law is given by the following equation (Eq. 2):
Here, C: concentration of chloride ions, X: penetration depth of chloride ions, C0: concentration of chloride ions on the exposed surface of mortars, t: immersion time in the solution, D: chloride ion diffusion coefficient, and erf: the error function.
An approximation of formula (2) is given by the following expression (Eq. 3) (Collepardi et al. 1972):
The graphs \(x - \sqrt t\) (Fig. 11) have negative intersections on the axis \(\sqrt t\)—depending on the type of composite mortar and the ratio WPLA/natural aggregate. This means that the graphs \(x - \sqrt t\) do not follow Fick’s second law because of the reaction of chloride ions with certain cement hydrates (Gouasmi 2012). In addition, our results are consistent with those reported by Benosman et al. (2008) and Omrane et al. (2016). Therefore, the following equation (Eq. 4) may be recommended to be used for the evaluation of the apparent coefficient of chloride ion diffusion (Goto et al. 1979). The use of formula 4 (Eq. 4) instead of formula 3 (Eq. 3) has been widely justified and discussed by the research team of Collepardi et al. (1972) and Goto et al. (1979):
Da: apparent diffusion coefficient of chloride ions; it may be calculated from the slope of the line, k: empirical constant.
The apparent diffusion coefficient of chloride ions in each specimen was obtained from the slopes of the graphs \(x-\sqrt{t}\), in Fig. 11a–e.
The above-mentioned apparent diffusion coefficient decreased with the substitution rate of the composite aggregate WPLA in the mixture. Thus, for control mortar WPLA0, containing the conventional aggregate only, this coefficient was found equal to 1.125 × 10−8 cm2/s. However, this value was 0.685 × 10−8 cm2/s for WPLA25, 0.286 × 10−8 cm2/s for WPLA50, 0.196 × 10−8 cm2/s for WPLA75, and 0.122 × 10−8 cm2/s for WPLA100, as shown in Fig. 12.
It was found that the apparent diffusion coefficients of chloride ions in mortars, containing the composite aggregate WPLA, dropped considerably with respect to WPLA0. For the first substitution rate, this coefficient decreased by 40% for WPLA 25, 75% for WPLA50, and 83% for WPLA75; however, for WPLA100, the drop was much more important, around 90%. This is probably due to the fact that the composite aggregate WPLA particles block or distract the transfer of chloride ions within the matrix of WPLAX mortars due to the impermeability of PET (water absorption of PET = 0%).
On the other hand, Alqahtani et al. (2014) reported that the chloride ion permeability (Rapid Chloride Permeability Test or RCPT) of concrete based on synthetic recycled plastic was 3% and 5% lower than that of control concrete and lightweight concrete, respectively, at 100% replacement rate of natural aggregate by the recycled plastic aggregate (RPA) based on linear low-density polyethylene (LLDPE). According to these authors, RPA particles allow cement to bond into the surface of the aggregate, thus blocking or distracting the transfer of chloride ions (Alqahtani et al. 2014).
In addition, Alqahtani (2017a) indicated that the charge passing through the concrete samples, containing RPA based on recycled plastic PET, was lower than that observed in normal concrete and conventional lightweight concrete. Alqahtani (2017a) suggested that the chloride ion penetrability for RP3F1C, RP3F2C, RP3F3C, RP4F1C, RP4F2C, and RP4F3C decreased by 30, 66, 36, 36, 69, and 40%, respectively, as compared to normal concrete.
The results obtained in this study are significantly better than those of Alqahtani et al. (2014) and Alqahtani et al. (2017c). Our results showed a decrease in the penetrability of chloride ions of the order of 90% for a 100% substitution rate of natural aggregate by WPLA lightweight composite aggregates based on PET plastic waste in screed mortars.
In general, the apparent diffusion coefficient tends to decrease with the increase in the composite aggregate content. Thus, the higher the substitution rate, the lower the apparent diffusion coefficient. It then becomes clear, from the data obtained, that the resistance to chloride ion penetration in mortars modified by the composite aggregate is much greater than that in the unmodified mortar (WPLA0). The results obtained make it possible to deduce that mortars containing the composite aggregate PET-siliceous sand (WPLA) can be used for the protection of reinforcements in reinforced concrete structures. In addition, the recycling and valorization of plastic bottles certainly help to protect the nature from this type of pollution.
The relationship between the compressive strength and the apparent chloride ion diffusion coefficient of the composite mortars is illustrated in Fig. 13. One can easily note a linear relationship between the apparent chloride ion diffusion coefficient and the compressive strength, with a correlation coefficient of about R2 = 0.85. In this study, the diffusion coefficient decreased as the compressive strength went down. This may be attributed to the waste PET lightweight composite aggregate particles that obturate the transfer of chloride ions.
