Abstract
With the development of technologies and the change of consumer attitudes, the amount of waste electrical and electronic equipment (WEEE) is increasing annually. As the core part of WEEE, the waste printed circuit board (WPCB) is a dangerous waste but at the same time a rich resource for various kinds of materials. In this work, various WPCB treatment methods as well as WPCB recycling techniques divided into direct treatment (landfill and incineration), primitive recycling technology (pyrometallurgy, hydrometallurgy, biometallurgy and primitive full recovery of NMF-non metallic fraction), and advanced recycling technology (mechanical separation, direct use and modification of NMF) are reviewed and analyzed based on their advantages and disadvantages. Also, the evaluation criteria are discussed including economic, environmental, and gate-to-market ability. This review indicates the future research direction of WPCB recycling should focus on a combination of several techniques or in series recycling to maximize the benefits of process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Electrical and electronic equipment items (EEE) including MP3, cell phones, and tablets have been the indispensable necessity of peoples’ daily lives while past decades have witnessed the rapid growth of EEE manufacturing industries. Currently, the production rate of EEE is estimated to be between 8.3 and 9.1 million tons per year in 2005 with an increasingly rapid growth rate due to technology updates, making it the dominant manufacturing industry in the world [1, 2]. The rapid development of EEE has given rise to the blooming of the printed circuit board manufacturing industry, which produces the core components of electrical and electronic equipment [3, 4]. Meanwhile, the accelerating upgrading of these industries also shortens the life span of EEE from around 5 years to as short as 2 or 3 years [5, 6]. This is an accelerating process, with an annual rate of 3–5% according to an EU report in 2000 [7]. Consequently, it is not difficult to realize the fact that the amount of waste electrical and electronic equipment (WEEE), as the result of EEE jettisoning, is increasing at an overwhelming rate. It is estimated that currently the amount of WEEE production is nearly 45 million tons yearly worldwide with an annual growth rate of 7–10% [8].
The printed circuit board (PCB) is considered to be the core part in most kinds of EEE (see Table 1). It is estimated that global production of printed circuit boards (PCBs) was around $50 billion in 2010 and reached nearly $60 billion in 2012 with a market growth rate of 1.7% [10, 11]. However, the mass disposal of WEEE generates a huge stream of waste printed circuited board (WPCB) into municipal solid waste, which was produced in large amounts which increase significantly every year (Fig. 1). Although WPCB only occupies a small portion around 3–6% of the total WEEE generated worldwide, the immense total WEEE amount still made it considerable [8, 12]. WPCB can be either directly treated, which includes landfill and incineration, or the recycling of WPCB by various kinds of technologies is also an alternative [13–18]. Compared to direct treatments, recycling is more favorable due to both environmental and economical consideration given the enrichment of materials in WPCB. It is regarded as a secondary resource since the concentration of precious metals, organic resins, or polymers in WPCB is normally ten times higher than rich non-renewable are (sometimes even hundred times higher) [19]. Therefore, recycling is not simply reduction of waste but the reuse of resources with better economic feasibility and less environmental impact.
PCBs increasing based on the global gross [11]
The USEPA (United States Environmental Protection Agency) has identified seven major benefits when scrap iron and steel are used instead of virgin materials (Table 2). Using recycled materials in place of virgin materials results in significant energy savings (see Table 3 [20]). Also, the European Commission launched the 2002/96/EC Directive, known as the WEEE Directive, which came into effect on February 13, 2003, with the aim of achieving up to 70–80% recovery of electrical and electronic equipment [15]. Therefore, recycling is not only for the purpose of resource refining, more importantly, it can reduce the toxic pollution emitted in the initial manufacturing process, which will make a significant benefit difference in remediation costs.
Current recycling methods includes pyrometallurgy and hydrometallurgy, which have a long history and wide application [21–23]. Biometallurgy as an emerging technology also takes up a certain share of the WPCB recycling market [24–26]. Many studies have been done to improve the performance or cut the cost of these techniques. However, the inherent drawbacks are very obvious for these techniques, including the irritation to the environmental intensive energy consumption. Therefore, new advanced technologies are in great demand due to the requirement of technology with high safety and economic feasibility. Mechanical separation was developed due to the requirement for a high-purity metallic fraction of WPCB (MF) and it can achieve good separation performance for real-use applications [27–30]. Recently, the use of the non-metallic fraction (NMF), which was forgotten in the past, is becoming the concern of researches including both the direct use or the modification of NMF [31–35].
However, a unanimous blind spot among the majority of works is the environmental emission analysis, which is a very serious problem derived from WPCB treatment. Several studies have been done on the evaluation of the emissions or discharge [5, 15]. Common pollutants generated from WPCB treatment or recycling include heavy metals, secondary particulates, PBDD/Fs, and PCDD/Fs, which have been studied and their toxicities are regarded as obstacles to WPCB recycling. However, emissions with lower toxicity, especially bromide in the form of hydrogen bromide or bromide, did not receive adequate attention. Despite the relatively low harm these emissions possess compared to dioxin-like organic compounds or heavy metals, the jeopardy they show is still a significant threat to human health that requires detailed studies and further treatment. Moreover, the complexity of WPCB’s composition creates the scenario that specific analysis targeting certain pollutants is available while an overall review of emissions impact is still absent. Therefore, this overall emission impact study and strategy needs more effort.
Other than the discussion of emission, another issue mentioned previously is concerned with the techniques that focus mainly on general WEEE treatment methods. Nevertheless, WPCB shares several similarities with WEEE in terms of composition and chemical characteristics, the minor differences between them still requiring the development of better process techniques for WPCB treatment [36–38]. The common composition of EEE is shown in Table 4 while the detailed discussion of WPCB will be presented in a later section. Therefore, the techniques for WEEE can be applied to WPCB but may not acquire ultimate efficiency. Actually, compared with WEEE, the composition of WPCB is more or less more consistent, since the outer casing for WEEE varies with different kinds of EEE, for example, the refrigerator or television while most WPCB has no relationship with the outer casing, making WPCB simpler for treatment or recycling. Thus, the specific design and analysis for WPCB treatment or recycling is indispensable in order to obtain the maximum sustainable environmental and economical benefits.
Herein, the lack of a summary on current techniques due to the upgrading of techniques as well as the absence of systematic emission analysis claims that an overall review of this field is imperative. Also, with the development of technologies, the overlapping of chemical, mechanical and physical treatments is very obvious, thus a new criteria is necessary for sorting and evaluating the interdisciplinary recycling and treatment techniques. In this work, the treatment and recycling techniques of WPCB are categorized according to their recovery rate and both existing and emerging techniques are reviewed and discussed. The performance of WPCB recycling techniques is evaluated from both an economic and environmental perspective. Moreover, the concept of “gate to market ability” is introduced for a more profound understanding of the feasibility of WPCB recycling techniques.
2 Characterization of WPCB
The composition of WPCB varies, since the source, components, and manufacturing processes of the WPCB maybe very different [40]. Generally, the composition of currently used PCBs is classified as glass fiber reinforced epoxyresin (FR-4), which is normally used for small electronic equipment or a paper laminated phenol is resin (FR-2) used in home appliances [41, 42]. Also, during the manufacturing process, carbonaceous compounds including brominated flame retardants and dyes are added in order to improve the flame resistance and visual recognition ability. In Table 5, it is obvious that WPCB mainly consists of MF and NMF. It is generally accepted that WPCB contains around 30% metallic fraction and 70% non-metallic fraction by weight [43, 44]. However, what needs to be clarified is that the percentage of noble metals in WPCB has been decreasing in recent years with the upgrading of technologies [45].
It is widely accepted that WPCB is comprised of a heterogeneous mixture [39, 46–48]. The heterogeneity originates from two aspects, which are the heterogeneity of different PCB brands including the use of cellulose paper reinforced phenolic resin or glass fiber reinforced epoxy resin and heterogeneity in the morphology of a PCB due to the presence of metal plating [49]. However, if an adequate amount of WPCB is collected and pulverized into fine powder, empirically rules of the composition of WPCB can still be identified by comparing them globally. This is the so-called “pseudo-homogenous” effect, which means that the individual composition of a certain WPCB item will vary from others, while the bulk composition of all WPCB items will still share similarities, as shown in Table 6.
Generally, the uncertainty of WPCB composition hampers the systematic treatment or recycling of WPCB, since the complexity of it interferes with each component [59]. Therefore, pulverization is a very important pretreatment part for mixed WPCB recycling and treatment techniques since the higher pseudo-homogeneity is more favorable for easier application in overall treatment.
3 Direct treatment methods—landfill and incineration
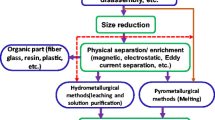
The types of technologies for WPCB recycling are increasing with the development of research and thus the old category for technologies sorting is no longer applicable. Some processes divide the technologies to thermal or non-thermal, or some divide into chemical treatment or mechanical treatment, others divide them by the atmosphere of the reaction or some divide them into hydrometallurgical processing or pyrometallurgical processing [16, 60, 61]. In this review, the treatment and recycling techniques for WPCB are classified according to their recovery degree (Fig. 2). Direct treatment including landfilling and incineration means that no or only the energy of WPCB was recovered while primitive recycling means the simple recycling of MF, while the NMF ended up in disposal or limited non-hazardous treatment. Advanced recycling includes two parts: separation methods without damage to the NMF and the direct use or modification of NMF. It is highly convincing that this classification can better describe the current situation of WPCB recycling and provide a basis for an overall treatment perspective instead of focusing on only one or two areas.
3.1 Landfill
Landfill is a WPCB treatment method with a long history and wide application worldwide because of simplicity in operation. However, the land for WPCB disposal is thereby regarded as wasteland and normally incapable of being exploited again in predictable decades, which is not suitable for countries/communities where there is a lack of land space. In addition, the environmental issues aroused by WPCB landfilling such as the leachate formation in landfill sites and evaporation of hazardous substances raise safety concerns. It is reported that almost 70% of the heavy metal in landfill sites come directly from WEEE, which indicates that a considerable part of heavy metals actually come from WPCB [62]. Concurrently, the co-landfilling of WPCB with various kinds of municipal solid waste makes the formation of leachate containing brominated toxic compounds and heavy metals possible due to the reactions during the landfilling process [13, 14]. The synergic pollution will contaminate the atmosphere, soil, and ground water, and this has been proven by several research groups.
