Abstract
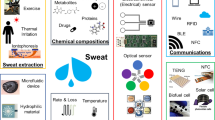
Sweat-based diagnostics offer an exciting avenue to noninvasively monitor analytes which had previously only been available through painful blood draws. Sweat is enriched with physiologically valuable information, and recent proteomic studies have identified numerous critical analytes which have highly correlated levels in blood, interstitial fluid and sweat. However, usage of sweat for health monitoring has not been studied extensively due to the substantial challenge of assembling a composite clinic-ready device. Recent advances in soft electronics have made this goal realizable, as these devices can perform electronic or optical monitoring on a flexible substrate using small volumes of liquid. While this field is still in its infancy, this review examines the physiological composition of sweat, various improvements in material science that improve sensor design, existing FDA approvals, methods of extracting sweat and comparisons to blood-based tests. Furthermore, this review assesses the critical challenges which must be overcome for this type of technology to make it out of research laboratories and into continuous clinical use. We believe that once properly harnessed, sweat-based diagnostics can provide patients a painless monitoring tool which can be customized to track a wide variety of medical conditions from the comfort of a patient’s own home.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sweat is enriched with electrolytes (Na+, Cl−, K+, NH4+, Ca2+, H+), metabolites (lactate, glucose, urea, uric acid and creatinine), proteins and peptides (interleukins, tumor necrosis factor) and xenobiotics (drugs, heavy metals and ethanol) [1, 2]. By collecting sweat, it is possible to monitor these numerous types of physiologically important molecules without any invasive procedure in real time. Thus, a sweat sensor when used as an in vitro diagnostic (IVD) device can be an excellent tool for continuous monitoring of disease markers.
IVD devices are the most convenient tools for a patient or clinical professional to evaluate, prevent or monitor a disease. IVD products are defined by the Food and Drug Administration (FDA) as any reagents, instruments and systems used for diagnosing disease or conditions. Here, the “diagnose” term encompasses the spectrum of cure, prevent or heal a condition [3]. The FDA further narrowed their definition of IVD by placing it under the umbrella of medical devices which can be used to determine health status. Those devices can be used for screening, risk assessment, staging and prognosis. The specimen types used in these devices are mainly whole blood, serum and cerebrospinal fluid. However, to diagnose cystic fibrosis the FDA recently cleared an IVD system which can induct and collect sweat to diagnose the disease [4].
The FDA closely monitors and regulates IVD devices since the introduction of the Medical Device Amendments of 1976. Many devices since then have received FDA approval and have been commercialized. For example, a single-lead ECG device to monitor eight different physiological features such as heart rate, heart rate variability, respiratory rate, skin temperature and posture has recently received FDA approval [5]. This patch can collect clinical-grade measurements and can transmit the data wirelessly to computers or cell phones. The Stat-Strip Glucose Hospital Meter System, used to measure glucose level in intensive care units (ICUs) patients, has also been recognized by the FDA as a valid platform to monitor blood glucose level [6]. Additional implantable electronic devices for cardiac applications have been reported since [7].
2 Sweat: An Introduction
The human body possesses 3 to 4 million eccrine sweat glands, which are distributed throughout the dermis layer of the skin [8]. However, at any given time only a small portion of these glands becomes active, indicating an enormous potential to secrete a large volume of sweat under critical circumstances. In a healthy individual, the sweat rate can range from 0.1 L/Hr to a maximum of 1.0 L/Hr [9]. On average, the sweat gland is 2–4 mm in length with an external diameter ranging from 30 to 60 μm [10]. The secretion of sweat can seem ordinary; however, the whole process is rather complex and requires participation of several receptors.
Sweat can be secreted from the sweat gland due to two different responses: thermoregulatory (via the hypothalamus) and limbic [12]. The sympathetic nerves distributed to sweat glands mainly consist of an abundant network of cholinergic nerve terminals and a scarce number of adrenergic terminals [13]. The amount of sweat release is determined by the amount of blood carried by the capillaries surrounding the sweat glands. Figure 1 shows the location of the sweat glands under skin.
