Abstract
Due to various scientific expeditions and tourism, there has been an increase in anthropogenic activities during the past 2–3 decades in Antarctica. Moreover, global contamination due to several freshly introduced chemicals like pesticides is on use since the previous century and even several issues contribute to pollution in Antarctica. Persistent organic pollutants (POPs) are persistent in nature and include some pesticides, polychlorinated biphenyls (PCBs) and dioxins. Due to volatile nature, these pollutants are transported across the earth by the “grasshopper effect”. During a 34th Indian Scientific Expedition to Antarctica in the austral summer of December 2014 to February 2015, a total of forty-five water samples from fifteen different lakes was collected from Grovnes peninsula, Larsemann Hill, East Antarctica. In these samples, twenty-seven compounds of POPs were estimated. POP’s level was found in the water samples from 10.00 to 75.00 pg/mL in different lakes. Occurrence of p,p′-DDT was identified in all lakes, but maximum concentration was found in L1E NG lake. This research confirms that Grovnes peninsula has a trace level of POPs and it is the initial state and needs to investigate further to preserve the pristine Antarctica. This manuscript provides an overview of the available sample preparation methods, analytical techniques and the concentration of POPs in the lakes of the Grovnes peninsula in a Larsemann Hill area, East Antarctica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
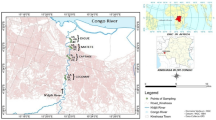
The Larsemann Hill (69o24′ S, 76o13′ E) is a series of islands and rocky peninsulas in Prydz Bay (Gillieson 1991). It contains two major peninsulas, the western named Stornes and the eastern Broknes, four minor peninsulas and ~ 130 near-shore islands. It is ~ 50 km2 polar oasis without ice on the Ingrid Christensen Coast, Princess Elizabeth Land, East Antarctica, located halfway between the southern boundary of the Vestfold Hill (410 km2) and the eastern boundary of the Amery Ice Shelf of Prydz Bay (Fig. 1). It is the second major among four ice-free oases found in East Antarctica’s coastline (Hodgson et al. 2005). Gillieson et al. (1990) stated that ~ 150 freshwater lakes are found in different peninsulas and islands of Larsemann Hill. Bolingen Island is the closest significant ice-free area, and it is situated ~ 25 km to the west-south-west, while Rauer Island is ~ 60 km to the northeast from Larsemann Hill. The hills are dissected by steep valleys lying between the ice sheet and the coast (Hodgson et al. 2001a) and include McLeod Island, Fisher Island, Broknes peninsula, Grovnes peninsula, Stornes peninsula and numerous other islands and nunataks. Grovnes peninsula is situated between the two major peninsula named as Stornes and Broknes. Bharati research station (69o24.41′ S, 76o11.72′ E) is India’s third Antarctic research facility and located on the Northern Grovnes between Quilty Bay and Thala Fjord, east of Stornes peninsula.
According to Bharti and Niyogi (2015), lakes are important creations on earth and provide water and valuable habitat for plants and animals. In Antarctica, some of the lakes are ice-free in the summertime from December to February when water temperature frequently exceeds from + 4 °C to + 8 °C. While in the winter due to the low temperature, lakes are covered with thick ice (~ 2 m) (Roberts and McMinn 1996; Hodgson et al. 2001b) and make Antarctica the largest stock of freshwater on the globe. Hodgson et al. (2001a) also described the closed system of lakes which contained V-shaped valleys normally around 50–100 m deep within a 1 km range. These freshwater lakes are found in basement rock basins in the Larsemann Hills and constitute a natural laboratory to investigate the anthropogenic impact on an untouched environment (Gasparon and Burgess 2000). Except some major lakes, most of the lakes near Larsemann Hills are recently formed (Gillieson et al. 1990).
According to the study of Abubakar (2007), the total size of Antarctica (14 million km2) is larger than the footprints of humans on that continent. While Legrand and Mayewski (1997) described that the atmosphere of Antarctica is affected by human activities, however, the impacts are not uniformly spread throughout the continent. Due to the increased anthropogenic activities and climate change, the environmental conditions of the continent are changing rapidly. Zhang et al. (2015) used the term final sink of many POPs for Antarctica because POPs are frequently detected in Antarctic biota. Corsolini et al. (2011) stated that POPs are known as the pollutants and elicit a dangerous effect on the Antarctic ecosystem.
