Abstract
Background/Objectives:
Age-related muscle and fat mass (FM) changes are ethnicity specific. We aimed to develop a cut-point for the muscle mass component of sarcopenia for black South African (SA) women, and to assess its predictive value, in comparison to established cut-points, to identify functional ability among older black SA women.
Subjects/Methods:
In a cross-sectional study, a sarcopenia cut-point was calculated from dual energy X-ray absorptiometry (DXA)-derived appendicular skeletal muscle mass (ASM) indexes (ASMI) from two young black SA reference groups. The new cut-point was compared with the most recent Foundation for the National Institutes of Health (FNIH) criteria (ASM <15.02 kg; and ASMBMI <0.512), an internationally accepted cut-point (ASMI <5.5 kg/m2) and a residual method adjusting for FM. All cut-points were then applied to 221 older black women to predict gait speed and handgrip strength.
Results:
A cut-point of ASMI <4.94 kg/m2 was derived from the young SA reference groups. Using this cut-point, 9.1% of older women were classified as sarcopenic, compared with 16.7–38.7% using other cut-points. The only cut-points that significantly predicted low functional ability (low gait speed and low handgrip strength) in older black women were the new SA cut-point and the FNIH ASM criterion. Multivariate logistic regression models for both these cut-points significantly predicted low handgrip strength (odds ratio (OR)=3.71, P=0.007 and OR=3.42, P=0.001, respectively) and low gait speed (OR=9.82, P=0.004 and OR=8.71, P=0.008, respectively).
Conclusions:
The new SA cut-point had similar or greater odds of predicting reduced functional ability in older SA women when compared with other internationally accepted cut-points.
Similar content being viewed by others
Introduction
Age-related loss of muscle mass and function, known as sarcopenia, is receiving increasing attention with ageing of the global population.1 Various cut-points for appendicular skeletal muscle mass (ASM), as well as the ASM index (ASMI, ASM divided by height squared), together with measures of muscle strength (handgrip strength) and physical performance (gait speed) have been proposed,2, 3, 4, 5 which identify sarcopenia, a condition associated with decreased physical functioning, frailty, falls and mortality in older adults.6 However, Batsis et al.,7 have identified a high degree of variability in the prevalence of sarcopenia depending on the criteria used. This is further compounded by the fact that muscle quantity and quality declines with age, whereas fat mass (FM) increases.1 Several methods have attempted to account for obesity when identifying sarcopenia, with adjustment of ASM for FM and height5, 8 or for body mass index (BMI).9 In addition, height and therefore muscle mass, as well as adiposity, differ in different populations,10 suggesting that standard criteria for sarcopenia may not be appropriate globally. In order for sarcopenia to be identified as a clinical condition, standard criteria, which are independently validated in different populations, are required.9
Cut-points to classify sarcopenia have not been determined in black African individuals, which is of particular relevance as the body composition of Africans differs considerably from Caucasians.10, 11 Specifically, height, body fat and body fat distribution differ between African and Caucasian populations.10 The aim of this study was therefore to develop an appropriate cut-point for sarcopenia for black South African (SA) women, which includes adjustment for differences in body size, and to assess the ability of the newly derived cut-point, in comparison to established cut-points, to categorise older women according to functional ability, the other important component of sarcopenia.
Subjects and methods
Dual energy X-ray absorptiometry (DXA) data from two reference groups of black SA women aged 18–40 years from Cape Town (n=238)12 and Soweto (n=371)13 were used for the determination of a sarcopenia cut-point. As the women from the two regions may represent different SA tribes,14 and different DXA machines were used, reference data were not pooled. The body composition data of the SA reference groups were compared with the reference group of 229 non-Hispanic white male and female participants in the Rosetta Study, aged 18–40 years,2 used to derive the first widely adopted ASMI-based sarcopenia cut-point of <5.5 kg/m2 in women.11 The SA women were also compared with 349 Brazilian women, aged 18–40 years, selected as a reference from a developing country.3 These women served as a young healthy reference group in a study to define sarcopenia in older Brazilian women.3
The ability of the derived cut-points to predict physical performance and strength was examined in a random sample of 221 black SA women, aged 45–84 years, recruited from Tlokwe municipality in the North-West province, SA.15 This study focused on a cut-point for women, because DXA data for female participants only were available. Inclusion criteria were as follows: (i) apparently healthy and living independently, with no disabilities and (ii) not pregnant or lactating. A similar demographic questionnaire at all three sites assessed socioeconomic status on the basis of education and access to water and sanitation.