Eccrine sweat glands in thick skin (left) and thin skin (right) [11]
Eccrine sweat glands have secretory coils and a duct [14]. The secretory coil consists of single layer of epithelial cells which has dark cells (granular), clear cells (agranular) and myoepithelial cells. Figure 2 shows the histology of the sweat glands [15]. The osmiophilic granules are released from the cells in order to produce sweat, whereas the clear cells are believed to be the core source of the production of sodium-chloride-rich fluid.
Histology of human sweat glands [15]
The secretory coils merge into a glandular duct. The duct is lined with epithelial cells and close to the surface of the skin it changes dramatically to a metabolically inactive opening to facilitate exit of the fluid [16]. Histology image of human sweat glands is shown in Fig. 2.
3 Sweat Generation
The plasma membrane of the sweat glands initiates a complex series of biochemical processes which alters the level of calcium within the cells. The Na+–K+ pump utilizes ATP to transport 3 Na+ ions out of the cells in exchange of 2 K+ ions into the cells. This pump ensures a low level of Na+ intracellularly. A blocker of the Na+–K+ pump has been found to block the secretion of sweat [17]. The outward movement of Cl− ions through calcium-activated chloride channels makes the lumen of the duct electronegative with respect to the surrounding cells. When Na+ combines with localized Cl− ions, it forms NaCl. The presence of NaCl generates an osmotic gradient and facilitates the movement of water through the duct.
4 Biomarkers Within Sweat
In a proteomic analysis, Yu et al. [18] found 861 unique proteins of which 57 were proteases and 38 were protease inhibitors. They also identified 32,818 endogenous peptides. Table 1 represents the concentration of commonly found ions and proteins in the blood and sweat.
4.1 Wearable Biosensors for Sweat Test
To create a skin-contact-based sensor, the electronics have to be flexible and soft. Soft electronics significantly expand the capabilities of conventional rigid electronics in sensing, monitoring and diagnosing functions. Figure 3 represents a sweat-based sensor on the skin surface. In the field of wearable soft electronics sensors, there are various materials of interest that could provide an ideal sensing platform. Hydrogels, nanomaterials and liquid metals are three fundamental materials used in the development of soft electronics.
4.1.1 Hydrogels
Applications of hydrogels in soft electronics have emerged in the past few years. Unique physicochemical properties such as stretchability, self-healing, biocompatibility and conductivity of hydrogels make them extremely suitable for wearable soft electronic sensors [25]. Additionally, major innovations in polymer chemistry, composite physics and micro- and nanofabrication have vastly expanded the capabilities of hydrogels. For example, the fundamental methods of synthesizing cellulose-based hydrogels are to dissolve the cellulose fibers and to introduce a physical and/or chemical cross-linking in order to form a 3-D hydrophilic network [26].
The physical process used during fabrication may include an interaction between ions or a hydrogen bond. Chemical processes are more complex and include different polymerization techniques, including chain-growth polymerization, irradiation polymerization and step-growth polymerization. Figure 4 demonstrates hydrogel design and structure under FESEM. Chemical routes have their own disadvantages, such as strong and irreversible covalent bonds which can lead to slow stimuli response, limited stretchability, and poor self-healing properties [28]. In contrast, the physical cross-linking methods are flexible and suitable for proper self-healing. However, it is critical to keep in mind that the physically cross-linked method exhibits excess stiffness, limiting its applications in soft electronics (Table 2).
The produced hydrogel rigidity increased after cross-linking (a–b). After infiltrating the hydrogel with phenol red, it appeared red in color (c). Field-emission scanning electron microscopy (FESEM) image representing the dense fibrous network structure in the gel (d) [27]
4.1.2 Nanomaterials
Nanomaterials have become an attractive tool to fabricate stretchable electronics. Nanomaterial-based conductors are made by the mixing of conductive fillers in a stretchable polymeric matrix [29]. The nanofillers allow for an electrical current pathway toward the composite since the nanofillers form a percolated network in the elastomer, allowing for the flow of current. The nanofillers can be categorized into the following three groups: 0D nanoparticles (NPs); 1D nanowires (NWs) and nanotubes (NTs) with high aspect ratios; and 2D nanosheets with large junction areas. Researchers also show interest to employ natural nanomaterials in designing new type of sensor. Figure 5 shows an example of a naturally occurring nanomaterial.
