Abstract
As technology advances and auxiliary electrical and electronic equipment expands, waste printed circuit boards are among the quickest growing sources of waste. Throughout the world, the exploitation of waste printed circuit boards has become one of the lucrative commercial enterprises in the recycling production company. Additionally, it can also cause a variety of effects on humans and the environment in terms of metal ions. In order to facilitate the recovery and recycling of printed circuit board, several innovative techniques have been developed, including pyrometallurgy, hydrometallurgy, and biometallurgy. It is possible to recover and recycle precious metals through the hydrometallurgy process simply and conveniently. Economic efficiency, environmental friendliness, and durability make this technology auspicious. On the other hand, there are few comprehensive studies on the hydrometallurgy and chemical processing of waste printed circuit board. As a result, in this work, a mini-review was performed in order to assess different chemical leaching methods, optimize parameters, and examine future investigation pathways.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
E-waste has grown into foremost worldwide issue as a result of the rapid advancement of technology, which has resulted in a decrease in the lifespan of electrical and electronic equipments (EEE) and materials (Qiu et al. 2020; Yao et al. 2018). There has also been a significant decrease in the life expectancy of EEE. There is a large amount of EEE that is near the end of its useful life (EOL). As estimated by the United Nations (UNs) in 1992, there were 14 million tons of waste electrical and electronic equipment (WEEE) created global level, whereas in 2002 there were 24 million tons and in 2012 there were 49 million tons, and today there are over 50 million tons, with an exponential increase in the quantity of waste produced each year and an increase of 10% in the rate of e-waste generation (Sakunda 2013). It is estimated that WEEE production is increasing exponentially at a rate of more than 3–5% per year. In 2014, approximately 41.8 million tons of total e-waste (5.9 kg per person) were generated around the world (Baldé, et al., 2017). E-waste production in Canada has been estimated at 20.4 Kg per capita, with total waste production of 725 kilograms in 2014. In 2016, the universal capacity of e-waste is about 44.7 million tons (Mt) then it will reach 57 million tons in 2021 (Baldé et al. 2017; Wang et al. 2020a, b). An estimated e-waste is growing very rapidly at the global level and producing huge amounts of waste in relation to volume, which has a negative impact on the environment. Most electronic products are constructed using PCBs, which are the foundation of electronics. The PCB is a key component of many types of EEE. As a result of the increased utilization in modern life (Qin et al. 2020), discarded PCBs are responsible for about 3–6 wt% of entire e-waste production (Korf et al. 2019, Kaya et al., 2020). In addition, an increased output of EEE and based e-waste consumer recycling are challenging in waste disposal industry. In the absence of suitable environmental management, waste PCBs are contributing to environmental pollution. Several precious metals like Au, Ag, Pd, and Pt, have been found in waste PCBs. PCBs are an essential part of most electronic devices and they are normally examined in end-user electronics. It is estimated that PCBs account for 3% of all e-waste. From PCB scrap, metal content can only be recovered as low as 30% in most practical recycling methods. Over 70% of PCB waste cannot be competently recycled, and it must be disposed of in landfills (Li et al. 2020a, b; Han et al. 2019). For emergencies, it is necessary to develop efficient, environmentally friendly, and cost-effective treatment technologies. Recycling PCBs not only prevents environmental pollution but also contributes to resource conservation. This is a noticeable suggestion for endless growth of recovery of resources to human beings. Each year, 3,657 households can save energy by reprocessing 1 million laptops. Around 1 g of gold (Au) is contained in 41 smartphones. Yearly 1 million smartphones are reprocessed, recovering 16 tons of copper (Cu), 350 kg of silver (Ag), 34 kg of gold (Au), and 1.5 kg of Pd (Kaya et al., 2016a). PCBs waste can be categorized into different grades according to their Au content, such as high (400 g/t), medium (100–400 g/t), and low (100 g/t) (Oguchi et al. 2011). The capital value of expensive metals has differed from €6.8 million for the mid-range to €63 million of high-end and the analysis has been explained in Fig. 1 (D’Adamo et al. 2019).
NPV is described in million € and cores are divided into three groups: i high-grade WPCBs, ii medium-grade WPCBs and iii low-grade WPCBs, (Adamo et al., 2019)
As PCBs become smaller and more complex, the amount of material they contain changes constantly. The gold content of a tonne of PCBs may range from 80 to 1500 g, and the copper content varies from 160 to 210 kg. These concentrations are 40 to 800 times higher than those found in gold ores and 30 to 40 times higher than those found in copper ores. Worldwide, 267.3 tonnes of gold (Au) and 7275 tonnes of silver (Ag) were disbursed yearly by the electronics factory (Vats and Singh 2015). According to the 2014 UN e-waste report, approximately 4,444,300 tonnes of Au are recovered annually, making the e-waste market worth $4,444,52 billion. Despite the steadily decreasing content of valuable metals viz., Au, Ag, and Pd, e-waste has proven worth recycling (http: //unu.edu/ news/ news/ewaste-2014-unu-report.html). E-waste rapidly pollutes groundwater, acidifies the soil, produces toxic fumes and gases after the combustion process, collects fastest in municipal waste areas, and discharges carcinogens, so e-waste is banned in all countries due to proper disposal conditions and safety (Kaya et al., 2016b). Globally, the disposal of e-waste, especially PCBs waste, has become a serious problem. In general, mobile phones last for one year and computers last for two years. As a result of equipment failures and outdated technology, approximately one hundred million mobile phones and seventeen million computers are scrapped each year. New PCB waste recycling technologies must consider several factors, including innovation, societal and environmental impacts, assimilated waste management policies, and procedure economics (Kaya et al., 2016a; b; Isıldar et al. 2018). Activities like acid dispossess and open igniting have a substantial impact on the environment. The eminence of polybrominated diphenyl ethers (PBDEs) pollution of e-waste landfill in Lagos and Nigeria has also been noticed. There has been an increase in PBDE levels in soil profiles (depth 0–45 cm) resulting from PBDE aggregation in the surface soil and relocation to the subsoil. It has been revealed that toxic metal concentrations can lead to cancer and risk assessment is needed. The average concentration of metal has been noticed for Pb (0.0693 ppm) and Cu (0.0525 ppm) and Cd (0.0042 ppm) (Abubakar et al. 2022). During the non-formal incineration of e-waste, substantial levels of air pollution have been observed, which poses health and environmental risks. As a result of hydrometallurgical technology, enormous amounts of waste water have been produced. Post-leaching hydrometallurgical techniques have been successfully used to recover precious metals from minerals and secondary resources. Hydrometallurgical processes include ion exchange, solvent extraction, reduction, and precipitation methods (Das 2010). These methods generate huge amounts of secondary waste and require the recovery of low concentrations of dissolved PM. For recycling waste PCBs, efficient and eco-friendly techniques are needed in the direction to create the method more efficient and eco-friendly., The aim of the present review, the current status of leaching and recovery of valuable metals using various waste PCB materials through hydrometallurgical process are reviewed. This techniques involves different chemical leaching processes as well as the detailed recovery of metal in a sustainable e-waste recycling process.
