Abstract
The present study characterized and evaluated the antifungal, nematicidal, acaricidal and repellent activities of Ridolfia segetum essential oil (EO), against the fungus Botrytis cinerea Pers., the nematode Meloidogyne javanica and the mite Tetranychus urticae Koch under laboratory conditions. The EO exerted inhibition of the mycelial growth of B. cinerea with median inhibitory concentration (IC50) of 3.63 μL/mL and 0.92 μL/mL air, under contact and vapor phase conditions, respectively. EO application against M. javanica revealed a strong nematostatic effect on second-stage juveniles (J2) and eggs of the root knot nematode with median lethal concentration (LC50) of 10.37 and 9.26 μL/mL, respectively. The oil was toxic to T. urticae by residual contact with median lethal dose (LD50) of 6005 µL/L and exhibited a repellent effect with a repellency index (RI) of 55%. Results suggest that R. segetum oil has potential to be formulated in biopesticides to control the targeted pest and diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Apiaceae (formerly Umbelliferae) is one of the largest families of flowering plants, with over 4000 species classified into 434 genera (Li et al. 2020). They are widely distributed, though are found predominantly in northern temperate regions and high altitudes of the tropics (Sayed-Ahmad et al. 2017). The family contains well-known plants used as foods, garnishes or spices (Knothe and Steidley 2019). Plants of the Apiaceae family also possess medicinal properties and have traditionally been used as household remedies. They are rich in phytochemicals and secondary metabolites and potential sources of flavonoids, terpenoids, triterpenoid saponins, coumarins, polyacetylenes and steroids (Sayed-Ahmad et al. 2017). Previous studies have highlighted the species of this family as natural agrochemicals, with biological activities against various plant pests and diseases (Oka et al. 2000; Tabanca et al. 2007; Ebadollahi 2013). In the family, the genus Ridolfia Moris is represented by one species, Ridolfia segetum (L.) Moris (Cabral et al. 2015).
R. segetum is an annual plant widely distributed throughout the Mediterranean basin in areas such as Morocco (Gattefossé and Igolen 1946), Tunisia (Jabrane et al. 2010), Portugal (Cabral et al. 2015), Sardinia (Marongiu et al. 2007), Spain and the Canaries (Palá-Paúl et al. 2002), where it grows as a weed in cereal fields (Pottier-Alapetite 1979). It is used in traditional medicine to prevent coughing, constipation, respiratory tract infections and for treatment of gastric acidity (Cabral et al. 2015). It has carminative, antispasmodic, antihaemorrhoidal and emmenagogue properties. Additionally, the dried fruits of the plants are used as a eupeptic digestive in atony and digestive difficulties (Marongiu et al. 2007).
Chemical characterization of the essential oil (EO) has been reported for the ecotypes of R. segetum grown in different countries and in different localities of the same country, enabling the recognition of two types of oils: those dominated by monoterpene hydrocarbons: α-phellandrene, terpinolene and p-cymene (Palá-Paúl et al. 2002, 2005; Marongiu et al. 2007; Bicchi et al. 2009; Jabrane et al. 2009; Poças et al. 2014; Cabral et al. 2015), and those which also contain phenylpropanoids (typically myristicin and dillapiol) as major compounds (Gattefossé and Igolen 1946; Gattefosse and Igolen 1951; Palá-Paúl et al. 2002, 2005; Jannet and Mighri 2007; Marongiu et al. 2007; Jabrane et al. 2010; El Karkouri et al. 2017). Biological activities of R. segetum EO include antioxidant (Jabrane et al. 2010; Cabral et al. 2015), antibacterial (Jannet and Mighri 2007; Jabrane et al. 2009, 2010), insecticidal (Badalamenti et al. 2021), anti-inflammatory (Cabral et al. 2015), anticancer (Poças et al. 2014) and HIV-1-inhibiting activities (Bicchi et al. 2009). The efficacy of the EO as a biopesticide against plant pathogens and pests has not been reported. Thus, the present study was designed to test the hypothesis that R. segetum possesses activities within the scope of crop protection against plant attacking fungi, nematodes and mites.
The gray mold agent Botrytis cinerea, the root knot nematode Meloidogyne javanica and the two-spotted spider mite Tetranychus urticae are the key pests that cause significant yield losses in agricultural crops, including fruits, vegetables and ornamentals (Stumpf et al. 2001; Javed et al. 2007; Angelini et al. 2016) especially in the Souss-Massa region in the south of Morocco. The aim of this study was to assess the in vitro antifungal, nematicidal, acaricidal and repellent activities of the oil against the gray mold agent B. cinerea, the root knot nematode M. javanica and the two-spotted spider mite T. urticae, respectively.