Natural nanomaterial formed by silica nanoparticles (150–300 nm) can interfere and diffract light propagation, resulting in extraordinary colored object [30]
4.1.3 Liquid Metals
Liquid metals are soft electronic sensors which have an ideal combination of conductivity and deformability properties. These two properties provide an environment in which the electromechanical properties of the device can be enhanced using this type of material. The most common liquid metals used are Ga and its alloys such as EGaIn (75% gallium and 25% indium) and galinstan (68.5% gallium, 21.5% indium and 10% tin) which are some of the least toxic liquid metals.
The liquid metals are typically embedded in fluidic channels of elastomers such as Ecoflex, poly(dimethylsiloxane) (PDMS), polyacrylates and block copolymer elastomers to form an intrinsically stretchable conductor [31]. The liquid metals do not add any mechanical loading to the carrying elastomers due to their fluidity. Thus, mechanical properties of the resulting conductor mainly depend on the carrier elastomeric matrix.
4.2 FDA-Approved Wearable Sensor
The revolutionary Abbott’s system is capable of real-time glucose monitoring without the hurdle of finger pricking [32, 33]. A small sensor is fixed on to the upper arm, and when scanned with a reader, it can reveal if a person is experiencing hypoglycemia (low level of blood glucose) or hyperglycemia (high blood glucose level). Figure 6 shows similar representation of a glucose monitoring system.
In 2002, the FDA approved GlucoWatch® G2 Biographer an automatic glucose biographer [34]. It can detect trends in collected data and track patterns of glucose levels when used in diabetic patient both at home or in clinical settings. The device is comfortable and can be worn as a wrist watch. When in use, it can send a low level of current to draw fluid onto the top of the skin. The interstitial fluid saturates the hydrogel disk and glucose reacts with glucose oxidase, generating hydrogen peroxide. A platinum sensor sensing the hydrogen peroxide produces electric current equivalent to the glucose level. An inbuilt warning system is capable of warning the user if glucose levels are abnormally low or high. However, most patient complaints about the GlucoWatch are due to it being extremely uncomfortable, painful and irritating to skin. Overall, this device could not meet patient’s satisfaction and now it is no longer in market.
5 Portable Sweat Biosensors in the Scientific Literature
5.1 Sweat Collection Approaches
5.1.1 Automated Micro-Pump
To analyze a sweat sample, a continuous flow of fluid in the sensor system is desirable. To assist this process, Bellando et al. [41] designed their sensor assembly with an autonomous micro-pump. This micro-pump is capable of driving continuous unidirectional flow of sweat in the amount of ~ 120 pL/minute. This pump is autonomous and requires zero energy for pumping sweat due to the use of passive channels. SU-8 passive micro/nanofluidic channels on SOI FETs were fabricated which collected sweat and transported it to a reservoir on the skin surface. Finally, a miniaturized Ag/AgCl electrode was used to measure biomarkers in sweat including pH, Na + and K + concentrations in sweat in real time.
5.1.2 Passive Perspiration
A PDMS-based soft microfluidic device reported by Koh et al. can retain 50 μl of sweat. Fundamentally the device is compatible with skin, and upon contact with skin, it forms a sealed barrier and forces a vertical flow of sweat. The device additionally contains reservoirs for reagents to produce a colorimetric response due to the presence of chloride, glucose and lactate and the sweat pH. To transfer the signal from the device, a near-field communication system is incorporated. Choi et al. [42] similarly relied on the passive movement of sweat in their developed skin-like microfluidic device. This device bonds onto the skin and transports sweat into a micro-reservoir. A 1.8-μl sweat sample was collected in 0.8 min and stored in a zone of size 0.03 cm2. Collected sweat was analyzed for lactate, sodium and potassium concentrations. Figure 7 demonstrates how a colorimetric sweat sensor can be used to detect analyte presence.