Heavy metals including lead and mercury are identified in landfill sites. Lindberg et al. identified total gaseous mercury (TGM), monomethyl mercury (MMM), and dimethyl mercury (DMM) in the landfill gas in Florida, USA [63]. The results show that the mean concentration of TGM is 7190 ng/m3 and MMM is 6 ng/m3. It should be emphasized that DMM, a form of extremely toxic organic mercury, was also observed in landfill gas with a concentration of 30 ng/m3. Spalvins et al. also identified lead in the leachate in landfill sites with a concentration of 0.007–0.066 mg/l [64]. Other than heavy metals, PCDE(polychlorinated diphenyl ethers)/PBDE(polybrominated diphenyl ethers) were also discovered in landfill sites by Osako et al. [65]. Samples from seven different landfill sites in Japan were collected and analyzed. Landfill sites in operation or closure within 1 year show the presence of several kinds of hazardous compounds, especially obvious is the higher concentration of brominated flame retardants and the derivatives. TBBPA with concentration from n.d. to 620,000 pg/l and PBDE-47, -99, and -100 with the total concentration from n.d. to 4000 pg/l were detected in the raw leachate from different landfill sites. More dangerously, the hazards of landfill leachate and gas are not only limited to the landfill site because they will propagate though aquatic systems or rain fall and cause the spread of contamination. Wong et al. found that even in the downstream of the small-scale landfill site of a Chinese processing village, a certain amount of metals can still be detected including Cd (n.d. to 10.3 mg/kg), Cu (17.0–4540 mg/kg), Ni (12.4–543 mg/kg), Pb (28.6–590 mg/kg), and Zn (51.3–324 mg/kg) [66]. This indicates the possibility of toxic substance migration, which makes the condition of landfill sites much worse.
It summary, landfilling is currently considered an improper way to treat WPCB due to the environmental concerns it brings about, and actually the function of it is to use land resources to remedy hazardous WPCB, which is a waste of resources for both soil and WPCB. Therefore, stricter policies and regulations have been imposed on simply landfill WPCB in many countries and more environmental benign treatment methods are required [67–71].
3.2 Incineration
Incineration means the combustion of WPCB by converting its calorific value to energy and emitting the gas directly or after treatment with the purpose mainly to remove the non-metallic fraction part (around 70 wt%) [72, 73]. It has the advantages of significantly reducing the volume of WPCB by 50%, and also the calorific value of WPCB is relatively high compared to municipal solid waste, which is around 9.9 × 104 kJ/kg [15, 16]. Therefore, it readily satisfies the minimum incineration calorific value for waste, which is roughly 5000 kJ/kg. Currently in the world, incineration is still widely used in American, Asia, and Europe due to the simplicity of the process. However, during the combustion of WPCB, toxic emissions including heavy metals, fly ash, polychlorinated dibenzo-p-dioxins/polychlorinated dibenzofurans (PCDD/Fs) and polybrominateddibenzo-p-dioxins and dibenzofurans (PBDD/Fs) are released into the atmosphere in the absence of post-purification [74, 75]. Cadmium, copper, nickel, lead, and zinc will be vaporized according to the order of their melting points and released into the atmosphere [76]. Unfortunately, incineration is an ideal place for the formation of PCDD/Fs and PBDD/Fs due to the presence of halogens and an oxidizing atmosphere as well as incomplete combustion [77–79]. Additionally, in incineration, copper in WPCB will play the role of catalyst for dioxin formation in the presence of brominated flame retardant [5]. This all contributed to the high emission amount of PBDD/Fs, as high as 130,000 ng/g, which is very high compared to other investigations [80]. Also, the amount of PBDD/Fs exceeds PCDD/Fs significantly due to the abundance of bromide in WPCB. Other gaseous pollutants including Br2, HBr, CO, and NOx also attract considerable concern about them.
Ni et al. has conducted high-temperature WPCB incineration in a tube furnace and found that high-temperature combustion favors the removal of PBDD/Fs, which can release maximum 99.9% bromide into flue gas in the form of HBr or Br2 with much lower toxicity compared with PBDD/Fs [79]. Also, the temperature needs to exceed 1200 °C in order to reduce the formation of CO and ensure the completely decomposition of brominated flame retardant (Fig. 3). However, the high temperature will slightly favor the formation of NOx from 690 mg/Nm3 (800 °C) to 790 mg/Nm3 (1400 °C) that exceeds the emission standard value of 500 mg/Nm3. In spite of HBr and Br2having a lower toxicity, they still need further treatment like adsorption to avoid the emission to local atmosphere.
Other than the gaseous emission that incineration releases, there is still the solid-phase residue of the incineration that is termed “bottom ash,” which will be landfilled or processed for further treatment. There are significantly enriched heavy metals in the bottom ash including copper (35.8–1295 mg/kg), Pb (226.3–606.6 mg/kg), and Cd (0.87–7.46 mg/kg), which makes the safe disposal of it become a troublesome issue [76]. Normally, a vitrification process at a temperature of 1500–1600°C is typical for the treatment of bottom ash [82].
Therefore, incineration as a treatment method for WPCB is not an environmentally friendly option considering the toxic emissions. Also, the construction of an incineration plant is a major expenditure for local governments. Actually, as these side effects were revealed by the emerging researches, the use of existing incineration plant or planning for future incineration plants was seriously affected in many countries. Public concerns and acceptability were more attracted to the recycling instead of simple treatment of WPCB.
4 Primitive Recycling Techniques
4.1 Partial Recovery of Metallic Fraction from WPCB
4.1.1 Pyrometallurgy Recycling Techniques
Pyrometallurgy recycling techniques are conducted by decomposing WPCB in the absence of air or the presence of an inert atmosphere. It is a very important and mature technology for WPCB recycling and numerous works have been done on this. Although pyrometallurgy shares similarities with incineration, they are still distinct and separate techniques. The main difference between pyrometallurgy and incineration is that the purpose of incineration is simply reducing the volume of WPCB and to, achieve partial heat recovery though the incineration of WPCB when the heat released is adequate, whereas the purpose of pyrometallurgy focuses more on resource recovery, which includes the concentrating of MF and other materials out of the WPCB feedstock by decomposing NMF to low molecular weight organic compound-formed liquid or gas. This also leads to the result that unlike incineration, the operation cost for pyrometallurgy is normally much higher than incineration due to maintaining the absence of oxygen from the process. More comparisons of incineration and pyrolysis in detail are listed in Table 7.
In pyrometallurgy, the process is typically divided into two stages. In the first stage, the WPCB starts to decompose due to the intensive heat input and the release of volatile organic compounds. The second stage is the formation of char due to the pyrolysis of polymer inside the structure [6]. Therefore, temperature is a very critical variable in pyrometallurgy such that low temperatures (<400 °C) will favor the formation of liquid products while high temperatures (>800 °C) will help the breakage of high molecular weight compounds, thus producing more small organic molecular [15, 83, 84]. Also, the pyrolysis of WPCB is extremely dangerous while the temperature is low (lower than 800 °C) and the absence of an inert atmosphere since there will be PBDD/Fs formation [85]. Normally, the absence of air is relatively difficult to ensure, especially in large-scale applications, thus the production of PBDD/Fs is a serious issue for low-temperature pyrolysis.
As for the pyrolysis products, some pyrolysis reactions can produce pyro-oil or pyro-gas, which has the potential to be used as fuel or chemical material source, but the complexity and uncertainty of it greatly hampers its application [15, 16]. Also, the products of pyrometallurgy have been characterized by several groups and HBr, by-products of brominated phenols, PCDD/Fs, PBDD/Fs have been found in pyrolysis products, which has the side effects of aquatic toxicity, carcinogenicity, and mutagenicity [17, 18, 86–89]. As for the pyro-solid, which is the inorganic components left after pyrolysis normally generates a huge amount of acidic effluents, will jeopardize the aquatic environment and cause side effects to human health [90]. As a result, the majority of research only focuses on the recovery rate of WPCB without overall consideration of the environmental impact analysis.
Pyrolysis of commercial WPCB has been done in an autoclave at 500 °C for 30 min and the pyro-liquid, pyro-gas, as well as pyro-solid were collected [15]. The yields of pyro-liquid, pyro-gas, pyro-solid, and char derived from the pyrolysis of polymeric materials were 16.2, 7.3, 76.5, and 5.8%, respectively. However, the value of these pyrolysis products is very limited since the pyro-solid contains a high content of heavy metals including Cu (36.4 wt%) and Pb (3.8 wt%), which is not capable of being recovered and actually requires further treatment. Also, the pyro-liquid contains a certain amount of Br and Cl, which is hazardous to human health, and is not clarified in the paper. Guan et al. also studied the pyrolysis products of WPCB and the GCV value of pyro-liquid was found to be around 29 MJ/kg, which is around the same level as coal, which is normally 30 MJ/kg [16]. However, the Br inside the pyro-liquid will hamper the usage of it, which must undergo removal before application. The main components of pyro-gas are hydrogen, carbon monoxide, carbon dioxide, and methane at 500 °C. However, there still are some bromorganic molecules inside, which will affect the usage of the pryo-gas. Therefore, we can conclude that the failure of separation with Br is a serious challenge for the application of pyro-liquid and pyro-gas. Thus, Terakado et al. used metal oxide including ZnO, Fe2O3, La2O3, CaO, and CuO as the fixation of Br in pyrolysis process and the results show a significant reduction of HBr as well as bromorganic compounds emission [91]. The deficiency is that metal oxide was used as additive and still in the end bromide salt requires further treatment.
Guo et al. has done the pyrolysis in 500 °C and 71.60 wt% solid residue, 18.23 wt% tar, and 10.71 wt% gas were obtained from this process [92]. The main component of the pyro-gas is CO, CO2, propylene, and small bromorganic molecules like bromomethane and bromopropane. The calorific value of pyro-gas is 2.386 MJ/kg, which is quite low comparing to natural gas (38.93 MJ/kg), together with the difficulty in direct application due to bromide inside the gas. The pyro-tar and pyro-solid have been investigated and been found to have a certain amount of metal and bromide, reducing its environmental friendliness. The energy balance was calculated and it shows that only 35% of the heat required for pyrolysis is capable of recovery from the pyrolysis products, which means an additional heat source is necessary for the process.
Cayumil et al. has done high temperature pyrolysis from 800 to 1350 °C [93]. After this process, they obtained a slag phase, tin–lead (~40% Sn, ~8% lead, and ~45% copper)-rich metallic phase and copper-rich phase (~85% copper), which has relatively high purity. However, there are still 22–25 wt% of slag phase which has not be recycling and the crucial point is that there is still certain percentage of metal inside this slag phase, which means that although this process is very energy-intensive, there is still a considerable amount of toxic solid waste produced (Fig. 4).
Typical design of pyrolysis equipment [91]
To remedy the drawbacks of a high pyrolysis temperature and side reaction occurrence, vacuum pyrometallurgy techniques have recently been developed as an advanced technology for pyrometallurgy recycling techniques, since it has the advantages of low operation temperature and a reduction in the intensity and occurrence of side reaction or reformation of hazardous materials [94–96]. However, the strict vacuum requirement calls for a separation before vacuum metallurgical methods.
Anjan Kumari et al. conducted vacuum pyrometallurgy followed by solvent extraction to recycle various kinds of product after vacuum pyrolysis at 300 °C for 4 min due to the enhanced performance of pyrolysis under high vacuum [88]. This is a very systematic process that has the advantage to recycle fuel oil, fuel gas, sulfuric acid, Cu, Ni, and Fe. Therefore, the emission of toxic gas will be reduced significantly since the recovery section takes a big percentage of the total composition of WPCB feedstock. Details about this process are shown in Fig. 5. However, attention to the bromide is still not be given, since the process does not contain a step for bromide removal and TEHA dissolved in kerosene is used as the extraction solvent, which will also have the side effect of emission.