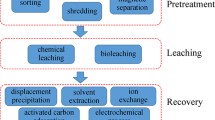
Different types of technologies for the PCBs waste treatment process
In recent years, more advanced techniques have been established to assist recovery of metals from PCB waste, comprising pyrometallurgy, hydrometallurgy, separation of physico-mechanical process, supercritical fluids, electrolysis, and bioleaching (Becci et al. 2020; Yu et al. 2019).
Physico-mechanical separation
Commonly, physico-mechanical and mechanical separators are utilized for the regulation of physics and assets for material separation of resources. Gravity and sorting are the two main methods of separation. The physico-mechanical splitting includes gravity + magnetic + electrostatic together. Separation of mechanical processes is an old physico-mechanical separation and progressive processing technique. Different parts of PCBs materials are affected by the separation process, which includes neither separation process, produces a pure material stream and it only increases purity. Total industrial recoveries for mechanical separations vary from 80 to 95% (https://wedocs.unep.org/handle/20.500.11822/45394). Physicomechanical processes include shredding and crushing mixed e-waste, concentration, and separation utilizing magnetic separators, gravity separators, and electrostatic separators. As a result, mechanical processes do not result in greater recoveries, particularly for precious metals (PM). A flow chart of the mechanical separation technique of a complex WEEE metallic retrieval plant feature is a physical sorting route to eliminate batteries, toner/ink, paper, and exterior cables. Motors, capacitors, and transformers of a large size were removed using line shredders and tape shredders. Magnetic crack separation is used to remove porous ferrous metals. Micronization was surveyed by eliminating adequate metals of ferrous and steel. Eddy Current Separator (ECS) for separating metals (Cu and Al) and non-metals (plastic, rubber, glass, wood, stone) and Al-rich and Cu-rich materials has been detached by density separation Kellner (2009). Waste recycling plants around the world use mechanical processing actions viz., gravity air separation, magnetic separation, and eddy current separation (Ogunniyi et al. 2009).
Electrolysis
Metal scraps from PCB waste are the most complex. Scholars on direct electrolysis efforts on copper 90-99.9% of purity could be recovered. Various factors such as recovery, current efficiency, purity, and electrolyte design are essential to understanding the mechanism and performance of electrolysis of multi-metals. Restoration after that coexisting metal assets cannot be mined proficiently. Using direct electrolysis as a starting point, a complete recycling technique can be established (Song et al. 2023).
Some advantages of this process include low energy consumption and low environmental pollution. It has been observed that a few disadvantages exist, such as the need for pre-leaching or a supercritical water oxidation process to handle PCB waste (Liu et al. 2022; Qi et al. 2019,). In addition, an environmentally friendly method has been described to separate gold (Au) from waste PCBs using suspension electrolysis and chlorination (Wang et al. 2020a, b). Using slurry electrolysis, high-purity, ultra-fine copper residues have been recovered from PCB waste metal powders. The obtained results have revealed that the purity of copper has gradually been enhanced and reduced with the rise in the concentration of (polyvinylpyrrolidone) (PVP), oleic acid, sulphuric acid, and current density. Copper powder particles increase the density of the current, which gradually becomes coarser over time (Zhang et al. 2017).
Supercritical fluid
Supercritical fluid extraction (SCF) using CO2 is a small process to ensure normal products which are not contaminated with solvents, contaminants, or synthetic substances (Bougnoux et al. 2010). In contrast to liquid–liquid extraction, solvent recovery occurs by the rapid expansion of the gas within the SCF containing CO2 and is, therefore, the consumption of lower energy (Azevedo et al. 2008). The major advantage of this technique is more efficient. However, more energy consumption and long processing duration are the main disadavantages. In general, the majority of the polymeric-based solid residue is obtained during the ewaste separation process.WPCBs typically include 50–70% nonmetallic components and 30–50% metallic components. The common polymeric solid residues was found in WPCBs such as phenolic resin, epoxy resin, glass fibers, inorganic fillers and brominated flame retardant etc. (Wan et al. 2023). Figure 2 explains the flow chart of reclamation of useful materials from discarded e-waste (Li et al., 2019; Chen et al. 2022).
Flow chart for reclamation of waste electronic wastes by SCW, (Chen et al. 2022)
After pretreatment with supercritical fluids, expensive metals like Au, Ag, and Pd are leached from PCBs in mobile cellphones (Xiu et al. 2015). The process of recovery of silver from PCB is a simple and environmentally friendly technique. The computer scrap materials was done by the presence of SCF because it contain CO2 which enhance the solubility of silver in SC-CO2 (Fayaz et al. 2021). In contrast to traditional methods such as hydrometallurgy and pyrometallurgy, the SCF method can remove harmful constituents without causing secondary pollution. Therefore, SCF technology has shown some advantages in retrieving valuable substances from e-waste in an environmentally friendly manner (Li and Xu 2019). More recovery rate of significant metals has been revealed in this article such as Pt, Pd, and Rh (Islam et al. 2020).