Body composition (FM; fat-free soft tissue mass; and body fat percentage) was measured using DXA (Hologic Discovery-W, software version 12.7 for Cape Town, South Africa; QDR-4500A software version 12.5:7 for Soweto, South Africa; and Hologic Discovery W, APEX system software version 2.3.1 for North-West, South Africa). FM and fat-free soft tissue mass for the whole body, trunk and limbs were derived using standard DXA cut-off lines. ASM was determined by summing the arm and leg data and ASMI by dividing ASM by height (metres) squared. ASMBMI was calculated as ASM divided by BMI. A cut-point for sarcopenia was calculated as the ASMI two s.d. below the mean for each reference group. In addition, the residual method (RM) was used to define a cut-point for ASM, adjusting for height (m) and FM (kg).8
To assess physical performance in the older group from North-West, grip strength and gait speed were measured by trained post-graduate Biokinetics students around mid-morning. Grip strength was measured for the dominant hand using a dynamometer, using the maximum of three repeated measurements (Jamar, Bollingbrook, IL, USA).16 Time to complete a 6 m walk test was recorded with a stopwatch to calculate gait speed in m/s.17 Participants were tested for HIV using the HIV card test (PMC Medical, India), with pre-test and post-test counselling and referral for treatment if tested positive.
Outcomes
The proportion of the sarcopenic women in the older group was calculated using five methods: (i) the Foundation for the National Institutes of Health (FNIH) criteria for sarcopenia of ASMBMI <0.512 (FNIH ASMBMI), (ii) ASM <15.02 kg (FNIH ASM),9 (iii) the European Working Group on Sarcopenia in Older People ASMI cut-point (5.5 kg/m2) (EWGSOP ASMI),11 (iv) the RM cut-point8 and (v) the new cut-point calculated from the SA reference groups (SA ASMI).
The Research Ethics Committees of the Universities of Cape Town, Witwatersrand and North-West approved the studies, and written informed consent was obtained from all participants.
Statistical analysis
The Kruskall Wallis and χ2-tests were used to compare variables between the two SA reference groups. Differences in body composition, ASMI and sarcopenic cut-points between the SA groups, the Rosetta study reference,2 and a Brazilian group3 were determined using the means, s.d. and samples sizes to derive a z-statistic.
The proportion of sarcopenic women according to each of the five cut-points, who also had gait speed <0.8 m/s and handgrip strength <16 kg, was determined by cross-tabulation.4 Receiver operating characteristic curves were created for the ASM, ASMBMI, ASMI and residuals, against low gait speed and low grip strength as gold standards for low functional ability. The area under the curve (AUC) and the sensitivity and specificity were determined for each method to discriminate between sarcopenic women who had low versus normal functional ability. Univariate odds ratios were calculated for sarcopenic women according to each cut-point to have low gait speed or low handgrip strength. Multivariate odds ratio were then calculated for the SA cut-point (SA ASMI) and the FNIH ASM cut point, adjusting for age, HIV status, tobacco use, education level and self-reported osteo-arthritis in backward stepwise logistic regression. Statistical analyses were performed using SPSS, version 22 (SPSS, Chicago, IL, USA).
Results
Reference groups
Approximately half of the SA women from the young reference groups had water inside the house (40.2%), flush toilets (47%) and high school education (56%). Table 1 shows that both SA groups had a higher BMI and body fat percentage compared with the international reference groups. ASMI was not different between the SA and Rosetta2 groups, but the Brazilian group3 had the lowest ASM and ASMI. The mean sarcopenia cut-point for the two SA groups was 4.94 kg/m2. The RM (linear regression of FM and height on ASM) yielded the following equations for the references:
Cape Town: ASM (kg)=8.57+0.78 × FM (kg) −0.09 × height (m), adjusted R2=0.60, P<0.001.
Soweto: ASM (kg)=7.93+0.79 × FM (kg) −0.09 × height (m), adjusted R2=0.63, P<0.001.