The relationship between sorptivity and the apparent chloride ion diffusion coefficients of the composite mortars is shown in Fig. 14. A polynomial correlation was found between sorptivity and the apparent diffusion of chloride ions; it is represented by the equation y = 6.4497 x2 − 4.0417 x + 4.521, where y is the sorptivity and x is the apparent chloride ion diffusion coefficient. This polynomial seems to give the best value of the correlation coefficient R2 (R2 = 80.16%); this value is greater than 0.80. The addition of WPLA as sand substitute led to an improvement in the apparent chloride ion diffusion coefficient of the composite mortars. This may be attributed to the WPLA aggregate particles that prevent the transfer of aggressive solution (chloride ions). It can be concluded, therefore, that lower sorptivity means lower chloride ion diffusion. This finding is in agreement with that of Ganesan et al. (2008) and Siad et al. (2014), who found a good correlation between sorptivity and diffusivity. According to GCI (2009), chloride ions in solution are first “drawn” into the pores along with the water absorbed. Beyond the absorption zone, chloride penetration occurs by diffusion.
Correlation between the durability and mechanical properties
Figure 15 summarizes the physico-mechanical and durability properties of the WPLAX composite mortars. Finally, it can be said that the waste PET lightweight composite aggregate WPLA can be used for the replacement of natural aggregates in screed mortars, as well as in mortars and concretes in general. The use of WPLA aggregate-based composite mortars in the construction and building sector is very attractive from the ecological or economic point of view; these mortars are also highly recommended for the protection of structural structures containing reinforcement and even for the repair of various reinforced concrete structures exposed to severe chemical attack, such as chloride ion penetration (saline environment, seas, oceans, etc.). In addition, this type of composite mortar is able to withstand even the harmful effects of de-icing salts.
Conclusions
The effects of replacing conventional sand by WPLA composite aggregates on the physico-mechanical and durability properties of composite mortars are summarized in Fig. 15. Based on the results obtained, it can be concluded that WPLA composite aggregate contributes to:
-
the good adhesion between the PET polymer phase and the mineral phase (siliceous sand); it is worth noting that there is no chemical interaction between the compounds of these two phases.
-
The compressive strength of WPLAX decreases when the substitution rate of the conventional aggregate by the light composite aggregate increases.
-
Capillary pores are blocked due to the water-repellent effect of PET (PET is impervious to water; its water absorption coefficient is zero).
-
The permeability (sorptivity) of composite screed mortars decreases. Thus, a reduction of 51% was found for WPLA25, 43% for both WPLA50 and WPLA75, and 65% for WPLA100 with respect to WPLA0.
-
The apparent coefficient of chloride ion diffusion decreases. There was a reduction of up to 40% for WPLA 25, 75% for WPLA50, and 83% for WPLA75. For WPLA100, this drop was much greater, since it was around 90% with respect to control mortar WPLA0.
-
The penetration of chloride ions decreased significantly, giving composite mortars protective characteristics for structural structures containing reinforcement. This should help to reduce the rate of corrosion, especially in environments with high salt concentrations.
The adhesion test of composite mortar as a substrate on concrete proves to be very useful for a future study that could be the subject of a research project. This is another innovative technique for the realization and application of WPLAX composite eco-materials in the field of construction. Moreover, this approach allows the management and recycling of PET plastic wastes; it helps to preserve the environment and make the production of modified mortars more profitable.
References
2iE. (2013). Collection Actes de conférences, “ECOMATERIAUX de construction: Pilier de la croissance verte en Afrique?,” Conférence Internationale, edn. Sud Sciences et Technologies. Ouagadougou, Burkina Faso, 10–12 Juin.
Adam Neville, M. (2000). Propriétés des Bétons. Paris: Traduit par le CRIB, Editions Eyrolles.
Adedayo Badejo, A., Adebola Adekunle, A., Olusola Adekoya, O., Julius Ndambuki, M., Kehinde Kupolati, W., Babatunde Bada, S., et al. (2017). Plastic waste as strength modifiers in asphalt for a sustainable environment. African Journal of Science, Technology, Innovation and Development, 9(2), 173–177. https://doi.org/10.1080/20421338.2017.1302681.
AFPC-AFREM. (1997). Méthodes recommandées pour la mesure des grandeurs associées à la durabilité. Compte rendu des Journées Techniques AFPC-AFREM “Durabilité des Bétons”, 11–12 décembre, Toulouse, France.
