Abstract
Persistent organic pollutants (POPs) are banned in almost all countries due to their adverse health effects while they are still present in the environment due to their persistence. As the dissipation and the emission factors of POPs change by temperature and other environmental factors current study aimed to determine selected POPs, including polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides (OCPs) in water and sediment samples collected from 12 stations located in Ankara River, Turkey, for 12 months. C-18 solid-phase extraction technique was used to extract organic pollutants and the analysis were performed using a validated gas chromatography-mass spectrometry method. DDE was the most frequently detected contaminant in water samples. Even though no PCB residues were present in water samples, PCB101 was the most common contaminant in sediment. Although both matrices had the least load of pollutants in winter, there was an increase in presence and concentration of pollutants from late spring to autumn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persistent organic pollutants (POPs) are chemical substances that persist in the environment, bioaccumulate and bio-magnify through the ecosystems including the food web, and pose significant risks through adverse effects on human health and environment. The most common POPs are organochlorine pesticides (such as DDT), industrial chemicals (such as polychlorinated biphenyls, PCBs), and unintentional by-products (such as dioxins and furans) (WHO 2018). Due to their potential for adverse health effects in human including neurological disorders, reproductive toxicity, endocrine disruption, and cancer, POPs received increasing attention worldwide (Yurdakok et al. 2015). As POPs are able to be transferred to water sources, monitoring programs for detecting the seasonal changes of their presence and possible sources of these pollutants have great importance for the environmental and public health risk management (Kuzukiran et al. 2016a).
Geographic differences and possible climatic and seasonal variation have a major influence in the POP release, along with distribution and degradation in the environment (Murayama et al. 2003). These environmental factors include temperature, pH, ionic strength, and the type of the sediments, where the hydrolysis rates of POPs affecting vapor pressure and water solubility are altered. On the other hand, photodegradation had only a few influence due to the nature of the compounds (Liu et al. 2017). Seasonal changes influence the fate and degradation of pesticides in hydrological systems mainly through temperature along with soil erosion, surface runoff, and other salinity changes. Intensive use of pesticides during certain seasons to get rid of insect-borne diseases also have an influence on the concentration differences. During warmer and wetter seasons, these compounds dissipate more through air and water resources (Unyimadu et al. 2017).
Anthropogenic activities due to habitation and agriculture play an important role in POP pollution in rivers. In this study, Ankara Stream was chosen as a natural model to determine the frequency of POPs and how they are affected by the environment in urban and rural areas. Ankara Stream is a small river with 140-km long flowing through the city of Ankara, Turkey, and splits the city nearly in half. This stream was polluted by municipal, industrial, and agricultural discharges for many years (Chambers of Environmental Engineers 2009). Several efforts for the improvement of this river including urban river restructuring such as greenways (Baris et al. 2010), waste water irrigation facilities in organized industrial districts, and application of good agricultural practices have been implemented. Along with these, Middle East’s largest sewage treatment plant facility was located along the flow direction of Ankara stream in Tatlar village of Sincan and operates since 2002 (Ankara Metropolitan Municipality 2014). Attempts for long-term monitoring pesticides and other persistent organic pollutants was implemented by the Ministry of Environment and Urbanization, Republic of Turkey (National Implementation Plan 2018). However, relevant data on POP loads in the major river of Ankara with regard to the seasonal changes and urban-rural differences is still missing. Therefore, in order to evaluate the seasonal changes and urban-rural differences, the aim of this study was to determine selected POPs including some polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), and organochlorine pesticides (OCPs) from water and sediment samples collected from 12 stations in Ankara River for 12 months.
