Abstract
Combining microflow chemistry and photoreaction technology has shown to be a viable option to intensify and significantly improve photochemical processes in terms of control and efficiency. Chemical actinometry allows to measure the actually incident photon flux in a specific reactor, but is not trivial to perform. Especially under flow conditions. Numerous errors can occur, not only in the experimental and analytical procedure, but also in the subsequent calculations before finally receiving the incident photon flux. Nevertheless, knowledge of this metric is of fundamental importance to determine the efficiency of photochemical reactor setups. Consequently, this work illustrates, comments and explains various possible pitfalls of chemical actinometry. To avoid adulterated results, a standard measurement and calculation procedure is proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research in the field of photochemistry covers a wide range of concepts for improvement. Main focuses are the development of new synthesis pathways, catalyst optimization, identification of the most suited absorption band, or the adaptation of reaction conditions. Possible objectives are identifying the most efficient use of resources, increasing the yield in a specific reactor setup, or minimizing the reaction time [1,2,3,4,5]. In all these cases, changing the operation mode from batch to flow can be a crucial factor to achieve these goals. The complementary application of microstructured devices to leverage short optical path lengths for a better light penetration can lead to intensified microphotoreactors.
In practice, a symbiosis of photochemistry and microreactor technology yields advantages such as better process control, higher selectivity and significantly increased reproducibility and productivity [6,7,8,9]. Moreover, the challenges of scaling-up photochemical processes and creating an economic added value can be addressed more systematically [10, 11]. Analogous to thermal reactions, the use of appropriate figures of merit is still a pivotal key aspect to drive holistic photochemical reaction engineering forward. The wavelength dependence of photochemical reactions renders this even more relevant. Consequently, it is of great interest to measure the spectral photon flux incident in the reactor volume and to relate this number to the photon flux emitted by the light source. The obtained data allows for a reasonable and unbiased evaluation as well as objective comparison of the overall performance of complete photochemical setups and concepts based on photonic quantities [12,13,14].
The only tool that is flexible enough to receive an accurate result for the actually incident photon flux in the irradiated volume is actinometry. For this measurement technique, a chemical reaction with well-known characteristics but insensitive towards thermally driven reactions is performed directly in the reactor. In addition to the obvious advantages of the implicitly included effects of transmission, reflection, and scattering, the experiments seem to be basically simple.
However, it is not trivial to choose the appropriate parameters for the actinometric measurement and subsequently to evaluate the measured data correctly. Many studies within the last decades identify possible sources of error in the experimental procedure and assess the deviation to the accurate result caused by unawareness or ignorance [15, 16]. Furthermore, most of them propose a different, sometimes contradictory redesign of the experimental steps and analytics [17,18,19,20]. Nevertheless, even if solutions to overcome those obstacles are identified, often new problems arise but are supposed to be less influential than the original issue [21, 22]. Another attempt to enhance the quality of the calculated actinometry result is to refine specific physical characteristics or to introduce more probable reaction mechanisms and kinetics [23, 24]. The creation of models with different simplification levels, e.g. for the irradiated spectrum or the time dependent absorbance, finally leads to in itself conclusive results, while the grade of detail understandably questions the comparability between different setups [25,26,27].
Hence, systematical analytical criteria are missing. In order to enable comparison between different studies, the data must at least be presented together with a precise description of the experiment as well as the subsequent modeling and evaluation steps, as stated by the IUPAC report from Kuhn et al. [28]. Despite this, exact preparation and methodological information associated with practical recommendations and calculational details are only rarely published [29,30,31,32,33].
It is elementary to gain understanding of photon pathways to proceed to systematical photoreactor development instead of conducting empiric experiment series in specific or improvised photoreactors. Most established actinometers, represented e.g. by the popular ferrioxalate actinometer originating from 1956 [34], have been invented and optimized for application in batch reactors. In recent decades, various reactors with flexible volumina and numerous light sources have been combined. Changing the operation mode from batch to flow in intensified microphotoreactors makes photochemical processes interesting for industrial application, but requires a supporting systematic evaluation and optimization procedure. For that reason, a sophisticated measurement and calculation procedure for actinometry needs to be established. Depending on the reactor setup, process intensification exposes additional pitfalls that need to be circumvented [35].
To bridge the currently existing gap between the required experimental characterization of intensified photoreactors and generation of valid data, this work will discuss possible pitfalls during experimental and theoretical handling of actinometric experiments. A generic discussion will be underlined by examples to raise awareness for a well-planned photonic characterization of high-performance reactors.
