Abstract
Tuta absoluta Meyrick originates in South America and is now one of the most important insect pests of Solanaceae in different parts of the world, including Africa. Its control has relied primarily on chemical insecticides, which are associated with negative ecological effects. In the present study, essential oils of Ocimum gratissimum and O. kilimandscharicum were tested for repellence and fumigant toxicity on the adult stages under laboratory conditions. The oil of O. gratissimum was more repellent, but its toxicity was comparable with that of O. kilimandscharicum. The major constituents of O. gratissimum were methyl eugenol (39.5%) and eugenol (29.7%). Those of O. kilimandscharicum were camphor (47.1%) and 1.8-cineole (19.3%). Eugenol (LC50 of 0.24 μl/ml, 83.3%, RI50 = 0.15) and camphor (LC50 of 0.23 μl/ml, 89.5%, RI50 = 0.13) were more toxic (at 1 μl/ml for 24 h) and repellent than the other constituents. The results show potential of the essential oils for use in integrated management of the tomato pest.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The tomato borer, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae), is an oligophagous and very harmful leaf-mining moth which feeds on Solanaceae crops, particularly tomato (Lycopersicon esculentum Mill.), but also on eggplant (Solanum melongena L.), potato (Solanum tuberosum L.), common bean (Phaseolus vulgaris L.), sweet pepper (Solanum muricatum L.) and Datura spp. (Garcia and Espul 1982). The moth was first known as a plant pest in many South American countries (Korycinska and Moran 2009). Now, it has spread rapidly throughout Afro-Eurasia and Middle Eastern countries (Sylla et al. 2017; Xian 2017; Biondi et al. 2018) and is considered as a global economic pest on tomato and other Solanaceae plants (Desneux et al. 2011). This high speed of colonisation is associated with the ability of the pest to adapt to varying climatic conditions and its high biotic potential. Each adult female of T. absoluta may lay 200 to 300 creamy coloured eggs, and 10 to 12 generations can be produced per year. After hatching, the larvae (the most destructive stage) can penetrate different parts of the whole plant, but prefer apical buds, tender new leaflets, flowers and green and ripe fruits. These larvae feed on mesophyll tissue of leaves creating mines, which affect the photosynthetic capacity of the plant. They can also form extensive galleries in the stems and fruits, which can provide entry points for secondary pathogens (Desneux et al. 2010). The pest damages tomato and other Solanaceae crops which may cause significant production losses ranging from 50 to 100% (Biratu 2018).
Control of T. absoluta is traditionally based on spraying crop plants with synthetic insecticides, including organophosphates, pyrethroids, thiocarbamates, diamides and acylurea growth regulators (Abbes and Chermiti 2012; Roditakis et al. 2015). However, these have low to moderate efficiency due to the high biotic potential and cryptic nature of the larvae of T. absoluta (Lietti et al. 2005; Haddi et al. 2012). The high reproduction of tomato borer leads to increase in spray frequency per crop cycle, which accelerates the evolution of resistance to the insecticides (Biondi et al. 2018; Roditakis et al. 2015). Moreover, these increasing and indiscriminate applications of synthetic pesticides have several environmental drawbacks and negative impact to natural biological control and disrupt pollination processes (Desneux et al. 2007).
Thus, there is an urgent need of finding new eco-friendly tools for the control of T. absoluta. Botanical-based insecticides have long been considered as attractive alternatives to synthetic chemicals for pest management (Zhu et al. 2008). These products are less harmful to the natural environment and human health (Isman 2006). Pyrethrum and neem are well established commercially and pesticides based on plant essential oils have recently entered the marketplace (Isman 2019). Insecticides based on essential oils and its constituents have been proved effective against many stored-grain insect pests. These have been formulated and applied variously as repellant, antifeedants, growth inhibitors, oviposition inhibitors and ovicides (Said-Al Ahl et al. 2017).
Tropical and subtropical plants such as Ocimum spp. belonging to the Lamiaceae family are recognised sources of bioactive essential oils (Clemente et al. 2003; Umerie et al. 1998). Essential oils of Ocimum spp. have been found to possess bio-pesticidal efficacy such as fungicidal, adulticidal, larvicidal, nematicidal, antihelminthic, ovicidal, oviposition deterrence, repellency, acaricidal, antileishmanial, trypanocidal and antimalarial (Bekele and Hassanali 2001; Ntonga et al. 2014; Chowdhary et al. 2018; Benelli et al. 2019). Furthermore, the essential oil of O. gratissimum and its constituents have demonstrated fumigant toxicity against many adults of insects (Kéita et al. 2001; Ogendo et al. 2008). Recently, researchers reported the efficacy of botanical plants against the tomato borer such as the essential oils of Shirazi thyme (Chegini et al. 2018) and Citrus peel (Campolo et al. 2017), and the oviposition deterrent activities of two basil plants (Yarou et al. 2017). In sensitive environments or enclosed areas like food processing, storage facilities and greenhouses, fumigant method is ideal to limit the residue problem, get to those hard to reach areas where pest may be hiding and acted in all life stage of the insect. For example, fumigant toxicity of cinnamaldehyde obtained from Cinnamomum verum J. Presl essential oil has shown potential activity against adults of Sitophilus oryzae L. (Lee et al. 2008). However, knowledge remains limited on how the essential oils of these aromatic plants (O. gratissimum and O. kilimandscharicum, and some of their constituents) affect the T. absoluta moths.
Mostly constituted with terpenoid, the essential oils of the two chosen species have shown different effects on varied insects (Cruz et al. 2017; Lima et al. 2018; Benelli et al. 2019) and post-harvest pest (Nguemtchouin et al. 2013; Bekele and Hassanali 2001). Given the use of these plants, no reports on their potential fumigant toxicity and repellency effects on T. absoluta have been reported so far. Thus, the paper present information on the repellent effects and fumigant toxicity of essential oil concentration of two ethno-botanical Ocimum plants (O. gratissimum L. and O. kilimandscharicum Gürke) and some of their constituents against T. absoluta.
