Key summary points
To determine the diagnostic cut-offs and the prevalence of sarcopenia in India.
AbstractSection FindingsIndians have lower muscle strength and muscle mass than Caucasians. The prevalence of ‘probable sarcopenia’, ‘sarcopenia’, and ‘severe sarcopenia’ is 14.6%, 3.2%, and 2.3%, respectively; corresponding values are higher using well-established Western cut-offs.
AbstractSection MessageIndigenous and not Western cut-offs should be used to define sarcopenia in Indians.
Abstract
Purpose
Comprehensive data on diagnosis and prevalence of sarcopenia in India are lacking. The present study was undertaken to determine cut-offs for low muscle strength (MS) and low muscle mass (MM), and find out the prevalence of sarcopenia in Indians.
Methods
Apparently healthy individuals aged ≥ 20 years with no prior history of any co-morbidities were recruited from community by door-to-door survey. Participants eligible for study underwent blood sampling. Individuals identified as having biochemical abnormalities that could potentially affect MS and MM were excluded. Enrolled participants underwent DEXA. Muscle mass, MS, and physical performance were expressed as appendicular skeletal muscle index (ASMI), dominant handgrip strength (HGS), and usual gait speed (GS), respectively. Cut-offs for low MS and MM were defined as HGS and ASMI 2SD < mean of young reference population (20–39 years). A GS ≤ 0.8 m/s defined poor physical performance. Using them, the prevalence of sarcopenia was estimated as per EWGSOP2 recommendations.
Results
After exclusion, 804 participants were enrolled (mean age = 44.4 years). Peak HGS, ASMI, and GS were achieved in the 3rd/4th decades. Muscle strength/mass was lower than Caucasians. A HGS < 27.5 kg (males)/18.0 kg (females) and an ASMI < 6.11 kg/m2 (males)/4.61 kg/m2 (females) defined low MS and MM, respectively. Accordingly, prevalence of ‘probable sarcopenia’, ‘sarcopenia’, and ‘severe sarcopenia’ was 14.6%, 3.2%, and 2.3%, respectively. Corresponding values were higher when European cut-offs were used. Only serum testosterone positively predicted HGS/ASMI/GS in males.
Conclusions
Indians have low MS/MM, and hence, indigenous and not Western cut-offs should be used to define sarcopenia in Indians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with a progressive loss of skeletal muscle mass and strength—a phenomenon called ‘sarcopenia’. It is a “progressive and generalised skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability, and mortality” [1]. Till the dawn of the twenty-first century, sarcopenia was regarded as an age-related decline in muscle mass with little or no emphasis on muscle strength and function [2]. It was in 2010 that the European Working Group on Sarcopenia In Older People (EWGSOP) laid down a proper operational definition of sarcopenia and recommended the presence of both low muscle mass and low muscle function (strength or performance) for its diagnosis [3]. Still, low muscle mass remained the cornerstone for the diagnosis of sarcopenia. However, revised guidelines were published by the EWGSOP2 in 2018 wherein muscle strength was given the upper hand as strength is a better predictor of adverse outcomes than muscle mass [1].
The prevalence of sarcopenia in the community varies, ranging from as low as 1.6% [4] to as high as 36.6% [5] amongst healthy aging adults. The marked variation can be explained on the basis of the population being studied and the cut-offs used to define the three components of sarcopenia. EWGSOP recommends the use of normative (healthy young adult) rather than any pre-specified reference population, with cut-off points at two standard deviations (SD) below the mean reference value [3]. There is a dearth of comprehensive data on the diagnosis and prevalence of sarcopenia in India. Most of the available literature is based on Caucasian cut-offs [6]. A few available studies that have developed indigenous cut-offs have concentrated only on low muscle mass [7, 8]. One such study by Marwaha et al. has reported a prevalence of low muscle mass of 15% among healthy Indian females [7].
Hence, the present study was undertaken to determine the cut-offs for defining low muscle strength and low muscle mass, and to find out the prevalence of sarcopenia as per the latest EWGSOP2 recommendations in a group of ostensibly healthy Indian adults randomly chosen from the community.