Complete process flow sheets for recovery of base metals from waste PCBs [116]
Zhou et al. found that the assistance of pre-separation will improve the efficiency of the process [49, 97]. The pretreatment is mainly aimed at separate solder and other electronic components. They performed the vacuum pyrolysis at 600 °C for 30 min under a vacuum lower than 1.5 kPa. However, the pretreatment was conducted at 183 °C, which needs an energy input as they are using an oil bath, which is not comparable to the result of separation of solder although the composition of solder remains almost unchanged. For the pyrolysis part, the pyro-oil it produced has unknown composition, which cannot be used for any refinery. Li et al. studied the vacuum pyrometallurgical process at low temperatures and concluded that vacuum (less than 1000 Pa) will significantly improve the performance of recycling by significantly reducing the activation energy from 127.87 and 115.36 kJ mol−1to 53.59 kJ mol−1, which significantly improves the outcome of the process [60].
However, the drawbacks of vacuum pyrometallurgy techniques are very obvious, namely, the requirement of a high vacuum. Although vacuum equipment is used in industry, the vacuum degree needed for the vacuum pyrometallurgy technique calls for expensive equipment and good air tightness, since the failure to guarantee high vacuum degree will lead to the incomplete pyrolysis of WPCB, causing hazardous emissions and side reactions in the process. Therefore, the application of this process is seriously hindered due to its low economic feasibility.
Summarily, pyrometallurgy recycling techniques can recycle most of the MF at the cost of ruining the NMF. Also, the burn off of the nonmetallic fraction will not only produce bromorganic compounds and other toxic emissions, but it will also give around 20–25% ash content, which will have a certain percentage of heavy metals and need a further workload to perform disposal or refining [51]. A remediation method is to do the dehalogenation either no later than the pyrolysis process, or in the upgrading of the pyrolysis products [82, 98]. However, it may add further workload and cause the reduction of the yield and value of the pyrolysis products. Moreover, copper or lead in the feedstock will function as collectors of other metals, which will result in the output of unknown alloy from pyrometallurgy instead of pure metal that require further refining [99].
4.1.2 Hydrometallurgy recycling techniques
Hydrometallurgy recycling techniques make use of solvent leaching using cyanide, thiourea, thiosulfate, halide, and recycling the metallic fraction (mainly copper and gold) from WPCB feedstock [100–102]. Compared to pyrometallurgy, hydrometallurgy recycling techniques are more accurate, highly predictable, and easily controlled, which makes it the most competitive technique for WPCB recycling currently with the assistance of mechanical crushing as pretreatment [103].
Kinoshita et al. use nitric acid as a leaching reagent and they used two rounds of leaching in the first round Ni was extracted with a concentration of 279 mg/l at 363 K for 72 h and in the second round Cu was extracted with a concentration of 3220 mg/l at 363 K for 6 h [102]. The Au was obtained by filtration of the leachant and separated in the form of flakes. The metal-rich solution was forward extracted and backward extracted by organic solvent and 4.0 M nitric acid, respectively. The recovery rate is high and selective recovery was achieved. However, the process takes a long time and it produces waste water containing an organic phase and a strong acid, which is a common issue in hydrometallurgy recycling techniques. Similarly, Park et al. use aqua regia as the leaching agent to extract palladium, silver, and gold from WPCB feedstock [50]. The recovery of palladium in the form of Pd(NH4)2Cl6 precipitation is 93 wt%. Gold nanoparticles were obtained by organic solvent extraction using dodecanethiol and sodium borohydride with a recovery rate of 97 wt%. Silver stays stable in aqua regia and was recovered without further treatment with a recovery rate of 98 wt%. The recovery rate is very high as well as the purity of the metal they collected. However, the usage of strong acid and organic solvent decrease the environmental evaluation of this process.
Therefore, despite the advantages of the hydrometallurgical treatment methods, a serious issues is that they produce a lot of highly toxic waste water containing cyanides or halides, which is extremely hazardous for both soil and water bodies [104]. Moreover, non-cyanide or non-halide leaching solvents like thiourea or thiosulfate have disadvantages including low-stability, high cost, and high consumption of extracting reagent [21–23]. Corrosion also causes problems for the equipment when several kinds of leaching reagents were applied. More details are shown in Table 8 below.
Also, the leaching process normally takes a long time to obtain metal-rich solution due to the slow leaching rate, which makes the process a time-consuming one. Therefore, studies that reinforce the driving force and accelerate the leaching rate have been studied including using electrochemistry, pre-pyrolysis, supercritical extraction, and mechanical treatment [52, 54, 99, 117, 118]. Havlik et al. studied the hydrometallurgical process with pre-combustion, in which WPCB was leached in 1 M HCl solution after combustion in air for 15 to 60 min in 500–900 °C [99, 119]. The results showed that the pre combustion at 900 °C will significantly improve the leaching of copper from the WPCB feedstock due to the conversion of Cu to Cu2O, which dissolves more preferable in HCl. However, the pre-combustion leads to the difficulty in extracting tin since Sn is oxidized to form SnO2, which is very stable in acid with minimal leaching. Therefore, the efficiency of hydrometallurgy was seldom enhanced even without considering the emission during combustion process.
Fogarasi et al. used mediated electrochemical oxidation to recover copper from WPCB and high purity copper (99.04%) was obtained with 63.84% current efficiency [52]. However, a sludge was still produced with certain concentrations of copper and lead (49.37 and 32.49 wt%, respectively); this issue needs further work. Also, the fate of NMF was not clarified, as well as the bromide inside the NMF. Kim et al. use electro-generated chlorine to leach metals from WPCB feedstock [120, 121]. Copper as well as noble metal was obtained together due to the high oxidation potential they gained in the process. In the first stage, the recovery of Cu is 94.91% and in the second stage the recovery rate of Au is 93.06%. Then the metal ions can be collected by an ion-exchanger with efficiency around 97%. However, the feedstock used is already after dismantling and sorting therefore it has very high metal concentration (Cu 66 wt% and Au 0.045 wt%), which is not comparable to the raw WPCB feedstock, making the research not representative for all kinds of WPCB. Also, although the acid solutions as well as the low concentration solution are recycled back after ion exchange, the problem of discharge still exists since the halide is concentrated in the process if it is applied to WPCB with a high NMF content as well as the low pH of the solution. Silvas et al. used magnetic separation before the leaching test and they recovered 90 wt% Al, 40 wt% Zn, 8.6 wt% Sn in the first leaching stage using sulfuric acid media and 100 wt% Cu, 60 wt% Zn, and 10 wt% Al in the second stage using oxidant [54]. It is supposed that the recovery rate is very high due to the assistance of mechanical separation and this could be a possible way for future work. However, the fate of NMF was not mentioned in this paper and remains a potential pollutant.
Summarily, hydrometallurgy recycling techniques are easy to apply and simple to operate. However, the problem that cannot be avoided or ignored is the discharge of leachate as well as the pollutants since these techniques do not incorporate the recycling of NMF. The process is mainly undermining the structure of NMF and emits them, which not only wastes the useful part of NMF but also converts them into pollutants. Another issue that should be highlighted is that the recovery rate for hydrometallurgy recycling techniques is full recovery. Therefore, there will be a certain amount of heavy metal present in the effluent in the form of free ions, which will reinforce the hazardous content of it. So an upgrading of current hydrometallurgical recycling techniques is urgently required to stop the wild application of it.
4.1.3 Biometallurgy recycling techniques
Biometallurgy recycling techniques use microorganisms including bacteria or fungi to treat WPCB [122]. Commonly used bacteria include mesophilic chemolithotrophic, cyanogenicor moderately thermophilic bacteria [25, 26, 123, 124]. The mechanism of biometallurgy recycling techniques is similar to hydrometallurgy recycling techniques since they all incorporate the process of leaching. However, instead of adding leaching reagents, biometallurgy recycling techniques normally use the chemicals produced by the microorganism itself, including organic/inorganic acids, cyanide, or sulfate ions. After leaching, the metal ions will form complexes or precipitates and thus they are separated from the culture broth for direct use or further refining. It has the advantages of only a small volume of waste water discharge and is environmental benign compared to hydrometallurgy, which generally requires a high dosage of toxic chemical reagents. Besides, certain kinds of bacteria are capable of reducing brominated flame retardants in the pathway shown in Fig. 6, which is rarely achieved in other recycling techniques [122, 125].
Liang et al. used a mixed culture of Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans to recover copper from WPCB fine powder [123]. The bacteria can oxide elemental sulfur added to sulfuric acid for bioleaching and then extract the metal from the broth. The highest recovery rate (98.37%) was obtained with the condition of pH value 1.56, elemental sulfur S0 5.44 g/l and 16.88 g/l FeSO4·7H2O concentration. However, the increase of WPCB addition in culture from 18 to 32.4 g/l will cause a sharp drop in the copper recovery rate from 98.3 to 87.2%. Also, the fate of NMF was not mentioned in this work and remains a potential hazardous material. Ilyas et al. used moderately thermophilic bacteria to recover metals and conducted the process in a column test [124]. The recovery rate for Zn, Al, Cu, and Ni is 80, 64, 86, and 74%, respectively, which already meets the requirements of industrial-scale implementation for recycling of MF of WPCB. It is also noticed that NMF will contribute to the alkalinity which will affect the leaching of metal ions, requiring washing before conducting the culture stage. However, in this process, Pb and Sn along with other metals will form precipitates in the column, not only preventing the recycling of metals but also causing potential blockages of the column. Yang et al. used A. ferrooxidans to study the factors affecting the mobilization of copper in the bioleaching process [125]. The higher concentration of Fe3+ from 0.64 to 2.13 g/l in the stock solution will bring to the increase of copper leaching in 12 h from 34.53 to 79.75%. As a similar result was observed that copper recovery decreased from 99.06 to 88.40% in 48 h when the pH value increased from 1.5 to 2.0. Therefore, it is noticed that the concentration of Fe3+ as well as the pH value have a very obvious effect on copper leaching. Ting et al. used two cyanide-producing bacteria, Pseudomonas fluorescens and Chromobacterium violaceum to extract gold and copper from WPCB and the recovery rate was around 27 and 20% for gold and copper [126]. In a two-step extraction, the recovery rate for gold and copper was increased to around 30 and 24%, respectively.
However, biometallurgy recycling techniques require a lot of nutrients for microorganism enrichment and metal extraction. The addition of an Fe source is necessary for copper extraction since the solubilization of copper needs the presence of Fe3+ according to reaction (1). Also, the ferrous ion is the energy source for A. ferrooxidans, which is an aerobic and autotrophic bacterium according to reaction (2).
Also, the low extraction rate in high WPCB dosage due to the limitation in air distribution and oxygen mass transfer hinder the application of the biometallurgical treatment method [127]. Moreover, normally microorganisms are vulnerable to heavy metals, thus the growth of them will be inhibited due to the toxicity of metals [128]. Although some bacteria or fungi can adjust to the condition after prolonged adaptation time and achieve a good recovery rate, the time required for this adaption is extremely long (more than a week) [129]. Furthermore, the recovery period for biometallurgical treatment is much longer than pyrometallurgy or hydrometallurgy recycling techniques, which affected the positive evaluation of process. Also, normally the WPCB feedstock needs to be a fine powder with a particle size around 100 µm or even lower to ensure adequate surface contact, which will consume a lot of energy in the early stage [130].