Materials and methods
Plant materials
The aerial parts of O. gratissimum L. and O. kilimandscharicum Gürke (Lamiaceae) were collected in Naivasha (0°43′S/36°26′E) and Njoro (0°11′S/35°30′E), respectively, near Egerton University, Nakuru County, Kenya, in December 2017. The plants were identified and authenticated at the Department of Botany, University of Nairobi, where voucher specimens were deposited: EEFR-2017/1 and EEFR-2017/2 for O. gratissimum and O. kilimandscharicum, respectively. The plant materials were dried under shade for 1 week before extraction.
Insects
The insects used for all bioassays originated from a colony reared at the International Centre of Insect Physiology and Ecology (ICIPE), Duduville Campus, Nairobi, Kenya. The original population from pupae and larvae of T. absoluta was collected in October 2016 from tomato plants (Lycopersicon esculentum) in the field in Meru County, Eastern Kenya, without any history of exposure to pesticides. The insects collected were initially maintained under quarantine to identify any parasitized individuals before the establishment of the colony, which was maintained at 26 ± 2 °C, relative humidity ranging from 60 to 70% and photoperiod of 16/8 (light:day). In the rearing cages, the sex ratio was 2:1 and insects were randomly sampled.
Isolation of essential oils and preparation of different concentrations for bioassays
Each dried plant material (1000 g) was steam-distilled using a Clevenger apparatus for over 4 h. The oils obtained were separated from water, dried using anhydrous sodium sulphate and weighed. The oils were stored in closed amber-coloured vials at 4 °C in the dark until used. For bioassays, 10 μl of each essential oil was dissolved in 5 ml of acetone and successively diluted with acetone to give 0.031, 0.063, 0.125, 0.25, 0.5 and 1 μl of the oil in 1 ml of the solution.
Analyses of the essential oils
The two essential oils were analysed using a gas chromatograph (GC) (HP-7890A, Agilent Technologies, Wilmington, USA) linked to mass spectrometer (MS) operated in the electron impact mode (HP 5975 C, Agilent, Wilmington, USA). The apparatus was equipped with a non-polar HP-5MS capillary column (30 m × 0.25 mm i.d.; 0.25-μm film thickness, with 5% phenyl methyl silicone as the stationary phase; J & W Scientific, Folsom, USA). Helium (1.2 ml min−1) was used as the carrier gas. The oven temperature was programmed at 35 °C (for 5 min) to 280 °C at 10 °C min−1 and then held isothermally at 280 °C for 10.5 min. An aliquot of 1 μl of each oil (100 mg of each sample was dissolved in 10 ml of dichloromethane) was injected in the splitless mode (column effluent was split 1:1 for simultaneous detection). The ion source temperature was 230 °C; electron ionisation mass spectra were acquired at 70 eV within a mass range of 38–550 Da (Da) during a scan time of 0.73 scans s−1. Compounds were identified using ChemStation software (Agilent) by comparison of mass spectral data of their retention time with library data: Adams (Adams 2017) and NIST 05 (NIST 2008). Identities of some constituents were confirmed by co-injections with commercially available authentic standards. Quantification was based on calibration curves (peak area vs. concentration) generated from authentic standards of identified compounds and also by flame ionisation gas chromatography (CG/FID), under the same conditions as in the GC/MS analysis.
Standards of constituents of essential oils that were sourced
Authentic standards of eugenol (purity 99%), E-caryophyllene (purity 97%), 1,8-cineole (purity 99%), (1S) (−)-β-pinene (purity 98%), l-(−)-fenchone (purity 98%), α-terpineol (purity 97%) and (+)-limonene (purity 97%) were purchased from Acros Organics, NJ, USA. α-Pinene (purity 98%) and methyl eugenol (purity 98%) were purchased from Sigma-Aldrich, Steinheim, Germany. Acetone (GC analytical grade 97%) was purchased from Sigma-Aldrich Chemical, Milwaukee, WI, USA. Camphor (purity 97%) and (−)-camphene (purity 97%) were purchased from abcr GmbH, Karlsruhe, Germany.
Repellence assays on adults
Repellence of O. gratissimum and O. kilimandscharicum oils
The repellent effect of the essential oils and some of their constituents on adult tomato borer was evaluated in a two-choice cuboidal plexi-glass wind tunnel (25 × 25 × 200 cm), which was a slight modification of the one described by Gikonyo et al. (2003) (Fig. 1). The wind tunnel had three square windows (5 cm × 5 cm) on the top side; two rear ones were for introducing sample dispensers while the middle one, which divided the tunnel symmetrically into the left and right arms, was for introducing insect release cages. Each side of the tunnel had a tube (20 cm long and 3.3 cm internal diameter) fixed with a white plexi-glass gauze on the inner side, which prevented the moths from getting out of the tunnel. These tubes were connected to an air cylinder by rubber tubing via air flow metres (air was cleaned with activated charcoal). The middle window was covered with a white plexi-glass wire mesh to facilitate air flow from both arms. Light was supplied from fluorescent tubes suspended 2 m above the tunnel giving about 1000 lx incident light. A white sheet of paper marked with black stripes 5 cm apart was fixed on the wooden frame of the tunnel to allow correct recording of fly distances during chemical triggering. The system was tested with clean air before experiment. All assays were performed with twelve adults (more than 3-day-old males and females, which were randomly sampled from the rearing population) of tomato leaf-miner in a laboratory kept at 25 ± 2 °C. One millilitre of each concentration of each essential oil (containing 0.031, 0.063, 0.125, 0.25, 0.5 and 1 μl of the oil, prepared as outlined in “Isolation of essential oils and preparation of different concentrations for bioassays”) was dispensed from a piece of clean cotton wool held in a rolled copper wire at the bottom of one of the plexi-glass rods attached to the lids covering the rear window. The other rod carried cotton wool treated with 1 ml of acetone, which served as control. The rods were covered with pieces of clean aluminium foil, which were replaced after every assay. The rear windows were closed tightly and air was allowed to flow (25 cm/s) for 2 min before the moths were released. The upwind flight behaviour of the moths was observed, and after 30 min, the number of moths in the two sides (beyond 20 cm from the centre) of the tunnel was recorded. Moths that were located closer to the centre were not classified. After each assay, air was blown into the wind tunnel at 50 cm/s to clear any residual odour. Four replicates were carried out for each concentrate, and the repellence index (RI) was calculated using the McDonald et al. (1970) formula, % (RI) = [(Nc−Nt)/Nc + Nt] × 100.