Akinyele, J. O., Salim, R. W., & Kupolati, W. K. (2016). Effect of rubber crumb on the microstructural properties of concrete. African Journal of Science, Technology, Innovation and Development, 8, 467–474.
Alqahtani, F. K. (2017a). Recycled plastic aggregate for use in concrete, Patent No. US 2017/0088463 A1. Washington, U.S. Patent and Trademark Office.
Alqahtani, F. K., Ghataora, G., Iqbal Khan, M., & Dirar, S. (2017b). Novel lightweight concrete containing manufactured plastic aggregate. Construction and Building Materials, 148, 386–397. https://doi.org/10.1016/j.conbuildmat.2017.05.011.
Alqahtani, F. K., Iqbal Khan, M., & Ghataora, G. (2014). Synthetic aggregate for use in concrete, Patent No. US 8,921,463 B1, Washington, DC: 634 U.S. Patent and Trademark Office.
Alqahtani, F. K., Iqbal Khan, M., Ghataora, G., & Dirar, S. (2017c). Production of recycled plastic aggregates and its utilization in concrete. Journal Materials of Civil Engineering, 29(4), 04016248. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001765. (ASCE).
ASTM C1437-01. (2001). Standard test method for flow of hydraulic cement mortar. West Conshohocken, USA: American Society for Testing and Materials.
ASTM C511-06. (2006). Standard specification for mixing rooms, moist cabinets, moist rooms, and water storage tanks used in the testing of hydraulic cements and concretes. West Conshohocken, USA: American Society for Testing and Materials.
ASTM C109/C109M. (2011). Standard test method for compressive strength of hydraulic cement mortars (using 2-in. or [50-mm] cube specimens). West Conshohocken, USA: American Society for Testing and Materials.
Badache, A., Benosman, A. S., Senhadji, Y., & Mouli, M. (2018). Thermo-physical and mechanical characteristics of sand-based lightweight composite mortars with recycled high-density polyethylene (HDPE). Construction and Building Materials, 163, 40–52. https://doi.org/10.1016/j.conbuildmat.2017.12.069.
Benazzouk, A., Douzane, O., & Queneudec, M. (2004). Transport of fluids in cement–rubber composites. Cement & Concrete Composites, 26, 21–29. https://doi.org/10.1016/S0958-9465(02)00119-1.
Benosman, A. S., Mouli, M., Taibi, H., Belbachir, M., & Senhadji, Y. (2011). Resistance of polymer (PET)—mortar composites to aggressive solutions. International Journal of Engineering Research in Africa, 5, 1–15. https://doi.org/10.4028/www.scientific.net/JERA.5.1.
Benosman, A. S., Mouli, M., Taibi, H., Belbachir, M., Senhadji, Y., Bahlouli, I., et al. (2017). The chemical, mechanical and thermal properties of PET-Mortar composites containing waste PET. Environmental Engineering and Management Journal, 16(7), 1489–1505. https://doi.org/10.30638/eemj.2017.162.
Benosman, A. S., Taibi, H., Mouli, M., Belbachir, M., & Senhadji, Y. (2008). Diffusion of chloride ions in polymer–mortar composites (PET). Journal of Applied Polymer Science, 110(3), 1600–1605. https://doi.org/10.1002/app.28587.
Choi, Y. W., Moon, D. J., Chung, J. S., & Cho, S. K. (2005). Effects of waste PET bottles aggregate on the properties of concrete. Cement Concrete Research, 35, 776–781. https://doi.org/10.1016/j.cemconres.2004.05.014.
Choi, Y. W., Moon, D. J., Kim, Y. J., & Lachemi, M. (2009). Characteristics of mortar and concrete containing fine aggregate manufactured from recycled waste polyethylene terephthalate bottles. Construction and Building Materials, 23, 2829–2835. https://doi.org/10.1016/j.conbuildmat.2009.02.036.
Collepardi, M., Marcialis, A., & Turriziani, R. (1972). The penetration of de-icing agents in cement pastes. IL Cemento, 69, 143–150.
Crank, J. (1956). The mathematics of diffusion (p. 347). Oxford: Clarendon Press.
Debska, B., & Licholai, L. (2017a). Analysis of bending strength of resin mortars that are at risk of long-term exposure to environmental corrosives. IOP Conference Series: Earth Environmental Science, 95(1–9), 042015. https://doi.org/10.1088/1755-1315/95/4/042015.
Debska, B., & Licholai, L. (2017b). Environmental factors affecting the strength characteristics of modified resin mortars. IOP Conference Series: Earth Environmental Science, 95(1–7), 042016. https://doi.org/10.1088/1755-1315/95/4/042016.