Discussion
This section covers the entire procedure of actinometer measurements, including the data flow. Besides, the mathematical method to calculate the incident photon flux from of the conversion measured by actinometry is derived. Influencing factors of the experimental setup are discussed and a universally applicable working procedure for actinometric measurements is described. Selected steps are explained to highlight the most important pitfalls of the whole procedure and to illustrate identifiers with fictitious data as needed. The discussion of pitfalls will follow the actual working sequence and can be tracked in the flow diagram of Fig. 1.
Choice of the actinometric system (Step  )
)
The first criterion to find a suited actinometer is the overlap of the incident wavelength and the reaction-inducing absorption spectrum of the actinometric system. Based on measurement sensitivity in the desired wavelength range, the availability of actinometer system components, and chemical experience, the suited actinometer should be chosen. Figure 2 depicts the absorption coefficient of three selected actinometers that cover the most interesting wavelength ranges for photochemical reactions. Their reaction equations are given in Fig. 3.
The photochemical reaction of the last system is reversible, thus two absorption spectra are shown. For a meaningful evaluation of experimental actinometric results, the processes of light absorption must be reliably calculable. The Beer-Lambert’s law
correlates the absorbance A of a dissolved species with its concentration c and is linearly proportional to the substance-specific molar absorption coefficient ελ (wavelength dependent) and the optical path length l.
To record the absorbance properly, care should be taken of the kind of absorption coefficient which can be either decadic (namely ελ) or napierian (κλ, see Eq. 3) following the IUPAC definition [28]. The transmission T scales inversely exponential with the path length:
Verification of the physical and chemical properties of the actinometric system (Step  )
)
Quantum Yield
The quantum yield Φλ of actinometric reactions varies with different wavelengths. Sometimes, the spectral range of an actinometer’s sensitivity is narrow enough to neglect the change, but e.g. for the most widely used ferrioxalate actinometer, an incorrect quantum yield significantly affects the calculated results [18, 23, 24]. This dependence is convincingly illustrated by Fig. 4. The only ways to get information about the wavelength-resolved quantum yield is to either rely on available literature or to generate missing data with measurements incorporating a tunable, strictly monochromatic light source.
Dependence of the quantum yield on the wavelength for the ferrioxalate actinometer according to Murov et al. [18]
Absorption Spectrum
Prior to all actinometric measurements, the precise determination of the absorption spectra of the relevant actinometer species (for both the reactant and the product) must be ensured. The reactant spectrum is required for the ascertainment of the initial concentration of the actinometer as well as for the mathematical prediction and practical monitoring of its change (see also section for the adjustment of f). The absorbance spectrum of the product is required to evaluate shadowing effects during irradiation and furthermore plays an important role in the final calculation of the photon flux. Although the absorption spectra of actinometers are all available in literature, they should also be checked experimentally to exclude spectrometer or preparation specific errors, e.g. missing the absorbance maximum or a wrong initial concentration of the actinometer solution.
Actinometer Calibration
Required calibration data can be gathered either during online measurement of the concentration of the relevant species during irradiation or by an experimental series during which the irradiation time is varied over a broad range. Figure 5 shows how the calibration curves should look like to perform a trustworthy quantification of the respective actinometer product (circles). Examples for systematic errors, e.g. wrong pH-range for the formation of the ferroin complex in case of the ferrioxalate actinometer (top) or an already degenerated Reinecke’s salt as reactant (bottom) leading to a lower maximum conversion are indicated by crosses.
Correlation Range
In combination with a reliable calibration, the conversion range of linear correlation to the irradiation time is an absolute applicability criterion, i.e. the conversion rate must remain constant regardless of the degree of conversion achieved to this time. Otherwise, if the actinometer conversion rate is no longer quantitatively proportional to the irradiated photon flux, measured values have no significance as no accurate calculation of the irradiated photon flux is possible. Especially in photoreactors with an inhomogeneous irradiation field, the deviation in the conversion rate of different reactor sections would have an additional biasing effect on the detected photon flux.
Physical Limits
Attention must be paid to the physical and chemical limits of the actinometer. A critical aspect is the solubility of all species within the correlation range that must be high enough to keep all components in the liquid phase. Precipitation does not only cause shadowing of the reactants but also affects the chemical equilibria of the actinometer system. Also gas-formation as observed for some actinometric systems can become a problematic issue, especially in flow reactors with small capillary diameters. The actinometer volume is alterated by gas bubbles that cannot escape and thus the measured results are falsified.