5.1.3 Iontophoresis Sweat Extraction Approach
To extract sweat on demand is a major hurdle in wearable sweat sensing technologies. To overcome this challenge, Emaminejad et al. [43] constructed an electrochemically enhanced iontophoresis device. This wearable platform can be used to induce sweat for a real-time analysis. The iontophoresis process involves delivery of stimulating agonists to the sweat glands with the aid of an electrical current. The system caused no discomfort or burning sensation when used on subjects.
Various drugs for sweat gland stimulation have been reported in the past, including acetylcholine, carbachol, methacholine and pilocarpine. Sonner et al. [44] used carbachol gel as a sweat-stimulating agent and developed a corresponding sensor to examine sweat. A pH-sensitive dye (bromophenol blue) was used to monitor the sweat secretion. The concept of iontophoresis and reverse iontophoresis is shown in Fig. 8.
Release of sweat by iontophoresis and reverse iontophoresis. The drive of current results in movement of pilocarpine drug under the skin which stimulates sweat glands to release sweat (left), whereas current can be used to release interstitial fluid without any drugs by reverse iontophoresis technique
5.2 Current Sweat Testing Applications
Noninvasively obtainable physiological information has been a holy grail for biomedical applications. Examining sweat can be a valuable method for noninvasive, continuous health monitoring in real time, and numerous physiological and clinical investigations can be performed. For example, in sports or other outdoor activities the bodily water content may deplete. A sweat sensor can hence be utilized to monitor the level of hydration by monitoring the level of sweat Na+ ions. Gao et al. [45] developed a fully integrated wearable sensor arrays to evaluate the level of sweat metabolites (such as glucose and lactate) and electrolytes (such as sodium and potassium ions). Their study showed that doses of methylxanthine drug can be monitored by evaluating sweat through a wearable sweat sensor [46]. Of additional interest is the measurement of cortisol, since it has been identified as the “stress biomarker.” A significant amount of cortisol is released in sweat [47], and wearable skin-like microfluidics devices have been developed to take advantage of this phenomenon to measure cortisol from sweat [48].
5.3 Challenge of Sweat Testing Compared to Blood-Based Test
Even though sweat is an excellent candidate for continuous health monitoring, sweat sensing still encompasses many major challenges. First, inducing sweat on demand is often challenging whereas blood can be withdrawn from the patient at any time. Second, the markers present in sweat are at very low concentrations compared to their concentrations in blood. Third, in a given period of time only a very small amount of sweat can be collected, and the amount can be further reduced by evaporation. Fourth, sweat on the epidermis can be easily contaminated with other entities present on the skin, reducing the accuracy of the results. Blood test, however, does not suffer from this degree of potential contamination. Due to these challenges, the number of sweat sensors which can monitor biomarkers accurately in real time is still very few. There are an insufficient quantity of studies on sweat sensors for monitoring peptides, hormones and DNAs/RNAs—despite the markers being readily available in sweat at low concentrations. It is also important to consider the substantial challenge that the sensing, analyzing and communication process often requires an energy source. A lightweight, flexible and self-powered battery to drive sensors is highly desired but is often hard to incorporate into the flexible, wearable device. Challenges arise when electronic devices generate excessive heat during operation, and the accumulation of heat can shorten device lifetime and lead to catastrophic failure [49]. Especially for soft electronics, elastomers are known to be poor thermal conductors, so heat dissipation of soft electronics becomes critical.