EN 196-1. (2005). Methods of testing cement—Part 1: Determination of strength. Brussels: European Committee for Standardization, CEN.
Fraj, A. B., Kismi, M., & Mounanga, P. (2010). Valorization of coarse rigid polyurethane foam waste in lightweight aggregate concrete. Construction and Building Materials, 24, 1069–1077. https://doi.org/10.1016/j.conbuildmat.2009.11.010.
Ganesan, K., Rajagopal, K., & Thangavel, K. (2008). Rice husk ash blended cement: assessment of optimal level of replacement for strength and permeability properties of concrete. Construction and Building Materials, 22, 1675–1683. https://doi.org/10.1016/j.conbuildmat.2007.06.011.
GCI-714. (2009). Durabilité et Réparations du Béton. Sherbrooke: Université de Sherbrooke.
Ge, Z., Huang, D., Sun, R., & Gao, Z. (2014). Properties of plastic mortar made with recycled polyethylene terephthalate. Construction and Building Materials, 73, 682–687. https://doi.org/10.1016/j.conbuildmat.2014.10.005.
Ge, Z., Sun, R., Zhang, K., Gao, Z., & Li, P. (2013). Physical and mechanical properties of mortar using waste polyethylene terephthalate bottles. Construction and Building Materials, 44, 81–86. https://doi.org/10.1016/j.conbuildmat.2013.02.073.
Ghernouti, Y., & Rabehi, B. (2012). Strength and durability of mortar made with plastics bag waste (MPBW). International Journal of Concrete Structures and Materials, 6(3), 145–153. https://doi.org/10.1007/s40069-012-0013-0.
Goto, S., Tsunetani, M., Yanagida, H., & Kondo, R. (1979). Diffusion of chloride ion in hardened cement paste. Yogyo-Kyokai-Shi, 87(3), 126–133. https://doi.org/10.2109/jcersj1950.87.1003_126.
Gouasmi, M. T. (2012). Effet d’agrégats légers à base de polytéréphtalate d’éthylène sur les propriétés des mortiers. Oran: Mémoire de Magister, Université d’Oran 1.
Gouasmi, M. T., Benosman, A. S., Taibi, H., Belbachir, M., & Senhadji, Y. (2015). Effect of a composite aggregate on the durability of mortars. Journal of Chemistry and Materials Research, 3, 26–31.
Gouasmi, M. T., Benosman, A. S., Taïbi, H., Kazi Tani, N., & Belbachir, M. (2017). Destructive and non-destructive testing of an industrial screed mortar made with lightweight composite aggregates WPLA. International Journal of Engineering Research in Africa, 33, 140–158. https://doi.org/10.4028/www.scientific.net/JERA.33.140.
Gu, L., & Ozbakkaloglu, T. (2016). Use of recycled plastics in concrete: A critical review. Waste Management, 51, 19–42. https://doi.org/10.1016/j.wasman.2016.03.005.
Kou, S. C., Lee, G., Poon, C. S., & Lai, W. L. (2009). Properties of lightweight aggregate concrete prepared with PVC granules derived from scraped PVC pipes. Waste Management, 29, 621–628. https://doi.org/10.1016/j.wasman.2008.06.014.
Latroch, N., Benosman, A. S., Bouhamou, N.-E., Senhadji, Y., & Mouli, M. (2018). Physico-mechanical and thermal properties of composite mortars containing lightweight aggregates of expanded polyvinyl chloride. Construction and Building Materials, 175, 77–87. https://doi.org/10.1016/j.conbuildmat.2018.04.173.
Marzouk, O. Y., Dheilly, R. M., & Queneudec, M. (2007). Valorisation of post-consumer plastic waste in cementitious concrete composites. Waste Management, 27, 310–318. https://doi.org/10.1016/j.wasman.2006.03.012.
Omrane, M., Benosman, A. S., Mouli, M., & Senhadji, Y. (2016). Use of thermoplastic polymer in mortar composites to improve its chloride penetration resistance. International Journal of Engineering Research in Africa, 22, 33–44. https://doi.org/10.4028/www.scientific.net/JERA.22.33.
Onuaguluchi, O., & Panesar, D. K. (2014). Hardened properties of concrete mixtures containing pre-coated crumb rubber and silica fume. Journal of Cleaner Production, 82, 125–131. https://doi.org/10.1016/j.jclepro.2014.06.068.
Planetoscope. (2012). Statistiques: Production mondiale de plastique. https://www.planetoscope.com/petrole/989-production-mondiale-de-plastique.html. Accessed 2 Jan 2018.