Bioleaching
Different types of microorganisms have been utilized for the bioleaching process, including chemolitrophic prokaryotes, heterotrophic bacteria, and fungi. There are three main factors: (1) conversion of inorganic or organic acids, (2) reduction and oxidation reactions, and (3) elimination of complex agents (Asghari et al 2013). This bioleaching method has the potential to retrieve elements from PCBs, since the method avoids an issue of high energy consumption, complicated operations, and heavy pollution (Awasthi et al. 2019). In recent times, various leaching methods have been established. Columns, percolators, and submerged leaching are being used for laboratory-scale studies. The following leaching processes have been utilized for company purposes: underground and tank leaching processes (Bosecker et al., 1997). The 68.5% of Au and 33.8% of Ag were obtained at pH 9.0 using gram negative Pseudomonas balearica bacterium mixed in the suspension of Au and Ag with other parameters such as pulp density 10 g/L, temperature 30 °C, and glycine concentration 5 g/L (Kumar et al. 2018). The comprehensive recycling of non-metallic residues and biological detoxification from copper-arrayed laminate waste have also been noticed (Zhou et al. 2020). In addition to being eco-friendly and low-cost, bioleaching also has some disadvantages, including a long time consumption and low efficiency. The SEM–EDS images of bioleaching electronic waste explained that the three typical genetic modification and engineering species, Escherichia coli, Bacillus subtilis and Saccharomyces cerevisiae, were collected from not dirty soil and evaluated for cadmium sensitivity (Gu et al. 2017, Trivedi et al., 2020).
Pyrometallurgy
As a result of their unique advantages, pyrometallurgical processes, such as metal recovery processes and chlorinated evaporation processes, have become the dominant flow for treating spent autocatalysts. For precious metals to be obtained, it is necessary to separate more active constituents from the other components. It is very important to stimulate the complete recovery of spent secondary resources of autocatalysts for the sustainable development of industry (Han et al. 2023). There are drawbacks associated with the pyrometallurgy technique, including its high energy consumption and high cost (Xia et al. 2018). High treatment efficiency and significant volume sizes are the advantages, while high energy consumption and equipment costs are the disadvantages. (Yao et al. 2020; Xiong et al. 2020).
Conventionally, more valuable metals have been recovered from electronic waste utilizing pyrometallurgical processes. As a result, dioxin gases are dangerously released into the environment through this method (Li et al. 2024; Castro and Bassin (2022).
Hydrometallurgy
Recent studies have shown that hydrometallurgical processes have numerous advantages over pyrometallurgy, such as a low capital cost, a reduced environmental impact, and ease of management. Among the key elements of hydrometallurgical processes are the leaching of metals, refining, and recovering precious metals (Manikkampatti Palanisamy et al. 2022). Over the past twenty years, the engineering field has learned that proper development is very important for recycling e-waste. A growing interest is being shown in the application of hydrometallurgical techniques to retrieval of metals from waste electronic items. Unlike pyrometallurgy, hydrometallurgy is more environmentally friendly and can be used for the recycling of old PCBs and electronics. In hydrometallurgical processes, cyanide solution or aqua regia leaching is unsustainable due to its toxicity and non-recyclability (Islam et al. 2020,; Kamberovic´ et al. 2011).
Currently, both industrialists and scholars are interested in recovering PM through hydrometallurgical processes. This technique offers a wide range of advantages, including operational flexibility, ease of use, neat working environments, minimal capital requirements, and little emission of poisonous gases. The hydrometallurgy process can be applied directly to liquid sources of PM. Before hydrometallurgy can be performed on solid PM sources, soluble materials must be extracted from solid components by using appropriate solvents. This procedure is called leaching. The hydrometallurgical technique begins with this crucial and significant step. Chemical leaching involves the use of different types of reagents. In this process, hydrogen peroxide (H2O2), aqua regia, cyanides, halides, thiosulfate, and thiourea were used as leaching agents. PM dissolves more quickly in cyanide solution. It is a widely used technique for recovering PM from secondary sources. However, cyanide is poisonous and causes harmful effects on natural drinking water resources. For this reason, other leaching agents viz., halides, thiourea, and thiosulfates were selected for recovery of PM and they have been used as a substitute for agents Recently, a new technique using chlorinated hydrochloric acid (Cl2/HCl) has been developed based on practical indications (Shen et al. 2011). As a result of this process, hydrometallurgy has emerged as a more efficient and effective method to recover PM from the second solution. In the form of a chlorine complex, this lye consists of a high concentration of chloride. As a result of a sequence of precipitation events, PM is mainly isolated. Despite its advantages, this method has many drawbacks: relatively poor selectivity over several stages of precipitation, long purification times, and high chemical consumption and labor costs (Won et al. 2014). Hydrometallurgical processes have involved recovering metals using various chemicals, such as cyanides, halides, thioureas, and thiosulfates, which have produced large amounts of waste liquids and waste chemical reagents. Shorter processing time and greater efficiency are the major advantages. One of the major disadvantages is the high amount of chemical waste and effluent water generated (Birloaga et al., 2018; Zhu et al. 2019; Zhihui et al. 2021).
Hydrometallurgical techniques
E-waste recycling has played a significant role in engineering development over the past 20 years. A growing interest has been shown in recycling e-waste and reclaiming metals by hydrometallurgy. Compared to pyrometallurgy, hydrometallurgy is more environmentally friendly when it comes to processing PCB waste and electronic scrap applications (Kamberovic' et al. 2009, 2011). Chemical reagents are the most important components of hydrometallurgical processes. Base metals can be leached using strong acids, while precious metals can be leached from electronic waste using cyanides, halides, thioureas, and thiosulfates (Liu et al. 2016; Li et al. 2020a, b). This process is simpler to regulate when it relates to selectively concentrating a specific metal with the right quantity, acid, and acid concentration (Ghosh et al. 2015). The various types of hydrometallurgical processes with different chemical leaching flow charts are explained in Fig. 3(Pant et al. 2012).
Types of hydrometallurgical techniques (Pant et al. 2012)
As a result, these processes require large quantities of chemical reagents and result in large quantities of waste water and by-products. It is noteworthy that cyanide leaching can be used to reclaim precious metals from rocks and e-waste, and it can result in high recovery rates and low costs. On the other hand, this process has a number of disadvantages, such as an increased need for wastewater treatment prior to discharge and cyanide is one of the regulated chemicals due to the fact that it is toxic (Faramarzi et al. 2020). The recovery of metals from e-waste by hydrometallurgy
is an old process. It offers a greater return on investment and is a better method than conventional operation (Yazici and Deveci 2014). Despite hydrometallurgical techniques being technologically mature, environmental impacts and economics of the process remain concerns, especially when toxic reagents are used (Tuncuk et al. 2012).