Materials and methods
Plant material and essential oil isolation
Aerial parts of R. segetum (flowers, stems, fruits) (1 kg) were collected in April, May and June of 2017, at flowering and ripening period, from the region of Souss-Massa (South-west of Morocco) coordinates (N 30° 0′ 13.6″ W 9° 38′ 19.6″). Identification of plant material was verified by one of the authors (Dr James Furze), Royal Geographical Society of London, UK. A voucher specimen (no. RS-17) was deposited in the herbarium of the Laboratory of biotechnology, National School of applied sciences, Ibn Zohr University, Agadir, Morocco.
Essential oil was obtained from dried aerial parts (250 g) by hydrodistillation using a Clevenger apparatus for 4 h until total recovery of oil. Hydrodistillation was repeated four times, and the average yield was calculated. The oil was weighed and stored in hermetically sealed dark vials at 4 °C for further analysis.
Test organisms
B. cinerea was isolated directly from infested rotten beans (Phaseolus vulgaris). The fungus was maintained on PDA (Biokar diagnostics) at 4 °C. Eggs of the nematode M. javanica were extracted from infected bean roots. Second-stage juveniles (J2) were obtained from the water suspension of infected roots containing hatched eggs and were stored at 4 °C before use. The two-spotted spider mite was obtained from a mass-rearing trial on bean plants (Microbials Production Unit, Omnium Agricole du Souss, Agadir, Morocco).
Antifungal activity
Poisoned food technique
The poisoned food technique (PF) was used according to Rhayour et al. (2003), with modifications. The EO was dispersed as an emulsion in sterile PDA using 0.1% Tween 80. The concentrations tested ranged from 0.25 to 16 μL/mL. Controls consisted of sterile PDA. The tested fungi were inoculated using a 6 mm mycelial plug from a 7-day-old culture. Three replicate plates were inoculated for each treatment, and plates were incubated for 7 days at 25 ± 2 °C. The test was conducted three times.
Volatile phase technique
Volatile activity assay (VA) was performed following Soylu et al. (2010), with modifications. Petri dishes (9 cm) were filled with 20 mL of PDA medium and seeded with a mycelial disk (6 mm diameter) cut from the periphery of a 7-day-old culture of the fungi. Petri dishes were inverted, and sterile Whatman No. 1 filter paper disks (10 mm diameter) impregnated with different volumes of EO were deposited on the inverted lids to obtain final concentrations of 0.05, 0.1, 0.2, 0.4, 0.8, 0.16 and 0.32 μL/mL air and incubated for 7 days at 25 ± 2 °C. Controls of sterilized filter paper disks impregnated with 20 μL/disk of distilled water were used. Three replicate plates were inoculated for each treatment, and plates were incubated for 7 days at 25 ± 2 °C. The test was conducted three times.
The fungitoxicity of the EO was expressed as percent inhibition of mycelial growth (I%) and evaluated according to the formula of Pandey et al. (1982), Eq. 1.
where Dc is mycelial growth diameter in control, Dt is mycelial growth diameter in treated petri plates.
Nematicidal activity
Egg hatch inhibition and nematicidal activity on second-stage juveniles (J2) of M. javanica
The experiment was carried out in 96-well microplates following a modified method of Sosa et al. (2012). Approximately 50 eggs or 50 freshly hatched J2s of M. javanica were placed in each well prior to adding the EO. The oil was diluted with water containing 0.1% Tween 20 v/v, as the surfactant to obtain the desired oil concentrations. Tested concentrations were 0.8, 8, 10, 12, 14 and 16 µL/mL. Distilled water and a 0.1% Tween 20 solution were included as controls. Microplates were maintained at 25 °C. Hatching percentage was recorded for 10 days, and the percentage of immobilized J2 was recorded for 3 days using an inverted microscope. Lack of mobility was taken as evidence of the effect of the tested solutions. Four replicates of each treatment were made, and the test was performed three times. Data of mortality and hatch inhibition (Crd) were corrected according to Abbott’s formula (Abbott 1925) Eq. 2.
where Pmtr is egg hatching percentage or dead J2 percentage in treated wells, Pmc is egg hatching percentage or dead J2 percentage in the control.
Demonstration of the nematostatic effect
Second-stage juveniles of M. javanica (approximately 50) were exposed to the action of the EO at the highest concentration of 16 µL/mL. The suspension of the treated J2 was passed through a 50 micron sieve while being washed with distilled water to eliminate the effect of the EO. Larvae suspended in distilled water were recovered in petri plates. The vitality of immobile nematodes was evaluated using Meldola Blue staining technique as per Ogiga and Estey (1974) and Mayad et al. (2019). A few drops of Meldola blue dye were added to each plate to distinguish dead J2 from the paralyzed ones. Dead larvae were stained purple-blue, while paralyzed larvae remained uncolored. Four replicates were performed. Two controls were used: the first consisted of J2 larvae suspended in distilled water and the second were J2 treated with heat (65 °C for 24 h). After one hour, dead and paralyzed larvae were counted under an inverted microscope. The percentage of J2 resuming their mobility was determined after 24, 48 and 72 h to study the persistence of paralysis and mobility recovery of J2. The test was performed three times.