Materials and methods
The index study was a part of the Chandigarh Urban Bone Epidemiological Study (CUBES), an observational cross-sectional study conducted in Chandigarh, wherein ostensibly healthy adult volunteers were recruited from the community by door-to-door survey over a period of two and half years (December 2016–June 2019). Chandigarh was specifically chosen for the study as its per capita income ranks fourth amongst all the states and Union Territories of India. All selected participants were duly pre-informed about the study and written informed consent was obtained from them. The study was approved by the Institute Ethical Committee, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
Details of the CUBES have been published elsewhere [9]. In short, apparently healthy individuals aged 20 years and above were recruited in the study. Individuals were chosen from four sectors of Chandigarh; these sectors were in turn selected by simple random sampling. Houses within each sector were chosen by systematic random sampling, selecting every fifth house from a random starting point. Household members eligible for enrollment were selected using the ‘Kish Selection Method’ [10]. A pre-prepared proforma comprising of questions on demography, menstrual history, co-morbidities, addictions, and drug intake was filled for the selected individual. In addition, a dietary history was obtained using the 24-h dietary recall method [11] and physical activity assessed using the Global Physical Activity Questionnaire (GPAQ) [12]. Participants eligible for the study underwent blood sampling after an overnight fast. Laboratory investigations included hemoglobin, creatinine, bilirubin, alanine transaminase, aspartate transaminase, serum albumin, total calcium, inorganic phosphorous, alkaline phosphatase, fasting blood glucose, glycated hemoglobin, testosterone, thyroid function test, 25-hydroxyvitamin D, intact parathyroid hormone, and IgA tissue transglutaminsase [IgA tTg) antibody. Volunteers without obvious biochemical abnormalities underwent dual-energy X-ray absorptiometry (DEXA) scan using the HOLOGIC Discovery A (QDR 4500; Hologic, Inc., Bedford, MA) scanner for assessment of body composition. All DEXA scans were performed by a dedicated, International Society of Clinical Densitometry (ISCD)-certified technician. Quality control procedures were carried out in accordance with the manufacturer’s recommendations. A thorough physical examination and anthropometry were performed prior to DEXA scan. Height of the participant was measured three times by a standard stadiometer to the nearest centimeter and the mean of the three readings was taken as the final height. Similarly, weight was measured three times using a digital weighing machine to the nearest of 0.1 kg and the mean of the three readings was considered as the final weight. The accuracy of the weighing machine was checked every day using an ISI standardized weight of 5 kg. Finally, body mass index (BMI) was calculated using formula weight (in kg)/height (in meter)2.
Handgrip strength was measured in each participant using the Jamar Plus Digital Hand Dynamometer (Jamar®, Patterson Medical). Participants were asked to sit in a standard chair with the shoulder adducted and neutrally rotated, the elbow flexed at right angle, forearm being neutral, and wrist at 0º–30º of dorsiflexion. The investigator (RP) demonstrated the use of the dynamometer before handing it over to the participants. Handgrip strength was measured six times, three in each arm. The volunteers were advised to press on the handle as tightly as possible for 3–5 s each and every time; the maximum reading obtained from each arm was reported as the final result. A rest period of at least 1 min was given between two consecutive measurements on the same arm. The instrument was calibrated every year as per the manufacturer’s guidelines.
Usual gait speed was measured using a 4-min walk test. A distance of 4 m was marked on the floor. The study participants were advised to walk at a comfortable speed from the start point to the stop point. The time taken to traverse the distance was measured using a stopwatch. Each participant was given two walking trials and the average of the two gait speeds (in m/s) was considered for final analysis.
Inclusion criteria comprised of all participants chosen by ‘Kish Selection Method’ who were willing to provide written informed consent for the study. Exclusion criteria were exhaustive and were applicable at each and every stage of the survey: (a) At the time of initial proforma filling, individuals with a history of hepatic, renal, neoplastic, respiratory, rheumatological, gastrointestinal, dermatological, endocrine (notably diabetes mellitus, hyperthyroidism, Cushing’s syndrome, hyperparathyroidism), systemic infective disorders, chronic drug intake (especially steroids, statins, complementary and alternative medications, and protein supplements), addictions, total calorie intake < 2100 kcal/day (minimum required calorie level for a healthy and active life as proposed by Indian Council of Medical Research) [13], physical inactivity (defined as < 250 MET-minutes/week) [14], and contraindications to DEXA scan (pregnancy, implant placement) were excluded at the outset; (b) Following biochemical investigations, participants identified as having anemia (as per WHO definition) [15], renal dysfunction (estimated glomerular filtration rate calculated by CKD-EPI formula < 90 ml/min/1.73 m2), low serum albumin (< 3.5 gm/dl), diabetes mellitus (as per ADA definition) [16], hyperthyroidism (subclinical or overt), overt hypothyroidism, hypercalcemia, 25-hydroxyvitamin D < 10 ng/ml, low serum testosterone in males (defined as testosterone levels below the lower limit of age-specific reference range) [17, 18], and elevated IgA tTg antibody (> 10 U/ml) were excluded prior to DEXA scan. Participants with pre-diabetes and subclinical hypothyroidism were, however, not excluded; (c) Following anthropometry, participants with a BMI < 18.5 kg/m2 were excluded.
Hemoglobin was estimated using Coulter LH 780 Automated Analyzer (Beckman Coulter, Inc., Brea, CA, USA). Creatinine, liver function test, serum albumin, total calcium, inorganic phosphorous, and fasting blood glucose were measured using Modular P800 Analyzer (Roche Diagnostics, Mannheim, Germany). HbA1c was measured using Bio-Rad D10 analyzer (DCCT standardized). Serum testosterone, thyroid function test, 25-hydroxyvitamin D, and iPTH were measured by electrochemiluminescence using Elecsys 2010 Analyzer (Roche Diagnostics, Mannheim, Germany). IgA tTg antibody was measured using fluoroenzyme assay.
Statistical analysis
Sample size was calculated using the formula n = 4pq/l2, where p = population proportion of low muscle mass, q = 1-p, and L = allowable error. For this study, L was presumed to be 20% of p giving a power of (1-L), i.e., 80% to study. p was taken as 15% based on the prevalence of low muscle mass in females according to the study of Marwaha et al. [7]. This yielded a value of 567. Keeping in mind the ‘design effect’, we had applied a correction factor of 1.2, and thus, the sample size after correction came out to be 680. The final sample size was kept as 750 to account for non-responders.
Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS) 23.0 software program (SPSS Inc., Chicago, IL, USA). Normality of data was checked using Kolmogorov–Smirnov test. Muscle mass was assessed using appendicular skeletal muscle mass index (ASMI) which was calculated as the sum of lean mass at arms and legs in kilograms divided by square of height in meters. Muscle strength was expressed in terms of dominant handgrip strength. All participants were divided into 10-year age groups. Appendicular skeletal muscle index, dominant handgrip, and usual gait speed in each age group were reported as mean ± standard deviation (SD). Comparisons of HGS, ASMI, and GS between males and females were made using Independent-Student T Test. HGS and ASMI of the study participants were compared with Caucasian counterpart using well-established NHANES data [19, 20]. For the purpose of generating cut-offs, individuals belonging to the 20–39 years age group were considered as the young reference population. Based on the EWGSOP recommendation, cut-off for low muscle strength was defined as dominant HGS two SD below the mean of young reference population. Similarly, low muscle mass was defined as ASMI two SD below the mean of young reference population [3]. Cut-offs were sex-specific. Usual gait speed ≤ 0.8 m/s defined low physical performance [1, 3, 21, 22]. Based on these cut-offs, the prevalence of probable sarcopenia, sarcopenia, and severe sarcopenia was estimated [1]. Likewise, prevalence was established based on the Western (as mentioned in the EWGSOP 2010 consensus statement) [3, 23, 24], EWGSOP2 (2018) [1], International Working Group on Sarcopenia (IWGS) [25], and Asian Working Group for Sarcopenia (AWGS 2014 and 2019) guidelines [22, 26]. Finally, correlations between biochemical parameters and components of sarcopenia were made using Pearson/Spearman correlation followed by multiple linear regression analysis with backward elimination. A p value < 0.05 was considered significant.
Results
Following door-to-door survey, 1186 participants were initially enrolled. After laboratory investigations, 382 volunteers had to be excluded (286 were found to have 25-hydroxyvitamin D < 10 ng/ml, 59 had anemia, 12 were diagnosed as having diabetes mellitus, 10 had hypoalbuminemia, 5 had elevated IgA tTg antibody levels, 5 male participants had low serum testosterone, 3 had hypercalcemia, and 2 had estimated glomerular filtration rate < 90 ml/min/1.73 m2). Amongst the 59 enrollees having anemia, 8 had a BMI < 18.5 kg/m2. Rest of the 804 participants was deemed healthy; they underwent DEXA scan and were included in the final analysis. The group included 339 male participants (M:F = 1.13:1.55). Out of the 465 female participants, 302 were premenopausal. The mean age of the group was 44.4 ± 15.4 years (range 20–85 years). There was no significant difference in age between males and females (p = 0.548). The mean BMI of the group was 26.5 ± 2.7 kg/m2; females had a higher BMI than males (26.8 vs. 26.0 kg/m2, p < 0.001). The decade-wise distribution of the participants has been shown in Fig. 1. Forty-three percent of the participants belonged to the 20–39 year age group. The biochemical parameters of the participants are summarized in Table 1.
Dominant handgrip strength
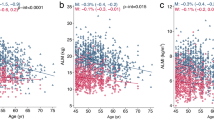
The mean dominant handgrip strength (HGS) of the group was 27.7 ± 8.5 kg (males vs. females being 34.7 vs. 22.6 kg, p < 0.001). The decade-wise distribution of HGS has been depicted in Fig. 2. Peak HGS was achieved in 3rd decade and 4th decade in males and females, respectively following which there was a steady decline. HGS in the 5th decade was significantly lower compared to peak HGS in both males (p = 0.001) and females (p < 0.001).
The comparison of dominant HGS of the study participants with those of Caucasians (derived from the NHANES data) is shown in Table 2. The HGS at all age groups was lower in our participants compared to Caucasians; the difference is more marked in the 8th and 9th decades.
Appendicular skeletal muscle index (ASMI)
The mean ASMI of the group was 6.96 ± 1.12 kg/m2. Males had a significantly higher ASMI than females (7.62 vs. 6.49 kg/m2, p < 0.001). Decade-wise distribution of ASMI has been depicted in Fig. 3. Peak ASMI was achieved in the 4th decade in both males and females. It declined thereafter, albeit less rapidly, with ASMI in the 6th and 7th decade being significantly lower than peak ASMI in males (p = 0.012) and females (p = 0.017), respectively.
The comparison of ASMI of the study participants with those of Caucasians has been shown in Table 3. The ASMI at all age groups was lower in our participants compared to Caucasians; the difference being more marked in males.
Usual gait speed
The mean usual gait speed (GS) of the participants was 1.07 ± 0.20 m/s. Males had a higher GS than females (1.13 vs. 1.02 m/s, p < 0.001). Peak GS was achieved in the 3rd decade in both males and females and declined thereafter with each decade (Fig. 4). The decline was more marked in males with GS in the 4th decade being significantly lower than the peak GS (p = 0.001), while in females, the GS in the 5th decade was significantly lower than corresponding peak GS (p < 0.001).
Cut-offs to define sarcopenia
For the purpose of generating cut-offs, individuals belonging to the 20–39 year age group were considered as the young reference population as peak muscle strength and mass were attained in the 3rd or 4th decades. Out of 345 participants in this group, 157 were males and the rest being females.