The sarcopenia cut-points (2 s.d. below the mean residuals) were −1.42 and −1.56, respectively, with a mean of −1.49.8
ASMI was significantly greater among the overweight/obese compared with the normal weight women in both SA reference groups (Soweto: 7.80±0.96 kg/m2 versus 6.11±0.65 kg/m2, P<0.001; Cape Town: 8.33±0.84 kg/m2 versus 6.35±0.62 kg/m2, P<0.001, respectively). Accordingly, the sarcopenia cut-points varied considerably between overweight/obese and normal weight groups (Soweto: 5.88kg/m2 versus 4.81 kg/m2; Cape Town: 6.65 kg/m2 versus 5.11 kg/m2, respectively).
Older women
Most of the older women in this study had access to water and sanitation, but the majority had only primary school education. Few (7.4%) of the older women were underweight, 26.1% were normal weight, 23.9% were overweight and 42.6% were obese. The older women were recruited as apparently healthy women, but on the study day 9.5% tested HIV positive. The performance of different sarcopenia cut-points to predict low gait speed and handgrip strength among older black SA women is presented in Table 2. FNIH ASM9 defined the largest proportion of the older women as sarcopenic (38.7%), with the SA cut-point (4.94 kg/m2) classifying the smallest proportion (9.1%). The two cut-points that included adjustment for adiposity yielded similar results, 21.4% and 20.8%, respectively (Table 2). Except for the FNIH ASM cut-point, all cut-points had relatively low sensitivity to identify sarcopenic women with low gait speed (23.1–30.8%) or low grip strength (19.1–27.7%). Specificity was high for all cut-points for identifying non-sarcopenic women with normal gait speed (94.2–98.6%) and grip strength (66.9–93.1%). The FNIH ASM cut-point had better sensitivity and specificity compared with the other cut-points, followed by the SA ASMI (Table 2).
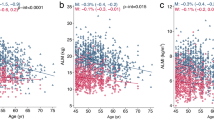
The AUCs derived from the receiver operating characteristic curves showed that ASM and ASMI had good ability to discriminate between those with a low versus normal gait speed (AUC=0.76 and 0.74, respectively, P<0.05, Figure 1A), as well as low versus normal handgrip strength (AUC=0.67 and 0.64, respectively, P<0.05, Figure 1B). The corresponding AUCs for the RM and ASMBMI to discriminate between those with a low versus normal gait speed, as well as for low versus normal handgrip strength, reflect insufficient discriminative power. (Figure 1A and B).
Only the new SA and the FNIH ASM9 cut-points significantly predicted both low gait speed and handgrip strength (Table 2) in older women. In multivariate logistic regression models with adjustment for possible confounders, the SA and the FNIH ASM cut-points still significantly predicted low gait speed (OR=9.82 and 8.71, both P<0.05) and low handgrip strength (OR=3.71 and 3.42, both P<0.05) (Table 3).
Discussion
A sarcopenia cut-point of ASMI <4.94 kg/m2 derived from two young SA reference groups classified 9.1% of older SA women as sarcopenic. This cut-point had similar or better predictive value compared with other established international cut-points to identify low functional ability in older black SA women. However, the recently derived FNIH ASM cut-point, which does not take into account any measure of body composition, had the greatest specificity and sensitivity to detect low functional ability in older black SA women.
Despite the adoption of international cut-points, there is no consensus regarding their appropriateness in different populations.18 Several groups have developed population-specific sarcopenia cut-points.19, 20 EWGSOP recommends normative reference populations of healthy young adults, instead of convenience samples.11 However, there are no clear criteria for the selection of the reference group, with sample size and composition varying greatly between studies, namely 122 women in the Rosetta Study2 compared with 2392 in a Korean study.20
An important finding of this study was the wide variation in sarcopenia prevalence when different cut-points are applied. The major variability in ASMI and sarcopenia cut-points may be attributed to the variation in FM between the groups. Compared with the two international reference groups, the SA reference women had higher body fat percentage and BMI. These results suggest that if overweight and obese individuals are included in the young reference group to derive a cut-point for sarcopenia, a larger variation in ASM and ASMI will be observed. This will lead to lower cut-points, resulting in fewer individuals being identified as sarcopenic. These findings suggest that obese persons should be excluded from healthy reference groups used to calculate sarcopenia cut-points. However, this may pose challenges in most developed, as well as many developing countries, such as SA, in which the majority of adult women are classified as overweight or obese.