Three parameters were determined: the larval mortality rate (Mr), the paralysis rate (Pr) and the percentage of J2 which resumed their mobility (Rm). The three parameters were calculated and corrected against the control according to Abbott's formula, Eqs. 3, 4 and 5.
Acaricidal activity
Toxicity effect
A modified leaf dip bioassay was used to test acaricidal activity, according to Qessaoui et al. (2020). Five concentrations including 10, 100, 1000, 5000 and 8000 µL/L of the EO were prepared with Tween-80 at 0.1% acting as an emulsifier. The bioassay was conducted in Petri dishes (9 cm diameter). Fresh bean leaves were collected from unsprayed plants growing in a greenhouse (INRA experimental farm, Agadir, Morocco). Leaves were dipped in prepared concentrations for 10 s. After drying at room temperature, each leaf was individually placed in the bottom of a Petri dish atop a 9 cm diameter disk of Whatman paper wetted with distilled water. Fifteen T. urticae adults were introduced into each Petri dish, put on top of the leaf and then covered. In control groups, mites were held on leaves dipped in distilled water mixed with the adjuvant (Tween-80). Four replicates for each treatment were used, and mortality rate was assessed at 1, 2, 3, 6, 7 and 8 days after treatment. The bioassay was replicated three times, and mortality rates (CrrM) were corrected using Abbott’s formula (Abbott 1925), Eq. 6.
where dmn is the number of dead mites in treatments, dmnc is the number of dead mites in control and mtn is the total mite number.
Repellent activity
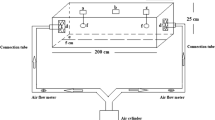
A modified choice test was used for a repellent activity assay following Qessaoui et al. (2017). Two boxes were used. One contained the treated leaves, and the other represented the control (untreated leaves). The two boxes were connected with an 8-cm-long translucent hose (1 cm in diameter) that was pierced in the middle, to allow the introduction of T. urticae adults. Test solutions were prepared by diluting the EO in 0.1% Tween-80. The concentrations of 10, 100, 1000, 5000 and 8000 µL/L of the EO were tested. Fresh bean leaves were immersed for 10 s in either an EO solution or 0.1% Tween-80, air-dried for 5 min and then placed in the corresponding box. Fifteen T. urticae adults were transferred gently through the hole made in the center of the linked-hose, after which the hole was sealed. This structure allowed mites to move freely to both boxes (control or treated box). The repellent effect was assessed at 24, 48 and 72 h after release. Four replicates were made for each treatment, and the bioassay was replicated three times. A repellency index (RI) was determined for each treatment periodically (24, 48 and 72 h) using the formula of Qessaoui et al. (2017) (Eq. 7).
where C is the number of mites in the control box and T is the number of mites in the treated box.
Statistical analysis
The data collected were subjected to one-way analysis of variance (ANOVA), followed by Newman–Keuls test. A minimum significance level of p < 0.01 was considered for differences between means. Data were analyzed by STATISTICA 6 software. Probit analysis was used to calculate median inhibitory concentration IC50, median lethal concentration LC50 and median lethal dose LD50 values at 95% confidence limit, using the Polo-PC software (LeOra-Software 1987).
Results
Antifungal activity
The inhibitory effects of R. segetum EO on mycelial growth of B. cinerea are shown in Fig. 1 (a) and (b). Using the PF technique, concentrations equal to and above 2 μL/mL produced significant inhibition, with percent inhibition increasing with concentration. The highest inhibition (98%) was obtained when the EO was applied at 16 μL/mL. Similarly, using the VA technique, volatiles of the oil exhibited concentration-dependent inhibition of mycelial growth, with the highest inhibition of 92% achieved at 0.32 μL/mL air. IC50 values using PF and VA techniques were 3632.841 μL/L and 73.618 μL (0.92 μL/mL air), respectively (Table 1).
Nematicidal activity
Egg hatch inhibition and nematicidal activity against J2
Results of hatch inhibition of M. javanica eggs are presented in Table 2, where use of letters (a–i) denote grouped values with no significant difference using the Newman–Keuls test (p < 0.01). The EO strongly suppressed egg hatching after 10 days of incubation. The hatch inhibition rate had a linear relationship with the oil concentration, reaching up to 75.35% inhibition at 16 μL/mL concentration, with an LC50 value of 10,376 μL/L (Table 3). The effect of the EO on percent immobility of M. javanica juveniles at different concentrations and durations is summarized in Fig. 2. Low immobility of juveniles occurred after a 24 h exposure to concentrations of the oil. Juvenile immobility gradually increased with concentration and time of exposure. After 72 h, 16 μL/mL produced the highest immobility rate of 71% immobility, and an LC50 value of 9269 μL/L (Table 3).