POPs are toxic and semi-volatile in nature and persist in the environment for a long time. They may exist in natural as well as anthropogenic form and are used worldwide for most of the applications like fire suppressants, flame retardants, heat transfer agents, surfactants and pesticides. According to Wild et al. (2014), these anthropogenic chemicals are ubiquitous and resist from photolysis, biological and chemical degradation. Some POPs are now used in industrial processes, disease control and agriculture and contaminate all provinces of the globe, while some are endocrine disrupters and can damage the body systems like the endocrine system, reproductive system, immune system and nervous system (United Nations Environmental Program 2009). Long-term exposure to these chemicals can cause allergy, while exposure to high concentrations in the short-term may result in illness as well as death. Through direct exposure and industrial accidents, these pollutants have a harmful effect on the human. POPs have fatal properties and have a tendency to accumulate toward the pole (Wania et al. 1996). These pollutants migrate from one continent to another by the process of grasshopper effect, and in this mechanism, pollutants evaporate from a warmer place and condense in a colder place (Hund 2014). The long-range transport mechanism of POPs through the air, water and migratory species is described by Barrie et al. (1992) and Fuoco et al. (2009).
Several researchers have reported the occurrence of POPs in the Antarctic abiotic components such as lake water (Dickhut et al. 2005; Fuoco et al. 2005, 2009; Bharti and Niyogi 2015; Vecchiato et al. 2015), marine atmosphere/air (Montone et al. 2005; Gambaro et al. 2005; Choi et al. 2008; Li et al. 2012; Piazza et al. 2013; Kallenborn et al. 2013; Wang et al. 2017), soil/sediment (Borghini et al. 2005; Negri et al. 2006; Klanova et al. 2008; Hale et al. 2008; Park et al. 2010; Cabrerizo et al. 2012; Zhang et al. 2013; Vecchiato et al. 2015; Mwangi et al. 2016) and Snow (Dickhut et al. 2005) as well as in the biotic components of Antarctica such as lichen (Park et al. 2010; Mwangi et al. 2016), mosses (Borghini et al. 2005; Cipro et al. 2011; Zhang et al. 2015), krill (Corsolini et al. 2006; Bengstone et al. 2008), penguin (Corsolini et al. 2006; Corsolini et al. 2011; Vanden Brink et al. 2011; Wolschke et al. 2015; Montone et al. 2016) and skua blood (Bustnes et al. 2006; Taniguchi et al. 2008; Wolschke et al. 2015).
Bhardwaj et al. (2018) have presented a review on POPs, which were studied worldwide in biotic and abiotic components of Antarctica. Corsolini et al. (2011) stated that the scientific literature on the presence of POPs in the Antarctic ecosystem is still very limited due to the high cost of the scientific expedition and the difficulty of collecting samples. In this framework, the aim of this study is to contribute to the assessment of POP’s level in the Antarctic lake's water, which was collected during the austral summer of 2014–2015 from Grovnes peninsula. This study is one of the very few studies which analyzed many contaminants from this area. This datum will be helpful to understand the contamination level and monitoring of POPs in Grovnes peninsula of Larsemann Hill.
2 Materials and Methods
2.1 Study area
The sampling sites for the analysis of POPs were chosen on the Grovnes peninsula, and these sites are shown in Fig. 1.
2.2 Sampling
Total of 45 samples were collected randomly from fifteen lakes of the Northern Grovnes peninsula: L1C NG (69°24′25.43″ S and 76°11′17.66″ E), L1D NG (69°24′22.41″ S and 76°11′22.26″ E), L1E NG (69°24′23.51″ S and 76°11′25.20″ E), L3 NG (69°24′27.72″ S and 76°11′2.70″ E), L5 NG (69°24′32.83″ S and 76°10′45.75″ E), L6 NG (69°24′37.30″ S and 76°11′5.13″ E), L7 NG (69°24′34.32″ S and 76°11′39.41″ E), L7A NG (69°24′32.78″ S and 76°11′57.96″ E), L7B NG (69°24′30.05″ S and 76°11′57.38″ E), Murk Water Lake NG (69°24′53.37″ S and 76°12′46.16″ E) and Southern Grovnes peninsula: L1 SG (69°25′13.70″ S and 76°13′18.33″ E), L2 SG (69°25′ 5.10″ S and 76°12′45.05″ E), L3 SG (69°25′09.07″ S and 76°12′36.1″ E), L4 SG (69°25′04.46″ S and 76°12′19.93″ E), L5 SG (69°25′08.65″ S and 76°11′53.9″ E) in the month of December 2014 to February, 2015.
One liter of lake water sample was collected in amber color sterile polypropylene bottles in triplicate from each lake. Samples were immediately stored at 4 °C and then transported to the analytical laboratory for the analysis of POPs. The sampling sites of Northern Grovnes and the Southern Grovnes peninsula are given in Figs. 2 and 3.
2.3 Equipment and Reagents
GC–MS/MS (gas chromatography–mass spectrometry) (Model No.-7000C, Agilent), rotary evaporator and electronic balance (0.01 mg to 220 g) were used for the chemical analysis.