Repellence tool. (a, c) Square windows for samples introduction. (b) Square window for insect introduction. (d) White plexi-glass gauze at the inner side to prevent moths from getting out to the tunnel. (e) White sheet of paper marked with black stripes of 5 cm to allow correct recording of fly distances. (f) Samples and control set; 20 cm: distance between the window side and the end of the tunnel; 25 cm: width of the tunnel; 200 cm: length of the tunnel
Repellence of blends of available constituents and assessment of their relative contributions in subtractive assays
Synthetic blends of available constituents were prepared in relative amounts found in each essential oil, or with each component missing in the same oil. Each blend was tested following the experimental design outlined in “Repellence of O. gratissimum and O. kilimandscharicum oils” at the following concentrations: 0.031, 0.063, 0.125, 0.25, 0.5 and 1 μl ml−1. Acetone alone was used as control. Each dose of each blend was tested in four replicates, and the repellence index of each blend was calculated.
Fumigant toxicity assays on adults
Effects of different concentrations of O. gratissimum and O. kilimandscharicum oils
The effects of exposing T. absoluta adults to 1 ml each of different concentrations (0.031, 0.063, 0.125, 0.25, 0.5 and 1 μl ml−1) of O. gratissimum and O. kilimandscharicum oils, respectively, and some of their constituents, were studied in small cage chambers (20 × 20 × 35 cm) according to the method described by WHO (1996). The top window of the cage was covered with white plexi-glass wire gauze to facilitate the introduction of moths. Test materials in acetone applied to Whatman filter paper (70 mm diameter) in the Petri dish (80 mm) acted as the sources of fumigants. These Petri dishes were covered with wire gauzes to prevent the leaf-miner to directly contact the filter papers. Control cages were similarly set with acetone only. Rolled filter papers (5 × 10 cm) dipped into honey served as sources of food for the insect. In each assay, six adult male and female moths (> 3-day-old) were released in both treated and control cages, and the number of live and dead insects was counted after 24 h. Each test was replicated four times.
Time-course effects of higher concentrations of O. gratissimum and O. kilimandscharicum oils
The time-course mortality of adult T. absoluta exposed to three concentrations of each essential oil (1 ml of 0.25, 0.5 and 1 μl ml−1) was monitored after 6, 12, 18 and 24 h. The assays were carried out in the same setups as in “Effects of different concentrations of O. gratissimum and O. kilimandscharicum oils”.
Fumigant toxicity of some constituents and assessment of contribution of each to synthetic blends in subtractive assays
Dose-response effects of some constituents were assessed. Also, synthetic blends of available constituents in each essential oil and blends with missing component were made. Each of these assays was setup as outlined in “Effects of different concentrations of O. gratissimum and O. kilimandscharicum oils” at the concentration of 0.031, 0.063, 0.125, 0.25, 0.5 and 1 μl ml−1, each in four replicates.
Statistical analyses
Mortality was corrected using Abbott’s formula and probit analysis was used to estimate lethal concentration (LC50 and LC90), lethal time (LT50) and the repellence index at 50% (RI50). A time-course effect at different exposure times was estimated using GLM model and the Log rank test was performed using the Kaplan-Meier survival method to compare the surviving at any specific time for each group (Kleinbaum and Klein 2012). The mean numbers of repelled or dead insects were analysed using analysis of variance and compared by the Student-Newman-Kuels (SNK) test. Results giving P value < 0.05 were considered significantly different. All statistical analyses were implemented in a custom script R 3.3.2 software (R Core Team 2015).
Results
Essential oils of O. gratissimum and O. kilimandscharicum
Repellence on T. absoluta adults
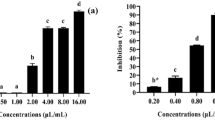
Figure 2 depicts the repellence effects of the two essential oils on adult leaf-miner. Both oils were found to be repellent to T. absoluta, with O. gratissimum essential oil showing higher activity than that of O. kilimandscharicum. The repellence index at 50% was RI50 = 0.13%, F = 69.28, df = 5 and P < 0.0001 for O. gratissimum oil, and RI50 = 0.50%, F = 92.62, df = 5 and P < 0.0001 for O. kilimandscharicum.
Repellency indices (RI) (%) of Ocimum gratissimum (Og) (df = 5, F = 69.28, P < 5 0.0001) and Ocimum kilimandscharicum (Ok) (df = 5, F = 92.62, P < 0.0001) essential oils on 6 adults of Tuta absoluta. For each oil, means at different concentrations marked by same lowercase letter(s) are not significantly different; and for each concentration of the two oils, means marked by the same uppercase letters are not significant
Fumigant toxicity on T. absoluta adults
Effects of different concentrations of the oils
Although, O. gratissimum demonstrated somewhat higher fumigant toxicity compared with O. kilimandscharicum to T. absoluta at most concentrations following 24 h of exposures (Table 1), the differences were not statistically significant. The lethal concentrations were LC50 = 0.24 and LC90 = 0.66 for O. gratissimum oil, and LC50 = 0.43 and LC90 = 1.83 for O. kilimandscharicum oil.
Time-course effects of selected concentrations
Table 2 summarises mortality of the adults following exposures to higher concentrations of the two oils after 6, 12, 18 and 24 h. The results show not significant effect of exposure time for O. gratissimum oil (with P = 0.05, LT50 = 15.49) and significant effect for O. kilimandscharicum (with P = 0.01, LT50 = 17.39) at 1 μl ml−1.