6 Conclusions
Numerous advantages of sweat monitoring platforms have been identified. A wearable, biocompatible, flexible and real-time monitoring device incorporating sweat measurement can clearly be a tremendous aid within the field of healthcare. Compared with rigid electronics, wearable soft electronics do not have air gaps at the interface between the device and the object, which reduces noise and artifacts. Furthermore, close contact between the soft device and the nonplanar object (human skin) allows collection of high-quality data. A sensing platform made by soft electronics, due to its mechanical properties, will furthermore cause minimal irritation to the human skin. Hence, these soft electronics can be essential in the development of new technologies for continuous healthcare sensing. However, trying to implement all of these sensing components while maintaining the flexible structure of the sensor is a recurrent issue in the assembly of soft electronics. This field still requires extensive research to reveal the true potential of the wearable sensing platform, and to tackle these challenges, a highly sensitive sensor with a preconcentration technology will be the ideal next target for research groups.
References
Robinson S, Robinson AH. Chemical composition of sweat. Physiol Rev. 1954;34(2):202–20. https://doi.org/10.1152/physrev.1954.34.2.202.
Watabe A, Sugawara T, Kikuchi K, Yamasaki K, Sakai S, Aiba S. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci. 2013;72(2):177–82. https://doi.org/10.1016/j.jdermsci.2013.06.005.
Težak Ž, Kondratovich MV, Mansfield E. US FDA and personalized medicine: in vitro diagnostic regulatory perspective. Per. Med. 2010;7(5):517–30. https://doi.org/10.2217/pme.10.53.
Warren A. ELITechGroup Biomedical Systems announces the release of the Macroduct® advanced—a sweat induction and collection method cleared by the FDA. https://www.elitechgroup.com/north-america/news/elitechgroup-biomedical-systems-announces-the-release-of-the-macroduct-advanced-a-sweat-induction-and-collection-method-cleared-by-the-fda/. Accessed 28 Jan 2019.
Ghafar-Zadeh E. Wireless integrated biosensors for point-of-care diagnostic applications. Sensors. 2015;15(2):3236–61. https://doi.org/10.3390/s150203236.
Gutierrez A. Novabiomedical. https://www.novabio.us/press/082514.php. Accessed 26 Jan 2019.
Rome BN, Kramer DB, Kesselheim AS. FDA approval of cardiac implantable electronic devices via original and supplement premarket approval pathways, 1979–2012. JAMA. 2014;311(4):385. https://doi.org/10.1001/jama.2013.284986.
Kuno Y. Physiology of human perspiration. London: J. & A. Churchill; 1934.
Bates GP, Miller VS. Sweat rate and sodium loss during work in the heat. J Occup Med Toxicol. 2008;3:4. https://doi.org/10.1186/1745-6673-3-4.
Sato K, Sato F. Individual variations in structure and function of human eccrine sweat gland. Am J Physiol Integr Comp Physiol. 1983;245(2):R203–8. https://doi.org/10.1152/ajpregu.1983.245.2.R203.
Komorniczak M. Layers of the skin. https://commons.wikimedia.org/wiki/File:Skin_layers.svg. Accessed 28 Jan 2019.
Schlereth T. Hyperhidrosis. Dtsch. Aerzteblatt. 2009. https://doi.org/10.3238/arztebl.2009.0032.
Shibasaki M, Crandall CG. Mechanisms and controllers of eccrine sweating in humans. Front Biosci (Schol Ed). 2010;2:685–96.
Bovell D. The human eccrine sweat gland: structure, function and disorders. J Local Glob Heal Sci. 2015;2015(1):5. https://doi.org/10.5339/jlghs.2015.5.
Elson, JA. Human skin sweat glands. https://commons.wikimedia.org/wiki/File:Humanskinsweatglands100x1.jpg. Accessed 10 Oct 2018.
Montgomery I, Jenkinson DM, Elder HY, Czarnecki D, Mackie RM. The effects of thermal stimulation on the ultrastructure of the human atrichial sweat gland. II. The duct. Br J Dermatol. 1985;112(2):165–77. https://doi.org/10.1111/j.1365-2133.1985.tb00080.x.