Standards for POPs from AccuStandard (Batch No.-211111006 for PCBs and 214061235-03 for pesticides) were procured for the analysis of POPs. Hexane and dichloromethane were purchased from Merck. Sodium sulfate and silica gel of mesh size (100/200) were purchased from Fisher Scientific. Prior to their use, sodium sulfate was kept at 400 °C for 4 h and silica gel was kept at 130 °C for 16 h.
2.4 Sample Extraction and Cleanup
2.4.1 Extraction Process
Before extraction, the lake water samples were filtered through a filter paper (Whatman No. 41) and were stored at 4 °C until the analysis. By using USEPA-3510B (1994) method, persistent pollutants were extracted from the water sample. Each one liter lake water sample was taken into a separatory funnel of 2000 mL with the help of one liter graduated measuring cylinder, and then, 60 mL dichloromethane was added. The funnel was clapped for 1–2 min and then kept for 10 min to allow the separation of the organic layer from the water phase. The organic layer was then filtered through the filter paper into a round-bottom flask of 250 mL. This procedure was repeated two more times, and total solvent extracts were collected in the same flask. The total volume of the organic layer was evaporated at 45 °C up to approx. 2 mL by rotary evaporator. Then, the final 2 mL residue solution was ready for the cleanup.
2.4.2 Cleanup Process
By using USEPA-3630C (1996) method, final solutions of the lake water samples were cleaned up. Approx. 5 g silica gel was filled into a glass column (10 mm width × 250 mm length) after that sodium sulfate was transferred into a glass column. Before passing the samples, a mixture of 50 mL solution of hexane and dichloromethane (1:1) was passed through the column; then, the final extracted solution was transferred into the glass column. After that 50 mL, hexane was transferred into the column and the process was repeated again. The column elution was collected into 250 mL of the round-bottom (RB) flask. The collected fraction of the sample was evaporated at 45 °C through the rotary evaporator, and then, the sample was re-dissolved into 1 mL with hexane. After that, the sample was transferred into a GC vial for the estimation of POPs.
2.5 Instrumental Analysis
GC–MS/MS (Agilent) system was used for the analysis of POPs. The MS system was operated in an EI + mode at the resolution > 10,000. The final solution of the sample (1µL) was introduced into the GC–MS/MS. The capillary column HP-5MS (30 m × 0.25 mm × 0.25 µm) was used for the analysis. The carrier gas was the helium with a constant flow of 1 mL/min. The injector mode was a pulsed splitless with a pressure of 27.5 psi and a temperature of 280 °C. The GC programming was as: The primary temperature was 70 °C and hold for 2 min, then ramped up to 150 °C at 25 °C/min, then ramped up to 200 °C at 3 °C/min, finally ramped up to 280 °C at 8 °C /min and hold for 10 min. The time of the GC cycle was 41.87 min. The detector makeup gas was nitrogen, and the temperature was 280 °C. The temperature of the transfer line was 280 °C. The temperature of the quadrupoles Q1 and Q2 was 180 °C. Nitrogen was the collision gas with a flow rate—1.5 mL/min. MRM (multiple reaction monitoring) of these compounds is shown in Table S.
2.6 Quantitative Analysis
POP’s compounds were analyzed relative to their standards through the GC–MS/MS system. One blank with every set of samples was analyzed throughout the procedure to check for interference and laboratory contamination. Every sample was examined in triplicate, and recovery rates were 40–100%, and this lies between the limit of the USEPA-1699 (2007) method. The analytical limit of detection (LOD) was based on the signal to noise ratio. The detection limit for POPs was evaluated as mean blank + 3SD (standard deviation), and the value was 10.00 pg/mL. The average value of the replicates was taken.