4.2 Summary of partial recovery of metallic fraction from WPCB
From the above-mentioned techniques, partial recovery of the metallic fraction from WPCB processes will benefit metal recovery, which incorporates the most valuable components inside WPCB. The application of partial recovery of metallic fraction from WPCB still exists in many developing countries trapped by the fact of lacking funding. Differences still exist among pyrometallurgy, hydrometallurgy, and biometallurgy in terms of cost, efficiency, harmful effect, as well as the time for recycling as shown in Table 9.
Pyrometallurgy shows the best performance in efficiency and time for recycling since it can significantly remove the non-metallic fraction in WPCB by thermo-decomposition. However, the high energy consumption and construction cost also make it the most expensive technique. Additionally, the toxic emissions and residues generated in the process cause considerable harmful effects to the environment if performed without proper treatment. Compared to pyrometallurgy, hydrometallurgy and biometallurgy have much less environmental impact since the pollution is confined mostly in the liquid phase. Nevertheless, the driving force in hydrometallurgy and biometallurgy is not comparable to pyrometallurgy, thus making them extremely time-consuming techniques although their cost is several times lower than pyrometallurgy. Summarily, the partial recovery of metallic fraction from WPCB by the above methods has obvious drawbacks. It is imperative to develop further full recovery techniques for WPCB to improve the current methods of WPCB recycling.
4.3 Primitive full recovery from WPCB
Despite diversity possessed by MF recycling techniques, a common drawback shared by them is the absence of NMF treatment, which will give rise to the toxic emission including brominated organic compounds, secondary particulates, and other kinds of pollutants. A simple mass balance concept implies the less recycled, the more emitted. Therefore, techniques focusing not only recycling the MF but also achieving the safe disposal of NMF simultaneously are reviewed next including supercritical fluid extraction, plasma treatment and hydrothermal method [131–133].
Huang et al. used DC arc plasma to treat printed circuit boards, which decomposed the WPCB in molten bath at 1400–1800 °C in a DC arc furnace [131]. The product for this process is homogenous and vitreous slag and small molecular gases including HCl, H2S, NOX, and SO2. Although the safe disposal of solid residue is achieved, it produces a certain amount of air pollution and the energy consumption is huge due to the high temperature and long duration time. Xiu et al. has conducted a series of studies using supercritical fluid including supercritical water (SCW) and supercritical methanol (SCM) to recycle WPCB [134–138]. For the SCM recycling, most kinds of heavy metals are converted to metal oxides with around 100% conversion, except for Mn and Ni, since near 50% of them enters in liquid phase. The copper extracted from WPCB will undergo an eletrokinetic (EK) process and can be synthesized to nanoparticles, which functions as a photocatalyst [139]. The degradation of NMF also happens with the formation of phenol and phenol derivations, which is the main composition of liquid product (~55%). Xing et al. also used SCW to detoxify bromorganic compounds inside WPCB and achieved maximum debromination rate of 97.8% [55, 140]. The bromide was concentrated in water in the form of hydrogen bromide. Also, the bromine-free oil with main components consisting of phenol (58.5%) and 4-(1-methylethyl)-phenol (21.7%) was collected. Moreover, copper was recovered in the purities of near 95% with a recovery rate as high as 98.11%. However, supercritical technologies need high temperature (above 400 °C) and high pressure (higher than 20 MPa). Therefore, the utilization of this process needs a good-quality equipment, which increases the cost significantly. Yin et al. used hydrothermal technology to decompose brominated epoxy resin in WPCB and achieved more than 80% decomposition rate and obtained a liquid with the main composition of phenol, which can be used as a chemical material [141]. Also, after this treatment, WPCB can be used to recover MF with a much lower emission. However, the feature of hydrothermal technology determines that the operation capacity is very limited; in this work, 0.1 g WPCB per batch, which is not applicable for large-scale operations.
Summarily, despite the fact that the previously mentioned technology can recycle MF with the safe disposal of NMF, the energy and effort put into this process is not proportional to the output, which is only the MF. Also, although the safe disposal of NMF will not cause further pollution, a considerable amount of resource inside NMF is wasted after all. Therefore, the separation of MF and NMF and further recycling of NMF is necessary for the consideration of sustainable resource recovery. For this, the effective separation for MF and NMF is indispensable for the purpose of more detailed and efficient recycling.
5 Advanced Recycling Techniques
5.1 Mechanic Separation of MF and NMF
Based on the previous Sects. 1, 2, 3, 4, it can be concluded that a common drawback shared by the primitive recycling technologies is the lack of attention given to the NMF-70% by weight of the WPCB, which fundamentally blocks the recycling or upgrading of NMF due to the loss of mechanical or chemical structure and properties. Therefore, separation of MF and NMF without damage to the structure of NMF is the precondition for NMF recycling and itself is also regarded as one type of recycling since the MF separated from the feedstock can be sold to the market directly. With the development of technologies, the method for MF and NMF separation has changed from manual disassembly to more advanced technologies. Mechanical separation methods take the advantages of the differences in density, magnetism, and electro-conductivity, and have become increasingly important in the recycling of WPCB [27–30]. Since the composition of NMF indicates that most chemical separation techniques will damage the properties of NMF and bring difficulties to further treatment operations, mechanical separations stand out although in some situations they were regarded as assistance processes for pyrometallurgy or hydrometallurgy techniques. Still it is an efficient recycling method which offers great potential, especially with the development of electrical conductivity-based separation [53]. Also, with the development of the automation industry, the mechanical separation also involves automatic or semi-automatic application [11]. These techniques can relieve the situation a lot by separating the MF and NMF of WPCB with a high efficiency and good purity. Therefore, it makes it possible to treat MF and NMF separately and it brings about the selling of high-purity MF to the market and also directing recycling and modification of NMF from WPCB as two potential ways to treat the NMF part of WPCB. Also, the separation of MF and NMF avoid potential interactions of NMF in further treatment stages because the metals in MF can catalyze unwanted side reactions of NMF.
5.2 Density-based Separation
Density-based separation uses the differences of particles size and density to separate the MF and NMF and various equipment items were developed, as shown in Fig. 7 [27]. This method has a long history dating back to the jigging method, which used a plunger to hit the bottom of the feedstock to give a force to kick the light particles out, thus preserving the heavier ones. Sarvar et al. used wet jigging to separate WPCB feedstock and the recovery rate of metal content for coarse-size, middle-size, and small-size fractions are 95.6, 97.5, and 85%, respectively [142]. However, low grades around 70% were obtained and a significant part of gold is lost at the flotation process. Currently, air classification is a popular method based on the fact that the particles suspended in the gas stream will be separated due to the gravity and drag forces experiencing in opposite directions [28]. The heavier particles will gain larger terminal settling velocity and move towards the bottom of air steam while light particles go to the opposite side, which is the top of the column. Eswaraiah et al. used an air classifier to separate the WPCB and recover 96.7% copper in the sink and 98% plastics and glass fibers in the float [143]. However, there is still 70.94% of other materials in the sink, which makes the purity of copper only 26.77%. Habib et al. [144] also use vertical vibration to separate MF and NMF from WPCB feedstock and they obtained a MF fraction as a combination of Cu (~50%), Fe(~10%), Sn(~10%), Zn(~8%), Pb(~8%), as well as some NMF. Also, the NMF fraction includes 65% NMF and 25% Cu as well as a certain amount of metals. The advantages of density-based separation are the simplicity of its equipment and low energy consumption while the common problem is low product purity, adding workload and difficulty to further refine [145].
5.3 Magnetic-based separation
Magnetic-based separations can achieve the recovery of ferromagnetic metals such as aluminum from non-ferrous metals and other non-magnetic contents with a permanent or electro-magnet. An eddy current separator is a mature technology for magnetic-based separation that can efficiently separate aluminum from non-ferrous contents, as shown in Fig. 8 [29]. When the feedstock passes over the separator, aluminum will be affected by the eddy current generated by the rotation of the magnets inside the shell at high speed, which causes them to be surrounded by a magnetic field. Therefore, the particles will be repelled away from the magnet much further due to the polarity between the two magnetic fields, thus the separation between them and NMF is achieved. Zhang et al. used an eddy current separator to recovery aluminum from electronic scrap and achieved more than 90% aluminum recovery rate with a purity around 85% for a single pass [146]. Yoo et al. used a two-step magnetic separation to recover MF from WPCB and from the first magnetic separation at 700 Gauss, 83% of the nickel and iron was recovered in the magnetic fraction. The second magnetic separation at 3000 Gauss increased the total amount of nickel recovery but caused a drop of the nickel purity from 76 to 56% [58].
However, not all MF can be separated out through eddy current separation because only material with a high σ/ρ can be separated out, where ρ is the density of the material and σ is the electrical conductivity of material. Table 10 shows the materials that can be separated by an eddy current separator, from which it is obvious that aluminum is the most easily separated material while stainless-steel, plastic, and glass have a zero value for the conductivity-to-density ratio, meaning it is not applicable to separate them by an eddy current separator [147].
5.4 Electronic conductivity-based separation
Electronic conductivity-based separation focuses on the difference in the electrical conductivity between MF and NMF, which is normally related to corona electrostatic separation in PCB recycling that fits the condition well, as shown in Fig. 8 [30]. The sample feed in the separator will be bombarded by the high-voltage electrostatic field generated by a corona electrode and electrostatic electrode. Now, the MF will be neutralized quickly as they contact the earthed electrode and leave the rotating roller while the charged NMF are pinned by the electric image force to the rotating roller and move with the rotating roller, finally falling into the holding tanks.
Li et al. used corona electrostatic separation with the pretreatment of pulverization of WPCB feedstock to successfully separate the MF and NMF of WPCB and achieved a high recovery rate (more than 90%) along with high capacity (0.5-1 t/h) with no obvious side effects compared to fluidized bed separation, which has wastewater discharge or air-current separation with a dust-releasing issue [19, 148]. The MF has very high purity and can be sent to a smelting plant directly or with minimal refining. Jiang et al. has designed a new two-roll electrostatic separator that takes advantage of the force of gravity to pass the mixture to the second step for recycling of metals and nonmetals from waste printed circuit board [149]. The production capacity was significantly increased for maximum 50% with 45% reduction of middling products. However, mechanical processes for recycling WPCB normally needs to undergo at least two steps, including coarse crushing and fine pulverizing and especially during fine pulverizing, the temperature will increase up to almost 300 °C [150]. Dust and ash is a common issue for mechanical separation and although it can be avoided by using personnel protective equipments, it still adds to the cost of the process, which makes the economical feasibility of this energy-intensive process even worse. Therefore, an upgrading of NMF to make value-added products is necessary to remedy the expenditure of energy and equipments cost. Also, noble metals will be lost in the crushing process since they will attach on the surface of nonmetallic fraction and cannot be recycled [49].
5.5 Directly Recycling of Nonmetallic Fraction from WPCB
At the beginning, the purpose of recycling of NMF from WPCB is simply to avert the potential hazardous environmental impacts when they end up in landfill or incineration. The composition of NMF is a random combination of epoxy resin and glass fiber. The reuse of NMF is hindered due to understanding of the chemistry of them. Therefore, it is common to be directly used as filler, concrete, and modifier, which means the NMF was used without any forms of modification and is a low value-added material [31–35, 151–154].