The goal of this research is to develop an environmentally friendly metal leaching technology from e-waste (Sikander et al. 2022). In hydrometallurgical techniques, acid leaching or caustic leaching of stable materials are the most important stages (Iannicelli-Zubiani et al. 2017). As far as marketability is concerned, precious metals contribute the most value to e-waste, as well as being the most attractive and active sector. For recovering valuable metals from waste electronic items, hydrometallurgical techniques are the most convenient and simple (Macaskie et al. 2007; Safarzadeh et al. 2007). Using solid acid or caustic solutions is one of the foremost stages in hydrometallurgical refining. Afterward, the metals are separated and purified through solvent removal, adsorption, ion exchange, and precipitation of impurities. In order to retrieve the elements from these solutions, chemical reductions, electrorefining processes, and crystallization processes are employed (Safarzadeh et al. 2007). Because of this, it may be incredible to quantitatively percolate maximum elements from e-waste, especially when chemical leaching methods are selected. A lot of attention should be paid to the consumption of non-toxic materials from e-waste chemicals (Gurung et al. 2013). Since hydrometallurgy produces a lot of secondary toxic waste, pre-treatment is necessary before they can be disposed of in the environment because of their environmental degradation (I¸sıldar et al. 2018; Liu et al 2016).
Classification of chemical leaching
The chemical leaching process can be classified into many types according to its properties, nature, and binding ability.
Chemical leaching
A chemical leaching medium is used to dissolve precious metals, and it is a common method of recovering metals and other materials. Waste PCBs have been subjected to several studies where valuable metals were separated from them. The literature has used a variety of inorganic acids including H2SO4 because of their effectiveness and low reagent costs. On the other hand, acid leaching uses chemicals like H2SO4, HNO3, and HCl, and aqua regia uses high amounts of chemicals and water and produces secondary waste. A treatment that takes place in the order of reduction, recovery, and isolation of metals (Xie et al. 2009). Thiourea, thiosulfate, and halide scavengers, as well as cyanide scavengers, were used for chemical leaching. The purpose of cyanide percolating agents has enabled mines to recover valuable metals, viz., gold (Au) and silver (Ag), more efficiently and at a lower cost (Akcil et al., 2010). Chemical leaching comprises a leaching process using either acids or ligand-assisted complexation such as cyanides, halides, thioureas, and thiosulfate leaching is described as follows. Figure 4 indicates the retrieval of valuable metals from scrap resources (Zhong et al. 2006; Wu et al. 2009; Ha et al. 2010).
Suggested mechanism for the retrieval of valuable metals from scraped electronic materials (Cui and Zhang, 2008)
Cyanide leaching
This process is mainly utilized to leaching of PM from PCBs. This method was used for centuries due to advantages of its high recovery efficiency, low cost, and ease of use. Nearly 90% of Au, Ag, Pd & Pt metals has been recovered by this method (Udayakumar et al. 2022). Further, hydrogen cyanide (HCN) is a feeble acid which is used to recover gold and silver metals by limiting its use. The risks posed by cyanide have not been eliminated. Cyanide volatilization occurs under pH 8.5 (Akcil et al. 2015). In contrast, this cyanide technique poses serious hazards to its natural habitat as it contaminates the environment is the major disadvantages. Researhers and inventors have worked with the industry to test alternative leaching agents like halides, thiosulfate, thiocyanates, and thiourea in response to increased pressure on the environment and the use of cyanide. Figure 5 shows the anodic cyanidation process of gold (Akcil et al. 2015). Non-cyanide leaching agents offer a safer and more robust solution than cyanide.
Anodic cyanidation model for gold; boundary i: gold-film interface, boundary o: film-solution interface, (Akcil et al. 2015)
In order to retrieve gold (Au) and silver (Ag) using cyanide solution, a reduction method was used. Dissolving Au and Ag on PCBs requires an alkali metal cyanide solution. The cyanide process is an efficient and effective method of leaching PCBs, but it is difficult to liquefy PCBs due to the cyanide process (Zhou and Zhu 2003). There is still a large use of cyanide leaching in the gold removal industry, but the process produces large amounts of cyanide-containing effluent that is hazardous to workers and the environment. Moreover, cyanide has a slow leaching rate, which results in longer production cycles. Thus, metallurgical investigators have deployed several non-cyanide leaching routes in current years, and a few methods have advanced significantly to the point where they have almost been deployed and marketable for production. Several non-cyanide leaching methods are now being explored, including the leaching of thiourea, thiosulfate, and halide leaching processes (Zhang et al. 2012).
Halide leaching
The halide leaching process consists of chloride, bromide, and iodide leaching techniques. This method has the major advantage of a faster leaching rate. This method was used to recover 97.5% of Au (Udayakumar et al. 2022). According to the solution environments, gold forms both Au+ and Au3+ complexes with iodide, chloride, and bromide.
The application of chlorine/chloride has been widely used in industry and also acting a vital part in the deposition of halides. The chloride leaching method, however, requires exceptional stainless steel equipment and rubber-lined machines to operate under more corrosive environments. The bromide leaching is a dangerous technique. An unusual equipment is required to handled the vapour pressure of bromine due to safety and health hazards, limiting its industrial uses. These are the main disadvantages of bromide leaching process (Moses and Petersen 2000). The leaching of iodide has shown some advantages, including rapid leaching rates, high selectivity, and no toxicity associated with the process. Furthermore, highly stable complexes have been formed by the combination of gold and iodine (Zhang et al. 2009). The simple leaching technique was utilized for the retrieval of gold and iodide from the rejected PCBs (Xu et al. 2010). The use of iodide leaching is considered an auspicious technology, and 95% gold has also been leached during this process.
Thiourea leaching
Additionally, thiourea is widely utilized for the leaching of elements, since it has main merits of low toxicity, excellent recoveries, faster kinetics, and greater selectivity. The diagram explain the technique of retrieval of valuable metals from waste PCBs from mobile phones can be found in Fig. 6(Gurung et al. 2013).