3 Result
The concentrations of POPs varied from 10.00 to 75.00 pg/mL in fifteen different lake water samples, and these concentrations are shown in Figs. 4 and 5 and Tables 1 and 2. The main pollutant was p,p ′-DDT, and the concentration was varied from 15.67 ± 2.08 to 75.00 ± 4.00 pg/mL, followed by endosulfan I (alpha isomer) 11.33 ± 1.53 to 46.33 ± 4.51 pg/mL, aldrin 10.67 ± 0.58–40.33 ± 1.15 pg/mL, PCB 31 10.33 ± 0.58–30.33 ± 0.58 pg/mL, PCB 143 10.33 ± 0.58–30.33 ± 1.53 pg/mL, o,p′-DDT 11.33 ± 1.53–30.00 ± 2.65 pg/mL, endosulfan II (beta isomer) 10.67 ± 0.58–27.67 ± 1.53 pg/mL, PCB 180 10.33 ± 0.58–27.67 ± 3.51 pg/mL, PCB 44 20.33 ± 0.58–23.67 ± 2.08 pg/mL, PCB 118 10.33 ± 0.58–23.67 ± 1.53 pg/mL, hexachlorobenzene 12.67 ± 2.08–23.00 ± 2.00 pg/mL, endrin 10.33 ± 0.58–22.00 ± 1.00 pg/mL, PCB 28 21.67 ± 3.06 pg/mL, PCB 138 12.67 ± 1.53–21.67 ± 1.15 pg/mL, PCB 105 10.33 ± 0.58–19.00 ± 2.00 pg/mL, dieldrin 11.00 ± 1.00–17.00 ± 1.73 pg/mL, α-HCH 11.67 ± 0.58–14.33 ± 2.31 pg/mL, PCB 18 11.00 ± 1.00–13.67 ± 1.15 pg/mL, γ-HCH 10.33 ± 0.58–13.00 ± 2.65 pg/mL, β-HCH 10.33 ± 0.58–12.67 ± 3.06 pg/mL, and other pollutants were below their LOD in all the different fifteen lakes of Northern and Southern Grovnes peninsula. A general trend of the POP’s residue is shown in bar diagram with error bars in Figs. 4 and 5.
4 Discussion
The outcome of the present study specifies that lakes of Grovnes peninsula have been polluted by traces of contaminants. DDT was detected earlier by several scientists in varying concentrations (5 to ~ 15,000 pg/L or pg/g) from the water, soil, sediment, air, snow, mosses and lichen samples (Borghini et al. 2005; Zhang et al. 2007, 2015; Cabrerizo et al. 2012). Several researchers stated that DDT was banned in 1972, but in some countries, it is still in use to control malaria (Klanova et al. 2007; Ali et al. 2014; Geisz et al. 2008). According to UNEP (2008) in 2003, more than 6300t of DDT was produced only in India. So it is not unexpected to find the trace amount of DDT in the ecosystem of Antarctica. DDT is released from stockpiles due to global warming and transported through migrating seabirds UNEP/AMAP (2011). According to Li and Macdonald (2005), the usage of DDT has declined significantly from the late 1960s. Our findings of p,p′-DDT from the lakes of Grovnes peninsula re-confirm the continental migration of the contaminants.
However, many researchers observed that the presence of POPs in oceanic water samples is due to the melting of the glacial (Corsolini and Focardi 2000) and also due to recent climate changes (Geisz et al. 2008). Corsolini (2009) has studied the concentration of some pesticides and PCBs in emerald rockcod (Trematomus Bernacchii) of Terra Nova Bay in Ross Sea and observed that concentration declined from the 1980s to 1995s and then increased from 2000s. Our result has also found a higher level of endosulfan I (alpha isomer) after DDT. Apart from these two, aldrin, dieldrin, hexachlorobenzene, endrin, endosulfan II (beta isomer), γ-HCH, β-HCH and PCBs have also been found in trace amounts. Zhang et al. (2007) have reported the isomers or metabolites of HCH in Antarctica. Wu et al. (2013) explained that α and γ isomers of HCH are more volatile than β and they travel a long distance through the air, while β and δ isomers of HCH are environmentally and chemically stable compounds (Raina et al. 2007).
Several researchers have explained the accumulation and biomagnification character of POPs in a lower trophic level organism like krills, small fishes, etc., to the higher trophic level organism of the food chain (Bustnes et al. 2006; Corsolini et al. 2007; Geisz et al. 2008; Bengstone et al. 2008; Wolschke et al. 2015). Lakaschus et al. (2002) have reported that HCHs travel a long distance through the atmosphere and then accumulate in fish-eating seabirds. The POPs such as p,p ′-DDE have been reported in southern elephant seal (Mirounga leonina) and Weddell seal (Leptonychotes weddellii) by Goerke et al. (2004). However, the present study does not have the occurrence of any isomer of DDT and HCH in the lakes of Antarctica. This may be due to the non-degradation of the parent molecule in the low temperature of Antarctica. Like DDT, a higher amount of other POPs is also reported by several scientists in higher trophic level organisms such as PCB in brown skuas, HCH in Adélie penguins, HCB in south polar skuas and snow petrel (Taniguchi et al. 2008; Corsolini et al. 2011; Mwangi et al. 2016; Montone et al. 2016).