Because of the high prevalence of obesity in the SA reference groups and the older women, both ASMBMI and the RM, with adjustment of ASM for BMI, or height and FM, respectively, were also applied.8, 9 The rationale for these methods is that excessive FM rather than poor muscle strength is the major determinant of physical disability among older women.3, 21 Using these adjustment methods, we identified more women with sarcopenia compared with two of the methods not taking obesity into account (21.3 and 20.8% versus 16.7 and 9.1%, respectively). However, both FNIH ASMBMI and the RM, proposed as more appropriate methods for overweight populations,8, 9 were not better predictors of functional ability of black SA women compared with the cut-points based on ASM and ASMI only. Our results differ from the results of Oliveira et al.3 who found that sarcopenic women, identified using the RM, had weaker quadriceps muscle strength compared with non-sarcopenic women; however, our study used different tests for functional ability. In another study from Brazil, the authors regarded the RM as more appropriate to diagnose sarcopenia among overweight and obese compared with normal weight women.21 The reasons why these methods were not better predictors of functional ability compared with those based purely on ASMI in our study are unknown. DXA-derived fat-free soft tissue mass of the arms and legs may not be sufficiently indicative of their function. The proportion of connective tissue in muscle increases with increasing adiposity, thereby reducing lean tissue density, and hence the quality of the muscle.22
Another reason for the large variance in ASM and hence sarcopenia cut-points may be the differences in height between the reference groups (Table 1). As there is a strong positive association between ASM and height, the Baumgartner2 and Newman8 definitions adjust ASM for height. The shorter stature of the SA and Brazilian participants compared with the Rosetta reference group may be genetically determined or linked to early undernutrition. Low socioeconomic status of the SA women may have contributed to early undernutrition and a shorter adult height and smaller ASMI.23 Childhood stunting remains a public health problem in SA and Brazil.24, 25 These findings indicate that height standardisation may be necessary for populations in transition, such as SA and Brazil, in which both undernutrition and overnutrition exist in the same community.24, 25
Although the FNIH ASM definition does not correct for height or BMI, it identified a higher prevalence of sarcopenia when compared with height-adjusted methods (38.7% versus 16.7% and 9.1%). This cut-point, together with the newly derived SA cut-point, was able to predict both low gait speed and handgrip strength in the older group of SA women. Compared with other internationally accepted cut-points, the FNIH ASM cut-point had the highest sensitivity and specificity to identify reduced functional ability. This finding is surprising given that 90% of the participants in the FNIH study were white, with a very small proportion being of African descent.9 Indeed, it is well recognised that body composition, in particular height and body fat, differs considerably between Africans and Caucasians.10 In addition, in contrast to our study, and the previously established cut-points that were derived from young reference groups, the FNIH cut-point was based on data from a very large and diverse sample (over 15 000) of community-dwelling older women.9 The results of our study suggest that it may be more appropriate to define sarcopenic cut-points using older adults, rather than a young healthy reference population. However, longitudinal studies are required to confirm the predictive value of these different cut-points, in particular focusing on the main consequences of sarcopenia, namely functional decline, falls and mortality.6 Although low gait speed and low grip strength were used as gold standards for low functional ability in our study, standards in line with more advanced consequences of sarcopenia, namely falls, could be useful.
On the basis of the new SA cut-point of ASMI<4.94 kg/m2 and the new FNIH criteria, older black women identified with sarcopenia had similar odds (9.8 versus 8.7) of having low gait speed and low handgrip strength (3.7 versus 3.4) compared with those without sarcopenia. Surprisingly, these odds are similar to those reported in the FNIH study, on which the cut-points were derived.4 However, compared with the new SA cut-point, the FNIH criteria had significantly higher sensitivity and identified more than four times (38.7% versus 9.1%) as many older black women with sarcopenia. Despite variation in the numbers of sarcopenic women identified by the different cut-points, low counts were recorded for women with low gait speed and handgrip strength, resulting in relatively low sensitivity to identify sarcopenic women with low functional ability.