Guo et al. has done a series of studies on the feasibility of replacing wood flour in the production of phenolic moulding compound (PMC) using the NMF of WPCB [151, 152]. The addition of NMF can significantly improve the impact strength and heat deflection temperature (HDT) and reduces flexural strength and Raschig fluidity of the phenolic moulding. Notably, the increase of the content of NMF will sharply reduce Raschig fluidity. Therefore, the optimum content for adding NMF is considered to be 20 wt%, in which condition the flexural strength is 70 MPa, the Charpy notched impact strength is 2.4 kJ/m2, the heat of deflection temperature is 168 °C, the dielectric strength is 3.9 MV/m and a Raschig fluidity of 103 mm. These criteria all meet the national standard. In addition, they did economic analysis, which shows that the addition of NMF can save the cost for the producers of PMC. However, if the cost-saving can cover the energy consumption of corona electrostatic separation is not clear. The authors claim the market price of NMF is for free, which is not correct in the current market. They also study the possibility of producing nonmetallic plate (NMP) from NMF of pulverized WPCBs by hot-press forming with the addition of a resin paste as a bonding agent [31, 32]. The maximum content of NMF in reproduction nonmetallic plate (RNMP) can be as high as 40 wt%. However, the RNMP showed excellent mechanical properties with impact strength of 5.8 kJ/m2 and flexural strength of 65.1 MPa when NMF added was 20 wt%. Yokoyama and Iji also investigated the use of NMF as filler in the reproduction of resin-type construction materials and the comparison of mechanical properties of these materials with those of reference materials with silica powder was conducted [33]. The NMFs in the materials show reinforcement in mechanical strength and thermal expansion properties of the epoxy resin mold, which has better performance than talc, calcium carbonate, and silica. This is probably because of the compatibility between the NMFs and the epoxy resin matrix, and also the incorporation of glass fiber.
Niu and Li used recycled waste PCBs for cement solidification, which is actually a method to use the waste PCBs as a raw material for concrete [154]. It is proved that the cement solidification can be significantly improved in terms of the compressive strengths (4.89 MPa for slag cement and 7.93 MPa for Portland cement) and the impact resistance of 200 (maximum) of NMF can turn it into strong monoliths. The leaching test shows that the leaching of Pb in the raw material can be effectively prevented (<5 mg/l) even under an acidic environment.
Zheng et al. used NMFs as reinforcing fillers in PP composites [153]. The NMFs are modified by a silane-coupling agent KH-550 and PP powder S1003 is applied as the matrix polymer. NMF and PP powder are pre-mixed and the products are obtained by extruding. The mechanical characterization shows that the ensile strength, tensile modulus, flexural strength, and flexural modulus are significantly improved 28.4, 62.9, 87.8, and 133.0%, respectively, by the adding of NMF. The optimum content of the NMF added is 30 wt% based on technical, environmental, and economical consideration.
However, Lu et al. found that PCB can be liberated effectively with a size between 0.5 and 1.2 mm while for a better recovery ratio the over-pulverized phenomenon is very serious. This limits the further use of the glass fibers that are the support material of PCB (~50 wt%) [155]. The mechanical properties of NMF were sacrificed in order to achieve a high metal recovery rate. Therefore, the direct reuse of NMF is seriously influenced due to this phenomenon. A more advanced technology of NMF modification is indispensable.
5.6 Modification of Nonmetallic Fraction from WPCB
The direct recycling of NMF can help solve the disposal problem of NMF. However, it is still weak for them to compensate for the high operation cost of the MF and NMF separation due to the low value-added products they produced by NMF. With the requirement to better use NMF, which takes more than half of the weight of NMF, the modification of NMF followed by the upgrading was studied by many research groups [156–160].
One thought for the modification is based on the thought that NMF is a carbon source for potential reuse considering the high content of carbon in its composition due to the addition of epoxy resin or other polymers. Normally, the carbon content in NMF is between 30 and 40%, which varies with the source of WPCB [161]. Ke et al. first pyrolyzed the NMF of WPCB in the temperature range 500–800 °C and used physical or chemical activation to obtain activated carbon [156]. Physical activation with H2O as an activation reagent produced granular activated carbon with a surface area as high as 1019 m2/g and pore volume 1.1 cm3/g while chemical activation with KOH as activation regent obtain the same product with higher surface area of 3112 m2/g and a pore volume of 1.13 cm3/g.
Rajagopal et al. used the activated carbon prepared by physical activation with CO2 subsequent to pyrolysis of NMF of WPCB to and apply it in supercapacitor [157]. The NMF activated at 850°C for 5 h shows the highest surface area of 700 m2/g as well as 0.022 cm3/g pore volume. Electrochemical characterization of the activated carbon prepared under optimum conditions, shows a specific capacitance of 220 F/g at the current density of 30 mV/s and 156 F/g at the current density of 100 mV/s, which is comparable to activated carbon prepared by other methods. Also, the activated carbon has an energy density of 15.84 Wh/kg at 850 W/kg, which is very high compared to commercially available supercapacitors based on activated carbons that have an energy density ranging from 4 to 5 Wh/kg with power density values of 1 to 2 kW/kg [162]. Moreover, the retention value of the activated carbon was studied to be 98% for over 1000 cycles.
Hadi et al. has developed a thermal-alkaline activation process to functionalize NMF to produce an aluminosilicate adsorbent for heavy metals uptake from waste water [158]. The NMF was mixed with potassium hydroxide solution and activated in 300 °C to develop the porosity of NMF which is a non-porous material. After activation, equilibrium isothermal adsorption tests show that the modified novel material called ANMF had a high uptake capacity for Cu(2.9 mmol/g), Pb(3.4 mmol/g), Zn(2.0 mmol/g), which is much higher than commercial adsorbents used in industry. Xu et al. studied the factors affecting the adsorption capacity including contact time, initial cadmium ion concentration, pH, and adsorbent dosage when this material is used for cadmium uptake [159]. The results showed that pH has an important effect on the uptake capacity for cadmium and the maximum uptake capacity for cadmium is 2.1 mmol/g obtained when pH = 4. Modeling of the adsorption was also done in this work and several isothermal equations have been studied while the Redlich–Peterson model shows the best fitting. Moreover, the activation mechanism as well as the optimization of reaction condition was done by Ning et al. [160]. The factors affecting the pore-development process were studied including reaction temperature, impregnation ratio, and reaction time, and the results shows that reaction temperature and impregnation ratio has a significant effect on the structure of ANMF during the reaction while reaction time has no significant effect. This provides a possibility for the porous structure tuning for the NMF of WPCB, which will significantly enlarge the potential applications of NMF as a catalyst, adsorbent, and filter support.
Summarily, the modification of NMF to make value-added products can greatly improve the value of NMF. Thus, the value added to NMF can possibly compensate for the high cost derived from mechanical separation.
6 Perspectives on Recycling Technologies
6.1 Economy Perspective
The economy perspective is always very important, or of first priority for the evaluation and implementation of a recycling technique. The process, which is cost-effective, will have a better chance to be commercialized especially in developing countries due to the lack of sufficient funds for WPCB recycling. Obviously, landfill and incineration has limited economical benefit even without the concern about the treatment of the hazardous substances they emit. The construction and administration costs of landfill sites or incineration plants are large expenses with a scant return. This is also a reason for the reduction in the current application of these two techniques for WPCB treatment. Pyrometallurgy and hydrometallurgy recycling techniques can achieve partial recycling of WPCB by recovery of MF from the feedstock. However, the intensive energy consumption in pyrometallurgy is ascribed for the main expense in the whole process. The energy power in the process is around 0.2–4 kW, varying with the different processes, while sufficient data is not available for these techniques since most works were conducted at the laboratory scale and energy analysis is absent for most of the studies [148]. However, the normal pyrolysis temperatures are from 400 °C to 800 °C, and the post gas treatment equipment normally needs the temperature higher than 1200 °C and no doubt consumes a considerable amount of energy. For hydrometallurgy recycling techniques, the chemical reagents used including cyanide, halide, acid, or other chemicals takes a considerable and indispensable percentage of the cost. Also, in hydrometallurgy, water is used as leaching media and solvent on a large scale, which also contributed to the operation cost.
Furthermore, as the decrease of percentage of noble metal in the WPCB is due to manufacturing upgrading, the driving force for recycling MF from WPCB is decreasing, since previous works focus on the benefit of metal recovery. Recycling of NMF is a potential way to remedy the cost on energy and/or chemical cost. However, the precondition for NMF recycling is the homogenization of the WPCB, which means the pulverization of WPCB feedstock by shredders and hammer mills, which also requires energy and equipment cost. A fine size of NMF (around 0.1 mm) is typical for the recycling of NMF in many studies. While in both pyrometallurgy and hydrometallurgy recycling techniques, the structure of NMF was totally decomposed, the recycling of NMF is therefore not applicable. In some types of mechanical separation, the structure or composition of NMF was preserved with a meager effect on the chemical and mechanical properties. Nevertheless, the direct use is not enough to add adequate value to compensate for the cost required to preserve their structure. Therefore, the upgrading and recycling of the value-added non-metallic fraction are attracting more and more researchers.
6.2 Environmental Perspective
The environmental perspective is another important issue that affects the evaluation of the WPCB recycling techniques. A general rule for emissions is that the more material recycled, the less will be emitted. Therefore, the focus of a technique should not only be on the performance of the recovery rate of specific components like copper or noble metals, but also the fate of the remaining part due to the potential emissions they may have, especially for gaseous emissions. The environmental emissions can be divided into primary pollution, which comes from the WPCB, and secondary pollution, which is emitted during the treatment or recycling process.
For primary pollution, heavy metals including Cu, Pb, Cd are of great concern. They can enter the environment in the form of leachate in landfill or vapor in incineration or some pyrometallurgy recycling techniques without proper purification. It can cause the distortion of the human body as well as various other hazardous effects; the presence of PBDD/Fs and PCDD/Fs is a critical health issue when their presence in the vapor will cause cancer. They mainly form due to the oxidizing atmosphere and the presence of metals as catalyst.
However, the majority of public attention is still paid to the emissions of NOx, SOx, or VOCs, while some kinds of pollutants are ignored due to their relatively low toxicity, like Br and HBr. Yet low toxicity does not equal being environmentally friendly. The vast emissions still cause damage to individual hygiene [19]. Bromide is a hazardous material source with obviously inadequate attention, it is even claimed as harmless in a few studies [5, 15]. Bromide concentration in WPCB is relatively high (~ 4%) due to the addition of brominated flame retardants [163]. The decomposition of brominated flame retardants happens in the recycling process while bromide still remains in the form of several kinds of bromide or bromorganic compounds including HBr, bromomethane, and bromophenol (Fig. 9). However, research shows that bromide, especially HBr, has a very bad effect on human health, since it can cause health problems and is accumulated in the human body and is difficult to degrade. Therefore, to achieve complete recycling and elimination, emissions are the ultimate objective of the environmental impact of WPCB recycling techniques.