Some scientists have explained the bio-magnification and metabolic character of HCB in the Antarctic tropic webs (Norstrom 2002; Wania 2003). Corsolini et al. (2011) have described the PCBs in higher amounts in Adélie penguin, krills, snow petrel and eggs of south polar skua from East Antarctica. According to Fuoco et al. (2009), the highest concentration of PCBs was found in the water of the Ross Sea, while Zhang et al. (2013) have reported the PCB 28 in pore water. Klanova et al. (2008) have reported the POPs in soil/sediment samples of Antarctica. Schwarzenbach et al. (2003) have explained that PCBs and OCPs have a tendency to accumulate in higher amounts in lipids because lipids are the major accumulation compartment in biota. The findings of less chlorinated PCB congeners and higher contaminant in the samples of the lake water indicate that besides the anthropogenic activities in and around research stations, the transport due to the long range is the main cause of contamination in Antarctica. It also supports the evidence of the long-range atmospheric transport of contaminants in Antarctica.
From the present and previous study, it is concluded that the occurrence of persistent pollutants in the Antarctic residents because of biomagnification and the actions of these residents is further leading to pollution of the lake water, sediment and soil. Since the usage of most of the pesticides such as DDT and HCH was banned in various countries. Hence, “In The Future Research” besides the assessment of these contaminants, recently emerging contaminants need to be studied. Boekelheide et al. (2012) have explained that future research will diagnose the disease by changing the levels of persistent pollutants and exposure over a lifetime. As illustrated in this future brief, during the research on POPs complexities, a number of issues arise and are not compulsory that the class at the top of the food chain is mainly affected (European Commission 2017). POPs migration and impacts need to be studied carefully, and other newly pollutants should be considered.
This manuscript provides many explanations to be focused on persistent pollutant contamination and offers clear faith that researchers can decrease the use of persistent pollutants with the finding of the other replacements and these replacements may be other substances. Researchers should ensure that they cannot switch one chemical difficulty with another. Non-chemical alternatives may also be measured for the removal of these toxic pollutants from the globe.
5 Conclusion
POPs are a very dangerous part of the environment. The present report confirms the occurrence of persistent pollutants as informed prior to other different Antarctic areas. For the understanding of the contamination level of contaminants in a lower amount, we used to trace analysis instruments like GC–MS/MS with a lower detection limit. POPs are not used or manufactured in Antarctica, although they accumulate in Antarctic biodiversity and in its environment. If something is not done to decrease the amount of these contaminants soon, the pristine environment of Antarctica will suffer in the long run. For decreasing the risk of POPs, we need to rapidly phase out these pollutants and require a more effective monitoring system that could control the emission of these contaminates; otherwise, they can cause the death of humans and other animal species. The contamination level of the contaminants is highly diverse in different provinces of the same continent, and their level will change with the changing of the biogeochemical mechanism of the worldwide cycling, climate change and the destiny of these chemicals.
Anthropogenic activities in the Antarctica are increasing due to research and tourism, which affect its pristine environment. The fuel used by different vehicle resources is also a minor contributor of these pollutants. The use of plastics like water bottles, poly bags, personal care goods, etc. if these are not checked effectively, then they will pollute the Antarctic environment in future. Vegetables, fruits and other food items for the researchers and helpers also carry the POPs residue. Continued monitoring of these pollutants at a worldwide level is the main point to switch off its relocation from one continent to another continent and to protect the Antarctic biota as well as to decrease these contaminants’ contamination globally.
References
Abubakar B (2007) The impact of human of human activities in Africa, The north and south pole regions on the global climate change. In: AGU spring meeting abstracts
Ali U, Syed JH, Malik RN, Katsoyiannis A, Li J, Zhang G, Jones KC (2014) Organochlorine pesticides (OCPs) in South Asian region: a review. Sci Total Environ 476:705–717
Barrie LA, Gregor D, Hargrave B, Lake R, Muir D, Shearer R, Tracey B, Bidleman T (1992) Arctic contaminants: sources, occurrence and pathways. Sci Total Environ 122:1–74
Bengtson NSM, Poulsen AH, Kawaguchi S, Vetter W, Schlabach M (2008) Persistent organohalogen contaminant burdens in Antarctic krill (Euphausia superba) from the eastern Antarctic sector: a baseline study. Sci Total Environ 407:304–314
Bhardwaj L, Chauhan A, Ranjan A, Jindal T (2018) Persistent organic pollutants in biotic and abiotic components of antarctic pristine environment. Earth Syst Environ 2(1):35–54