Compositions of O. gratissimum and O. kilimandscharicum essential oils
The yields of O. gratissimum and O. kilimandscharicum essential oils that were obtained were 0.3% and 0.15%, respectively. A total of 41 and 35 constituents were identified by GC-MS in the two oils, respectively. The more abundant constituents (more than 1%) of O. gratissimum oil were methyl eugenol (39.5%), eugenol (29.7%), epi-β-santalene (4.7%), (E)–caryophyllene (3.7%), neo-allo-ocimene (3.1%), linalool formate (1.6%), (Z)-β ocimene (1.5%), (1S)-(−)-β-pinene (1.1%) and 1,8-cineole (1.1%) (Fig. 3(A)). Those of O. kilimandscharicum included camphor (47.0%), 1,8-cineole (19.3%), (−)-camphene (5.2%), l-(−)-fenchone (4.9%), terpinene-4-ol (2.7%), myrtenol (2.1%) and α terpineol (1.5%) (Fig. 3(B)). The identified constituents of the two oils are listed in Table 3.
Some commercially available constituents (methyl eugenol, eugenol, E-caryophyllene, 1,8-cineole, (1S)-(−)-β-pinene and α-pinene of O. gratissimum oil, and (−)-camphene, (%), l-(−) fenchone, α-terpineol, 1,8-cineole, E-caryophyllene and limonene of O. kilimandscharicum) were purchased and tested on T. absoluta. These compounds were prepared separately and also blended to obtain a mixture in relative amounts found in each oil.
Contribution of some constituents of O. gratissimum oil to fumigant toxicity and repellence
Repellence of six compounds of O. gratissimum essential oil
Table 4 summarises the individual repellence of six constituents of O. gratissimum oil after 30-min exposure. Significant differences (P < 0.0001) were found between the compounds, and the most repellent was eugenol with RI50 of 0.15.
Repellency of six-component blend and blends with each constituent subtracted
The effect of subtracting each of the six constituents on the repellence of the resulting blend is summarised in Table 5. Subtraction of eugenol (RI50 of 0.44) gave a blend with the lowest repellence at all concentrations. However, subtraction of α-pinene (RI50 of 0.07) showed a minimum reduction of repellence.
Fumigant toxicity of the six constituents
The dose-response fumigant toxicities of the six constituents are depicted in Fig. 4. The constituents showed varied level of mortality, with eugenol being most lethal (LC50 of 0.24; LC90 of 1.77 μl/ml and 83.3% at 1 μl/ml) followed by methyl eugenol (LC50 of 0.32; LC90 of 1.79 μl/ml and 79.1% at 1 μl/ml). The lowest mortality was recorded with α-pinene (LC50 of 1.17; LC90 of 3.09 μl/ml and 31.2% at 1 μl/ml). No mortality was observed in the control assay.
Effect of each constituent of the 6-component blend in subtractive fumigant toxicity bioassays
Effects of the synthetic blend of the six constituents of O. gratissimum (MOG) and those of five-component blends with each constituent subtracted are depicted in Fig. 5. A significant difference (P < 0.0001) was found between the treatments and the most lethal was the five-component blend with α-pinene missing (MOG-α-pinene) with LC50 of 0.19, LC90 of 1.82 μl/ml and 77.0% at 1 μl/ml. Subtraction of methyl eugenol led to the highest drop in the activity of the resulting blend (LC50 of 0.68, LC90 of 2.89 μl/ml, 54.16% at 1 μl/ml). No mortality was observed in the control assay (acetone alone).
Contribution of seven constituents of O. kilimandscharicum oil to fumigant toxicity and repellence
Repellence effect of each of the seven constituents
The repellence effects of the seven purchased constituents of O. kilimandscharicum oil are outlined in Table 6. Camphor was the most repellent compound followed by camphene and 1,8-cineole with RI50 of 0.13, 0.17 and 0.19, respectively.
Repellence effect of seven-component blend and blends with each constituent subtracted
The synthetic blends prepared with that seven compounds of O. kilimandscharicum oil were found to have varying levels of repellence against T. absoluta (Table 7). The repellencies of different blends were highly significant (P < 0.0001) and the interaction between the constituents of the blends was also significant (P = 0.002). Subtraction of limonene (MOK-limonene) with 100% at 1 μl/ml (RI50 of 0.09) gave the six-component blend with minimum reduction in repellence. Subtraction of 1,8-cineole or camphor led to significant drops (78.3%, RI50 of 0.23 and 79.8%, RI50 of 0.21 at 1 μl/ml, respectively) in the repellence.
Fumigant toxicity of each constituent
Significant differences (P < 0.0001) were also found between camphor, 1,8-cineole, (−)-camphene, l-(−)-fenchone, E-caryophyllene, α-terpineol and limonene present in O. kilimandscharicum oil (Fig. 6). The most toxic constituent was camphor (LC50 of 0.23; LC90 of 1.69 μl/ml and 89.5%) followed by 1,8-cineole (LC50 of 0.26; LC90 of 1.99 μl/ml and 72.9%), with limonene (LC50 of 1.10; LC90 of 3.05 μl/ml and 33.3%) being least effective at 1 μl/ml.
Contribution of each constituent to fumigant toxicity in subtractive assays
Fumigant toxicities of the synthetic blend of the seven compounds in proportion found in O. kilimandscharicum oil (MOK) and blends with each constituent missing are summarised in Fig. 7. Significant difference was found between the blends (P < 0.0001). The most lethal blend was one without limonene (MOK-limonene) (LC50 of 0.22; LC90 of 1.76 μl/ml and 83.3%) and the less one was MOK (LC50 of 0.92; LC90 of 2.86 μl/ml and 58.3%) at 1 μl/ml. No effect was found in the control set.