Possible decomposition pathway of brominated epoxy resin according to the products generated [51]
6.3 Gate-to-Market Ability Perspective
Previously, the evaluation of a recycling technique is always based on economic and environmental perspectives. However, for a real application, it is not a two-sided choice while sometimes a technique is both cost-effective and environmentally friendly and does not mean that this process has high feasibility since the product’s status is not well clarified. In previous research, a common mentioned concept is the recovery rate, which means the percentage of materials recovered from feedstock, in weight or volume percentage. Yet with deeper understanding and the real need for resources, the recovery rate is not comprehensive enough to describe the extent of recycling due to the fact that it is not capable of reflecting the required purity of the products, as for some techniques the recovery rate is very high (>90%) while the product purity is particularly low, including several components with concentrations between 20 and 40%. These products are actually incapable of being used or sold to the market directly, which need further separation or refining. However, in the evaluation of the process, the cost and environmental effects are not ascribed to the technique itself but are transferred to the uncertain downstream treatment. Thus, the performance of the technology can be overestimated or underestimated. Therefore, in this work, a new criteria was first raised named “gate-to-market ability”, which means the ability of the products to be sold or used in a real industry application after it has been produced by the technique. It is obvious that the purity of the products will be significantly affected by their gate-to-market ability of them, for some recycling produces even a slightly lower purity will cause them to be discarded.
The market price of copper, a common recycling product of WPCB, is seriously affected by their purity, as shown in Fig. 10 where the prices of copper were collected from several copper markets over the world on January 21, 2016. It can be concluded from the figure that a small decrease in purity (from 99 to 90%) will cause a sharp drop in the unit price of around 22.89% when the purity is decreased. However, the copper recovered from the above-mentioned technology is normally around 70%, except corona electrostatic separation, which means the margin of profit is cut a lot or even not applicable without further refinery. The following process will add both an economic and environmental burden to the techniques. Therefore, gate-to-market ability is a criterion independent of economical and environmental perspective and plays an important role in the evolution of the feasibility of the process.
Normally, hydrometallurgy recycling techniques will give low gate-to-market ability due to the form of products, which are normally alloys. Generally, hydrometallurgy recycling techniques and mechanical separation can provide products with good gate-to-market ability since the products of these processes can achieve high purity beyond 90%, which reduces the difficulty of further application. Still, co-precipitation in hydrometallurgy will reduce the gate-to-market ability since the product will have several kinds of components inside.
7 Conclusions
WPCB is dangerous waste, but at the same time a rich resource for various kinds of materials. The recycling technologies of WPCB divided into direct treatment (landfill and incineration), primitive recycling technology (pyrometallurgy, hydrometallurgy, biometallurgy and primitive full recovery of NMF), and advanced recycling technology (mechanic separation, directly use and modification of NMF) were studied and analyzed to access their advantages and disadvantages. This review shows that direct treatment has advantages on their simplicity of treatment but the environmental problem is a big issue for them. Primitive recycling techniques achieve a certain extent of recycling, mainly the MF of WPCB. However, the energy consumption as well as the waste gas and water emission still remain a problem for the application of them. Advanced recycling techniques can separate the MF and NMF of WPCB with minimum effects on the environment with a good gate-to-market ability while the energy consumption is huge for them. Therefore, the direct use and modification of NMF was studied to remedy the cost from the energy consumption and become the concern of WPCB recycling.
To summarize, a triangle figure of economy perspective, environmental perspective, and gate-to-market ability perspective can be set as shown in Fig. 11. However, the discussion indicates that the three criteria cannot be satisfied with one simple technique. Therefore, the future research direction of WPCB recycling should focus on the combination of several techniques or in series recycling since the drawback of a process could have a chance to be remedied by one of the techniques and the evaluation should be conducted on the whole process until the products satisfy the market standard.
Abbreviations
- EEE:
-
Electrical and electronic equipment
- WEEE:
-
Waste electrical and electronic equipment
- PCB:
-
Printed circuit board
- WPCB:
-
Waste printed circuit board
- MF:
-
Metallic fraction of printed circuit board or waste printed circuit board
- NMF:
-
Non-metallic fraction of printed circuit board or waste printed circuit board
References
Gu Y, Wu Y, Xu M, Mu X, Zuo T (2016) Waste electrical and electronic equipment (WEEE) recycling for a sustainable resource supply in the electronics industry in China. J Clean Prod 127:331–338
Williams PT (2010) Valorization of printed circuit boards from waste electrical and electronic equipment by pyrolysis. Waste Biomass Valorization 1:107–120
Hadi P, Ning C, Ouyang W, Lin SK, Hui CW, Mckay G (2014) Conversion of an aluminosilicate-based waste material to high-value efficient adsorbent. Chem Eng J 256:415–420
Lee CH, Chang SL, Wang KM, Wen LC (2000) Management of scrap computer recycling in Taiwan. J Hazard Mater 73:209–220
Flandinet L, Tedjar F, Ghetta V, Fouletier J (2012) Metals recovering from waste printed circuit boards (WPCBs) using molten salts. J Hazard Mater 213–214:485–490
Quan C, Li A, Gao N (2013) Combustion and pyrolysis of electronic waste: thermogravimetric analysis and kinetic model. Procedia Environ Sci 18:776–782
Draft proposal for a European parliament and council directive on waste electric and electronic equipment. Brussels
Ghosh B, Ghosh MK, Parhi P, Mukherjee PS, Mishra BK (2015) Waste printed circuit boards recycling: an extensive assessment of current status. J Clean Prod 94:5–19
He Y, Xu Z (2014) The status and development of treatment techniques of typical waste electrical and electronic equipment in China: a review. Waste Manag Res 32:254–269
Kumari A, Kumari A, Jha MK, Kumar V, Singh RP, Yoo K (2014) Copper recovery from small devices populated on waste printed circuit boards. J Metall Mater Sci 56:41–51
Zeng X, Zheng L, Xie H, Lu B, Xia K, Chao K, Li W, Yang J, Lin S, Li J (2012) Current status and future perspective of waste printed circuit boards recycling. Procedia Environ Sci 16:590–597
Das A, Vidyadhar A, Mehrotra SP (2009) A novel flowsheet for the recovery of metal values from waste printed circuit boards. Resour Conserv Recycl 53:464–469
Hadi P, Ning C, Ouyang W, Xu M, Lin SK, McKay G (2014) Toward environmentally-benign utilization of nonmetallic fraction of waste printed circuit boards as modifier and precursor. Waste Manag 35:236–246
Akcil A, Erust C, Gahan CS, Ozgun M, Sahin M, Tuncuk A (2015) Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—a review. Waste Manag 45:258–271
De Marco I, Caballero BM, Chomôn MJ, Laresgoiti MF, Torres A, Fernández G, Arnaiz S (2008) Pyrolysis of electrical and electronic wastes. J Anal Appl Pyrolysis 82:179–183
Jie G, Ying-Shun L, Mai-Xi L (2008) Product characterization of waste printed circuit board by pyrolysis. J Anal Appl Pyrolysis 83:185–189
Marsanich K, Zanelli S, Barontini F, Cozzani V (2004) Evaporation and thermal degradation of tetrabromobisphenol A above the melting point. Thermochim Acta 421:95–103
Luda MP, Balabanovich AI, Hornung A, Camino G (2003) Thermal degradation of a brominated bisphenol a derivative. Polym Adv Technol 14:741–748
Li J, Lu H, Liu S, Xu Z (2008) Optimizing the operating parameters of corona electrostatic separation for recycling waste scraped printed circuit boards by computer simulation of electric field. J Hazard Mater 153:269–275
Insititute of scrap recycling industries Inc. (2003) Scrap recycling: where tomorrow begins. Washington DC, USA
La Brooy SR, Linge HG, Walker GS (1994) Review of gold extraction from ores. Miner Eng 7:1213–1241
Heath JA, Jeffrey MI, Zhang HG, Rumball JA (2008) Anaerobic thiosulfate leaching: development of in situ gold leaching systems. Miner Eng 21:424–433
Yen W T XC (2008) Effects of copper minerals on ammionical thiosulfate leaching of gold. Proceeding XX IV Int. Miner. Process. Congr.