Bharti PK, Niyogi UK (2015) assessment of pollution in a freshwater lake at Fisher Island, Larsemann Hills over East Antarctica. Sci Intern 3:25–30
Boekelheide K, Blumberg B, Chapin RE, Cote I, Graziano JH, Janesick A, Lane R, Lillycrop K, Myatt L, States JC, Thayer KA (2012) Predicting later-life outcomes of early-life exposures. Environ Health Perspect 120(10):1353–1361
Borghini F, Grimalt JO, Sanchez-Hernandez JC, Bargagli R (2005) Organochlorine pollutants in soils and mosses from Victoria Land (Antarctica). Chemosphere 58:271–278
Bustnes JO, Tveraa T, Henden JA, Varpe Ø, Janssen K, Skaare JU (2006) Organochlorines in Antarctic and Arctic avian top predators: a comparison between the South Polar Skua and two species of northern Hemisphere Gulls. Environ Sci Technol 40:2826–2831
Cabrerizo A, Dachs J, Barceló D, Jones KC (2012) Influence of organic matter content and human activities on the occurrence of organic pollutants in Antarctic Soils, Lichens, Grass, and Mosses. Environ Sci Technol 46:1396–1405
Choi SD, Baek SY, Chang YS, Wania F, Ikonomou MG, Yoon YJ, Park BK, Hong S (2008) Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean Arctic and Antarctic research stations: implications for long-range transport and local pollution. Environ Sci Technol 42:7125–7131
Cipro CVZ, Yogui GT, Bustamante P, Taniguchi S, Sericano JL, Montone RC (2011) Organic pollutants and their correlation with stable isotopes in vegetation from king George Island, Antarctica. Chemosphere 85:393–398
Corsolini S, Focardi S (2000) Bioconcentration of polychlorinated biphenyls in the pelagic food chain of the Ross Sea. Ross sea ecology. Springer, Berlin, pp 575–584
Corsolini S, Covaci A, Ademollo N, Focardi S, Schepens P (2006) Occurrence of organochlorine pesticides (OCPs) and their enantiomeric signatures, and concentrations of polybrominated diphenyl ethers (PBDEs) in the Adelie penguin food web, Antarctica. Environ Pollut 140:371–382
Corsolini S, Borghesi N, Schiamone A, Focardi S (2007) Polybrominated diphenyl ethers, polychlorinated dibenzo-dioxins, -furans, and -biphenyls in three species of Antarctic penguins. Environ Sci Pollut Res 14:421–429
Corsolini S (2009) Industrial contaminants in Antarctic biota. J Chromatogr A 1216(3):598–612
Corsolini S, Borghesi N, Ademollo N, Focardi S (2011) Chlorinated biphenyls and pesticides in migrating and resident seabirds from East and West Antarctica. Environ Int 37:1329–1335
Dickhut RM, Cincinelli A, Cochran M, Ducklow HW (2005) Atmospheric concentrations and air-water flux of organochlorine pesticides along the western Antarctic Peninsula. Environ Sci Technol 39:465–470
European Commission (2017) Future brief: persistent organic pollutants: towards a POP’s-free future. Sci Environ Policy. https://doi.org/10.2779/170269
Fuoco R, Giannarelli S, Wei Y, Abete C, Francesconi S, Termine M (2005) Polychlorobiphenyls and polycyclic aromatic hydrocarbons in the sea-surface micro-layer and the water column at Gerlache Inlet, Antarctica. J Environ Monit 7:1313–1319
Fuoco R, Giannarelli S, Wei Y, Ceccarini A, Abete C, Francesconi S, Termine M (2009) Persistent organic pollutants (POP’s) at Ross Sea (Antarctica). Microchem J 92:44–48
Gambaro A, Manodori L, Zangrando R, Cincinelli A, Capodaglio G, Cescon P (2005) Atmospheric PCB concentrations at Terra Nova Bay, Antarctica. Environ Sci Technol 39:9406–9411
Geisz HN, Dickhut RM, Cochran MA, Fraser WR, Ducklow HW (2008) Melting glaciers: a probable source of DDT to the Antarctic marine ecosystem. Environ Sci Technol 42:3958–4396
Gillieson D (1990) An atlas of the lakes of the Larsemann Hills, Princess Elizabeth Land, Antarctica (vol. 74). Australian National Antarctic Research Expeditions, Antarctic Division, Department of the Arts, Sport, the Environment, Tourism, and Territories.
Gasparon M, Burgess J (2000) Human impacts in Antarctica: trace-element geochemistry of freshwater lakes in the Larsemann Hills, East Antarctica. Environ Geol 39:963
Gillieson DS (1991) An environmental history of two freshwater lakes in the Larsemann Hills, Antarctica. Hydrobiologia 214(1):327–331
Goerke H, Weber K, Bornemann H, Ramdohr S, Plotz J (2004) Increasing levels and biomagnification of persistent organic pollutants (POP’s) in Antarctic biota. Mar Pollut Bullet 48:295–302
Hale RC, Kim SL, Harvey E, La-Guardia MJ, Mainor TM, Bush EO, Jacobs EM (2008) Antarctic research bases: local sources of polybrominated diphenyl ether (PBDE) flame retardants. Environ Sci Technol 42:1452–1457
Hodgson DA, Noon PE, Vyverman W, Bryant CL, Gore DB, Appleby P, Gilmour M, Verleyen E, Sabbe K, Jones VJ, Ellis-Evans JC (2001a) Were the Larsemann Hills ice-free through the last glacial maximum? Antarct Sci 13(4):440–454
Hodgson DA, Vyverman W, Sabbe K (2001b) Limnology and biology of saline lakes in the Rauer Island, Eastern Antarctica. Antarct Sci 13:255–270
Hodgson DA, Verleyen E, Sabbe K, Squier AH, Keely BJ, Leng MJ, Saunders KM, Vyverman W (2005) Late quaternary climate-driven environmental change in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment core. Quat Res 64(1):83–99
Hund AJ (2014) Antarctica and the Arctic Circle: a geographic encyclopedia of the Earth’s polar regions. Santa Barbara 2: ABC-CLIO.