The mean (± SD) handgrip strength of the reference population was 39.3 ± 5.9 kg and 24.4 ± 3.2 kg for males and females, respectively. Accordingly, as per the EWGSOP recommendation [3], the cut-offs to define low muscle strength were 27.5 kg and 18.0 kg for males and females, respectively. Similarly, the mean (± SD) ASMI of the reference population was 7.83 ± 0.86 kg/m2 for males and 6.61 ± 1.00 kg/m2 for females. Likewise, the cut-offs to define low muscle mass in males and females were 6.11 kg/m2 and 4.61 kg/m2, respectively. A comparison of these cut-offs with those hitherto proposed by international organizations is shown in Table 4.
Prevalence of probable sarcopenia, sarcopenia, and severe sarcopenia
Applying these cut-offs to the entire study population (N = 804), we found that the prevalence of ‘probable sarcopenia’, ‘sarcopenia’, and ‘severe sarcopenia’, as proposed by EWGSOP2 [1] were 14.6% (males 16.8%. females 13.1%), 3.2% (males 4.7%, females 2.1%), and 2.3% (males 2.6%, females 1.9%), respectively. Likewise, the prevalence of sarcopenia in our study population was established as per internationally established cut-offs (Table 5).
Correlation between muscle strength, muscle mass, and physical performance with biochemical parameters
On univariate analysis (using Pearson/Spearman correlation), serum albumin was found to have significant positive correlation with HGS (r = 0.114, p = 0.001). Similarly, serum testosterone (only in males) was positively correlated with HGS (rs = 0.348, p < 0.001), ASMI (rs = 0.111, p = 0.041), and GS (rs = 0.366, p < 0.001). Serum calcium, 25-hydroxyvitamin D, and iPTH did not have any significant correlations with any of the three parameters. On multiple linear regression analyses, only serum testosterone (in males) was found to be a positive predictor of HGS, ASMI, and GS (supplementary Table 1).
Discussion
In this study, we have generated indigenous cut-offs to define low muscle strength and low muscle mass, and have estimated the prevalence of probable sarcopenia, sarcopenia, and severe sarcopenia in a population of ostensibly healthy adults randomly recruited from the community by door-to-door survey. We have demonstrated that muscle mass (expressed as appendicular skeletal muscle index) is lower in Indians compared to Caucasians across all decades, hence, applying Western cut-offs leads to an undue overestimation of sarcopenia in the Indian context. Using the cut-offs derived from the present study, we found that the prevalence of ‘probable sarcopenia’, ‘sarcopenia’, and ‘severe sarcopenia’ in the community were 14.6%, 3.2%, and 2.3%, respectively, figures that seem to be more realistic.
Sarcopenia is a geriatric syndrome characterized by progressive and generalized loss of muscle mass and function [3]. Sarcopenia eventually results in functional decline, physical disabilities, falls, fractures, poor quality of life, frequent hospitalizations, and mortality [27, 28]. Thus early diagnosis is critical to prevent the aforementioned adverse outcomes [29]. This entails the establishment of well-defined cut-offs to define sarcopenia. However, there exist no unanimous cut-offs that can be used to define sarcopenia for the global population. This is because of the inherent variations in muscle strength and muscle mass among populations of different ethnicities and different geographical locations. As an example, Asians have low muscle mass compared to Caucasians (even after adjustment for stature) and mean grip strength in developing countries is substantially lower than that in the developed nations [30, 31]. In addition, the rate of decline in muscle strength with aging is much more rapid in Asians compared to other ethnic populations [32]. Herein lies the need to generate region-specific cut-off values to define low muscle strength and low muscle mass as a component of sarcopenia. Indeed, the EWGSOP (and EWGSOP2) has recommended the use of indigenous cut-offs to define and stratify sarcopenia [1, 3].
India is home to 1.37 billion people accounting for 17.7% of the world population. As is the global scenario, India’s population is also aging at a rapid rate. As per the country’s latest census in 2011, 104 million people were aged above 60 years; according to estimates, the number is likely to cross 230 million in 2041. Likewise, sarcopenia is expected to increase. However, hitherto, there are no compendious data on the prevalence of sarcopenia in India; neither have comprehensive indigenous cut-offs been established to define this entity. For the first time, we undertook the endeavor to determine cut-offs to define sarcopenia and find out its prevalence as per the latest EWGSOP2 recommendations [1]. We have herein shown that muscle strength (expressed as dominant handgrip strength) and muscle mass (expressed as ASMI) are lower in native Indians than Caucasians across all age decades, thereby calling for new cut-offs that are expected to be lower than the well-established European ones [1, 3, 33,34,35,36]. Such a venture was undertaken by Marwaha et al. who defined low muscle mass as ASMI < 5.11 kg/m2 in a group of apparently healthy participants. However, the study included only females and did not include the assessment of muscle strength and physical performance [7]. Another study by Mohanty et al. found that the prevalence of sarcopenia (based on low total skeletal muscle index) was 15.3% and 20.5% in older males and females, respectively. Nonetheless, the study was conducted in a hospital setting, only 50 young participants were recruited as the reference population, and other parameters of sarcopenia were not assessed [8]. Other studies have used European cut-offs to define sarcopenia [6].