Materials and methods
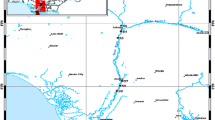
Water and sediment samples were collected from 12 stations (approximately 10 km distance from each other) between May 2014 and April 2015, with given coordinates of latitude/longitude as follows; region 1: 40,076017-32,954967; region 2: 39,880591-32,094051; region 3: 39,967106-32,865308; region 4: 39,845220-32,298216; region 5: 39,791209-32,373883; region 6: 39,888984-32,464936; region 7: 39,974548-32,579478; region 8: 39,948963-32,792255; region 9: 39,967011-32,865171; region 10: 39,983962-32,895106; region 11: 40,076046-32,955033; region 12: 40,224112-32,025506 (Fig. 1). Regions 1–6 represent rural section and 7–12 as urban sections. Due to the recreation, no sediment sample was available to be collected for regions 7–12. A total of 144 water and 72 sediment samples were collected and transferred to the laboratory at cold chain. Sediment samples were first dried at room temperature and analyzed within 48 h. Water samples are stored directly at − 20 °C, and analyzed within 48 h.
Standards of PCBs (PCB28, PCB30, PCB52, PCB101, PCB118, PCB138, PCB153, PCB180, PCB209) and OCPs [alfa- hexachlorocyclohexane (α-HCH), gamma-hexachlorocyclohexane (γ-HCH), hexachlorobenzene (HCB), heptachlor, p,p’-dichlorodiphenyl dichloroethane (p,p’-DDD), p,p’-dichlorodiphenyldichloro ethylene (p,p’-DDE), and p,p’- dichlorodiphenyl trichloroethane (p,p-DDT)] were purchased from Dr. Ehrenstorfer Laboratories (Augsburg, Germany) and PBDEs (PBDE17, PBDE47, PBDE66, PBDE100, PBDE153, PBDE183) were purchased from Wellington Laboratories (Guelph, Canada). PCB153-labeled 13C12 were purchased from Cambridge Isotope Laboratories (Andover, MA, USA). PCB 30, PCB 209, and PCB153-labeled 13C12 were used as the internal standards. Other chemicals and solvents used in the analysis were of analytical grade and from Merck (Darmstadt, Germany). Standard stock solutions and internal standards were prepared in acetonitrile and stored in the dark at ≤ 4 °C; while standard working dilutions were prepared from the stock daily.
Extraction and analysis of water and sediment samples were followed according to our previously adopted, optimized, and validated method (Kuzukiran et al. 2016b). In brief, homemade cartridge (10-mL glass syringe) for solid-phase extraction (SPE) filled with C18 (Agilent Technologies, Santa Clara, California-USA) were used for the extraction of water samples. SPE cartridge was conditioned with n-hexane, dichloromethane, methanol, and deionized water avoiding dryness (flow rate 5 mL/min). Following conditioning, 10 mL unfiltered water sample was passed and cartridge was rinsed with deionized water. It was then dried, using vacuum for 30 min and eluted using dichloromethane: hexane mixture (1:1, v/v). Extract was evaporated under nitrogen stream at 35 °C and resuspended in 90 μL isooctane. Samples were lastly fortified with 10 μg/L of injection internal standard of PCB30. Sediment samples were homogenized using a beaker and filtered through a 0.4-mm stainless steel sieve. Acetone mixed sample were kept in an ultrasonic bath (frequency 35 kHz, 0.32 kW, Super RK 510, Sonorex, Bandelin, Germany) for 10 min at 25 ± 2 °C and centrifuged at 4000 rpm for 10 min. Separated supernatant was dried under nitrogen at 35 °C and dissolved in acetone. Homemade SPE cartridge for sediment analysis included florisil (60–100 mesh), Bondesil-Primary Secondary Amine (PSA) (40 μm), and magnesium sulfate (Agilent Technologies, Santa Clara, California-USA). Before transferring the samples, cartridge was conditioned using ethyl acetate/acetone/hexane (5:2:1, v/v/v) and eluted with ethyl acetate/acetone/hexane (5:2:1, v/v/v) mixture. Collected eluate was evaporated, collected, and spiked as described in water extraction. Final samples were injected into the GC-MS. HP-5MS capillary column (30 m × 0.25 mm id, 0.25 μm film thickness) (Agilent Technologies, Palo Alto, CA, USA) was used for chromatographic separation.