Furthermore, for some actinometers, including the most used and established ones ferrioxalate and Reinecke’s salt, the pH of the reaction solution during the irradiation as well as in subsequent analytics is of importance. The amount of converted reactant may vary by a wrong pH-range due to protonation, reduction, or at least changed electronic conditions. In consequence, the quantum yield changes and implies a divergent irradiated photon flux. The same can happen in the analytical process where often dilution takes place or in particular quantitative reactions (e.g. ligand exchanges) are used for analytics. At non-ideal pH values, repeatedly wrong concentration measurements cause systematic errors (see also in the section about reproducibility).
As this issue concerning a systematic error of a wrong slope of the calibration curve (case 1) is not intuitively obvious, a closer look must be taken on the complete measurement procedure to further illustrate this potential pitfall. It arises for example in the application of the ferrioxalate actinometer, where the photochemical reaction of the ferrioxalate system is followed by dilution as well as buffering and addition of 1,10-phenanthroline as a selective coloring complexing agent for the photochemically generated Fe2+. Then, the concentration of Fe2+ can be spectrometrically determined after a certain time that ensures quantitative formation of the intensively red ferroin complex. Based on this measurement, the conversion rate and subsequently the incident photon flux is calculated. However, if the diluted actinometer solution is not sufficiently buffered and the pH value gets too low, a distinc ratio of ferroin is destroyed and the measured concentration systematically lowered.
Data acquisition of the experimental setup (Step  )
)
The arrangement of the experimental setup has a distinct influence on the incident photon flux. The fundamental irradiation concepts are shown in Fig. 6. Depending on the geometric emission characteristics of the light source, the parallel plate, cylindrical or annular irradiation concept might be suited best. The spherical shell reactor raises specific requirements to the light source since powering of the light source has to occur either wireless or with wires that are sufficiently encapsulated to be protected from liquids. This leads to a quite rare number of actual implementations. Distance and geometric overlap between emission of the light source and absorption area of the reactor in particular have a considerable influence on the overall efficiency and thus should be optimized prior to any experimental work.
Generic photoreactor setups: the parallel plate a, cylindrical b, annular c and spherical shell d geometry [14]
Transmission of the Reactor Material
The same applies for the transparency of the irradiated reactor component that ideally provides the highest transmission possible for the required wavelength range for initiating the reaction but still offers the necessary manufacturing properties. For instance, it is difficult to manufacture circular bent quartz glass tubes that are mechanically stable enough, so polyfluorinated plastic capillaries that are way easier to handle are often used.
The wavelength-dependent transmission of the reactor material \( T_{\lambda }^{\textup {mat}} \) is recommended to be determined individually and logged as an explicit spectrum. Pyrex (commonly used in batch photoreactors) or FEP capillaries (in flow photoreactors) for example are known to be not transparent for photons below 300 nm, nevertheless the transmission spectrum does not abruptly drop from 1 to 0 at this point. Hence, the change in transmission has to be considered accordingly.
Density Function of the Light Source
The last wavelength-dependent quantity to be introduced is the density function of the light source gλ. It specifies the spectrally resolved ratio of all emitted photons and usually is characteristic for a distinct type of light source (compare Fig. 7).
Emission density function of a 365 nm NICHIA NVSU233A UV SMD-LED and a medium pressure mercury vapor lamp (MVL) from Peschl Ultraviolet [37]
During evaluation of experimental raw data, the assumption of a monochromatic or discrete emission spectrum of the light source is an important restriction. All light sources used in photochemistry (apart from lasers) have a narrow to broad polychromatic emission spectrum. Even if the spectrum itself is exactly symmetric, the energy of a photon within this range changes with its wavelength, meaning that the photon flux is not distributed symmetrically around the main emission wavelength but is lower at shorter wavelengths. Even if the photon energy could still be mathematically averaged, the impact on the correlation with other wavelength dependent quantities may lead to major errors in calculation. For instance, changes of wavelength- dependent properties, e.g. the absorption coefficient or the quantum yield, are ignored within the real emission range and limited to the value of the wavelength of the incorrectly assumed monochromatic emission line. Consequently, the density function g calculated from the spectral distribution and the overall output power given by the data sheet of the light source considers the varying number and energy of photons emitted at different wavelengths and must not be neglected in the photon flux calculation. Ideally, the density function g is measured with a suited spectrometer.