Recommended flow chart for the leaching of valuable metals from PCBs of mobile waste and retrieval by adsorption technique, (Gurung et al. 2013)
The work suggests intervening electron pairs of nitrogen and sulfur atoms are likely to be coordinate bonds between Au and Ag associated with cyanide (Lacoste-Bouchet et al. 1998, Murthy et al., 1996).
Thiourea is also called urea sulfide and it is formed of a white complex crystal with several metal ions. In the existence of an oxidizing agent (commonly Fe3+), thiourea forms a soluble cationic complex with gold. Leaching with thiourea is simply less stable than other methods. Since it consumes a large number of chemical reagents is the major disadvantage, so it is not widely used in industry (Wu et al. 2009). Xu et al., reported the separating of gold and silver from scraped PCBs. This leaching process was affected by time, temperature and concentration of thiourea (Xu et al., 2011). Thio urea leaching has more advantage compared to cyanide leaching because of the leaching rate, and its efficiency are higher (86% Au and 71% Ag) (Udayakumar et al. 2022) This technique is an environmentally friendly technique. However, thiourea method is still in its infancy due to its high cost (Zhang et al. 2012). It is important to note that thiourea leaching is an environmentally friendly method. In addition to having fewer harmful effects, greater leaching rates, fewer interfering ions, and quicker kinetics, this method is widely accepted by scientists (Behnamfard et al., 2013; Jing-ying et al. 2012). PCBs contain a greater amount of copper, which accelerates the disintegration of thiourea.
Thiosulphate leaching
Thiosulfate solutions have been widely used in gold leaching research to replace conventional cyanide technology (Ha et al. 2014; Joda and Rashchi 2012). Generally, sodium thiosulfate and ammonium thiosulfate are used to leach gold.
In the presence of oxygen, a stable complex has been formed between gold and thiosulphate (Jiang et al. 1993). This method exhibits several advantages, including high selectivity, non-toxicity, and non-corrosive. A major disadvantage during extraction is the consumption of more reagents. Despite the potential environmental benefits, consumption makes it uneconomical for most thiosulfate systems and the flow chart of recovery of valuable metals has been noticed in Fig. 7 (Zhang et al. 2012).
The flow diagram of reclamation of valuable metals from waste PCBs, (Zhang et al. 2012)
The copper ions (Cu2+), ammonia (NH3) and thiosulfate are primarily employed to dissolve gold (Au). These catalysts are widely used to improve metal retrieval techniques (Akcil et al., 2010). Therefore, the ammonia concentration ratio has been altered as per the need of catalyst. The presence of thiosulfate in solution is a key factor in the successful reclamation of metals. Petter et al. also revealed the retrieval of Ag and Au metals (Petter et al. 2014). Despite the predictable benefits of thiosulfate leaching, there are severe problems related to the high use of thiosulfate and the low efficacy of the process. (Tanısalı et al. 2021). 95% of Au and 100% of Ag metals has been recovered by this technique (Udayakumar et al. 2022).
Chemical leaching (involving ligands)
This leaching process involves some of the chemicals directly as ligands, which allows the metals to be separated from the leachates.
EDTA leaching
The chelating agent of EDTA are highly stable which enhance the rate of extraction of metals. ( Vuyyuru et al. 2010; Chauhan et al. 2012).
However, higher stability is more resistant to the biodegradation process. Therefore, the presence of non-biodegardable chelating agent increases the stability of complex with metal ion residues. Chauhan et al., reported the theoretical outcomes of metal complex formation and chelators in the biodegradation process. The strong potential of extraction of metal and computational thermodynamics indicates the demonstration of experimental and theoretical work (Chauhan et al. 2012). More consumption of organic acid, inorganic acid and higher concentration of acid are the main disadvanges so it is not considered for the leaching process. Therefore, the successful leaching of copper (Cu) from waste PCBs has been achieved using an EDTA solution at 1000 °C (Jadhao et al. 2016). However, the EDTA and citric acid based organic reagents facilitate the extraction of copper but it was carried out in the presence of hydrogen peroxides. As a result of its minimum solubility, EDTA solutions have been shown to have a few disadvantages, including the ability to support additional high concentrations of metals in the solution (Torres et al., 2016).
DTPA leaching
The utilization of diethylenetriaminepentaacetic acid (DTPA) as an organic chelating agent for leaching selected metals such as nickel, copper, and zinc from scrap PCBs were examined. DTPA possesses very low solubility. Various parameters (pH, Temperature and Concentration) was involved for the optimization of experimental work. The pH has shown a significant role in the chelating reaction of DTPA. The DTPA is soluble in hot water and alkaline solution and it is insoluble in orgnic solvents like alcohol and ether. According to the obtained results, the DTPA ligand performed better in alkaline conditions compared to acidic and neutral conditions (Verma, et al., 2020). DTPA and nitrilotriacetic acid (NTA) are biodegradable ligands with the combination of chelating agents such as citrate, oxalate and the separation of elements like Cu, Pb, Cr, and Zn (Elliott and Shastri 1999). The DTPA possess tough binding tenedncey whereas, NTA possess strong sequestering tendencies. A small number of studies have shown promising outcomes about the leaching of Cu from PCBs utilizing the chelating agent of the EDTA method (Jadhao et al. 2016). Consequently, it is necessary to discover other harmless chelators for the extraction of metals using e-waste with minimal cross-contamination. Similarly, a small amount of material exists for the chelation removal of elements in e-waste. Furthermore, the performance of chelators in separation of elements from e-waste below oxidizing conditions has not yet been investigated. Against this background, in this study, PCBs on an outdated computer were utilizing DTPA as a safe ligand and optimization of the experimental process with parameters. Furthermore, the consequences of H2O2 as an oxidizing agent together with the chelating agent of DTPA in the selected chemical leaching process. Evaluation of metals from e-waste was also done.