Kallenborn R, Breivik K, Eckhardt S, Lunder CR, Manø S, Schlabach M, Stohl A (2013) Long-term monitoring of persistent organic pollutants (POP’s) at the Norwegian Troll station in Dronning Maud Land, Antarctica. Atmos Chem Phys 13:6983–6992
Klanova J, Eupr P, Kohoutek J, Harner T (2007) Assessing the influence of meteorological parameters on the performance of polyurethane foam-based passive air samplers. Environ Sci Technol 42(2):550–555
Klanova J, Matykiewiczova N, Macka Z, Prosek P, Laska K, Klan P (2008) Persistent organic pollutants in soils and sediments from James Ross Island, Antarctica. Environ Poll 152:416–423
Lakaschus S, Weber K, Wania F, Bruhn R, Schrems O (2002) The air sea equilibrium and time trend of hexachlorocyclohexanes in the Atlantic Ocean between the Arctic and Antarctica. Environ Sci Technol 36(2):138–145
Legrand M, Mayewski P (1997) Glaciochemistry of polar ice cores: a review. Rev Geophys 35(3):219–243
Li YF, Macdonald RW (2005) Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: a review. Sci Total Environ 342:87–106
Li Y, Geng D, Liu F, Wang T, Wang P, Zhang Q, Jiang G (2012) Study of PCBs and PBDEs in King George Island, Antarctica, using PUF passive air sampling. Atmos Environ 51:140–145
Montone RC, Taniguchi S, Boian C, Weber RR (2005) PCBs and chlorinated pesticides (DDTs, HCHs and HCB) in the atmosphere of the southwest Atlantic and Antarctic oceans. Mar Pollut Bull 50:778–782
Montone RC, Taniguchi S, Colabuono FI, Martinsn CC, Cipro CVZ, Barroso HS, Silva JD, Bicego MC, Weber RR (2016) Persistent organic pollutants and polycyclic aromatic hydrocarbons in penguins of the genus Pygoscelis in Admiralty Bay—an Antarctic specially managed area. Mar Pollut Bull 106:377–382
Mwangi JK, Lee WJ, Wang LC, Sung PJ, Fang LS, Lee YY, Chien GPC (2016) Persistent organic pollutants in the Antarctic coastal environment and their bioaccumulation in penguins. Environ Pollut 216:924–934
Negri A, Burns K, Boyle S, Brinkman D, Webster N (2006) Contamination in sediments, bivalves and sponges of McMurdo sound, Antarctica. Environ Poll 143:456–467
Norstrom RJ (2002) Understanding bioaccumulation of POP’s in food webs. Environ Sci Pollut Res 9(5):300–303
Park H, Lee SH, Kim M, Kim JH, Lim HS (2010) Polychlorinated biphenyl congeners in soils and lichens from king George Island, South Shetland Island, Antarctica. Antarct Sci 22(01):31–38
Piazza R, Gambaro A, Argiriadis E, Vecchiato M, Zambon S, Cescon P, Barbante C (2013) Development of a method for simultaneous analysis of PCDDs, PCDFs, PCBs, PBDEs, PCNs and PAHs in Antarctic air. Anal Bioanal Chem 405(2–3):917–932
Raina V, Hauser A, Buser HR, Rentsch D, Sharma P, Lal R, Holliger C, Poiger T, Müller MD, Kohler HPE (2007) Hydroxylated metabolites of β-and δ-hexachlorocyclohexane: bacterial formation, stereochemical configuration, and occurrence in groundwater at a former production site. Environ Sci Technol 41(12):4292–4298
Roberts D, McMinn A (1996) Relationships between surface sediment diatom assemblages and water chemistry gradients in saline lakes of the Vestfold Hills, Antarctica. Antarct Sci 8:331–341
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, 2nd edn. Wiley, Hoboken, p p1328
Taniguchi S, Montone RC, Bícego MC, Colabuono FI, Weber RR, Sericano JL (2008) Chlorinated pesticides, polychlorinated biphenyls and polycyclic aromatic hydrocarbons in the fat tissue of seabirds from King George Island, Antarctica. Mar Pollut Bull 58:129–133
UNEP (United Nations Environmental Program) (2008) Global status of DDT and its alternatives for use in vector control to prevent disease. Report of the UNEP expert group. Secretariat of the Stockholm Convention, Geneva, Switzerland. UNEP/ POP’s /DDT-BP,1/2.