Total Volume and Irradiated Volume
The inner volume of photochemical reactors to be evaluated by actinometry should be precisely determined, distinguishing between total volume and irradiated reactor volume if different. Referring specifically to capillary flow reactors, two measurement methods are possible: a standard steady state experiment and a stop flow experiment that might be easier to perform and needs less actinometer solution. In this latter case, feeding lines or windings that are filled with actinometer do not lie within the actual irradiation zone of the reactor light source. This actinometer volume later dilutes the sample solution, consequently causing errors in the concentration determination later on if not considered correctly.
Adjustment of the absorption fraction within experimental limits (Step  )
)
The absorption fraction f is another important photochemical quantity. It changes with the wavelength λ and, per definition, adds up to 1 with the transmission:
The actinometer concentration cact after a certain reaction time depends on the occurred conversion X and can be written as \( c_{_{0,\text {act}}} (1 - X) \). Combined with the Beer-Lambert law (Eq. 1), the absorption fraction turns to
Thus, the absorption fraction of the actinometer can decrease additionally during irradiation time as soon as the conversion X becomes large enough to make the assumption of a complete absorption of photons no longer valid (f < 1). Starting from this moment, the absorption fraction has to be considered during numerical evaluation of the experiments to avoid neglecting photons that are no longer absorbed. As this influence increases non-linear with time and is different for every wavelength, the absorption fraction needs to be evaluated differentially.
Since actually measuring photons is better than recovering them in the aftermaths, the absorption fraction of the applied actinometer should be close to f = 1 even during measurement if possible (compare Eq. 4). To avoid significant deviations due to this issue, a value of f > 0.99 is recommendable. This means that the resulting absorption fraction of a specific reactor setup needs to be mathematically predicted prior to the experiments, adapting the following parameters within the defined adjustment range (see sections about the correlation range and the physical limits):
-
light intensity (for controllable light sources),
-
concentration of the applied actinometer,
-
irradiation time in batch reactors, respectively the flow rate in continuous reactors.
Based on the made predictions, five suited irradiation times can be defined for the experiments in the photoreactor.
Actinometer preparation (Step  )
)
From the moment a liquid actinometer solution is prepared, it is important to check for solid particles. Concentration determination can be incorrect due to undissolved residues in the beginning or the actinometer can be shaded by precipitation during the measurement process (comp. section about physical limits). A further pitfall are the instruments and tools used during the whole experimental process as they must not influence the actinometer reaction system. For example, the acidic ferrioxalate solution is sensitive towards Fe2+, so the deployment of iron spatulas or insufficiently encapsulated iron stirring bars can already lead to an error. By using a simple standard reactor cell with fixed irradiation conditions prior to every set of measurement runs, absolute reproducibility can be guaranteed if the actinometer matches a series of independent measurements.
Experimental measurements (Step  )
)
Proposed Working Procedure
The experimental procedure proposed below already includes all strategies for avoiding measurement errors. After design of experiment, it is advised to work under dimmed red light. The use of microliter pipettes is suggested for preparation as well as in the potential dilution and development steps afterwards. The actinometer should be freshly prepared for every set of experiments without using metallic components that may affect the reaction. If it is planned to make stock solutions, awareness of the short- and long-term stability of the prepared solutions is necessary. Directly after that, a first non-irradiated zero sample should be taken to determine potential thermal conversion. This is followed by a reference measurement, e.g. irradiation under standard conditions to ensure the same concentration and reactivity of each solution. Then, the actual irradiation experiment in the reactor is carried out. This includes two measurement runs for every parameter combination including at least five flow rates (or reaction times). Subsequently, analyzing the second non-irradiated zero sample after the reactor measurements allows checking for light influence during the experiments. If required, the development steps are carried out (e.g. addition of a chemical developer and/or a buffer and waiting for the formation of the colored complex). As a last practical step, the concentration of the actinometer product is recorded, e.g. by triple UV/Vis measurements of all samples to rule out bad mixing, non-quantitative complexation or influence of the measurement method. Finally, script-based calculation the incident photon flux has to be conducted (see Eq. 9).
Multiple Samples for Reproducibility
The intrinsic characteristics of actinometers to integrally determine the photon flux independently of the irradiation time and intensity change should be verified by design of experiment. For this, zero samples are taken before the actual measurement to test for blind conversion at the beginning and afterwards to exclude undesired conversion by ambient light. While the reference measurement guarantees for general reproducibility of the actinometer setup, a series of runs yielding different conversions within an appropriate range (comp. section about the correlation range) proves the reproducibility of the irradiation setup. It is recommended to conduct at least two analytic evaluations in parallel for each of five irradiation time variation cycles per setup condition. Multiple sampling confirms the precision of the analytical steps, finally leading to a plot of the conversion at several irradiation times with almost no deviation of measurement points at the same conditions and a clearly straight fitting line (see Fig. 8, top). Apart from systematical errors, the precision of the slope is a promising basis for the subsequent photon flux calculation.