NTA leaching
Nitrilotriacetic acid (NTA) can be utilized for removing metals, including Cr, Cu, and Zn. The use of this method is not common (Elliott and Shastri 1999). It is widely used to separate valuable metals from their ores, such as Fe, Zn, Pb, Cu, and Cd (Hong et al. 2000). The use of NTA has been suggested in studies concerning increased phytoextraction (Grčman et al. 2003). In sequence to increase the plant removal of elements from MSW compost, biodegradable complexing agents and NTA were used. Peatgrass has been harvested many times each year and NTA has been applied before each harvest in direction to maximize the efficacy of plant extraction (Zhao et al. 2016). Cobalt has been recovered from used LIBs (lithium-ion batteries) using NTA with adipic acid and ascorbic acid (Nayaka et al. 2019). The mixture of NTA and choline chloride-urea deep eutectic solvent (ChCl urea DES) were used for leaching of zinc from zinc oxide by simple leaching technique.
A chemical leaching process has been used to remediate soil contaminated with heavy metal ions (Wang et al., 2016). The NTA chelating agent was used to estimate the leaching mechanism of cadmium (Cd) from polluted soils (Xie et al. 2021).
Oxalate leaching
In general, oxalic acid is an organic acid that dissolves readily in hot water and also serves as a metal precipitant.
It is possible to precipitate cobalt oxalate from cobalt ions in solution. Oxalate leaching can be used effectively to remove cobalt and lithium from a solution. The oxalate residual can be reprocessed and recycled in the next leaching process, and the effluent can be easily treated with the help of sulphuric acid, hydrochloric acid, and nitric acid in leaching process. In both cases, the metals have been reprocessed and separated into oxalates. So the developed method was eco-friendly and oxalates were used as a leaching and precipitating agent with LiCoO2. Hence, the tendency of LiCoO2 increases by increasing the concentration of oxalates even, a small changes of temperature and time. Whereas, the insignificant effect has been determined by the addition of H2O2. Therefore, more efficiency has been achieved under optimum conditions; 98% of LiCoO2 (Sun & Qiu 2012).
Chemical leaching (involving acid treatment)
The following chemicals such as hydrochloric acid, sulphuric acid, aqua regia and sodium hyphochloride were used in the chemical leaching process.
Sulphuric acid leaching (H2SO4)
As an inexpensive reagent, sulfuric acid is commonly utilized at industrial levels for the recovery of metals from minerals and is very effective in dissolving copper in the presence of hydrogen peroxide H2O2. 95% of copper metal was recovred by this process (Udayakumar et al. 2022). In view of this, several journalists have considered this system to be one of the most appropriate chemical leaching systems for dissolving copper from scrap PCBs (Yang et al. 2011). The effect of concentration of H2SO4 has been observed in the leaching of manganese. The obtained results has showed an increasing concentration of H2SO4 from 0.5 to 2 mol/L respectively. In addition, manganese recovery was improved from 28.8 to 37.3% and iron recovery was improved from 6.1 to 22.5%. On the other hand, manganese recovery reduced moderately when the acid concentration exceeded 2 mol/L.
Using anodic dissolution in acidic media with appropriate additives, a new method of recycling mechanical rank cemented carbide materials has been developed. The present innovation involved the use of H2SO4 with (NH4)2SO4 as the electrolyte in order to dissolve the scrap waste material effectively. The process involves placing scrap metal into vibrating titanium baskets that contain perforations and extracting the product of tungstic acid (Katiyar et al., 2020). The prepared spinel structure of CO3O4 is more stable and it is very difficult dissolve even by strong acid. The persistence of this study is to examine how precious metals can be recovered from LIB through a combined leaching process using sulfuric acid and L-ascorbic acid in a synergistic manner (Chen et al. 2022).
Hydrochloric acid leaching (HCl)
For dissolving metal ions from spent cathode materials, HCl is generally used (Joo et al., 2016). Rather than HCl and H2SO4, nitric acid from TV-WPCB waste was found to be most effective for leaching metals (Dhawan et al., 2009). The HCl leaching kinetics of inactivated waste and activated waste fluorophores were systematically studied with a focus on Tb leaching (corresponding to about 79% of the total REE value in the waste fluorophores). The purpose has been used to improve the large-scale application of formerly suggested recovery methods and give the maintainable use of REE resources from phosphor waste (Quanyin et al. 2017). By combining HCl acid leaching and photoreduction, selective metals like Y and Eu can be recovered from RETPP waste. Wu et al. 2019 obtained a Y-Eu mixed solution by selectively leaching the RETPP waste with HCl + H2O2. Metallurgical research began with the easy extraction of copper (Cu) and iron (Fe) from different ores and further sources utilizing HCl. By combining HCl and HNO3, a simple recovery of gold (99.2%) was also demonstrated. The leaching method involves large groups of ores and concentrated solutions. The recovery of metal has been improved by the additional leaching process (Jergensen, 1999). A number of studies have also been conducted using acids such as H2SO4, HCl, and HNO3 to analyze minerals for metal recovery (Madenog 2005). Hydrometallurgical processes commonly use leaching acids such as HNO3, HCl, and H2SO4 which received greater efficiency by control procedures. The leaching of metals from PCB flakes was achieved via the use of five different acids, namely HNO3, H2SO4, HCl, citric acid (C6H8O7), and acetic acid (C2H4O2). HCl is an effective leaching agent due to the short time required for the metal recovery process. Using a large piece of PCB for the recovery of metals makes it easier to recycle the rest of the board (Jadhav and Hocheng 2015).
Aqua regia leaching
The Aqua regia leaching process has been utilized to ample leach metals from PCB waste materials (Jadhav and Hocheng 2015). This process was used to recover 97% Au and 98% Ag(Udayakumar et al. 2022). Park and Frey reported the aqua regia leaching process of magnetic, non-magnetic, conductive and non-conductive materials (Park and Frey, 2009). These leaching process produced a certain amount of materials by chemical attack of aqua regia and HCl. Birloaga et al., also noticed the pre-roasting of materials for 1 h at 600 °C in a muffle furnace (Humboldt, H-30204F) and the collected product was grinded in ball milling machine (Birloaga et al. 2013). Akcil et al. also reported the usage of thiosulfate of cyanide, thiocyanate, thiourea and aqua regia solutions for the leacing of precious metals. The gold can be recovered by the development of an eco-friendly processes (Akcil et al. 2015).