Sato K, Taylor JR, Dobson RL. The effect of oubain on eccrine sweat gland function. J Invest Dermatol. 1969;53(4):275–82.
Yu Y, Prassas I, Muytjens CMJ, Diamandis EP. Proteomic and peptidomic analysis of human sweat with emphasis on proteolysis. J Proteomics. 2017;155:40–8. https://doi.org/10.1016/j.jprot.2017.01.005.
Patterson MJ, Galloway SDR, Nimmo MA. Variations in regional sweat composition in normal human males. Exp Physiol. 2000;85(6):S0958067000020583. https://doi.org/10.1017/S0958067000020583.
Johnson RE, Pitts GC, Consolazio FC. Factors influencing chloride concentration in human sweat. Am J Physiol Content. 1944;141(4):575–89. https://doi.org/10.1152/ajplegacy.1944.141.4.575.
Kenneth Walker H, Hall WD, Hurst JW. Clinical methods: the history, physical, and laboratory examinations. 1990.
Rastegar A. Serum potassium. 1990.
Wacharasint P, Nakada T, Boyd JH, Russell JA, Walley KR. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock. 2012;38(1):4–10. https://doi.org/10.1097/SHK.0b013e318254d41a.
Marques-Deak A, Cizza G, Eskandari F, Torvik S, Christie IC, Sternberg EM, Phillips TM. Measurement of cytokines in sweat patches and plasma in healthy women: validation in a controlled study. J Immunol Methods. 2006;315(1–2):99–109. https://doi.org/10.1016/j.jim.2006.07.011.
Fu L-H, Qi C, Ma M-G, Wan P. Multifunctional cellulose-based hydrogels for biomedical applications. J Mater Chem B. 2019. https://doi.org/10.1039/C8TB02331J.
Ranganathan N, Joseph Bensingh R, Abdul Kader M, Nayak SK. Synthesis and properties of hydrogels prepared by various polymerization reaction. Systems. 2018;or:1–25. https://doi.org/10.1007/978-3-319-76573-0_18-1.
Experimental hydrogel bandage.jpg. https://commons.wikimedia.org/wiki/File:Experimental_hydrogel_bandage.jpg. Accessed 8 Mar 2019.
Wang C, Wang C, Huang Z, Xu S. Materials and structures toward soft electronics. Adv Mater. 2018;30(50):1801368. https://doi.org/10.1002/adma.201801368.
Yao S, Zhu Y. Nanomaterial-enabled stretchable conductors: strategies, materials and devices. Adv Mater. 2015;27(9):1480–511. https://doi.org/10.1002/adma.201404446.
Mekis D. Nanomaterial. https://en.wikipedia.org/wiki/Nanomaterials. Accessed 8 Mar 2019.
Zhu S, So J-H, Mays R, Desai S, Barnes WR, Pourdeyhimi B, Dickey MD. Ultrastretchable fibers with metallic conductivity using a liquid metal alloy core. Adv Funct Mater. 2013;23(18):2308–14. https://doi.org/10.1002/adfm.201202405.
Johnson LA. FDA approves first blood sugar monitor without finger pricks. https://www.statnews.com/2017/09/28/fda-approves-blood-sugar-monitor-without-finger-pricks/. Accessed 28 Jan 2019.
Revolutionizing continuous glucose monitoring with freestyle libre. https://www.abbott.com/corpnewsroom/product-and-innovation/revolutionizing-cgm-with-freestyle-libre.html. Accessed 3 Feb 2019.
FDA. Summary of safety and effectiveness data; 2002. Accessed 3 Feb 2019.
Currano LJ, Sage FC, Hagedon M, Hamilton L, Patrone J, Gerasopoulos K. Wearable sensor system for detection of lactate in sweat. Sci Rep. 2018;8(1):15890. https://doi.org/10.1038/s41598-018-33565-x.
Hoekstra R, Blondeau P, Andrade FJ. IonSens: a wearable potentiometric sensor patch for monitoring total ion content in sweat. Electroanalysis. 2018;30(7):1536–44. https://doi.org/10.1002/elan.201800128.