Demonstration of the nematostatic effect
J2 of M. javanica exhibited a nematostatic response to R. segetum EO. Control (heat-treated J2), immobile J2 exposed to the EO at 16 μL/mL were not stained by the blue Meldola. The percentage of dead J2 was less than the paralyzed fraction, reaching 9.5% after three days of exposure. The recorded percentage of paralyzed larvae represented the majority, though showed a slight decrease over the three days of exposure. The mortality rate increased, reaching 90.5% on the third day (Fig. 3). The EO induced a direct nematostatic effect. Regarding the reversibility of J2 paralysis, juveniles subjected to treatment with the EO remained paralyzed during the three days of incubation. Dead juveniles did not recover mobility.
Acaricidal activity
Results of toxicity of the EO were presented as percentage of mortality of T. urticae adults at different doses and different times of exposure (Table 4). The oil exhibited contact toxicity to T. urticae adults. The earliest deaths occurred within 24 h after application. Response of T. urticae adults to treatment by the EO varied with increasing concentrations and periods after exposure. At the highest concentration (8000 ppm), the mortality rate exceeded 54% 72 h after application, and complete mortality was recorded 192 h after exposure to the oil. Probit analysis indicated an LD50 of 6005 µL/L (Table 5).
Repellent activity
The EO was screened for its repellent activity against T. urticae at doses of 10, 100, 1000, 5000 and 8000 µL/L, and the results as shown in Fig. 4. The EO exhibited moderate repellent action against T. urticae adults after 24, 48 and 72 h exposure. The level of repellency observed for each treatment was consistent at 24, 48 and 72 h. The EO had an attractive effect at concentrations 10, 100 and 1000 µL/L with a RI ranging from 65 to 8. After 72 h, moderate repellent action was observed on adult mites at the highest concentration tested (8000 µL/L) with a RI of 55%.
Discussion
Antifungal potency against the agent of gray mold disease has been identified in plant oils belonging to Apiaceae family members (Abo-El Seoud et al. 2005; Behdani et al. 2012; Fraternale et al. 2014). The current study is the first to investigate antifungal activity from the genus Ridolfia, further the oil produced different results of mycelial growth inhibition with different methods used. Greater inhibition was achieved using the volatile phase method than with the poisoned food method, in agreement with previous studies, in which the volatile fraction of Melissa officinalis L., Rosmarinus officinalis L., Origanum syriacum L. var. bevanii and Lavandula stoechas L. var. stoecha EOs showed better antifungal activity against B. cinerea compared to the direct contact method (Soylu et al. 2010; El Ouadi et al. 2017). Antifungal action of EO vapors depends on the presence of functional groups in the oil in a gaseous state, as well as the vapor pressure which allows them to cross through the fungal cell membranes (Belletti et al. 2007). Antifungal volatiles are penetrating and uniformly distributed. Consequently, relatively low concentrations of the oil are active at inhibiting fungal growth.
Antifungal potential of an EO depends mainly on its composition and the target fungi. The most abundant group of compounds in EOs are terpenes, which are known to be active against a wide range of fungi (Houicher et al. 2018). Further, Basaid et al. (2020a) showed that R. segetum EO was dominated by (z)-β-ocimene (19.7%), β-phellandrene (9.6%) and β-pinene (8.6%). In the latter study, other compounds were present in the oil at smaller percentages, such as cubenol, alloaromadendrene, γ-eudesmol, α-bisabolol and terpinen-4-ol. (Z)-β-ocimene and β-pinene have been reported to be antifungal against a wide variety of phytopathogenic fungi (Sekine et al. 2007; Saroj et al. 2015). Alternatively, Marei and Abdelgaleil (2018) reported that terpinen-4-ol had antifungal activity against B. cinerea, with an EC50 of 77 mg/L. Similarly, α-bisabolol was reported as antifungal against B. cinerea (Kamatou and Viljoen 2010). Terpinen-4-ol and α-bisabolol are proportionately small (2.4%) in R. segetum oil, but since they were reported as effective against B. cinerea, it is plausible that they contributed to the antifungal activity of the oil. We infer that the activity of the EO is linked to a synergism between major and minor constituents.