Discussion
The results showed significant levels of repellence and fumigant toxicity of the essential oils of both O. gratissimum and O. kilimandscharicum to the adults of T. absoluta. The repellence and fumigant toxicity of some individual constituents that were assayed, and subtractive assays with synthetic blends of these, showed that the activities of both oils are due to additive/synergistic effects of different constituents. However, these assays also showed differential intrinsic activities of the constituents. The leaves’ essential oils of the two oils were analysed using GC-MS method and methyl eugenol (39.5%), eugenol (29.7%) (for O. gratissimum), camphor (47.0%) and 1,8-cineole (19.3%) (for O. kilimandscharicum) were found to be the major constituents. Base on the GC profile, these oils belonged to methyl eugenol-eugenol and camphor-1,8-cineole chemotype, respectively. These major constituents were the most repellent and toxic, and the subtraction of them in the artificial blend leads to significant drop of effect which showed that they contributed most to the activity in both oils. O. gratissimum and O. kilimandscharicum plants growing in different agro-ecological areas have also been investigated by many researchers and variations associated with epigenetic, the different seasons and the extraction method may impact the composition of the oil and may significantly affect their activities (Beatovic et al. 2015; Hussain et al. 2007). In fact, a literature survey revealed that, in O. gratissimum, eugenol, thymol, citral, geraniol, ethyl cinnamate, linalool, linalool-methyl chavicol, methyl eugenol-eugenol and methyl cinnamate chemotype were found (Matasyoh et al. 2007; Asawalam et al. 2008; Faria et al. 2006; Vieira et al. 2001; Padalia et al. 2014). Likewise, in O. kilimandscharicum, camphor, linalool-camphor-1,8-cineole, camphor-limonene, camphor-1,8-cineole, 1,8-cineole, linalool, 1,8-cineole-eugenol, 1,8-cineole-methyl chavicol-eugenol, 1,8-cineole-β-bisabolene-(E)-α-bisabolene and 1,8-cineole-methyl chavicol-β-bisabolene chemotype were found (Lawal et al. 2014; Sarin et al. 2013; Ram et al. 2013; Ntezurubanza et al. 1984; Charles and Simon 1992).
Phenylpropanoids (⁓ 69.4%) and oxygenated monoterpenoids (⁓ 82%) are found to be the main compounds in the oils and could be responsible for the activities. Moreover, methyl eugenol was found to be more effective in term of knockdown activity, as well as repelling and killing effects (Ngoh et al. 1998), apart from larvicidal activity against Spodoptera litura F., (Bhardwaj et al. 2010). It is a potent inhibitor of the enzyme acetylcholinesterase (Lee et al. 2001), responsible for the hydrolysis of the neurotransmitter acetylcholine, which can eventually lead to paralysis in insects. Eugenol has been shown to exhibit insecticidal property toward Sitophilus zeamais Motsch (Huang et al. 2002) and be toxic and repellent to the beetle Dinoderus bifoveolatus Wollaston (Ojimelukwe and Adler 2000) and tick (Ixodes ricinus L.) (Bissinger and Roe 2010) and a fumigant toward Callosobruchus maculatus F. (Ajayi et al. 2014); it is also used in insect attractant formulation developed for oriental fruit flies, Bactrocera dorsalis Hendel, and melon flies, Bactrocera cucurbitae Coquillett (Gomez and Coen 2013; Hausen et al. 1999). Camphor has been found to possess insecticidal activity against wheat weevil, Sitophilus granarius L. (Mossa et al. 2017), and sheep bot fly, Oestrus ovis L. larvae (Mazyad and Soliman 2001), and fumigant and contact toxicity against cigarette beetle Lasioderma serricorne F. adults (Chen et al. 2014), red flour beetle Tribolium castaneum Herbst (Khiyari et al. 2014), armyworm Pseudaletia unipuncta Haworth (Isman et al. 2008) and the cowpea weevil, Callosobruchus maculatus (Khani and Asghari 2012). 1,8-Cineole is used as an insect repellent and insecticide to the American cockroach (Sfara et al. 2009); as a mosquito larvicide (Corbet et al. 1995); as a mosquito ovipositional repellent (Klocke et al. 1987) and as a fumigant toward adults of Callosobruchus maculatus, Rhyzopertha dominica F. and S. oryzae (Aggarwal et al. 2001), and has toxic effect on the two-spotted spider mite, Tetranychus urticae Koch (Isman et al. 2008), and inhibitor of the enzyme acetylcholinesterase (Ryan and Bryan 1988). Nevertheless, the presence of minor components might also play a role in the activities of the oils.
However, a number of research questions and challenges would need to be addressed in the downstream development and evaluation of the oils. The relatively high volatility of the essential oils means that they would have to be nano-encapsulated in a suitable inclusion complex like Alginate microsphere, which can facilitate their controlled-release and their performance over longer periods of time in the field (Soliman et al. 2013). Recently, micro- and nano-emulsions studies have shown capability of decreasing volatility and improving stability, water solubility and efficacy of essential oil formulations (Pavoni et al. 2020; Mossa et al. 2017). Also a number of reports to optimise the control released dynamic using cyclodextrins to enclose essential oils and other volatile compounds or blends have significantly controlled the rate of evaporation of volatiles with good efficacy in the field (Marques 2010; del Toro-Sánchez et al. 2010; Junnila et al. 2015; Khoshtinat et al. 2017). One of our follow-up objectives is to pursue this line of research to find out if the oils can be effectively deployed using the host plant. Further tests will have to be done to explain the mechanism of action of the oils and their effect on non-target organisms. However, tests on sublethal doses of the oils on fecundity, longevity of the next generation and vitality could justify the higher values of LC90 due to the long-term effect. In fact, previous reports of Benelli et al. (2018) and Pavela et al. (2020) have shown how sublethal concentrations can decrease longevity, fecundity, fertility and natality of insects. Also, the effect of the environmental factor like temperature when applied in field conditions could play a major role in the oil efficacy. The low or high temperature of both Ocimum spp. during application could eventually modify the action of the different constituents inside the oils. For example, Pavela and Sedlak (2018) reported the influence of temperature on three major constituents (thymol, carvacrol and p-cymene) on Spodoptera littoralis Boisd larvae mortality. In addition, it will be interesting to see if these perennial Ocimum plants naturally emit volatiles at sufficient quantities to have significant negative effects on the different stages of T. absoluta.
In conclusion, this study has shown potential effect of O. gratissimum and O. kilimandscharicum essential oils on T. absoluta. Follow-up studies highlighted above could shed more light on their full potential in their downstream applications.