We recruited apparently healthy adults from the community by door-to-door survey. Apart from medical history, we performed an array of laboratory investigations and anthropometry and diligently excluded participants with underlying anemia, hypoalbuminemia, renal and liver disease, diabetes mellitus, hypogonadism, hyperthyroidism, hypercalcemia, celiac disease, and who were underweight (BMI < 18.5 kg/m2), all of which can contribute to sarcopenia. These stringent measures ensured the exclusion of any ‘unhealthy’ individual. In addition, all the components of sarcopenia, namely muscle strength, muscle mass, and physical performance, were assessed. Decade-wise distribution of dominant handgrip strength showed that the peak was achieved in the 3rd decade in males and 4th decade in females; this is very similar to what is seen in Caucasians in whom the HGS usually peaks in the 4th decade in both males and females [19, 37]. Likewise, ASMI peaked in the 4th decades in both males and females similar to what has been reported by Marwaha et al. [7]. Data in Caucasians are inconsistent; the NHANES data showed that peak ASMI was achieved in the 5th decade in both males and females [20], and on the other hand, Imboden et al. reported that ASMI reached its peak in the 3rd decade in both the genders [38]. ASMI was lower in our study participants compared to Caucasians across all ages; however, the difference was more marked in males than in females. The decline in HGS and ASMI was stereotypical, with HGS showing a rapid decline as compared to ASMI; a phenomenon that is well described [39]. As far as physical performance was concerned, usual gait speed peaked in the 3rd decade in males and females and declined rapidly thereafter. A report by Bohannon et al. showed that usual gait speed (GS) peaked in the 4th decade among Caucasians [40]. Although the peak GS did not differ much between the present study and that reported by Bohannon et al. (1.31 vs. 1.21 m/s in males, 1.15 vs. 1.15 m/s in females), the rate of decline was much rapid in Indians as compared to Caucasians. Putting together, we can conclude than Indians have lower muscle mass and muscle strength but analogous physical performance compared to Caucasians; physical performance, however, declines at a much rapid rate.
Cut-offs to define low muscle mass and low muscle strength were derived based on the standard recommendation proposed by EWGSOP [3]. These cut-offs are much lower as compared to Caucasians [23, 24, 33,34,35,36]; however, when equated against the EWGSOP2 recommendation, the indigenous cut-offs to define low muscle strength were higher. This is because the EWGSOP2 recommendation for defining low handgrip strength is based on the data by Dodds et al. who defined weak grip strength as 2.5 standard deviations (instead of 2SD) below the gender-specific peak mean. When using 2SD, the cut-offs rose to 32 kg for males and 19 kg for females, which would surpass the cut-offs derived from the present study [37]. Similarly, the cut-points for defining low muscle strength proposed by the Asian Working Group for Sarcopenia (AWGS, initially proposed in 2014 based on data derived from South-East Asian population and revised in 2019) are very similar to those being proposed by us, although those for defining low muscle mass are still higher amongst the South-East Asians as compared to the Indians [22, 26]. On further dissection of the Asian data, we found that our cut-offs to define low ASMI are similar to those proposed by some authors [41,42,43], while others have reported higher thresholds [44,45,46]. This can only be explained on the basis of heterogeneity in the population studied by various authors. Finally, Marwaha et al. proposed a lower limit of ASMI as 5.11 kg/m2 for defining low muscle mass in females using a cut-off of less than 20% of sex-specific normal population. When using the standard definition of 2SD below peak mean, they found a value of 4.42 kg/m2 for defining low ASMI in females, a value very similar to what we had derived from the present study [7]. Of note, all the aforementioned studies had measured muscle mass using DEXA scan rather than by bioimpedance analysis (BIA). Accordingly, cut-offs based on BIA are different from those based on DEXA [3]. Bahat et al. conducted a study in Turkish population wherein body composition was measured using BIA; low muscle strength was defined based on skeletal muscle mass indices (SMMI), instead of ASMI. A SMMI < 9.2 kg/m2 in males and < 7.4 kg/m2 in females defined low muscle mass [47]. Another very recent study from Turkey using BIA found an ASMI cut-off of 8.33 kg/m2 in males and 5.70 kg/m2 in females; thresholds for HGS were 28 kg for males and 14 kg for females [48]. Although HGS cut-offs are very similar to the present study, the thresholds defining low muscle mass are higher than Indians.
Using the latest EWGSOP2 recommendation and applying the indigenous cut-offs, we found that the prevalence of ‘probable sarcopenia’, ‘sarcopenia’, and ‘severe sarcopenia’ was 14.6% (males 16.8%. females 13.1%), 3.2% (males 4.7%, females 2.1%), and 2.3% (males 2.6%, females 1.9%), respectively. The prevalence of sarcopenia in the community is highly variable, ranging from 1.6% to 36.6% amongst healthy aging adults [4, 5]. However, most of these estimates are based on the previous EWGSOP guidelines that laid more emphasis on muscle mass than muscle strength [3]. Limited data on the prevalence of probable sarcopenia, sarcopenia, and severe sarcopenia following the paradigm shift are available [49,50,51]. Based on the EWGSOP2 consensus, Kim et al. found that the prevalence of probable sarcopenia (only low MS based on HGS), sarcopenia (low HGS and low ASMI), and severe sarcopenia (low HGS, low ASMI and low GS) in community-dwelling older adults were 13.7%, 9.3%, and 1.9%, respectively [49]. Our results seem to be reasonable estimates, considering the fact that the study participants were healthy and meticulously screened for the absence of any underlying disease that could have contributed to (secondary) sarcopenia.