UNEP (United Nations Environmental Program) (2009) Stockholm convention on persistent organic pollutants. UNEP/POPs/COP.4/38.
UNEP/AMAP (United Nations Environmental Program/Arctic Monitoring and Assessment Program) (2011) Climate change and POP’s: predicting the impacts. Report of the UNEP/AMAP expert group. Secretariat of the Stockholm Convention, Geneva, Switzerland, p 62
USEPA (US Environmental Protection Agency)-3510B (1994) Separatory funnel liquid liquid extraction. Revision 2.
USEPA (US Environmental Protection Agency)-3630C (1996) Silica gel cleanup. Revision 3, December 1996.
USEPA (US Environmental Protection Agency) (2007) Method 1699: pesticides in water, soil, sediment, Biosolids and Tissue by HRGC/HRMS.
Van den Brink NW, Riddle MJ, van den Heuvel-Greve M, Van Franeker JA (2011) Contrasting time trends of organic contaminants in Antarctic pelagic and benthic food webs. Mar Pollut Bull 62(1):128–132
Vecchiato M, Zambon S, Argiriadis E, Barbante C, Gambaro A, Piazza R (2015) Polychlorinated biphenyls (PCBs) and Polybrominated diphenyl ethers (PBDEs) in Antarctic ice-free areas: influence of local sources on lakes and soils. Microchem J 120:26–33
Wang P, Li Y, Zhang Q, Yang Q, Zhang L, Liu F, Fu J, Meng W, Wang D, Sun H, Zheng S, Hao Y, Liang Y, Jiang G (2017) Three-year monitoring of atmospheric PCBs and PBDEs at the Chinese Great Wall Station, West Antarctica: levels, chiral signature, environmental behaviors and source implication. Atmos Environ 150:407–416
Wania F, Mackay D (1996) tracking the distribution of persistent organic pollutants. Environ Sci Technol 30:390–396
Wania F (2003) Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions. Environ Sci Technol 37(7):1344–1351
Wild S, McLagan D, Schlabach M, Bossi R, Hawker D, Cropp R, King CK, Stark JS, Mondon J, Nash SB (2014) An Antarctic research station as a source of brominated and perfluorinated persistent organic pollutants to the local environment. Environ Sci Technol 49(1):103–112
Wolschke H, Meng XZ, Xie Z, Ebinghaus R, Cai M (2015) Novel flame retardants (N-FRs), polybrominated diphenyl ethers (PBDEs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in fish, penguin, and skua from King George Island Antarctica, Mar Pollut Bull 96:513–518
Wu CW, Zhang AP, Liu WP (2013) Risks from sediments contaminated with organochlorine pesticides in Hangzhou, China. Chemosphere 90:2341–2346
Zhang HS, Wang ZP, Lu B, Zhu C, Wu GH, Walter V (2007) Occurrence of organochlorine pollutants in the eggs and dropping-amended soil of Antarctic large animals and its ecological significance. Sci China Ser D 50:1086–1096
Zhang L, Dickhut R, Demaster D, Pohl K, Lohmann R (2013) Organochlorine pollutants in western Antarctic peninsula sediments and benthic deposit feeders. Environ Sci Technol 47:5643–5651
Zhang Q, Chen Z, Li Y, Wang P, Zhu C, Gao G, Xiao K, Sun H, Zheng S, Liang Y, Jiang G (2015) Occurrence of organochlorine pesticides in the environmental matrices from King George Island, West Antarctica. Environ Pollut 206:142–149
Acknowledgments
We would like to thank to the director of the National Centre for Polar and Ocean Research (NCPOR) and Ministry of Earth Sciences (MoES) for allowing our participation in the 34th Indian Scientific Expedition to Antarctica (ISEA). We are also gratified to the leader of the expedition for his motivation and constant support. We thank to the staff of the Bharati research station and fellow Antarctic researchers for their valuable support during the expedition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhardwaj, L.K., Jindal, T. Persistent Organic Pollutants in Lakes of Grovnes Peninsula at Larsemann Hill Area, East Antarctica. Earth Syst Environ 4, 349–358 (2020). https://doi.org/10.1007/s41748-020-00154-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41748-020-00154-w