Plot of the measured correlation between the conversion of a ferrioxalate actinometer sample (0.16 molL− 1) irradiated with a mercury vapor lamp at different levels of shading (top). The calculated photon flux from these values via Eq. 9 is shown on the bottom
Following this instruction, the correct post-processing of measurement data can be verified if the calculated photon flux remains constant (see the horizontal fitting line in Fig. 8, bottom). Evaluating the precision of the fit, data handling can be further improved by identifying single errors in the procedure or subsequent modeling process.
Focusing on the detection of systematical errors in post-irradiational analysis (see also section Actinometer Calibration), again the ferrioxalate actinometer is taken as an example. Table 1 illustrates the influence of the sequence of adding chemicals as well as the impact of the pH-value during the development steps. While the addition sequence itself does not have an apparent effect on the measured absorbance and thus the formed amount of ferroin under the same irradiation conditions, a lower pH-value drastically lowers the detected absorbance. This is due to the destruction of a distinct fraction of the ferroin complex and can neither be countered by addition of the recommended amount of buffer solution or complexing agent nor by a longer development time. Actually, experimental results are systematically lowered and lead to wrong calibration or measurement curves as shown in Fig. 5 on the top. In contrast to that, the detected absorbance remains constant for low to medium acid concentrations (pH around 4.5) after 1 h if the developer/buffer is added before the dilution. Using 0.05 M H2SO4, the pH-value stays reliably in the optimal buffer range of 3.7 to 5.7. Even for the addition of pure demin. water, the concentration of the formed complex remains equally stable since the pH does not become too high. The apparently slightly better values are within the measurement error range, whereas the pH value is already shifted to higher values apart from the optimum of 4.7. Consequently, the dilution with 0.05 M H2SO4 is preferable.
An often underestimated factor in the analytics is the time between generation and evaluation of received conversion. Some photosensitive chemicals are also susceptible to thermal or time dependent degradation. This also accounts for the quantitative development of complexes formed with the photochemical products to get spectrometrically detectable species. Development times should be strictly obeyed to on the one hand guarantee an actually quantitative formation of the desired species and on the other hand to prevent degradation or other consecutive processes. To sum up, every analytical step must consider possible factors that may distort the results, e.g. pH-range, concentration of complexing agents, or development and storage time of the irradiated samples.
Work under Dimmed Red Light
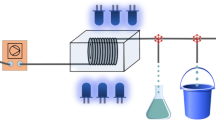
Since actinometers are sensitive towards light, working under exclusion of ambient and standard room light is inevitably. Even the use of reference samples or online-detection trying to measure the effect of ambient light cannot circumvent these experimental needs. In either way, the reaction rate of this pseudo-reference is retrospectively subtracted from all experimentally gained raw data. In detail, these two conceivable approaches dealing with ambient light are illustrated in Fig. 9.
On the left-hand side of Fig. 9, some actinometer is placed in a separated vessel (D) close to the reactor setup (C) to only quantify the ambient light irradiation (symbolized by light bulb A). This approach to conclude on the conversion generated exclusively by the isolated photoreaction is prone to miscalculation though [20]. Potential errors of the reference measurement are caused by unintentional irradiation from the light source of the experimental setup (B) or a difference in the ambient light conditions inside and outside the reactor. Additional sources of error are an actually different irradiation angle (α≠β) corresponding with the exact location of the reference (D) relative to the reactor (C), a difference in actinometer layer thickness of reference and sample, and the vessel materials. All of them add up to an inestimable deviation to the result of the intended, correct actinometer measurement.
On the right-hand side of Fig. 9, online-tracking of the irradiation chamber in a UV/Vis spectrometer (E) before and after turning on the light source of the setup to be photonically characterized (B) is shown. Alternatively to this setup, fiber optic cables can be installed instead of the spectrometer to adapt more sophisticated reactor geometries. Furthermore, it should be considered that the permanent irradiation by the spectrometer light source during the online measurement causes a conversion different to a reference sample. A fictional result of the online method is shown in Fig. 10. To receive the actually incident photon flux, the conversion rate within the actual irradiation time (dotted) is reduced by the slope outside this period (dashed). However, this method is not only highly biased by changes in ambient light conditions but also requires actinometer solutions with concentrations that have an absorption fraction of substantially less than f = 1, inducing the disadvantages referred to in the section about the adjustment of f.