We found a positive correlation between serum albumin and serum total testosterone (only in males) with HGS, ASMI, and GS. Multiple linear regression showed that only serum testosterone positively predicted HGS, ASMI, and GS in male. Positive correlation between serum albumin and testosterone with measures of muscle mass and strength is well documented in the literature [52,53,54]. The available data are also supportive of the fact that testosterone treatment has been reported to have beneficial effects on muscle mass and function, although results have been inconsistent [54]. We did not find any correlation between serum 25-hydroxyvitamin D and components of sarcopenia, even after the exclusion of participants who had been on calcium and vitamin D supplements over the past 6 months. Association between muscle mass, strength, and physical performance with serum 25-hydroxyvitamin D are highly variable; some studies have shown a positive correlation [55,56,57], while others have shown no significant correlation [58,59,60,61]. Moreover, the effect of vitamin D supplementation on improvement in muscle mass and strength is controversial [62].
The study did have certain limitations. First, the proportion of participants belonging to the 8th and 9th decades were relatively less; this was attributed to the scrupulous exclusion criteria that led to the elimination of a large number of older adults due to underlying co-morbidities (mostly anemia and diabetes mellitus). This ensured that the selected older participants were free from any underlying disease that could have contributed to secondary sarcopenia and falsify our findings. Second, we excluded patients with serum 25-hydroxyvitamin D < 10 ng/ml rather than those with a serum level < 20 ng/ml, the widely accepted definition of ‘vitamin D deficiency’. This is because of the fact that vitamin D deficiency (serum 25-hydroxyvitamin D < 20 ng/ml) is rampant in India and setting a lower limit of 20 ng/ml would have led to exclusion of 70–80% of the recruited population [63, 64]. We believe that a cut-point of 10 ng/ml was a sensible trade-off as it identifies patients with severe vitamin D deficiency; prior studies have shown a decline in physical performance and sarcopenia in older individuals with vitamin D levels only below 10 ng/ml [65, 66]. Third, we did not derive indigenous cut-offs to define low physical performance; instead, we used a unanimously accepted value of 0.8 m/s to define low gait speed [1, 3, 21, 22]. Cut-offs to define low gait speed are based on prospective studies that predict disability and reduced overall survival [67]. Such an endeavor could not be undertaken in our observational study. Finally, we lack follow-up data on the clinical outcomes of those participants who were categorized as having probable sarcopenia, sarcopenia, and severe sarcopenia as per our study.
In conclusion, we have generated cut-offs to define low muscle strength and low muscle mass in a population of ostensibly healthy Indian adults. The cut-offs to define low muscle mass are consistently lower compared to the European cut-offs. The thresholds to define low muscle strength are similarly lower when compared to the old Western cut-offs (as proposed by EWGSOP) but higher when compared to the new ones (as proposed by EWGSOP2). Applying these indigenous cut-offs, we found that the prevalence of ‘sarcopenia’ was 3.2%; an estimate that is lower than when using the Caucasian cut-offs. Considering the dearth of data on sarcopenia from the Indian subcontinent, we believe that the present study will pave the way for large-scale observational and interventional studies from this part of the world.
References
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Rosenberg IH (1997) Sarcopenia: Origins and clinical relevance. J Nutr 127:990S–991S
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Yu S, Appleton S, Adams R, Chapman I, Wittert G, Visvanathan T et al (2014) The impact of low muscle mass definition on the prevalence of sarcopenia in older Australians. BioMed Res Int 2014:1–7
Pagotto V, Silveira EA (2014) Applicability and agreement of different diagnostic criteria for sarcopenia estimation in the elderly. Arch Gerontol Geriatr 59:288–294
Anbalagan VP, Venkataraman V, Pradeepa R, Deepa M, Anjana RM, Mohan V. The prevalence of presarcopenia in Asian indian individuals with and without type 2 diabetes. Diabetes technol ther [Internet]. 2013 [cited 2019 Oct 28]; 15(9). https://www.liebertpub.com/doi/10.1089/dia.2013.0068
Marwaha RK, Garg MK, Bhadra K, Mithal A, Tandon N. Assessment of lean (muscle) mass and its distribution by dual energy X-ray absorptiometry in healthy Indian females. Arch Osteoporos [Internet]. 2014 [cited 2019 Oct 28];9. https://springerlink.bibliotecabuap.elogim.com/10.1007/s11657-014-0186-z
Mohanty L, Sahoo D (2016) Prevalence and risk factors of sarcopenia: a study in a tertiary care centre. Int J Adv Med 3:364–367
Aggarwal A, Ram S, Garg A, Pal R, Bhansali A, Singh P, et al. Metabolic bone profile of healthy adult north Indian population from Chandigarh Urban Bone Epidemiological Study (CUBES). Indian J Clin Biochem [Internet]. 2019 [cited 2019 Dec 6]. https://springerlink.bibliotecabuap.elogim.com/10.1007/s12291-019-00857-6
Kish Selection Method. In: Encyclopedia of Survey Research Methods [Internet]. 2455 Teller Road, Thousand Oaks California 91320 United States of America: Sage Publications, Inc.; 2008 [cited 2019 Oct 28]. https://methods.sagepub.com/reference/encyclopedia-of-survey-research-methods/n262.xml
Shim J-S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;e2014009.