Essential oil constituents have different modes of action dependent on the target fungal strain (Bakkali et al. 2008). In B. cinerea, morphological alterations of hyphae and conidia of the fungi exposed to EOs have been reported. Treated mycelium displayed reduced hyphal diameters, shriveled hyphal aggregates and lysis of hyphal wall occurred, effecting cytoplasmic leakage (Xueuan et al. 2018). In the same context, β-pinene, a major compound of the EO was reported to destroy cellular integrity and thereby, inhibiting respiration and ion transport processes (Uribe et al. 1985), in addition to inhibiting microbial phospholipase and esterase activities (Silva et al. 2012).
Nematicidal activity of Apiaceae plant oils against M. javanica is limited to a few studies (Oka et al. 2000; Sousa et al. 2015; Basaid et al. 2020b). R. segetum oil exhibited inhibition of egg hatch and J2 survival of the root knot nematode. Lethal effects of EO include immobility of nematodes. A permanent or temporary paralysis (nematostatic effect) may also occur. Previous reports of the latter effect of plant-derived substances using the Meldola Blue staining method have centered on plant extracts (Jourand et al. 2004; Mayad et al. 2019; Seenivasan 2019). To avoid confusion, a coloring test with Meldola Blue on J2 exposed to EO treatments was performed in the current study. The EO showed a strong nematostatic effect on J2 of M. javanica. This study is the first to use the Meldola Blue staining method in conjunction with EOs. The nematostatic effect may be attributed to the bioactive compounds in R. segetum. Major compounds of the oil (z)-β-ocimene and β-pinene, have been reported to reduce hatching and J2 mobility of Meloidogyne incognita (Adekunle et al. 2007; Echeverrigaray et al. 2010). EO constituents may cause irreversible changes to protein structures, particularly those located on the nematode surface. EOs disrupt membrane function and cause alteration of permeability; further, they act on nematode nervous systems by inhibiting acetylcholinesterase activity (Oka 2001).
Recently, Badalamenti et al. (2021) reported toxicity of R. segetum EO against different pests (Culex quinquefasciatus Say, Musca domestica L., and Spodoptera littoralis (Boisduval)). Further, the EO exhibited acaricidal activity against T. urticae. The current study agrees with previous work, reporting the toxicity of other plant EOs from the Apiaceae family against T. urticae (Attia et al. 2011; Amizadeh et al. 2013; Ebadollahi et al. 2014). Although, higher concentrations and greater exposure time were required to achieve complete adult mortality. Exploring other testing methods may ascertain improved toxicity of R. segetum EO; Araújo et al. (2012) reported the variability of the residual effect of Piper EO through fumigation and contact against T. urticae. Different modes of action operate according to the method employed. In contact bioassays, toxicity may occur through the tarsi and ingestion (Araújo et al. 2012); whereas if applied as a fumigant, volatile constituents penetrate the organism via the respiratory system, resulting in enhanced efficacy (Choi et al. 2004).
The potential repellent effects of EOs against T. urticae have been evaluated for many plant species (Araújo et al. 2012; Motazedian et al. 2012; Reddy and Dolma 2018). In the current paper, the EO of R. segetum exhibited moderate repellency against adult mites at high concentrations. It is probable if adult mites detected the presence of the oil, it was not interpreted by them as a cue to avoid exploitation of the site containing the EO (de Lira et al. 2015). The low repellent effect of the EO may be linked to a relatively high volatility of the oil (Liu and Ho 1999). Effects of EOs usually dissipate relatively quickly, in contrast to when they are freshly applied, given their high volatility. This may be compensated by development of nanoformulations that minimize loss by evaporation or degradation and establish conditions for controlled release (González et al. 2014).
Conclusion
The current investigation adds knowledge of biological activities of Moroccan R. segetum EO against key pest and diseases of great economic importance for many important crops. Bioassays have revealed a broad spectrum of activities of the studied EO at high concentrations. Further research is required to determine the activity of this EO against other plant pathogens and parasites, and to test the EO in combination with other biocontrol agents, keeping in view the potential synergistic effects which will allow development of sustainable tools and methods for crop protection.