Online Measurements
However, the limitations of online measurements under ambient light do not exclude online-analytics of photon fluxes in general. In flow photoreactors, online analysis is of great interest to implement simple and continuous measurements in the reactor system. As long as the actinometer fulfills the additional requirement to be an instantaneous reaction without the need of any developing agent or other additive, it can be a powerful tool to characterize even intensified flow reactors. A suitable example of the actinometers mentioned so far is Aberchrome 540 as it shows instant reactivity and the conversion can be detected via online UV/Vis spectrophotometry. The actinometer reported by Roibu et al. is another recent example [27].
Calculation of the actinometer conversion rate (Step  )
)
Another criterion to get reliable absolute values for the actually incident photon flux is to identify and consider blind conversion of the actinometer. In almost all actinometer systems, a small amount of actinometer has already reacted to the product prior to the application in the photoreactor and can be detected. Thus, a zero sample from the storage vessel is inevitable to be recorded, ideally before and after the measurement to make sure that no ambient light or thermal reactions have caused an additional conversion in the storage vessel while experiments are conducted. To calculate the conversion rate of the experimental runs, this initial conversion \( X_{_{0}} \) is subtracted from the value considering the starting concentration of the actinometer \( c_{_{0,\text {act}}}\), the dilution volume \( V_{_{\text {dil}}}\), the sample volume \( V_{_{\text {samp}}}\), the quantity for concentration determination (exemplary A for absorbance) and the slope of the calibration curve \( k_{_{\text {cal}}}\):
Even after this zero point calibration, it may occur that the linear regression line fits the irradiated measurement points very well, but does not cross the origin. A shift of the y-axis intercept is valid in this case, as the calculation of the received photon flux is based on the slope representing the conversion rate and which is still reliable if the correlation is strictly linear.
The y-axis intercept can also be shifted if parts of the actinometer remain unirradiated due to the reactor design and are not pumped within the reactor (comp. section about total and irradiated volume). This dilution of the irradiated actinometer solution must be taken into account with the blind conversion of the zero samples. Simply considering an additional virtual dilution step would neglect the existence of actinometer product and consequently lead to a systematic error. Therefore, the ratio of the total actinometer volume \( V_{_{\text {tot}}}\) and the actually irradiated actinometer volume \( V_{_{\text {irr}}}\) has to be factored in when calculating the conversion caused by intended irradiation. Equation 6 extends to:
Calculation of the photon flux (Step  )
)
Accurately calculating the photon flux relies on the physical and chemical characteristics of the actinometer system. Hence, their demands must be met before calculating any further numbers. Especially in highly intensified flow photoreactors, the calculation is quite sensitive towards slight changes in chemical substance data that will affect e.g. the linearity of the correlation or the degree of absorption. Multiple non-linear or wavelength-dependent factors can affect the final result. Calculations with the obtained raw data must be done carefully, in particular with regard to the declaration of measurement errors. An absorption or even conversion value seemingly being quite far from the linear regression line can still remain within strict reproducibility criteria. According to our experience, a reproducibility between 3 to 5% is achievable.
In addition, physical limits still play an important role as they can prevent a whole set of experimental data from being reasonably evaluated. A single species that exceeds the solubility product shades the subsequent photoreaction and distorts the equilibrium, especially if it remains in the capillary. This applies in particular for the precipitation occurring with the ferrioxalate actinometer that has been irradiated for too long, see Fig. 11.
In due consideration of these restrictions, the actual calculation can be executed. Incorporating the mentioned quantities so far, the equation for the reaction rate \( \frac {dX}{dt} \) to determine the incident photon flux \( q^{0}_{_{\mathrm {p}}}\) in the irradiated reactor volume Vr is:
Usually, Eq. 8 could be simplified as the absorption fraction can be kept close to f = 1 in batch reactors, but in highly intensified flow photoreactors, this is no longer valid by implication. Nonetheless, the absorption fraction f can be replaced by Eq. 5 to only include directly available parameter data. Self-determined experimental data and values from the literature for the wavelength-dependent quantities are most probably given in discrete wavelength steps. Discretizing the spectrum into elementary intervals Δλi finally gives the equation to calculate the incident photon flux:
Numerically solving the differential Eq. 9 for \( q^{0}_{_{\mathrm {p}}}\) yields the incident photon flux over the entire irradiated reactor volume. To apply the numerical findings to a reaction of interest, the spectral photon flux information can be used to predict the conversion rate by reversely entering \( q^{0}_{_{\mathrm {p}}}\) in Eq. 9. Combined with the known spectral data of the other relevant species, the conversion rate \( \frac {dX}{dt} \) can be predicted.