References
Abbes K, Chermiti B (2012) Failure of the biological control of Tuta absoluta using the predator Nesidiocoris tenuis in a protected tomato crop: analysis of factors. IOBC/WPRS Bull 80:231–236
Adams RP (2017) Identification of essential oil components by gas chromotography/mass spectrometry, 4th edn. Allured publishing, Carol Stream
Aggarwal KK, Tripathi AK, Prajapati V, Kumar S (2001) Toxicity of 1,8-cineole towards three species of stored product coleopterans. Int J Insect Sci 21:155–160
Ajayi OE, Appel AG, Fadamiro HY (2014) Fumigation toxicity of essential oil monoterpenes to Callosobruchus maculatus (Coleoptera: Chrysomelidae: Bruchinae). J Insects:1–7. https://doi.org/10.1155/2014/917212
Asawalam EF, Emosairue SO, Hassanali A (2008) Essential oil of Ocimum gratissimum (Labiatae) as Sitophilus zeamais (Coleoptera: Curculionidae) protectant. Afr J Biotechnol 7:3771–3776
Beatovic D, Krstić-Milošević D, Trifunovic S, Petrovic J, Glamoclija JM, Ristic M, Jelacic S (2015) Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec Nat Prod 9:62–75
Bekele J, Hassanali A (2001) Blend effects in the toxicity of the essential oil constituents of Ocimum kilimandscharicum and Ocimum kenyense (Labiatae) on two post-harvest insect pests. Phytochemistry 57:385–391
Benelli G, Pavela R, Giordani C, Casettari L, Curzi G, Cappellacci L, Petrelli R, Maggi F (2018) Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind Crop Prod 112:668–680
Benelli G, Pavela R, Maggi F, Wandjou JGN, Fofie NBY, Koné-Bamba D, Sagratini G, Vittori S, Caprioli G (2019) Insecticidal activity of the essential oil and polar extracts from Ocimum gratissimum grown in Ivory Coast: efficacy on insect pests and vectors and impact on non-target species. Ind Crop Prod 132:377–385. https://doi.org/10.1016/j.indcrop.2019.02.047
Bhardwaj A, Tewary DK, Kumar R, Kumar V, Sinha AK, Shanker A (2010) Larvicidal and structure–activity studies of natural phenylpropanoids and their semisynthetic derivatives against the tobacco armyworm Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Chem Biodivers 7:168–177
Biondi A, Guedes RNC, Wan FH, Desneux N (2018) Ecology, worldwide spread, and management of the invasive south American tomato pinworm, Tuta absoluta: past, present, and future. Annu Rev Entomol 63:239–258. https://doi.org/10.1146/annurev-ento-031616-034933
Biratu W (2018) Review on the effect of climate change on tomato (Solanum lycopersicon) production in Africa and mitigation strategies. J Nat Sci Res 8:62–70
Bissinger BW, Roe RM (2010) Tick repellents: past, present, and future. Pestic Biochem Physiol 96:63–79
Campolo O, Cherif A, Ricupero M, Siscaro G, Grissa-Lebdi K, Russo A, Cucci LM, Pietro PD, Satriano C, Desneux N, Biondi A, Zappalà L, Palmeri V (2017) Citrus peel essential oil nanoformulations to control the tomato borer, Tuta absoluta: chemical properties and biological activity. Sci Rep 7(1):13036. https://doi.org/10.1038/s41598-017-13413-0
Charles DJ, Simon JE (1992) Essential oil constituents of Ocimum kilimandscharicum Guerke. J Essent Oil Res 4:125–128
Chegini SG, Abbasipour H, Karimi J, Askarianzadeh A (2018) Toxicity of Shirazi thyme, Zataria multiflora essential oil to the tomato leaf miner, Tuta Absoluta (Lepidoptera: Gelechiidae). Int J Trop Insect Sci 38:340–347
Chen HP, Yang K, You CX, Lei N, Sun RQ, Geng ZF, Ma P, Cai Q, Du SS, Deng ZW (2014) Chemical constituents and insecticidal activities of the essential oil of Cinnamomum camphora leaves against Lasioderma serricorne. J Chem 2014:1–5. https://doi.org/10.1155/2014/963729
Chowdhary K, Kumar A, Sharma S, Pathak R, Jangir M (2018) Ocimum sp.: source of biorational pesticides. Ind Crop Prod 122:686–701. https://doi.org/10.1016/j.indcrop.2018.05.068
Clemente S, Mareggiani G, Broussalis A, Martino V, Ferraro G (2003) Insecticidal effects of Lamiaceae species against stored products insects. Bol San Veg Plagas 29:421–426
Corbet SA, Danahar GW, King V, Chalmers CL, Tiley CF (1995) Surfactant-enhanced essential oils as mosquito larvicides. Entomol Exp Appl 75:229–236
Cruz GS, Wanderley-Teixeira V, da Silva LM, Dutra KA, Guedes CA, de Oliveira JV, Ferraz Navarro DMA, Araújo BC, Teixeira ÁAC (2017) Chemical composition and insecticidal activity of the essential oils of Foeniculum vulgare Mill., Ocimum basilicum L., Eucalyptus staigeriana F. Muell. ex Bailey, Eucalyptus citriodora Hook and Ocimum gratissimum L. and their major components on Spodoptera frugiperda (Lepidoptera: Noctuidae). J Essent Oil Bear Plants 20:1360–1136
del Toro-Sánchez CL, Ayala-Zavala JF, Machi L, Santacruz H, Villegas-Ochoa MA, Alvarez-Parrilla E, González-Aguilar GA (2010) Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J Incl Phenom Macro 67:431–441
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narváez-Vasquez CA, González-Cabrera J, Ruescas DC, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbaneja A (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83:197–215
Desneux N, Luna MG, Guillemaud T, Urbaneja A (2011) The invasive south American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J Pest Sci 84:403–408
Faria TJ, Ferreira RS, Yassumoto L, Pinto de Souza JR, Ishikawa NK, Barbosa AM (2006) Antifungal activity of essential oil isolated from Ocimum gratissimum L. (eugenol chemotype) against phytopathogenic fungi. Braz Arch Biol Technol 49:867–871