Raharinaivo, A., Arliguie, G., Chaussadent, T., Grimaldi, G., Pollet, V., & Taché, G. (1998). La corrosion et la protection des aciers dans le béton, Ed. Marne-la-Vallée: Presse de l’école nationale des ponts et chaussées (ENPC).
Rashad, A. M. (2016). A comprehensive overview about recycling rubber as fine aggregate replacement in traditional cementitious materials. International Journal of Sustainable Built Environment, 5, 46–82. https://doi.org/10.1016/j.ijsbe.2015.11.003.
Saikia, N., & de Brito, J. (2012). Use of plastic waste as aggregate in cement mortar and concrete preparation: A review. Construction and Building Materials, 34, 385–401. https://doi.org/10.1016/j.conbuildmat.2012.02.066.
Senhadji, Y., Escadeillas, G., Benosman, A. S., Mouli, M., Khelafi, H., & Ould Kaci, S. (2015). Effect of incorporating PVC waste as aggregate on the physical, mechanical, and chloride ion penetration behavior of concrete. Journal of Adhesion Science and Technology, 29(7), 625–640. https://doi.org/10.1080/01694243.2014.1000773.
Senthil Kumar, K., & Baskar, K. (2015a). Development of ecofriendly concrete incorporating recycled high-impact polystyrene from hazardous electronic waste. Journal of Hazardous, Toxic, and Radioactive Waste, 19(3), 04014042-1. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000265.
Senthil Kumar, K., & Baskar, K. (2015b). Recycling of E-plastic waste as a construction material in developing countries. Journal of Material Cycles and Waste Management, 17, 718–724. https://doi.org/10.1007/s10163-014-0303-5.
Senthil Kumar, K., Premalatha, P. V., & Baskar, K. (2017). Evaluation of transport properties of concrete made with E-Waste plastic. Journal of Testing and Evaluation, 45(5), 1849–1853. https://doi.org/10.1520/JTE20160008.
Shanmugapriya, M., & Helen Santhi, M. (2017). Strength and chloride permeable properties of concrete with high density polyethylene wastes. International Journal of Chemical Sciences, 15(1), 108–116.
Sharma, R., & Bansal, P. P. (2016). Use of different forms of waste plastic in concrete—a review. Journal Cleaner Production, 112, 473–482. https://doi.org/10.1016/j.jclepro.2015.08.042.
Siad, H., Mesbah, H. A., Mouli, M., Escadeillas, G., & Khelafi, H. (2014). Influence of mineral admixtures on the permeation properties of self-compacting concrete at different ages. Arabian Journal of Science Engineering, 39, 3641–3649. https://doi.org/10.1007/s13369-014-1055-1.
Stanish, K.D., Hooton, R.D. & Thomas, M.D.A. (1997). Testing the Chloride Penetration Resistance of Concrete: A Literature Review. FHWA Contract DTFH61-97-R-00022 “Prediction of Chloride Penetration in Concrete”, University of Toronto, Toronto, Ontario, Canada.
Sulyman, M., Haponiuk, J., & Formela, K. (2016). Utilization of recycled polyethylene terephthalate (PET) in engineering materials: A review. International Journal of Environmental Science and Development, 7(2), 100–108. https://doi.org/10.7763/IJESD.2016.V7.749.
UNI 7928, (1978). Concrete-Determination of the Ion Chloride Penetration. Ente Nazionale Italiano Di Unificazione-UNI, Milano, piazza A. Diaz, 2, December.
Zuccheratte, A. C. V., Freire, C. B., & Lameiras, F. S. (2017). Synthetic gravel for concrete obtained from sandy iron ore tailing and recycled polyethyltherephtalate. Construction and Building Materials, 151, 859–865. https://doi.org/10.1016/j.conbuildmat.2017.06.133.
Acknowledgements
We would like to acknowledge the financial contribution of the Ministry of Higher Education within the framework of the Algerian project CNEPRU B00L01UN310120130068, as well as the HASNAOUI Group of Companies, TEKNACHEM Algeria, and the late Ahmed Taleb. We would like also to extend our deepest thanks to Professor M. MOULI, team leader at the LABMAT laboratory at ENP-Oran Maurice Audin, as well as to Dr. Y. SENHADJI and Dr. N. KAZI TANI.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Gouasmi, M.T., Benosman, A.S. & Taïbi, H. Improving the properties of waste plastic lightweight aggregates-based composite mortars in an experimental saline environment. Asian J Civ Eng 20, 71–85 (2019). https://doi.org/10.1007/s42107-018-0089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42107-018-0089-1