The varied prevalence of sarcopenia based on different cut-points among the older women in our study underlines the importance of consensus about an ethnic-specific cut-point for clinical practice. Using different cut-points within the same population may result in overdiagnosis and unnecessary treatment of sarcopenia in resource-limited settings or failure to diagnose and treat those who urgently need treatment. A recent study drew attention to the limitations of applying mathematical distributions to define sarcopenia from data of reference groups, often resulting in wider variations and consequently higher prevalence estimates.7 These cut-points should therefore be assessed on the basis of predictive relationships to functional outcomes.7
A limitation of this study is that only two data sets without proper characterisation of tribal descent were available as reference data to calculate an ASMI-based cut-point. DXA technology is not generally available in Africa, resulting in a lack of data to compile a reference database. Our findings were not limited by the small sample size, as the SA groups had more women (n=371 and n=238) compared with the Rosetta study (n=122),2 and similar numbers to the Brazilian study (n=349).3 Because of the high prevalence of obesity among SA women26 the available SA data included more overweight and obese compared with normal weight women. Ideally, a reference group with a normal body composition distribution should be recruited to assess a more appropriate cut-point for sarcopenia for the sub-Saharan black population. The skewed distribution of BMI and FM and greater s.d. of the ASMI of our reference groups resulted in a lower sarcopenia cut-point, irrespective of their mean ASMI. The lower counts that could impact their results were added as a limitation. Although the mean age of the older group in this study was 58.9 years, the average life expectancy of SA women is 58.3 years (http://www.worldlifeexpectancy.com); therefore, this sample reflects older SA women. In addition, although the older group were apparently healthy at recruitment, 9.5% of them were HIV positive. This did not, however, change our results.
In conclusion, the results indicate that the new SA cut-point was able to predict reduced functional ability in older SA women and outperformed established internationally accepted cut-points. The results of this study could have implications for the early identification of sarcopenia among black women in sub-Saharan Africa, with the view for more targeted early intervention in this high-risk and understudied population. Although the new SA cut-point and the FNIH ASM criterion had similar odds of predicting reduced functional ability in older black women, the FNIH ASM cut-point had greater sensitivity to detect sarcopenia. Further research in African populations is necessary to confirm the appropriateness of these cut-points.
References
Baumgartner R, Heymsfield S, Roche A . Human body composition and the epidemiology of chronic disease. Obes Res 1995; 3: 73–95.
Baumgartner R, Koehler K, Gallagher D, Romero L, Heymsfield SB, Ross RR et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763.
Oliveira RJ, Bottaro M, Junior JT, Farinatti PTV, Bezerra LA, Lima RM . Identification of sarcopenic obesity in postmenopausal women: a cutoff proposal. Braz J Med Biol Res 2011; 44: 1171–1176.
Cawthon PM, Peters KW, Shardell MD, McLean RR, Dam TTL, Kenny AM et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci 2014; 69: 567–575.
Fielding R, Vellas B, Evans W, Bhasin S, Morley JE, Newman AB et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–256.
Visser M, Schaap LA . Consequences of sarcopenia. Clinics Geriatr Med 2011; 27: 387–399.
Batsis JA, Barre LK, MacKenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ . Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc 2003; 51: 1602–1609.
Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003; 51: 1602–1609.
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69: 547–558.
Rush EC, Goedecke JH, Jennings C, Micklesfield L, Dugas L, Lambert EV et al. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes 2007; 31: 1232–1239.
Cruz-Jentoft A, Baeyens JP, Bauer J, Boirie Y, Cederholm T, Landi F et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423.
Evans J, Micklesfield LK, Jennings CL, Levitt NS, Lambert EV, Ollson T et al. Diagnostic ability of obesity measures to identify metabolic risk factors in South African women. Metab Syndr Relat Dis 2011; 9: 353–360.
Crowther NJ, Norris SA . The current waist circumference cut point used for the diagnosis of metabolic syndrome in sub-Saharan African women is not appropriate. PLoS One 2012; 7: e48883.
Lane AB, Soodyall H, Arndt S, Ratshikhopha ME, Jonker E, Freeman C et al. Genetic substructure in South African Bantu-speakers: evidence from autosomal DNA and Y-chromosome studies. Am J Phys Anthropol 2002; 119: 175–185.