Wavelength Range for Evaluation
Depending on the selected spectral range to calculate the incident and emitted photon flux, different objective assessments can be made with the obtained result. To determine the overall efficiency of a photochemical setup, obviously the whole wavelength range of the light source should be taken as reference. If, instead, only an independent characterization of the geometrical match of light source and irradiated reactor volume is of interest, the emission spectrum must be limited to the actually light absorbing regions of the actinometer. This way, the efficiency loss due to the spectral mismatch of the light source emission and the photon flux detectable by the actinometer is excluded.
Conclusions
While actinometry offers the possibility to accurately measure incident photon fluxes in photoreactors, it can be concluded that it is absolutely not trivial to perform reliable actinometry, in particular in flow reactors. As a consequence, it is of utmost importance to always incorporate elaborated preliminary considerations to avoid explicit or systematic errors. Acquiring test data to evaluate the appropriate actinometer concentration and irradiation times is unavoidable.
Affiliated thereto without claiming completeness, we propose sticking to the recommended measurement procedure. Considering all the mentioned points finally ensures accurate and trustworthy results. An on-hands assistance might be the working step chart in Fig. 1.
Finally, the awareness of the characteristic limitations of actinometry must be mentioned at this point. The experiment and calculation is substantially dependent on correctly determined data sets for all spectra (e.g. absorption and emission spectrum, quantum yield, and transmission) and absolute values (emitted radiant power, reactor volumes, starting concentration of the actinometer). Experimental conversions intrinsically are integral values that are mathematically transformed to a wavelength-resolved result. For objective optimization of photoreactors, a large set of experiments is required that strongly depend on the accuracy of various experimental information. A reasonable attempt to address this disadvantage is the combination of actinometry and radiometry. Radiometry offers the possibility to determine locally resolved information on the photon flux density within the photoreactor setup while its lack in correct distinction between actually absorbed and e.g. scattered photons is compensated by actinometry. Mutual validation allows for a significant contribution to a holistic understanding of reaction processes in photoreactors and for closing the photon balance within the setup.
References
Oelgemöller M (2012) . Chem Eng Technol 35(7):1144. https://doi.org/10.1002/ceat.201200009
Gilmore K, Seeberger PH (2014) . Chem Rec 14(3):410. https://doi.org/10.1002/tcr.201402035
Oelgemöller M, Hoffmann N, Shvydkiv O (2014) . Aust J Chem 67(3):337. https://doi.org/10.1071/ch13591
Krüger K, Lüdke V, Pettinger J, Ashton L, Bonnet L, Motti CA, Lex J, Oelgemöller M (2018) . Tetrahedron Lett 59(14):1427. https://doi.org/10.1016/j.tetlet.2018.02.074
Radjagobalou R, Blanco JF, Dechy-Cabaret O, Oelgemöller M, Loubiére K (2018) . Chem Eng Proc - Process Intensification 130:214. https://doi.org/10.1016/j.cep.2018.05.015
Gutierrez AC, Jamison TF (2012) . J Flow Chem 1(1):24. https://doi.org/10.1556/jfchem.2011.00004
Williams JD, Otake Y, Coussanes G, Saridakis I, Maulide N, Kappe CO (2019) . Chem Photo Chem 3(5):229. https://doi.org/10.1002/cptc.201900017
Abdiaj I, Horn CR, Alcazar J (2018) . J Org Chem 84(8):4748. https://doi.org/10.1021/acs.joc.8b02358