Currently, waste PCBs contain a high concentration of precious metals (Au, Ag, Pt, Pd, etc.). These metals are mainly extracted using rude and harsh methods, such as open acid cleaning (by dissolving the PCB directly in cyanide or aqua regia). An unregulated recycling process has resulted in severe environmental pollution as well as health hazards throughout the world (Wang and Xu 2015). There has been selective adsorption of trivalent gold (Au3+) in HCl in the PPF resin. The PPF resin can recover 100% of gold from leachate of aqua regia in HNO3 acidic system with acid concentration in S/L ratio of 2 (Fan et al. 2014). The process is labor-intensive and often involves the use of toxic or hazardous chemicals. In this investigation, DMF-CuCl2-CaCl2 was used and the (DMF: Dimethylformamide) system was called the "Mild Aqua" system. It is possible to use this reaction method cyclically without requiring any pretreatment or concentration steps (Wang et al. 2021). Similarly, Lin et al. (2010) reported high rate of dissolution for liquefied noble metals. The prospective medium of selective or moderately selective leaching of noticeable gold from PCBs has been done in the analysis. Additionally, the dissolution of iron and copper is being examined (Elomaa et al. 2017).
Sodium hypochlorite leaching
The WPCB was used to recover gold by sodium hypochlorite and hydrochloric acid leaching process. This investigation involved four steps for treating WPCB. (1) Elimination of copper by sulfuric acid leaching to (2) Leaching of gold by acidic thiourea leaching (85.75%) (3) Precipitating gold from step (2) utilizing sodium borohydride. (4) Leach residual gold (100%) by leaching with sodium hypochlorite and hydrochloric acid. This work has addressed the leaching of palladium in a NaClO-HCl-H2O2 leaching system. A three-stage leaching arrangement was also discussed (Benhamfurd et al., 2013), with two sulfate oxidation and one thiourea acid leaching stage. A pressure-leaching process using sodium hypochlorite was also used by Martinez-Ballesteros et al. to recover Ag, Au, and Pt from PCBs (Martinez-Ballesteros et al. 2021).
Hydrometallurgical leaching
A more significant parameter is the retrieval of precious metals like silver (Ag), lead (Pb), gold (Au), copper (Cu), and tin (Sn) from spent chemical solutions. The consumption of etching waste from each metal is also most important for the manufacturing process of different recovery processes of metals. This hydrometallurgical leaching process depends upon the metals separated from the electronic waste.
FeCl3 leaching
Ferric chloride (FeCl3) solutions have been widely utilized as a leaching and etching agent in hydrometallurgy. Iron(III) ions have been formed in complexes with chloride ions like FeCl3 and FeCl4. The development of these complexes can be exploited by recovering the FeCl3 solution from a disbursed FeCl3 solution using solvent extraction. Under high ionic strength conditions, this analysis examines the solvent extraction equilibrium of FeCl3 and TPB. (Zou et al. 1998).
CuCl2 leaching
Using a mixture of CuCl2 and HCl, 92% of Sn leaching was examined (Somasundaram et al. 2014). A study conducted on waste materials resulted in the leaching of copper and extra metals such as Ag, Pd, Fe, Ni, and Au. The waste PCBs in the HCl-CuCl2-NaCl medium were also examined (Yazici and Deveci 2015). Copper elements have been recycled from PCBs waste. A leaching agent was an ammonia-ammonium salt consisting of Cu(I) and Cu(II)-ammonia complex ions. Through the three processes of leaching, refining, and electrolysis, copper ions are produced in good quantities. Likewise, copper was oxidized by Cu(II) to a Cu(I) complex by leaching an basic ammonium salt solution from scrap circuit panels. Cu(II) complex ammonium can be used to improve the leaching rate (Xu & Liu, 2016). The etching of copper by CuCl2 was examined. The impact of the concentration of etchant, temperature of additives, and etching on the etching rate of copper was also investigated. Furthermore, this plays a significant role in recycling, while keeping the environment in mind. The process of regeneration of CuCl2 is also specified (Cakir 2006). Waste PCBs are an important by-product for the computational field of the WEEE disposal process, including a large amount of valuable, expensive, and dangerous metals. There have been serious environmental problems caused by CuCl2 released from etching waste in recent years. Due to the large amount of copper-bearing wastewater generated by the electronics industry, wastewater emissions are increasing dramatically. Copper discharge is extremely toxic and can cause cancer and genetic disorders in humans. (Gupta and Ali 2000).
HCl leaching
The complete dissolution of Sn, Ni, Cu, and Zn has been attained in a mixture of HCl with both H2O2 and NaClO systems, while little Ag leaching was also detected at lower than 10%. The H2O2-HCl leaching process has also been used for the removal of Au from waste PCB.
A process for recycling copper wastes using copper chloride solutions (HCl, NaCl, and CuCl2) was described by Yu et al. CuCl2 complexes were formed by oxidizing the associated copper to Cu+, Cu2+, and Cl−. Copper was then recycled by electrodeposition techniques (Yu et al. 1999). A solvent extraction method has been used to separate Pt and Pd from artificial chloride solutions. Pt and Pd ammonium salts influence the extraction behavior of amines at high concentrations of HCl (Nguyen et al. 2015). To obtain metallic copper, caustic hydrochloric acid solutions have been electrochemically treated. In order to prevent the release of chlorine into the workplace atmosphere, a preliminary solution has been proposed to eliminate chloride ions (Makovskaya et al. 2020).
Organic solvents leaching
IC compositions can contain up to 15% epoxy coatings. Since epoxy coatings are flammable organic coatings, they can be easily removed with organic acids (Senophiyah-Mary et al. 2018). The separation of waste PCBs utilizing organic solvents has grown very popular in many industries and it is an eco-friendly and effective technology. Conversely, they are relatively used more temperature at the time of separation method. Furthermore, there has been an increase in solvent consumption, an acceleration in solvent decomposition, and the emission of possible smoke. It has been claimed that physico-mechanical treatment offers a promising recycling rate that generates no dust. Economic analyses of leaching technology have shown that it could offer tremendous economic benefits (Sakunda 2013). Through ultrasonic waves and vibrations, low-temperature dissolution of organic solvents has significantly reduced toxic gas emissions and energy consumption. After entering the sandwich structure of the material, the organic solvent expands, cracks, and finally separates the glass fiber and metal foil layers (Gulgul et al., 2017). An organic solvent such as dimethylacetamide (DMA) was utilized as an excellent solvent because it has a high boiling point, comparatively more viscosity, and greater thermal stability through the ability of active temperature relating to other solvents viz., N-methyl-2-pyrrolidone (NMP), dimethylformamide (DMF) and dimethyl sulfoxide (DMSO). The stability is greater for DMSO at high temperatures and strangely stable in neutral and basic media, but decomposition is promoted in acidic media. The DMSO solvent can be recycled several times without the loss of its properties. The regeneration of DMSO organic solvent for dissolution has been indicated in Fig. 8 (Zhu et al. 2013).