Armstrong T, Bull F (2006) Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health 14:66–70
Srivastava SK, Chand R (2017) Tracking transition in calorie-intake among Indian households: insights and policy implications. Agric Econ Res Rev 30:23
Janssen I, Ross R (2012) Vigorous intensity physical activity is related to the metabolic syndrome independent of the physical activity dose. Int J Epidemiol 41:1132–1140
Blanc B, Finch C, Hallberg L, Herbert V, Lawkowicz W, Layrisse M (1968) Nutritional anaemias. report of a WHO scientific group. WHO Tech Rep Ser. 1–40.
American Diabetes Association (2019) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 42:S13–28
Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM et al (2018) Testosterone therapy in men with hypogonadism: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab 103:1715–1744
Livingston M, Kalansooriya A, Hartland AJ, Ramachandran S, Heald A (2017) Serum testosterone levels in male hypogonadism: Why and when to check-a review. Int J Clin Pract 71:e12995
Wang Y-C, Bohannon RW, Li X, Yen S-C, Sindhu B, Kapellusch J. Summary of grip strength measurements obtained in the 2011–2012 and 2013–2014 national health and nutrition examination surveys. J Hand Ther [Internet]. 2018 [cited 2019 Nov 1]. https://linkinghub.elsevier.com/retrieve/pii/S0894113017303897
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 4:e7038
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol Ser A 69:547–558
Chen L-K, Liu L-K, Woo J, Assantachai P, Auyeung T-W, Bahyah KS et al (2014) Sarcopenia in Asia: consensus report of the asian working group for sarcopenia. J Am Med Dir Assoc 15:95–101
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR et al (1998) Epidemiology of Sarcopenia among the Elderly in New Mexico. Am J Epidemiol 147:755–763
Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A et al (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 95:1851–1860
Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB et al (2011) Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 12:249–256
Chen L-K, Woo J, Assantachai P, Auyeung T-W, Chou M-Y, Iijima K et al (2020) Asian Working group for Sarcopenia: 2019 consensus update on Sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21(300–307):e2
Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O (2017) Health outcomes of Sarcopenia: a systematic review and meta-analysis Wright JM, editor. PLoS ONE 12:e0169548
Cruz-Jentoft AJ, Sayer AA (2019) Sarcopenia. The Lancet 393:2636–2646
Inouye SK, Studenski S, Tinetti ME, Kuchel GA (2007) Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept: (see Editorial comments by Dr. William Hazzard on pp 794–796). J Am Geriatr Soc. 55:780–791
Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB et al (2010) Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol 22:76–82
Dodds RM, Syddall HE, Cooper R, Kuh D, Cooper C, Sayer AA (2016) Global variation in grip strength: a systematic review and meta-analysis of normative data. Age Ageing 45:209–216
Wu Y-H, Hwang A-C, Liu L-K, Peng L-N, Chen L-K (2016) Sex differences of sarcopenia in Asian populations: the implications in diagnosis and management. J Clin Gerontol Geriatr 7:37–43
Tankó L, Movsesyan L, Mouritzen U, Christiansen C, Svendsen OL (2002) Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism 51:69–74
Gillette-Guyonnet S (2003) Body composition in French women 75+ years of age: The EPIDOS study. Mech Ageing Dev 124:311–316
Rolland Y, Lauwers-Cances V, Cristini C, van Kan GA, Janssen I, Morley JE et al (2009) Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr 89:1895–1900
Iannuzzi-Sucich M, Prestwood KM, Kenny AM (2002) Prevalence of Sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 57:M772–M777
Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM et al (2014) Grip strength across the life course: normative data from twelve british studies. Vina J, editor. PLoS ONE 9:e113637
Imboden MT, Swartz AM, Finch HW, Harber MP, Kaminsky LA (2017) Reference standards for lean mass measures using GE dual energy x-ray absorptiometry in Caucasian adults. van Wouwe JP, editor. PLoS ONE 12:e0176161
Keller K, Engelhardt M (2013) Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J 3:346–350
Bohannon RW, Wang Y-C (2019) Four-Meter gait speed: normative values and reliability determined for adults participating in the NIH toolbox study. Arch Phys Med Rehabil 100:509–513
Lau EMC, Lynn HSH, Woo JW, Kwok TCY, Melton LJ (2005) Prevalence of and risk factors for sarcopenia in elderly chinese men and women. J Gerontol A Biol Sci Med Sci 60(2):213–216
Wen X, Wang M, Jiang C-M, Zhang Y-M (2011) Are current definitions of sarcopenia applicable for older Chinese adults? J Nutr Health Aging 15:847–851
Kim TN, Park MS, Yang SJ, Yoo HJ, Kang HJ, Song W et al (2010) Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean sarcopenic obesity study (KSOS). Diabetes Care 33:1497–1499
Lee JSW, Auyeung T-W, Kwok T, Lau EMC, Leung P-C, Woo J (2007) Associated factors and health impact of Sarcopenia in older chinese men and women: a cross-sectional study. Gerontology 53:404–410