Vorster HH, Kruger A, Wentzel-Viljoen E, Kruger HS, Margetts BM . Added sugar intake in South Africa: findings from the Adult Prospective Urban and Rural Epidemiology cohort study. Am J Clin Nutr 2014; 99: 1479–1486.
Taekema DG, Gussekloo J, Maier AB, Westendorp RG, de Craen AJ . Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Aging 2010; 39: 331–337.
Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Makrides KS, Ostir GV et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000; 55: M221–M231.
Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L . Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas 2012; 71: 109–114.
Lau EM, Lynn HS, Woo JW, Kwok TC, Melton LJ 3rd . Prevalence of and risk factors for sarcopenia in elderly Chinese men and women. J Gerontol B Psychol Sci Soc Sci 2005; 60: 213–216.
Kim CH, Park HS, Park M, Kim H, Kim C . Optimal cutoffs of percentage body fat for predicting obesity-related cardiovascular disease risk factors in Korean adults. Am J Clin Nutr 2011; 94: 34–39.
Domiciano DS, Figuerdo CP, Lopes JB, Caparbo VF, Takayama. L, Menezes PR et al. Discriminating sarcopenia in community-dwelling older women with high frequency of overweight/obesity: the Sao Paulo Ageing & Health Study (SPAH). Osteoporos Int 2013; 24: 595–603.
Lebrun CE, van der Schouw YT, de Jong FH, Grobbee DE, Lamberts SW . Fat mass rather than muscle strength is the major determinant of physical function and disability in postmenopausal women younger than 75 years of age. Menopause 2006; 13: 474–481.
Brameld JM . The influence of undernutrition on skeletal muscle development. Br J Nutr 2004; 91: 327–328.
Kruger HS, Steyn NP, Swart EC, Maunder EM, Nel JH, Moeng L et al. Overweight among children decreased, but obesity prevalence remained high among women in South Africa, 1999-2005. Public Health Nutr 2012; 15: 594–599.
Horta BL, Santos RV, Welch JR, Cardoso AM, Dos Santos JV, Assis AM et al. Nutritional status of indigenous children: findings from the First National Survey of Indigenous People's Health and Nutrition in Brazil. Int J Equity Health 2013; 12: 1–23.
Shisana O, Labadarios D, Rehle T, Simbayi L, Zuma K, Dhansay A et al South African National Health and Nutrition Examination Survey (SANHANES-1). Human Sciences Research Council: Cape Town, South Africa, 2013.
Acknowledgements
The work was funded by the South African National Research Foundation, the South African Medical Research Council, the International Atomic Energy Agency and the University of Cape Town. LKM acknowledges funding from the MRC/DFID African Research Leader Scheme. The funders had no role in study design, in the collection, analysis or interpretation of data, in the writing of the report or in the decision to submit the paper for publication. Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors, and therefore the National Research Foundation does not accept any liability in regard thereto. Data analyses were conducted with assistance from Professor HS Steyn and S Ellis of the Statistical Consultation Service of the North-West University. We acknowledge the contribution to data analysis made by Treasure A Munonoka during his NRF internship at the UCT/MRC Research Unit for Exercise Science and Sports Medicine.
Author Contributions
The study was conceptualised and designed by HSK, JHG and LKM; HSK, JHG, LKM, HHW and LHN contributed to data collection; data analyses were performed by HSK, JHG and LKM, with assistance from Professor HS Steyn and S Ellis of the Statistical Consultation Service of the North-West University; and discussion of the data and writing of the manuscript was carried out by HSK, JHG, LKM, HHW and LHN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kruger, H., Micklesfield, L., Wright, H. et al. Ethnic-specific cut-points for sarcopenia: evidence from black South African women. Eur J Clin Nutr 69, 843–849 (2015). https://doi.org/10.1038/ejcn.2014.279
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2014.279
- Springer Nature Limited
This article is cited by
-
Relationship between DNA methylation changes and skeletal muscle mass
BMC Genomic Data (2023)
-
Understanding factors associated with sarcopenic obesity in older African women from a low-income setting: a cross-sectional analysis
BMC Geriatrics (2021)
-
Prevalence of Sarcopenia and Relationships Between Muscle and Bone in Indian Men and Women
Calcified Tissue International (2021)
-
Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients
Respiratory Research (2019)