Garcia MF, Espul JC (1982) Bioecology of the tomato moth (Scrobipalpula absoluta) in Mendoza, Argentine Republic. Rev Investig Agropecu 17:135–146
Gikonyo NK, Hassanali A, Njagi PGN, Saini RK (2003) Responses of Glossina morsitans morsitans to blends of electroantennographically active compounds in the odors of its preferred (buffalo and ox) and non preferred (waterbuck) hosts. J Chem Ecol 29:2331–2345
Gomez LE, Coen CE (2013) Insect attractant formulations and insect control. US Patent no. WO2013173300 A1
Haddi K, Berger M, Bielza P, Cifuentes D, Field LM, Gorman K, Rapisarda C, Williamson MS, Bass C (2012) Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta). Insect Biochem Mol Biol 42:506–513
Hausen BM, Reichling J, Harkenthal M (1999) Degradation products of monoterpenes are the sensitizing agents in tea tree oil. Am J Contact Derm 10:68–77
Huang Y, Shuit-Hung HOSH, Lee HC, Yap YL (2002) Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J Stored Prod Res 38:403–412
Hussain AI, Anwar F, Sherazi STH, Przybylski R (2007) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 108:986–995
Isman MB (2006) Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol 51:45–66
Isman MB (2019) Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem Rev 19:235–241. https://doi.org/10.1007/s11101-019-09653-9
Isman MB, Wilson JA, Bradbury R (2008) Insecticidal activities of commercial rosemary oils (Rosmarinus officinalis) against larvae of Pseudaletia unipuncta and Trichoplusia ni in relation to their chemical compositions. Pharm Biol 46:82–87. https://doi.org/10.1080/13880200701734661
Junnila A, Revay EE, Müller GC, Kravchenko V, Qualls WA, Xue d R, Allen SA, Beier JC, Schlein Y (2015) Efficacy of attractive toxic sugar baits (ATSB) against Aedes albopictus with garlic oil encapsulated in β-cyclodextrin as the active ingredient. Acta Trop 152:195–200
Kéita SM, Vincent C, Schmit JP, Bélanger A (2001) Essential oil composition of Ocimum basilicum L., O. Gratissimum L. and O. Suave L. in the Republic of Guinea. Flavour Frag J 15:339–341
Khani A, Asghari J (2012) Insecticide activity of essential oils of Mentha longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against two stored product pests, the flour beetle, Tribolium castaneum, and the cowpea weevil, Callosobruchus maculatus. J Insect Sci 12:73–10. https://doi.org/10.1673/031.012.7301
Khiyari MEA, Kasrati A, Jamali CA, Zeroual S, Markouk M, Bekkouche K, Wohlmuth H, Leach D, Abbad A (2014) Chemical composition, antioxidant and insecticidal properties of essential oils from wild and cultivated Salvia aucheri subsp. blancoana (Webb. & Helder), an endemic, threatened medicinal plant in Morocco. Ind Crop Prod 57:106–109
Khoshtinat K, Barzegar M, Sahari MA, Hamidi Z (2017) Encapsulation of Iranian garlic oil with β-cyclodextrin: optimization and its characterization. J Agr Sci Tech-Iran 19:97–111
Kleinbaum DG, Klein M (2012) Survival analysis - a self-learning text, 3rd edn. Springer Publishers, New York
Klocke JA, Darlington MV, Balandrin MF (1987) 1,8-Cineole (eucalyptol), a mosquito feeding and ovipositional repellent from volatile oil of Hemizonia fitchii (Asteraceae). J Chem Ecol 13:2131–2141
Korycinska A, Moran H (2009) Plant pest notice: South American tomato moth, Tuta absoluta (No. 56), pp1–4, Department for Environment. Food and Rural Affairs, Food Environment Research Agency
Lawal OA, Ogunwande IA, Omikorede OE, Owolabi MS, Olorunsola FF, Sanni AAO, Amisu KO, Opoku AR (2014) Chemical composition and antimicrobial activity of essential oil of Ocimum kilimandscharicum (R. Br.) Guerke: a new chemotype. Am J Essent Oil Nat Prod 2:41–46
Lee SE, Lee BH, Choi WS, Park BS, Kim JG, Campbell BC (2001) Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Manag Sci 57:548–553
Lee EJ, Kim JR, Choi DR, Ahn YJ (2008) Toxicity of cassia and cinnamon oil compounds and cinnamaldehyde-related compounds to Sitophilus oryzae (Coleoptera: Curculionidae). J Econ Entomol 101:1960–1966
Lietti MMM, Botto E, Alzogaray RA (2005) Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotropical Entomol 34:113–119
Lima AS, Milhomem MN, Monteiro OS, Arruda ACP, de Castro JAM, Fernandes YML, Maia JGS, Costa-Junior LM (2018) Seasonal analysis and acaricidal activity of the thymol-type essential oil of Ocimum gratissimum and its major constituents against Rhipicephalus microplus (Acari: Ixodidae). Parasitol Res 117:59–65
Marques HMC (2010) A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Frag J 25:313–326
Matasyoh LG, Matasyoh JC, Wachira FN, Kinyua MG, Muigai AWT, Mukiama TK (2007) Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L. growing in eastern Kenya. Afr J Biotechnol 6:760–765
Mazyad SA, Soliman M (2001) Laboratory evaluation of the insecticidal activity of camphor on the development of Oestrus ovis larvae. J Egypt Soc Parasitol 31:887–892
McDonald LL, Guy RH, Speirs RD (1970) Preliminary evaluation of new candidate materials as toxicants, repellents and attractants against stored product insects. Marketing Research Report No. 882, Agriculture Research Service, US Department of Agriculture, Washington
Mossa ATH, Abdelfattahn NAH, Mohafrash SMM (2017) Nanoemulsion of camphor (Eucalyptus globulus) essential oil, formulation, characterization and insecticidal activity against wheat weevil, Sitophilus granarius. Asian J Crop Sci 9:50–62. https://doi.org/10.3923/ajcs.2017.50.62