Caldara M, Colleoni C, Guido E, Re V, Rosace G. Optical monitoring of sweat pH by a textile fabric wearable sensor based on covalently bonded litmus-3-glycidoxypropyltrimethoxysilane coating. Sens Actuators, B Chem. 2016;222:213–20. https://doi.org/10.1016/j.snb.2015.08.073.
Zilberstein G, Zilberstein R, Maor U, Righetti PG. Noninvasive wearable sensor for indirect glucometry. Electrophoresis. 2018;39(18):2344–50. https://doi.org/10.1002/elps.201700424.
Kim J, De Araujo WR, Samek IA, Bandodkar AJ, Jia W, Brunetti B, Paixão TRLC, Wang J. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem Commun. 2015;51:41–5. https://doi.org/10.1016/j.elecom.2014.11.024.
Cho E, Mohammadifar M, Choi S. A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines. 2017;8:(9). https://doi.org/10.3390/mi8090265.
Bellando F, Garcia-Cordero E, Wildhaber F, Longo J, Guerin H, Ionescu AM. Lab on skin TM: 3D monolithically integrated zero-energy micro/nanofludics and FD SOI ion sensitive FETs for wearable multi-sensing sweat applications. IEEE Int Electron Dev Meeting (IEDM). 2017. https://doi.org/10.1109/IEDM.2017.8268413.
Choi J, Kang D, Han S, Kim SB, Rogers JA. Thin, soft, skin-mounted microfluidic networks with capillary bursting valves for chrono-sampling of sweat. Adv Healthc Mater. 2017;6(5):1601355. https://doi.org/10.1002/adhm.201601355.
Emaminejad S, Gao W, Wu E, Davies ZA, Nyein YYH, Challa S, Ryan SP, Fahad HM, Chen K, Shahpar Z, et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc Natl Acad Sci. 2017;114(18):4625–30. https://doi.org/10.1073/pnas.1701740114.
Sonner Z, Wilder E, Gaillard T, Kasting G, Heikenfeld J. Integrated sudomotor axon reflex sweat stimulation for continuous sweat analyte analysis with individuals at rest. Lab Chip. 2017;17(15):2550–60. https://doi.org/10.1039/c7lc00364a.
Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, Fahad HM, Ota H, Shiraki H, Kiriya D, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529(7587):509–14. https://doi.org/10.1038/nature16521.
Tai L, Gao W, Chao M, Bariya M, Ngo QP, Shahpar Z, Nyein HYY, Park H, Sun J, Jung Y, et al. Methylxanthine drug monitoring with wearable sweat. Sensors. 2018. https://doi.org/10.1002/adma.201707442.
Jia M, Chew WM, Feinstein Y, Skeath P, Sternberg EM. Quantification of cortisol in human eccrine sweat by liquid chromatography—tandem mass spectrometry. Analyst. 2016;141(6):2053–60. https://doi.org/10.1039/C5AN02387D.
Parlak O, Keene ST, Marais A, Curto VF, Salleo A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci Adv. 2018;4(7):eaar2904. https://doi.org/10.1126/sciadv.aar2904.
Xu S, Zhang Y, Jia L, Mathewson KE, Jang K-I, Kim J, Fu H, Huang X, Chava P, Wang R, et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science. 2014;344(6179):70–4. https://doi.org/10.1126/science.1250169.
Acknowledgements
The authors would like to acknowledge the financial support by the National Science Foundation under NSF Cooperative Agreement (Nos. EEC-1648451 and EEC-1647837). Dr. Li also thanks the support sponsored by NSF Independent Research/Development (IRD) Program.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sarwar, M., Rodriguez, P. & Li, Cz. Sweat-Based in Vitro Diagnostics (IVD): From Sample Collection to Point-of-Care Testing (POCT). J. Anal. Test. 3, 80–88 (2019). https://doi.org/10.1007/s41664-019-00097-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-019-00097-w