Availability of data and materials
All relevant data are within the paper.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Abo-El Seoud MA, Sarhan MM, Omar AE, Helal MM (2005) Biocides formulation of essential oils having antimicrobial activity. Arch Phytopathol Plant Prot 38:175–184
Adekunle O, Acharya R, Singh B (2007) Toxicity of pure compounds isolated from Tagetes minuta oil to Meloidogyne incognita. Aust Plant Dis Notes 2:101–104
Amizadeh M, Hejazi MJ, Saryazdi GA (2013) Fumigant toxicity of some essential oils on Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 39:285–289
Angelini RMDM, Pollastro S, Faretra F (2016) Genetics of Botrytis cinerea. In: Fillinger S, Walker A-S (eds) Botrytis–the fungus, the pathogen and its management in agricultural systems. Springer International Publishing, Switzerland, pp 35–53
Araújo MJC, Câmara CAG, Born FS, Moraes MM, Badji CA (2012) Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae. Exp Appl Acarol 57:139–155
Attia S, Grissa KL, Lognay G, Heuskin S, Mailleux AC, Hance T (2011) Chemical composition and acaricidal properties of Deverra scoparia essential oil (Araliales: Apiaceae) and blends of its major constituents against Tetranychus urticae (Acari: Tetranychidae). J Econ Entomol 104:1220–1228
Badalamenti N, Ilardi V, Bruno M, Pavela R, Boukouvala MC, Kavallieratos NG, Maggi F, Canale A, Benelli G (2021) Chemical composition and broad-spectrum insecticidal activity of the flower essential oil from an ancient sicilian food plant. Ridolfia Segetum Agric 11:304
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils–a review. Food Chem Toxicol 46:446–475
Basaid K, Chebli B, Bouharroud R, Furze JN, de Oliveira AL, Mayad EH (2020a) Chemical characterization of essential oils of Senecio glaucus ssp. Coronopifolius (Maire) Alexander and Ridolfia segetum (L.) Moris growing in Morocco. J Essent Oil Bear Plants 23:918–930
Basaid K, Chebli B, Mayad EH, Furze JN, Bouharroud R, Krier F, Barakate M, Paulitz T (2020b) Biological activities of essential oils and lipopeptides applied to control plant pests and diseases: a review. Int J Pest Manag 67:1–23. https://doi.org/10.1080/09670874.2019.1707327
Behdani M, Pooyan M, Abbasi S (2012) Evaluation of antifungal activity of some medicinal plants essential oils against Botrytis cinerea, causal agent of postharvest apple rot, in vitro. Int J Agric Crop Sci 4:1012–1016
Belletti N, Kamdem SS, Patrignani F, Lanciotti R, Covelli A, Gardini F (2007) Antimicrobial activity of aroma compounds against Saccharomyces cerevisiae and improvement of microbiological stability of soft drinks as assessed by logistic regression. Appl Environ Microbiol 73:5580–5586
Bicchi C, Rubiolo P, Ballero M, Sanna C, Matteodo M, Esposito F, Zinzula L, Tramontano E (2009) HIV-1-inhibiting activity of the essential oil of Ridolfia segetum and Oenanthe crocata. Planta Med 75:1331–1335
Cabral C, Poças J, Gonçalves MJ, Cavaleiro C, Cruz MT, Salgueiro L (2015) Ridolfia segetum (L.) Moris (Apiaceae) from Portugal: a source of safe antioxidant and anti-inflammatory essential oil. Ind Crops Prod 65:56–61
Choi W-I, Lee S-G, Park H-M, Ahn Y-J (2004) Toxicity of plant essential oils to Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae). J Econ Entomol 97:553–558
de Lira CS, Pontual EV, de Albuquerque LP, Paiva LM, Paiva PMG, de Oliveira JV, Napoleão TH, Navarro DMdAF (2015) Evaluation of the toxicity of essential oil from Alpinia purpurata inflorescences to Sitophilus zeamais (maize weevil). Crop Prot 71:95–100
Ebadollahi A (2013) Plant essential oils from Apiaceae family as alternatives to conventional insecticides. Ecologia Balkanica 5:149–172
Ebadollahi A, Jalali Sendi J, Aliakbar A, Razmjou J (2014) Chemical composition and acaricidal effects of essential oils of Foeniculum vulgare Mill. (Apiales: Apiaceae) and Lavandula angustifolia Miller (Lamiales: Lamiaceae) against Tetranychus urticae Koch (Acari: Tetranychidae). Psyche 2014:1–6
Echeverrigaray S, Zacaria J, Beltrão R (2010) Nematicidal activity of monoterpenoids against the root-knot nematode Meloidogyne incognita. Nematology 100:199–203
El Karkouri J, Amalich S, Drioiche A, Fadili K, Eto B, Khabbal Y, Zair T (2017) Phytochemical valuation of the umbels of Ridolfia segetum (L.) Moris of Morocco. Int J Adv Res 5:1780–1788