A Polaris Q External Ionization Ion Trap GC-MS was used with split/splitless injector equipped with a 12-cm × 5-mm i.d. Silcoseeve liner (Thermo Finnigan, San Joe, CA, USA) in a splitless mode with temperatures of 280 °C for the injector, 270 °C for the transfer line, and 250 °C for the external ion source; where 2 μL sample was injected. Helium was used as a carrier gas with a constant flow rate of 1.0 mL/min. GC oven program and mass spectrometric conditions for quantitative measurement in selected ion monitoring (SIM) mode along with method validation parameters including LOD and LOQ values, recovery, and linearity parameters were described previously (Kuzukiran et al. 2016b).
Comparison between the rural and urban areas according to the frequency distribution was conducted by Pearson chi-square analysis using SPSS 14.0 software (SPSS, Chicago, IL, USA).
Results and discussion
The first samples collected in May 2014, from region 1, showed foamy appearance, where strong sulphur odor was evident along with household pollution; meanwhile, in other areas, this foamy appearance was much less evident and was not found to be present in the following months. Foam formed through surfactants by increased surface tension is mostly natural, through decomposed organic materials and more evident during warmer seasons. On the other hand, synthetic surfactants through anthropogenic sources (household cleaning products such as detergents, shampoos, toothpaste, and cosmetic) are also another source for foam formation (Swisher 1986).
Monthly differences of POPs in water and sediment samples collected from Ankara River are described in Tables 1 and 2, respectively. Among tested POPs in water; p,p’-DDE residues were detected the most, followed by p,p’-DDT. No residues of PCB were present, while traces of PBDE100, PBDE17, and PBDE47 were found. In terms of frequency, residues of p,p’-DDE were found 44 times, PBDE100 and p,p’-DDT 4 times, PBDE17 and PBDE47 1 time in water samples. p,p’-DDE is the major metabolite of DDT, which is a banned organochlorine pesticide; due to its long half-life, residues are still present probably due to its past local use or originated elsewhere and transported through the atmosphere (Kuzukiran et al. 2016b).
In sediment, the most common compound as organochlorine pesticides was γ-HCH (6 times) followed by p,p’-DDE (2 times). Contrary to the fact that PCBs were absent in water, they were found in sediment at a frequency of 13 samples for PCB101, 4 samples for PCB28, and 2 samples for PCB138 and PCB153. Only residues of PBDE66 was present in sediment samples collected in July and August. Overall, the most common contaminants in sediments were PCB101 (13 times), γ-HCH (6 times), PCB28 (4 times), PBDE66 (3 times), PCB138 (2 times), PCB153 (2 times), and p,p’-DDE (2 times). Concentrations of POPs in general were much higher in sediment samples compared to water. For both matrices, POP concentrations were found to be lower in winter period (December, January, February) compared to the rest of the months, while an increase was evident in the May–August period. Even though the frequency distribution of POPs was 27 in rural areas and 26 in urban areas, the difference was not found to be significant (p > 0.05).
Temperature averages for months were calculated from the daily-recorded temperatures. Since Ankara is located in the middle of Turkey, surrounded by mountains, the climate is terrestrial, where temperature differences are high in day-night (Fig. 2). According to the Ministry of Environment and Urbanization, monthly averages for winter, spring, summer, and autumn were 10.12, 20.22, 6.74, and 1.61 °C, respectively, while the highest average temperature was recorded in August as 22.57 and the lowest in January as − 0.98 °C. The monthly difference of PCBs could also be attributed to the distribution of particles (including the size and source such as the amount of resuspended sediment in trapped settling material) and the role of diagenesis of the organic matter on particles in the sediment samples. As the particle size decreases, time-to-equilibrium partitioning between water and particle phases also decreases (Robinson et al. 2008).