Cambié D, Bottecchia C, Straathof NJW, Hessel V, Noël T (2016) . Chem Rev 116(17):10276. https://doi.org/10.1021/acs.chemrev.5b00707
Politano F, Oksdath-Mansilla G (2018) . Org Proc Res &, Dev 22(9):1045. https://doi.org/10.1021/acs.oprd.8b00213
Noël T (2017) . J Flow Chem 7(3–4):87. https://doi.org/10.1556/1846.2017.00022
Elliott LD, Knowles JP, Koovits PJ, Maskill KG, Ralph MJ, Lejeune G, Edwards LJ, Robinson RI, Clemens IR, Cox B, Pascoe DD, Koch G, Eberle M, Berry MB, Booker-Milburn KI (2014) . Chem Eur J 20(46):15226. https://doi.org/10.1002/chem.201404347
Cambié D, Zhao F, Hessel V, Debije MG, Noël T (2017) . React Chem Eng 2(4):561. https://doi.org/10.1039/c7re00077d
Sender M, Ziegenbalg D (2017) . Chem Ing Tech 89(9):1159. https://doi.org/10.1002/cite.201600191
Bowman WD, Demas JN (1976) . J Phys Chem 80(21):2434. https://doi.org/10.1021/j100562a025
Radjagobalou R, Blanco JF, da Silva Freitas VD, Supplis C, Gros F, Dechy-Cabaret O, Loubière K (2019) . J Photochem Photobiol A 382:111934. https://doi.org/10.1016/j.jphotochem.2019.111934
Taylor HA (1971) Analytical methods and techniques for actinometry. In: Dekker M (ed) Analytical Photochemistry and Photochemical Analysis: Solids, Solutions and Polymer
Murov SL (1993) Handbook of Photochemistry, vol. Sect 13 (Marcel Dekker)
Vincze L, Kemp TJ, Unwin PR (1999) . J Photochem Photobiol A 123(1-3):7. https://doi.org/10.1016/s1010-6030(99)00048-9
Lehóczki T, Józsa É, Ösz K (2013) . J Photochem Photobiol A 251:63. https://doi.org/10.1016/j.jphotochem.2012.10.005
Stookey LL (1970) . Anal Chem 42(7):779. https://doi.org/10.1021/ac60289a016
Kirk AD, Namasivayam C (1983) . Anal Chem 55(14):2428. https://doi.org/10.1021/ac00264a053
Demas JN, Bowman WD, Zalewski EF, Velapoldi RA (1981) . J Phys Chem 85(19):2766. https://doi.org/10.1021/j150619a015
Goldstein S, Rabani J (2008) . J Photochem Photobiol A 193(1): 50. https://doi.org/10.1016/j.jphotochem.2007.06.006
Sugimoto A, Fukuyama T, Sumino Y, Takagi M, Ryu I (2009) . Tetrahedron 65(8):1593. https://doi.org/10.1016/j.tet.2008.12.063
Aida S, Terao K, Nishiyama Y, Kakiuchi K, Oelgemöller M (2012) . Tetrahedron Lett 53(42):5578. https://doi.org/10.1016/j.tetlet.2012.07.143
Roibu A, Fransen S, Leblebici ME, Meir G, Gerven TV, Kuhn S (2018) . Sci Rep 8(1):5421. https://doi.org/10.1038/s41598-018-23735-2
Kuhn HJ, Braslavsky SE, Schmidt R (2004) . Pure Appl Chem 76(12):2105. https://doi.org/10.1351/pac200476122105
Aillet T, Loubière K, Dechy-Cabaret O, Prat L (2013) . Chem Eng Technol 64:38. https://doi.org/10.1016/j.cep.2012.10.017
Aillet T, Loubière K, Dechy-Cabaret O, Prat L (2014) . Int J Chem React Eng 12(1):1. https://doi.org/10.1515/ijcre-2013-0121
Rochatte V, Dahi G, Eskandari A, Dauchet J, Gros F, Roudet M, Cornet J (2017) . Chem Eng J 308:940. https://doi.org/10.1016/j.cej.2016.08.112
Wriedt B, Kowalczyk D, Ziegenbalg D (2018) . Chem Photo Chem 2(10):913. https://doi.org/10.1002/cptc.201800106
Reinfelds M, Hermanns V, Halbritter T, Wachtveitl J, Braun M, Slanina T, Heckel A (2019) . Chem Photo Chem 3(5):441
Hatchard CG, Parker CA (1956) . Proc Roy Soc A 235(1203):518. https://doi.org/10.1098/rspa.1956.0102
Valkai L, Marton A, Horváth AK (2019) J Photochem. Photobiol., A p 112021. https://doi.org/10.1016/j.jphotochem.2019.112021
Wegner EE, Adamson AW (1966) . J Am Chem Soc 88(3):394. https://doi.org/10.1021/ja00955a003
Peschl ultraviolet: Emission spectra mercury vapor lamp
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wriedt, B., Ziegenbalg, D. Common pitfalls in chemical actinometry. J Flow Chem 10, 295–306 (2020). https://doi.org/10.1007/s41981-019-00072-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-019-00072-7




 )
)

 )
)

 )
)

 )
) )
) )
)


 )
) )
)