Ilyas S, Anwar MA, Niazi SB, Afzal Ghauri M (2007) Bioleaching of metals from electronic scrap by moderately thermophilic acidophilic bacteria. Hydrometallurgy 88:180–188
Faramarzi MA, Stagars M, Pensini E et al (2004) Metal solubilization from metal-containing solid materials by cyanogenic Chromobacterium violaceum. J Biotechnol 113:321–326
Choi M-S, Cho K-S, Kim D-JD-S, Kim D-JD-S (2004) Microbial recovery of copper from printed circuit boards of waste computer by Acidithiobacillus ferrooxidans. J Environ Sci Heal Part A- Toxic/Hazardous Subst Environ Eng 39:2973–2982
Duan C, Sheng C, Wu L, Zhao Y, He J, Zhou E (2014) Separation and recovery of fine particles from waste circuit boards using an inflatable tapered diameter separation bed. Sci World
Shapiro M, Galperin V (2005) Air classification of solid particles: a review. Chem Eng Process Process Intensif 44:279–285
RI S (1996) Recycling and resources recovery engineering. Springer, Berlin
Li J, Xu Z, Zhou Y (2007) Application of corona discharge and electrostatic force to separate metals and nonmetals from crushed particles of waste printed circuit boards. J Electrostat 65:233–238
Guo J, Cao B, Guo J, Xu Z (2008) A plate produced by nonmetallic materials of pulverized waste printed circuit boards. Environ Sci Technol 42:5267–5271
Guo J, Guo J, Cao B, Tang Y, Xu Z (2009) Manufacturing process of reproduction plate by nonmetallic materials reclaimed from pulverized printed circuit boards. J Hazard Mater 163:1019–1025
Yokoyama S, Iji M (1995) Recycling of thermosetting plastic waste from electronic component production processes. In: Proceedings 1995 IEEE Int. Symp., pp 132–137
Mou P, Xiang DDG (2007) Products made from nonmetallic materials reclaimed from waste printed circuit boards. Tsinghua Sci Technol 12:276–283
Ban BC, Song JY, Lim JY, Wang SK, An KG, Kim DS (2005) Studies on the reuse of waste printed circuit board as an additive for cement mortar. J Env Sci Heal A Tox Hazard Subst Env Eng 40:645–656
Goosey M, Kellner R (2003) Recycling technologies for the treatment of end of life printed circuit boards (PCBs). Circuit World 29:33–37
Hadi P, Ning C, Ouyang W, Lin SK, Hui CW, McKay G (2014) Conversion of an aluminosilicate-based waste material to high-value efficient adsorbent. Chem Eng J 256:415–420
Hu SH, Wu JY, Yen FS, Tsai MS (2006) Alkaline leaching of printed circuit board sludge. Environ Prog 25:243–250
Cui J, Forssberg E (2003) Mechanical recycling of waste electric and electronic equipment: a review. J Hazard Mater 99:243–263
Huang K, Guo J, Xu Z (2009) Recycling of waste printed circuit boards: a review of current technologies and treatment status in China. J Hazard Mater 164:399–408
Hall WJ, Williams PT (2007) Separation and recovery of materials from scrap printed circuit boards. Resour Conserv Recycl 51:691–709
Yamane LH, de Moraes VT, Espinosa DCR, Tenório JAS (2011) Recycling of WEEE: characterization of spent printed circuit boards from mobile phones and computers. Waste Manag 31:2553–2558
Hadi P, Xu M, Lin CSK, Hui CW, McKay G (2015) Waste printed circuit board recycling techniques and product utilization. J Hazard Mater 283:234–243
Menad N, Björkman B, Allain EG (1998) Combustion of plastics contained in electric and electronic scrap. Resour Conserv Recycl 24:65–85
Malhotra SC (1985) Trends and opportunities in electronic scrap reclamation. Conserv Recycl 8:327–333
Vijayaram R, Nesakumar D, Chandramohan K (2013) Copper extraction from the discarded printed circuit boards by leaching. Res J Eng Sci 2:11–14
Jha MK, Kumari A, Choubey PK, Lee JC, Kumar V, Jeong J (2012) Leaching of lead from solder material of waste printed circuit boards (PCBs). Hydrometallurgy 121–124:28–34
Chancerel P, Rotter S (2009) Recycling-oriented characterization of small waste electrical and electronic equipment. Waste Manag 29:2336–2352
Zhou Y, Qiu K (2010) A new technology for recycling materials from waste printed circuit boards. J Hazard Mater 175:823–828
Park YJ, Fray DJ (2009) Recovery of high purity precious metals from printed circuit boards. J Hazard Mater 164:1152–1158
Evangelopoulos P, Kantarelis E, Yang W (2015) Investigation of the thermal decomposition of printed circuit boards (PCBs) via thermogravimetric analysis (TGA) and analytical pyrolysis (Py-GC/MS). J Anal Appl Pyrolysis 115:337–343
Fogarasi S, Imre-Lucaci F (2014) Copper recovery and gold enrichment from waste printed circuit boards by mediated electrochemical oxidation. J Hazard Mater 273:215–221
Behnamfard A, Salarirad MM, Veglio F (2013) Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste Manag 33:2354–2363
Silvas FPC, Jiménez Correa MM, Caldas MPK, Moraes VT, Espinosa DCR, Tenório JAS (2015) Printed circuit board recycling: physical processing and copper extraction by selective leaching. Waste Manag 46:503–510
Xing M, Zhang FS (2013) Degradation of brominated epoxy resin and metal recovery from waste printed circuit boards through batch sub/supercritical water treatments. Chem Eng J 219:131–136
Calgaro CO, Schlemmer DF, da Silva MDCR et al (2015) Fast copper extraction from printed circuit boards using supercritical carbon dioxide. Waste Manag 45:289–297
He W, Li G, Ma X, Wang H, Huang J, Xu M, Huang C (2006) WEEE recovery strategies and the WEEE treatment status in China. J Hazard Mater 136:502–512
Yoo JM, Jeong J, Yoo K, Lee JC, Kim W (2009) Enrichment of the metallic components from waste printed circuit boards by a mechanical separation process using a stamp mill. Waste Manag 29:1132–1137
Hadi P, Ning C, Kubicki JD, Mueller K, Fagan JW, Luo ZT, Weng LT, McKay G (2016) Sustainable development of a surface-functionalized mesoporous aluminosilicate with ultra-high ion exchange efficiency. Inorg Chem Front 3:502–513
Li J, Duan H, Yu K, Liu L, Wang S (2010) Characteristic of low-temperature pyrolysis of printed circuit boards subjected to various atmosphere. Resour Conserv Recycl 54:810–815
Hoffmann JE (1992) Recovering precious metals from electronic scrap. J Miner Met Mater Soc 44:43–48
Kopacek B, Kopacek P (2015) Extracting rare materials from electr(on)ic scrap. IFAC-PapersOnLine 48:157–161
Lindberg SE, Wallschläger D, Prestbo EM, Bloom NS, Price J, Reinhart D (2001) Methylated mercury species in municipal waste landfill gas sampled in Florida, USA. Atmos Environ 35:4011–4015
Spalvins E, Dubey B, Townsend T (2008) Impact of electronic waste disposal on lead concentrations in landfill leachate. Environ Sci Technol 42:7452–7458
Osako M, Kim YJ, Sakai SI (2004) Leaching of brominated flame retardants in leachate from landfills in Japan. Chemosphere 57:1571–1579
Wong CSC, Wu SC, Duzgoren-Aydin NS, Aydin A, Wong MH (2007) Trace metal contamination of sediments in an e-waste processing village in China. Environ Pollut 145:434–442
Kang HY, Schoenung JM (2005) Electronic waste recycling: a review of US infrastructure and technology options. Resour Conserv Recycl 45:368–400
Kang HY, Schoenung JM (2006) Economic analysis of electronic waste recycling: modeling the cost and revenue of a materials recovery facility in California. Environ Sci Technol 40:1672–1680
Davis G, Herat S (2008) Electronic waste: the local government perspective in Queensland, Australia. Resour Conserv Recycl 52:1031–1039
Tanskanen P (2013) Management and recycling of electronic waste. Acta Mater 61:1001–1011
USEPA (US (2007) Management of Electronic Waste in the United States
Guo J, Guo J, Xu Z (2009) Recycling of non-metallic fractions from waste printed circuit boards: a review. J Hazard Mater 168:567–590
Komilis D, Evangelou A, Giannakis G, Lymperis C (2012) Revisiting the elemental composition and the calorific value of the organic fraction of municipal solid wastes. Waste Manag 32:372–381
Oguchi M, Sakanakura H, Terazono A (2013) Toxic metals in WEEE: characterization and substance flow analysis in waste treatment processes. Sci Total Environ 463–464:1124–1132
Tange L, Drohmann D (2005) Waste electrical and electronic equipment plastics with brominated flame retardants—from legislation to separate treatment—thermal processes. Polym Degrad Stab 88:35–40
Long YY, Feng YJ, Cai SS, Ding WX, Shen DS (2013) Flow analysis of heavy metals in a pilot-scale incinerator for residues from waste electrical and electronic equipment dismantling. J Hazard Mater 261:427–434
Duan H, Li J, Liu Y, Yamazaki N, Jiang W (2011) Characterization and inventory of PCDD/Fs and PBDD/Fs emissions from the incineration of waste printed circuit board. Environ Sci Technol 45:6322–6328
Sakai SI, Watanabe J, Honda Y, Takasuki H, Aoki I, Futamatsu M, Shiozaki K (2001) Combustion of brominated flame retardants and behavior of its byproducts. Chemosphere 42:519–531
Ni M, Xiao H, Chi Y, Yan J, Buekens A (2012) Combustion and inorganic bromine emission of waste printed circuit boards in a high temperature furnace. Waste Manag 32:568–574
Oguchi M, Sakanakura H, Terazono A, Takigami H (2012) Fate of metals contained in waste electrical and electronic equipment in a municipal waste treatment process. Waste Manag 32:96–103
Bidini G, Fantozzi F, Bartocci P, D’Alessandro B, D’Amico M, Laranci P, Scozza E, Zagaroli M (2015) Recovery of precious metals from scrap printed circuit boards through pyrolysis. J Anal Appl Pyrolysis 111:140–147
Yang X, Sun L, Xiang J, Hu S, Su S (2013) Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): a review. Waste Manag 33:462–473
Ortuño N, Conesa JA, Moltó J, Font R (2014) Pollutant emissions during pyrolysis and combustion of waste printed circuit boards, before and after metal removal. Sci Total Environ 499:27–35
Barontini F, Marsanich K, Petarca L, Cozzani V (2004) The thermal degradation process of tetrabromobisphenol A. Ind Eng Chem Res 43:1952–1961
Barontini F, Cozzani V, Marsanich K, Raffa V, Petarca L (2004) An experimental investigation of tetrabromobisphenol A decomposition pathways. J Anal Appl Pyrolysis 72:41–53
Lai YC, Lee WJ, Li HW, Wang LC, Ping G, Chien C (2007) Inhibition of polybrominated dibenzo-p-dioxin and dibenzofuran formation from the pyrolysis of printed circuit boards. Environ Sci Technol 41:957–962
Kim YM, Han TU, Watanabe C, Teramae N, Park YK, Kim S, Hwang B (2015) Analytical pyrolysis of waste paper laminated phenolic-printed circuit board (PLP-PCB). J Anal Appl Pyrolysis 115:87–95
Kumari A, Jha MK, Lee JC, Singh RP (2016) Clean process for recovery of metals and recycling of acid from the leach liquor of PCBs. J Clean Prod 112:4826–4834
Terakado O, Ohhashi R, Hirasawa M (2013) Bromine fixation by metal oxide in pyrolysis of printed circuit board containing brominated flame retardant. J Anal Appl Pyrolysis 103:216–221
Guo X, Qin FGF, Yang X, Jiang R (2014) Study on low-temperature pyrolysis of large-size printed circuit boards. J Anal Appl Pyrolysis 105:151–156
Cayumil R, Khanna R, Rajarao R, Mukherjee PS, Sahajwalla V (2015) Concentration of precious metals during their recovery from electronic waste. Waste Manag 57:121–130
Benallal B, Roy C, Pakdel H, Chabot S, Poirier MA (1995) Characterization of pyrolytic light naphtha from vacuum pyrolysis of used tyres. Comparison with petroleum naphtha. Fuel 74:1589–1594
Long L, Sun S, Zhong S, Dai WC, Liu JY, Song WF (2010) Using vacuum pyrolysis and mechanical processing for recycling waste printed circuit boards. J Hazard Mater 177:626–632
Wu W, Qiu K (2015) Vacuum co-pyrolysis of Chinese fir sawdust and waste printed circuit boards. Part II: influence of heating conditions. J Anal Appl Pyrolysis 111:216–223