Woo J, Leung J, Sham A, Kwok T (2009) Defining sarcopenia in terms of risk of physical limitations: a 5-year follow-up study of 3153 chinese men and women: definition of sarcopenia by incident physical limitation. J Am Geriatr Soc 57:2224–2231
Sanada K, Miyachi M, Tanimoto M, Yamamoto K, Murakami H, Okumura S et al (2010) A cross-sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol 110:57–65
Bahat G, Tufan A, Tufan F, Kilic C, Akpinar TS, Kose M et al (2016) Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People (EWGSOP) definition. Clin Nutr 35:1557–1563
Ates Bulut E, Soysal P, Dokuzlar O, Kocyigit SE, Aydin AE, Yavuz I, et al. Validation of population-based cutoffs for low muscle mass and strength in a population of Turkish elderly adults. Aging Clin Exp Res [Internet]. 2020 [cited 2020 Mar 15]. https://springerlink.bibliotecabuap.elogim.com/10.1007/s40520-019-01448-4
Kim M, Won CW (2019) Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in older people 2: findings from the Korean frailty and aging cohort study. Age Ageing 48:910–916
Yang M, Liu Y, Zuo Y, Tang H. Sarcopenia for predicting falls and hospitalization in community-dwelling older adults: EWGSOP versus EWGSOP2. Sci Rep [Internet]. 2019 [cited 2020 Mar 15];9. https://www.nature.com/articles/s41598-019-53522-6
Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr [Internet]. 2019 Dec [cited 2020 Mar 15];19. https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-019-1338-1
Baumgartner RN, Koehler KM, Romero L, Garry PJ (1996) Serum albumin is associated with skeletal muscle in elderly men and women. Am J Clin Nutr 64:552–558
Schalk BWM, Deeg DJH, Penninx BWJH, Bouter LM, Visser M (2005) Serum albumin and muscle strength: a longitudinal study in older men and women: serum albumin and muscle strength in men and women. J Am Geriatr Soc 53:1331–1338
Shin MJ, Jeon YK, Kim IJ (2018) Testosterone and sarcopenia. World J Mens Health 36:192
Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B et al (2007) Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 62:440–446
Kuwabara A, Tsugawa N, Kondo H, Ao M, Fujiwara H, Hosokawa N et al (2017) Associations between serum 25-hydroxyvitamin D 3 level and skeletal muscle mass and lower limb muscle strength in Japanese middle-aged subjects. Osteoporos Sarcopenia 3:53–58
Mastaglia SR, Seijo M, Muzio D, SoMoza J, Nuñez M, Oliveri B (2011) Effect of vitamin D nutritional status on muscle function and strength in healthy women aged over sixty-five years. J Nutr Health Aging 15:349–354
Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ, Amin S (2011) Is vitamin D a determinant of muscle mass and strength? J Bone Miner Res 26:2860–2871
Mathei C, Van Pottelbergh G, Vaes B, Adriaensen W, Gruson D, Degryse J-M (2013) No relation between vitamin D status and physical performance in the oldest old: results from the Belfrail study. Age Ageing 42:186–190
Ceglia L, Chiu GR, Harris SS, Araujo AB (2011) Serum 25-hydroxyvitamin D concentration and physical function in adult men: vitamin D and physical function. Clin Endocrinol (Oxf) 74:370–376
Pramyothin P, Techasurungkul S, Lin J, Wang H, Shah A, Ross PD et al (2009) Vitamin D status and falls, frailty, and fractures among postmenopausal Japanese women living in Hawaii. Osteoporos Int 20:1955–1962
Gunton JE, Girgis CM (2018) Vitamin D and muscle. Bone Rep 8:163–167
Gupta R, Gupta A (2014) Vitamin D deficiency in India: prevalence. Causalities Interventions Nutr 6:729–775
Kamboj P, Dwivedi S, Toteja G (2018) Prevalence of hypovitaminosis D in India & way forward. Indian J Med Res 148:548
Wicherts IS, van Schoor NM, Boeke AJP, Visser M, Deeg DJH, Smit J et al (2007) Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab 92:2058–2065
Visser M, Deeg DJH, Lips P (2003) Low Vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (Sarcopenia): the longitudinal aging study Amsterdam. J Clin Endocrinol Metab 88:5766–5772
Studenski S (2011) Gait speed and survival in older adults. JAMA 305:50
Acknowledgements
The authors acknowledge the incessant efforts of Anil Saini, Ashutosh Kumar Arya, Harwinder Singh Saini, Rashi Goel, Anmol, Seema Dahiya, Amanpreet Kaur, Shalu, Chandana Dutta, and Dr. Reetu Nahar who had been an integral part of Chandigarh Urban Bone Epidemiological Study (CUBES) since its inception. The authors also acknowledge The Indian Society for Bone and Mineral Research (ISBMR) for providing intellectual support.
Funding
No funds in any form were used in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest in this work.
Ethical approval
The study was approved by the Institute Ethical Committee, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
Informed consent
All selected participants were duly pre-informed about the study and written informed consent was obtained from them.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pal, R., Aggarwal, A., Singh, T. et al. Diagnostic cut-offs, prevalence, and biochemical predictors of sarcopenia in healthy Indian adults: The Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES). Eur Geriatr Med 11, 725–736 (2020). https://doi.org/10.1007/s41999-020-00332-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-020-00332-z