Although several studies are available for the pollution levels in Ankara River and surrounding water sources (Karakoc et al. 2003; Kazanci and Girgin 1998; Ozyurek et al. 2013), data compromising various POPs and monthly differences was not present. In 2012, 4 of the 10 Organized Industrial District (OID) compromising many different forms of industrial production and labor did not or have poor waste water treatment facilities. During these years, several attempts regarding Ankara Water and Sewerage Authority for the improvement of facilities and storage conditions along with prevention of leakage were conducted. Following 2013, plans for a wastewater treatment facility in Sincan Organized Industrial District (SOID) were complete and in March 2014, this facility was put in use. These OIDs compromise various sectors including the power plants, transistors, dye and chemicals, packaging, construction, and plastic materials along with other electric-electronic machinery producing a valuable contribution to cities economy. Among the waste materials, PCBs are the most common, since these compounds were widely used as lubricants, heat transfer agents, paint additives, and insulating media in capacitors, and voltage regulations due to their chemical stability, including low flammability, and high dielectric constant, where they are used in closed applications including coolants and insulating fluids (transformer oil) for transformers and capacitors. Endocrine disrupting health effects of these bioaccumulating compounds are the main drive for local authorities for finding the sources of these existing PCBs in the environment and develop monitoring activities (National Implementation Plan 2018).
In the study by Ozyurek et al. (2013), surface sediment samples in Ankara river collected around SOID in 2008, which also compromises the same sampling site of our research (region 5), were found to contain PCB residues of Arachlor 1254 and 1016 at 12485.5 and 777.6 ng/g (ppb) concentrations. In our study, sampling was done after the wastewater treatment facility was structured and functioned which was from May 2014. No residues of PCB were present in water, while only PCB101 was found in May samples at 1.192 ppb concentrations. Although there are studies concerning the wastewater treatment plants still have presence of these stable compounds (Mantis et al. 2005), these facilities along with appropriate governmental measures produce promising results, since PCB levels were much lowered according to our results.
Cagdas et al. (2017) screened PCBs, OCPs, and PBDEs in Buyuk Menderes River in the Aegean Sea Region. Similar to our results, even though its ban, OCP (DDT) were found in water samples from all stations at lower concentrations, probably due to its past usage, while no PCB and PBDEs were detected. In that study, the metabolite DDE were not screened, which might have been found at a higher concentrations, as in our study. Recently a training regarding POP emissions by unintentional industrial production was held by The United Nations Industrial Development Organization (UNIDO), including general information, sampling, and analysis of industrial chimney emissions, which might have a potential beneficiary influence on the industrial emissions of these contaminants in near future (UN 2017).
Conclusion
The current study revealed monthly presence and/or concentration of selected POPs in water and sediment samples collected from 12 stations including urban and rural regions of Ankara River. p,p’-DDE was the most detected contaminant in water samples. No PCB residues were detected in water samples. However, PCB101 was the most common contaminant in sediment. No differences between urban and rural regions were observed. For both matrices, winter period was found to have least load of contaminants, while an increase in the presence and concentration was present from late spring until autumn. This could be related to the temperature dependence of emission factors of POPs. Longer-term surveillance along with a broader spectrum of POPs is required to be implemented by authorities globally for the conservation of environment and public health.
References
Ankara Metropolitan Municipality (2014). Water and Sewerage Authority:Tatlar Wastewater Treatment Plant, http://www.aski.gov.tr/en/Icerik.aspx?detay=821&id=817. Accessed 10 March 2018.
Baris, M. E., Erdogan, E., Dilaver, Z., & Arslan, M. (2010). Greenways and the urban form: city of Ankara, Turkey. Biotechnology & Biotechnological Equipment, 24(1), 1657–1664.
Cagdas, B., Kocagoz, R., Onat, I., Percin, F., Ozaydin, O., & Orhan, H. (2017). Periodic monitoring of persistent organic pollutants and molecular damage in Cyprinus carpio from Büyük Menderes River. Environmental Science and Pollution Research, 24(5), 4241–4251.
Chamber of Environmental Engineers (2009). Environmental Status Report for Ankara. http://www.cmo.org.tr/resimler/ekler/11c1b89c6d18cdc_ek.pdf. Accessed 10 March 2018.
Karakoc, G., Unlu-Erkoc, F., & Katircioglu, H. (2003). Water quality and impacts of pollution sources for Eymir and Mogan Lakes (Turkey). Environment International, 29(1), 21–27.