El Ouadi Y, Manssouri M, Bouyanzer A, Majidi L, Bendaif H, Elmsellem H, Shariati M, Melhaoui A, Hammouti B (2017) Essential oil composition and antifungal activity of Melissa officinalis originating from north-est Morocco, against postharvest phytopathogenic fungi in apples. Microb Pathog 107:321–326
Fraternale D, Flamini G, Ricci D (2014) Essential oil composition of Angelica archangelica L. (Apiaceae) roots and its antifungal activity against plant pathogenic fungi. Plant Biosyst-an Int J Dealing All Aspects Plant Biol 150:558–563
Gattefossé J, Igolen G (1946) Contribution à l’étude de la flore aromatique du Maroc: l’essence de fenouil des moissons (Ridolfia segetum). Bull Soc Chim Fr 13:361–363
Gattefosse J, Igolen G (1951) Essential oil of Ridolfia segetum. Industrie Parfum 6:305–307
González JOW, Gutiérrez MM, Ferrero AA, Band BF (2014) Essential oils nanoformulations for stored-product pest control–characterization and biological properties. Chemosphere 100:130–138
Houicher A, Hamdi M, Hechachna H, Özogul F (2018) Chemical composition and antifungal activity of Anacyclus valentinus essential oil from Algeria. Food Biosci 25:28–31
Jabrane A, Jannet HB, Harzallah-Skhiri F, Casanova J, Mighri Z (2009) GC, GC-MS and 13C NMR spectroscopy integrated analyses and in vitro antibacterial activity of Ridolfia segetum essential oils from Tunisia. J Essen Oil Bear Plants 12:521–530
Jabrane A, Ben Jannet H, Mastouri M, Mighri Z, Casanova J (2010) Chemical composition and in vitro evaluation of antioxidant and antibacterial activities of the root oil of Ridolfia segetum (L.) Moris from Tunisia. Nat Prod Res 24:491–499
Jannet HB, Mighri Z (2007) Hydrodistillation kinetic and antibacterial effect studies of the flower essential oil from the Tunisian Ridolfia segetum (L.). J Essent Oil Res 19:258–261
Javed N, Gowen S, Inam-ul-Haq M, Anwar S (2007) Protective and curative effect of neem (Azadirachta indica) formulations on the development of root-knot nematode Meloidogyne javanica in roots of tomato plants. Crop Prot 26:530–534
Jourand P, Rapior S, Fargette M, Mateille T (2004) Nematostatic effects of a leaf extract from Crotalaria virgulata subsp. grantiana on Meloidogyne incognita and its use to protect tomato roots. Nematology 6:79–84
Kamatou GPP, Viljoen AM (2010) A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc 87:1–7
Knothe G, Steidley KR (2019) Composition of some Apiaceae seed oils includes phytochemicals, and mass spectrometry of fatty acid 2-methoxyethyl esters. Eur J Lipid Sci Technol 121:1800386
LeOra-Software (1987) POLO-PC: a user’s guide to probit or logit analysis. LeOra Software, Berkeley, CA
Li M-Y, Feng K, Hou X-L, Jiang Q, Xu Z-S, Wang G-L, Liu J-X, Wang F, Xiong A-S (2020) The genome sequence of celery (Apium graveolens L.), an important leaf vegetable crop rich in apigenin in the Apiaceae family. Hortic Res 7:1–10
Liu Z, Ho S (1999) Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst). J Stored Prod Res 35:317–328
Marei GIK, Abdelgaleil SAM (2018) Antifungal potential and biochemical effects of monoterpenes and phenylpropenes on plant pathogenic fungi. Plant Prot Sci 54:9–16
Marongiu B, Piras A, Porcedda S, Tuveri E, Maxia A (2007) Comparative analysis of the oil and supercritical CO2 extract of Ridolfia segetum (L.) Moris. Nat Prod Res 21:412–417
Mayad EH, Basaid K, Furze JN, Heimeur N, Senhaji B, Chebli B, El Hadek M, Mateille T, Idrissi Hassani LA, Ferji Z (2019) Reversible nematostatic effect of Peganum harmala L. (Nitrariaceae) on Meloidogyne javanica. J AgriSearch 6:29–33
Motazedian N, Ravan S, Bandani AR (2012) Toxicity and repellency effects of three essential oils against Tetranychus urticae Koch (Acari: Tetranychidae). J Agric Sci Technol 14:275–284
Ogiga IR, Estey RH (1974) The use of Meldola Blue and Nile Blue A, for distinguishing dead from living nematodes. Nematologica 20:271–276
Oka Y (2001) Nematicidal activity of essential oil components against the root-knot nematode Meloidogyne javanica. Nematology 3:159–164
Oka Y, Nacar S, Putievsky E, Ravid U, Yaniv Z, Spiegel Y (2000) Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 90:710–715
Palá-Paúl J, Velasco-Negueruela A, Pérez-Alonso MJ, Ramos-Vázquez P (2002) Volatile constituents of Ridolfia segetum (L.) Moris gathered in southern Spain, Andalucia province. J Essent Oil Res 14:206–209