Zhou Y, Wu W, Qiu K (2010) Recovery of materials from waste printed circuit boards by vacuum pyrolysis and vacuum centrifugal separation. Waste Manag 30:2299–2304
Mitan NMM, Bhaskar T, Hall WJ, Muto A, Williams PT (2008) Effect of decabromodiphenyl ether and antimony trioxide on controlled pyrolysis of high-impact polystyrene mixed with polyolefins. Chemosphere 72:1073–1079
Havlik T, Orac D, Petranikova M, Miskufova A (2011) Hydrometallurgical treatment of used printed circuit boards after thermal treatment. Waste Manag 31:1542–1546
Liu JY, Duan NYHY (2010) Study on the metals dissolving from waste printed circuit boards by using nitric acid as leaching liquor. Environ Pollut Control 12:35–38
Jun W, Liang C, Lijuan LQ (2008) Study on selectively leaching gold from waste printed circuit boards with thiourea. Gold 6:17
Kinoshita T, Akita S, Kobayashi N, Nii S, Kwaizumi F, Takahashi K (2003) Metal recovery from non-mounted printed wiring boards via hydrometallurgical processing. Hydrometallurgy 69:73–79
Qiu KQ, Gu HCS (2008) Characteristics of metals resources and status of hydrometallurgical processes for recycling metals from waste printed circuit boards. Chinese J Nonferrous Met 18:s381–s385
Mankhand TR, Singh KK, Gupta SK, Das S (2013) Pyrolysis of printed circuit boards. Int J Metall Eng 1:102–107
Mecucci A, Scott K (2002) Leaching and electrochemical recovery of copper, lead and tin from scrap printed circuit boards. J Chem Technol Biotechnol 77:449–457
Sheng PP, Etsell TH (2007) Recovery of gold from computer circuit board scrap using aqua regia. Waste Manag Res 25:380–383
Chmielewski AG, Urbański TS, Migdał W (1997) Separation technologies for metals recovery from industrial wastes. Hydrometallurgy 45:333–344
Zhou QFZW (2003) Recovery of gold from waste computer and its accessories. China Resour Compr Util 7:31–35
Quinet P, Proost J, Lierde A Van (2005) Minerals and Metallurgical Processing, 22 (1):17. 994740
Becker E, Knothe MLJ (1983) Gold recovery from non-metallic secondary raw materials by leaching with thiourea and adsorption on ion exchangers. Hydrometallurgy 3:265–275
Xu XL LJ (2011) Experimental study of thiourea leaching gold and silver from waste circuit boards. J Qingdao Univ (E&T), 69–73
Moore DM, Zhang XR, Li CX. (2005) Using thiosulfate as a leach reagents instead of cyanide. Met Ore Dress Abroad 5–12
Ha VH, Lee J, Jeong J, Hai HT, Jha MK (2010) Thiosulfate leaching of gold from waste mobile phones. J Hazard Mater 178:1115–1119
Abbruzzese C, Fornari P, Massidda R, Vegliò F, Ubaldini S (1995) Thiosulphate leaching for gold hydrometallurgy. Hydrometallurgy 39:265–276
Xu Q, Chen DH, Chen L, Huang M (2010) Gold leaching from waste printed circuit board by iodine process. Nonferrous Met. 3:335–343
Birloaga I, De Michelis I, Ferella F, Buzatu M, Vegliò F (2013) Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manag 33:935–941
Devecci H. (2010) Extraction of copper from scrap TV boards by sulphuric acid leaching under oxidising conditions. In: GOING GREEN-CARE Innov. 2010 Conf. Vienna, p 45
Kumari A, Jha MK, Singh RP (2015) Recovery of metals from pyrolysed PCBs by hydrometallurgical techniques. Hydrometallurgy 165:97–105
Havlik T, Orac D, Petranikova M, Miskufova A, Kukurugya F, Takacova Z (2010) Leaching of copper and tin from used printed circuit boards after thermal treatment. J Hazard Mater 183:866–873
Kim EY, Kim MS, Lee JC, Pandey BD (2011) Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J Hazard Mater 198:206–215
Ehrlich HL (2001) Past, present and future of biohydrometallurgy. Hydrometallurgy 59:127–134
Bosecker K (1997) Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev 20:591–604
Rawlings DE (2002) Heavy metal mining using microbes. Annu Rev Microbiol 56:65–91
Morris PJ, Quensen JF, Tiedje JM, Boyd SA (1992) Reductive debromination of the commercial polybrominated biphenyl mixture Firemaster BP6 by anaerobic microorganisms from sediments. Appl Environ Microbiol 58:3249–3256
Liang G, Tang J, Liu W, Zhou Q (2013) Optimizing mixed culture of two acidophiles to improve copper recovery from printed circuit boards (PCBs). J Hazard Mater 250–251:238–245
Ilyas S, Ruan C, Bhatti HN, Ghauri MH, Anwar MH (2010) Column bioleaching of metals from electronic scrap. Hydrometallurgy 101:135–140
Yang T, Xu Z, Wen J, Yang L (2009) Factors influencing bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 97:29–32
Yen-Peng Ting, Chi Chong Tan VAP (2008) Cyanide-generating bacteria for gold recovery from electronic scrap material. J Biotechnol 136:S653–S654
Marhual NP, Pradhan N, Kar RN, Sukla LB, Mishra BK (2008) Differential bioleaching of copper by mesophilic and moderately thermophilic acidophilic consortium enriched from same copper mine water sample. Bioresour Technol 99:8331–8336
Sampson MI, Phillips CV (2001) Influence of base metals on the oxidizing ability of acidophilic bacteria during the oxidation of ferrous sulfate and mineral sulfide concentrates, using mesophiles and moderate thermophiles. Miner Eng 14:317–340
Brandl H, Bosshard R, Wegmann M (1999) Computer-munching microbes: metal leaching from electronic scrap by bacteria and fungi. Process Metall 9:569–576
Zhu N, Xiang Y, Zhang T, Wu P, Dang Z, Li P, Wu J (2011) Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria. J Hazard Mater 192:614–619
Search H, Journals C, Contact A, DC Arc Plasma Disposal of Printed Circuit Board * System of the DC arc plasma disposal of solid waste. 2423
Liu K, Zhang Z, Zhang F-S (2016) Advanced degradation of brominated epoxy resin and simultaneous transformation of glass fiber from waste printed circuit boards by improved supercritical water oxidation processes. Waste Manag 56:423–430
Wang H, Hirahara M, Goto M, Hirose T (2004) Extraction of flame retardants from electronic printed circuit board by supercritical carbon dioxide. J Supercrit Fluids 29:251–256
Xiu FR, Zhang FS (2010) Materials recovery from waste printed circuit boards by supercritical methanol. J Hazard Mater 178:628–634
Xiu F-R, Weng H, Qi Y, Yu G, Zhang Z, Zhang FS (2016) A novel reutilization method for waste printed circuit boards as flame retardant and smoke suppressant for poly (vinyl chloride). J Hazard Mater 315:102–109
Xiu FR, Qi Y, Zhang FS (2015) Leaching of Au, Ag, and Pd from waste printed circuit boards of mobile phone by iodide lixiviant after supercritical water pre-treatment. Waste Manag 41:134–141
Xiu FR, Zhang FS (2012) Size-controlled preparation of Cu2O nanoparticles from waste printed circuit boards by supercritical water combined with electrokinetic process. J Hazard Mater 233–234:200–206
Xiu FR, Zhang FS (2009) Preparation of nano-Cu2O/TiO2 photocatalyst from waste printed circuit boards by electrokinetic process. J Hazard Mater 172:1458–1463
Sanyal S, Ke Q, Zhang Y, Ngo T, Carrell J, Zhang H, Dai l (2013) Understanding and optimizing delamination/recycling of printed circuit boards using a supercritical carbon dioxide process. J Clean Prod 41:174–178
Xing M, Zhang F (2012) A novel process for detoxification of BERs in waste {PCBs}. Procedia Environ Sci 16:491–494
Yin J, Li G, He W, Huang J, Xu M (2011) Hydrothermal decomposition of brominated epoxy resin in waste printed circuit boards. J Anal Appl Pyrolysis 92:131–136
Sarvar M, Salarirad MM, Shabani MA (2015) Characterization and mechanical separation of metals from computer printed circuit boards (PCBs) based on mineral processing methods. Waste Manag 45:246–257
Eswaraiah C, Kavitha T, Vidyasagar S, Narayanan SS (2008) Classification of metals and plastics from printed circuit boards (PCB) using air classifier. Chem Eng Process Process Intensif 47:565–576
Habib M, Miles NJ, Hall P (2013) Recovering metallic fractions from waste electrical and electronic equipment by a novel vibration system. Waste Manag 33:722–729
Kumar V, Lee J, Jeong J, Jha MK, Kim BS, Singh R (2015) Recycling of printed circuit boards (PCBs) to generate enriched rare metal concentrate. J Ind Eng Chem 21:805–813
Zhang S, Forssberg E, Arvidson B, Moss W (1998) Aluminum recovery from electronic scrap by high-force eddy-current separators. Resour Conserv Recycl 23:225–241
WL. D (1990) Practical applications of eddy current separators in the scrap recycling industry. Second Int. Symp. Met. Eng. Mater
Li J, Lu H, Guo J, Xu Z, Zhou Y (2007) Recycle technology for recovering resources and products from waste printed circuit boards. Environ Sci Technol 41:1995–2000
Jiang W, Jia L, Zhen-ming X (2009) A new two-roll electrostatic separator for recycling of metals and nonmetals from waste printed circuit board. J Hazard Mater 161:257–262
Zhao M, Li J, Yu K, Zhu F, Wen X (2006) Measurement of pyrolysis contamination during crushing of waste printed circuit boards. J Tsinghua Univ Technol 46:1995–1998
Guo J, Li J, Rao Q, Xu Z (2008) Phenolic molding compound filled with nonmetals of waste PCBs. Environ Sci Technol 42:624–628
Guo J, Rao Q, Xu Z (2008) Application of glass-nonmetals of waste printed circuit boards to produce phenolic moulding compound. J Hazard Mater 153:728–734
Zheng Y, Shen Z, Cai C, Ma S, Xing Y (2009) The reuse of nonmetals recycled from waste printed circuit boards as reinforcing fillers in the polypropylene composites. J Hazard Mater 163:600–606
Niu X, Li Y (2007) Treatment of waste printed wire boards in electronic waste for safe disposal. J Hazard Mater 145:410–416
Lu MX, Zhou CH, Liu WL (2000) The study of recovering waste printed circuit board by mechanical method. Tech Equip Environ Pollut Control 10:30–35
Ke YH, Yang ET, Liu X, Liu CL, Dong WS (2013) Preparation of porous carbons from non-metallic fractions of waste printed circuit boards by chemical and physical activation. Xinxing Tan Cailiao/New Carbon Mater 28:108–114
Rajagopal RR, Aravinda LS, Rajarao R, Bhat BR, Sahajwalla V(2016) Activated carbon derived from non-metallic printed circuit board waste for supercapacitor application. Electrochim Acta 211:488–498
Hadi P, Barford J, McKay G (2013) Toxic heavy metal capture using a novel electronic waste-based material-mechanism, modeling and comparison. Environ Sci Technol 47:8248–8255
Xu M, Hadi P, Chen G, McKay G (2014) Removal of cadmium ions from wastewater using innovative electronic waste-derived material. J Hazard Mater 273:118–123
Ning C, Hadi P, Lin CSK, McKay G (2016) Valorization of an electronic waste-derived aluminosilicate—surface functionalization and porous structure tuning. ACS Sustain Chem Eng 6:2980–2989
Pimenta S, Pinho ST (2011) Recycling carbon fibre reinforced polymers for structural applications: technology review and market outlook. Waste Manag 31:378–392
Burke A (2007) R&D considerations for the performance and application of electrochemical capacitors. Electrochim Acta 53:1083–1091
Ludwig Christian, Hellweg Stefanie, Samuel Stucki E (2012) Municipal solid waste management: strategies and technologies for sustainable solutions. Springer, Berlin
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Chemistry and Chemical Technologies in Waste Valorization”; edited by Carol Sze Ki LIN.
Rights and permissions
About this article
Cite this article
Ning, C., Lin, C.S.K., Hui, D.C. et al. Waste Printed Circuit Board (PCB) Recycling Techniques. Top Curr Chem (Z) 375, 43 (2017). https://doi.org/10.1007/s41061-017-0118-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-017-0118-7