Kazanci, N., & Girgin, S. (1998). Distribution of Oligochaeta species as bioindicators of organic pollution in Ankara Stream and their use in biomonitoring. Turkish Journal of Zoology, 22, 83–87.
Kuzukiran, O., Yurdakok-Dikmen, B., Filazi, A., Sevin, S., Aydin, F. G., & Tutun, H. (2016a). Determination of polychlorinated biphenyls in marine sediments by ultrasound-assisted isolation and dispersive liquid–liquid microextraction and gas chromatography–mass spectrometry. Analytical Letters, 49(15), 2525–2536.
Kuzukiran, O., Yurdakok-Dikmen, B., Totan, F. E., Celik, C., Orhan, E. C., Bilir, E. K., et al. (2016b). Analytical method development and validation for some persistent organic pollutants in water and sediments by gas chromatography mass spectrometry. International Journal of Environmental Research, 10(3), 401–410.
Liu, C., Zhang, L., Fan, C., Xu, F., Chen, K., & Gu, X. (2017). Temporal occurrence and sources of persistent organic pollutants in suspended particulate matter from the most heavily polluted river mouth of Lake Chaohu, China. Chemosphere, 174, 39–45.
Mantis, I., Voutsa, D., & Samara, C. (2005). Assessment of the environmental hazard from municipal and industrial wastewater treatment sludge by employing chemical and biological methods. Ecotoxicology and Environmental Safety, 62(3), 397–407.
Murayama, H., Takase, Y., Mitobe, H., Mukai, H., Ohzeki, T., Shimizu, K., & Kitayama, Y. (2003). Seasonal change of persistent organic pollutant concentrations in air at Niigata area, Japan. Chemosphere, 52(4), 683–694.
National Implementation Plan (2018). The Ministry of Environment and Urbanization, Republic of Turkey, General Directorate of Environmental Management Chemicals Management Department http://chemicals.csb.gov.tr/monitoring-i-5255. Accessed 10 March 2018.
Ozyurek, N. A., Gedik, K., Siltu, E., & Imamoglu, I. (2013). Levels and sources of polychlorinated biphenyls in Ankara creek sediments, Turkey. Journal of Environmental Science and Health, Part A, 48(7), 800–808.
Robinson, S. D., Landrum, P. F., Van Hoof, P. L., & Eadie, B. J. (2008). Seasonal variation of polychlorinated biphenyl congoners in surficial sediment, trapped settling material and suspended particulate material in Lake Michigan, USA. Environmental Toxicology and Chemistry., 27(2), 313–322.
Swisher, R. D. (1986). Surfactant biodegradation, second edition, revised and expanded. Volume 18 of surfactant science. New York-USA: Taylor and Francis.
UN (2017). United Nations Turkey Newsletter. UNIDO provides training on Persistent Organic Pollutants in Turkey. https://www.bmdergi.org/language/en/unido-provides-training-on-persistent-organic-pollutants-in-turkey/. Accessed 10 October 2018.
Unyimadu, J. P., Osibanjo, O., & Babayemi, J. O. (2017). Selected persistent organic pollutants (POPs) in water of River Niger: occurrence and distribution. Environmental Monitoring and Assessment, 190(1), 6.
WHO (2018). World Health Organization. Food safety, Persistent Organic Pollutants https://www.who.int/foodsafety/areas_work/chemical-risks/pops/en/. Accessed 10 October 2018.
Yurdakok, B., Tekin, K., Daskin, A., & Filazi, A. (2015). Effects of polychlorinated biphenyls 28, 30 and 118 on bovine spermatozoa in vitro. Reproduction in Domestic Animals, 50(1), 41–47.
Acknowledgements
The collection of the samples of this study was supported by Ankara University Scientific Research Projects by project number 13B3338010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sevin, S., Kuzukiran, O., Yurdakok-Dikmen, B. et al. Selected persistent organic pollutants levels in the Ankara River by months. Environ Monit Assess 190, 705 (2018). https://doi.org/10.1007/s10661-018-7072-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-7072-x