Ngoh SP, Choo-LEW PFY, Huang Y, Kini MR, Ho SH (1998) Insecticidal and repellent properties of nine volatile constituents of essential oils against the American cockroach, Periplaneta americana (L.). Pestic Sci 54:261–268
Nguemtchouin MG, Ngassoum MB, Chalier P, Kamga R, Ngamo LS, Cretin M (2013) Ocimum gratissimum essential oil and modified montmorillonite clay, a means of controlling insect pests in stored products. J Stored Prod Res 52:57–62
NIST (2008) NIST Standard Reference Database, National Institutes of Standards and Technology, NIST/EPA/NIH Mass Spectral Library. http://www.nist.gov
Ntezurubanza L, Scheffer JJC, Looman A, Baerheim AS (1984) Composition of essential oil of Ocimum kilimandscharicum grown in Rwanda. Planta Med 50:385–388
Ntonga PA, Baldovini N, Mouray E, Mambu L, Belong P, Grellier P (2014) Activity of Ocimum basilicum, Ocimum canum, and Cymbopogon citratus essential oils against Plasmodium falciparum and mature-stage larvae of Anopheles funestus s.s. Parasite 21:33. https://doi.org/10.1051/parasite/2014033
Ogendo JO, Kostyukovsky M, Ravid U, Matasyoh JC, Deng AL, Omolo EO, Kariuki ST, Shaaya E (2008) Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J Stored Prod Res 44:328–334
Ojimelukwe PC, Adler C (2000) Toxicity and repellent effects of eugenol, thymol, linalool, menthol and other pure compounds on Dinoderus bifloveatus (Coleoptera: Bostrichidae). J Sustain Agr 2:47–54
Padalia RC, Verma RS, Chauhan A (2014) Analyses of organ specific variations in essential oils of four Ocimum species. J Essent Oil Res 26:409–419. https://doi.org/10.1080/10412905.2014.942808
Pavela R, Sedlak P (2018) Post-application as a factor influencing the insecticidal activity of essential oil from Thymus vulgaris. Ind Crops Prod 113:46–49. https://doi.org/10.1016/j.indcrop.2018.01.021
Pavela R, Maggi F, Petrelli R, Cappellacci L, Buccioni M, Palmieri A, Canale A, Benelli G (2020) Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem Toxicol 136:111037. https://doi.org/10.1016/j.fct.2019.111037
Pavoni L, Perinelli DR, Bonacucina G, Cespi M, Palmieri GF (2020) An overview of micro- and nanoemulsions as vehicles for essential oils: formulation, preparation and stability. Nanomaterials 10(135):1–24. https://doi.org/10.3390/nano10010135
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ram SV, Rajendra CP, Amit C, Sanjog TT (2013) Exploring compositional diversity in the essential oils of 34 Ocimum taxa from Indian flora. Ind Crop Prod 45:7–19
Roditakis E, Vasakis E, Grispou M, Stavrakaki M, Nauen R, Gravouil M, Bassi A (2015) First report of Tuta absoluta resistance to diamide insecticides. J Pest Sci 88:9–16
Ryan MF, Bryan O (1988) Plant-insect coevolution and inhibition of acetylcholinesterase. J Chem Ecol 14:1965–1975
Said-Al Ahl H, Hikal WM, Tkachenko KG (2017) Essential oils with potential as insecticidal agents: a review. J Environ Plann Man 3:23–33
Sarin RV, Narwal S, Bafna PA (2013) Anti-diarrhoeal activity of aqueous extract of Ocimum kilimandscharicum. J Ethnopharmacol 148:223–228
Sfara V, Zerba EN, Alzogaray RA (2009) Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. J Med Entomol 46:511–515
Soliman EA, Yel-M A, Smel-D M, Magdy AM (2013) Microencapsulation of essential oils within alginate: formulation and in vitro evaluation of antifungal activity. J Encapsul Adsorp Sci 3:48–55
Sylla S, Brévault T, Bal AB, Chailleux A, Diatte M, Desneux N, Diarra K (2017) Rapid spread of the tomato leafminer, Tuta absoluta (Lepidoptera : Gelechiidae), an invasive pest in sub-Saharan Africa. Entomol Gen 36:269–283
Umerie SC, Anaso HU, Anyasoro LJC (1998) Insecticidal potentials of Ocimum basilicum leaf-extract. Bioresour Technol 64:237–239
Vieira RF, Grayer RJ, Paton A, Simon JE (2001) Genetic diversity of Ocimum gratissimum L. based on volatile oil constituents, flavonoids and RAPD markers. Biochem Syst Ecol 29:287–304
WHO (1996) Report of the WHO Informal Consultation on the Evaluation and Testing of Insecticides. WHO/HQ, Geneva, 7 to 11 October. : 32–36; 50–52. https://apps.who.int/iris/handle/10665/65962
Xian X (2017) The potential invasion risk of the tomato leafminer Tuta absoluta in China. In Conference. Ottawa, Canada, 30th August 2017, 1–37
Yarou BB, Bawin T, Boullis A, Heukin S, Lognay G, Verheggen FJ, Francis F (2017) Oviposition deterrent activity of basil plants and their essentials oils against Tuta Absoluta (Lepidoptera: Gelechiidae). Environ Sci Pollut Res Int 25:29880–29888
Zhu J, Zeng X, O’neal M, Schultz G, Tucker B, Coats J, Bartholomay L, Xue RD (2008) Mosquito larvicidal activity of botanical-based mosquito repellents. J Am Mosquito Contr 24:161–168. https://doi.org/10.2987/8756-971X(2008)24[161:MLAOBM]2.0.CO;2
Acknowledgements
We gratefully acknowledge the financial support for the German Academic Exchange Service In-Region scholarship for funding FREE. SAM thanks the Federal Ministry of Cooperation and Development (BMZ), Germany for providing the financial support through ICIPE Tuta absoluta IPM project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Giovanni Benelli
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Essoung, F.R.E., Tadjong, A.T., Chhabra, S.C. et al. Repellence and fumigant toxicity of essential oils of Ocimum gratissimum and Ocimum kilimandscharicum on Tuta absoluta (Lepidoptera: Gelechiidae). Environ Sci Pollut Res 27, 37963–37976 (2020). https://doi.org/10.1007/s11356-020-09773-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-09773-2