Palá-Paúl J, Velasco-Negueruela A, Pérez-Alonso MJ, Vallejo MCG (2005) Volatile constituents of Ridolfia segetum (L.) Moris gathered in Central Spain: Castilla la Mancha province. J Essent Oil Res 17:119–121
Pandey DK, Tripathi NN, Tripathi RD, Dixit SN (1982) Fungitoxic and phytotoxic properties of the essential oil of Hyptis suaveolens. Zeitschrift Für Pflanzenkrankheiten Und Pflanzenschutz 89:344–349
Poças J, Lemos M, Cabral C, Cavaleiro C, Cruz MT, Salgueiro L, Pires I (2014) Assessment of the properties of the essential oil from Ridolfia segetum Moris (Portugal) on cancer cell viability. Planta Med 80:P1L44
Pottier-Alapetite G (1979) Flore de la Tunisie: angiospermes, dicotylédones, apétales, dialypetales. Ministère de l'enseignement supérieur et de la recherche scientifique et ministère de l'agriculture, Tunisia
Qessaoui R, Bouharroud R, Amarraque A, Ajerrar A, Mayad EH, Chebli B, Dadi M, Elaini R, El Filali F, Walters AS (2017) Ecological applications of Pseudomonas as a biopesticide to control two-spotted mite Tetranychus urticae: chitinase and HCN production. J Plant Prot Res 57:409–416
Qessaoui R, Amarraque A, Lahmyed H, Ajerrar A, Mayad EH, Chebli B, Walters AS, Bouharroud R (2020) Inoculation of tomato plants with rhizobacteria suppresses development of whitefly Bemisia tabaci (GENNADIUS)(HEMIPTERA: ALEYRODIDAE): agro-ecological application. PLoS ONE 15:e0231496
Reddy SGE, Dolma SK (2018) Acaricidal activities of essential oils against two-spotted spider mite, Tetranychus urticae Koch. Toxin Rev 37:62–66
Rhayour K, Bouchikhi T, Tantaoui-Elaraki A, Sendide K, Remmal A (2003) The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J Essent Oil Res 15:356–362
Saroj A, Pragadheesh V, Yadav A, Singh S, Samad A, Negi A, Chanotiya C (2015) Anti-phytopathogenic activity of Syzygium cumini essential oil, hydrocarbon fractions and its novel constituents. Ind Crops Prod 74:327–335
Sayed-Ahmad B, Talou T, Saad Z, Hijazi A, Merah O (2017) The Apiaceae: Ethnomedicinal family as source for industrial uses. Ind Crops Prod 109:661–671
Seenivasan N (2019) Nematostatic activity of root extracts of banana (Musa spp.) genotypes as pre-infectional resistance mechanism against the burrowing nematode, Radopholus similis. J Hortic Sci Biotechnol 94:49–62
Sekine T, Sugano M, Majid A, Fujii Y (2007) Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J Chem Ecol 33:2123–2132
Silva ACRD, Lopes PM, Azevedo MMBD, Costa DCM, Alviano CS, Alviano DS (2012) Biological activities of a-pinene and β-pinene enantiomers. Molecules 17:6305–6316
Sosa ME, Lancelle HG, Tonn CE, Andres MF, Gonzalez-Coloma A (2012) Insecticidal and nematicidal essential oils from Argentinean Eupatorium and Baccharis spp. Biochem Syst Ecol 43:132–138
Sousa RMO, Rosa JS, Silva CA, Almeida MTM, Novo MT, Cunha AC, Fernandes-Ferreira M (2015) Larvicidal, molluscicidal and nematicidal activities of essential oils and compounds from Foeniculum vulgare. J Pest Sci 88:413–426
Soylu EM, Kurt Ş, Soylu S (2010) In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int J Food Microbiol 143:183–189
Stumpf N, Zebitz CP, Kraus W, Moores GD, Nauen R (2001) Resistance to organophosphates and biochemical genotyping of acetylcholinesterases in Tetranychus urticae (Acari: Tetranychidae). Pestic Biochem Physiol 69:131–142
Tabanca N, Demirci B, Baser KHC, Mincsovics E, Khan SI, Jacob MR, Wedge DE (2007) Characterization of volatile constituents of Scaligeria tripartita and studies on the antifungal activity against phytopathogenic fungi. J Chromatogr B 850:221–229
Uribe S, Ramirez J, Peña A (1985) Effects of beta-pinene on yeast membrane functions. J Bacteriol 161:1195–1200
Xueuan R, Dandan S, Zhuo L, Qingjun K (2018) Effect of mint oil against Botrytis cinerea on table grapes and its possible mechanism of action. Eur J Plant Pathol 151:321–328
Funding
None.
Author information
Authors and Affiliations
Contributions
Khadija Basaid performed the investigation and wrote the first draft; all other authors contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Basaid, K., Chebli, B., Bouharroud, R. et al. Biocontrol potential of essential oil from Moroccan Ridolfia segetum (L.) Moris. J Plant Dis Prot 128, 1157–1166 (2021). https://doi.org/10.1007/s41348-021-00489-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-021-00489-0