A flowchart for dissolution of epoxy resin and regeneration of DMSO, (Zhu et al. 2013)
Comparison of different leaching process
Different leaching processes utilized for the retrieval of precious elements from WPCBs have been discussed in this section. This is claimed that economic, environmental, and technological affordability are the most important variables in determining the best procedure for extracting metal from WPCBs. The comparison of different valuable metals from WPCBs showed in Table 1. All the variables are depends upon the specific experimental conditions. Hence, the upcoming research technology and necessity of environmental needs to reduce the environmental impacts such as toxicity and safety. The old process will decline in the area of recycling and recovery process due to updating e-waste laws. In general, the presence of metal composition was more complex and difficult, high selectivity of leaching technique. Besides, the reagent with more recyclability can significantly decrease the costs of reagent expended, which makes the leaching process is simple and efficient.
Future perspective
As a result of current leaching methods, important metals cannot be separated from e-waste without affecting natural resources, in a simple and pollution-free manner. In future research, more attention should be paid to techniques that reduce costs, use less energy, and are simple and pollution-free. Combining other processes with the extraction of precious metals may provide society with a more efficient and useful method. The recycling of new strategic techniques is an important method. In recent years, promising results have been observed in reclamation of precious elements from waste PCBs. However, so far, no attempts have been made to analyze the recycling and recovery of precious metals using β-Cyclodextrin-Hyaluronic acid-MWCNTs hydrogel adsorbent. The fundamental ideas were involved in the elimination of toxic metal ion processes from industrial waste water. Complex metallurgy with multiple important resources may benefit from this process. In order to retieval metals from spent discarded water, a chemical leaching process will be used, which has some drawbacks associated with heavy or hazardous metal ions in the water. There will be a number of impacts on the environment and on humans as a result of that. Recycled polymeric materials can be used to prepare polymeric hydrogel adsorbents that are more effective against waste water treatment. Through this kind of activity or work, metals, and polymeric materials adsorbent could be recycled and recovered more efficiently. The goal is to reduce pollution, such as zero waste in the industry, and benefit society and the environs. The proposed schematic mechanism for the removal of precious metals using polymeric adsorbent materials has been reflected in Fig. 9.
Conclusion
This mini-review illustrates the recycling of spent PCBs through hydrometallurgical chemical leaching methods. The chemical leaching process has shown more advantages in terms of being simple, convenient, low-cost, and eco-friendly. Furthermore, it is an efficient way to recover metals and polymers from waste PCBs. In comparison to other leaching techniques, the chemical leaching technique is more advantageous. The maximum recovery of metals as cyanide (90% Au, Ag, Pd & Pt), thiourea (86% Au & 71% Ag), thiosulphate (95% Au & 100%nAg), Halide (97.5% Au), sulphuric acid (95% Cu) and aqua regia (97% Au & 98% Ag). A variety of scientific studies have demonstrated that different chemical leaching techniques can be adapted to different leaching conditions and utilize to increase the leaching efficacy and recovery of metals. However, there is still a lot of focus on improving chemical leaching, as well as additional research need not only for industrial applications and also recovery of elements from the waste treated water using adsorbent hydrogel. In addition, e-waste typically contains many complex substances that can adversely affect different ecosystems and natural resources such as water, soil, and food interfere with metals. Sorting and collecting various types of e-waste is an essential initial step in the pretreatment of waste PCBs. Dismantling and crushing is other significant process for eliminating toxic constituents and enhancing the efficacy of chemical leaching process. It is necessary to build a new type of excellent polymeric adsorbent hydrogel out of recycled materials. It is important to test the addition of different types of adsorbents to the chemical leaching process and to increase the retrieval of valuable metals.
Data Availability
Data available with the paper: Data availability is not applicable to this review article as no new data were created or analysed in this study.
Abbreviations
- DMA:
-
Dimethylacetamide
- DMF:
-
Dimethylformamide
- DMF:
-
Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- DTPA:
-
Diethylenetriaminepentaacetic acid
- ECS:
-
Eddy Current Separator
- E-waste:
-
Electronic Waste
- H2SO4 :
-
Sulphuric acid
- HCl:
-
Hydrochloric acid
- HCN:
-
Hydrogen cyanide
- HNO3 :
-
Nitric acid
- NMP:
-
N-methyl-2-pyrrolidone
- NTA:
-
Nitrilotriacetic acid
- PCBs:
-
Printed Circuit Board
- PM:
-
Precious metal
- PNDEs:
-
Polybrominated diphenyl ethers
- PPM:
-
Parts per million
- REE:
-
Rare earth elements
- SCF:
-
Supercritical fluid extraction
- WEEE:
-
Electrical and electronic equipment
References
Abubakar A, Zangina AS, Maigari AI, Badamasi MM, Ishak MY, Abdullahi AS, Haruna JA (2022) Pollution of heavy metal threat posed by e-waste burning and its assessment of human health risk. Environ Sci Pollut Res 29(40):61065–61079
Akcil A (2010) A new global approach of cyanide management: international cyanide management code for the manufacture, transport, and use of cyanide in the production of gold. Miner Process Extr Metall Rev 31(3):135–149
Akcil A, Erust C, Gahan CS, Ozgun M, Sahin M, Tuncuk A (2015) Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants–a review. Waste Manage 45:258–271
Anand B, Jha A, Kumar MK, Sahu R (2013) Recycling of precious metal gold from waste electrical and electronic equipments (WEEE): a review. In: Proceedings of the XIII international seminar on mineral processing technology, 916–923). MPT
Asghari I, Mousavi SM, Amiri F, Tavassoli S (2013) Bioleaching of spent refinery catalysts: a review. J Ind Eng Chem 19(4):1069–1081
Awasthi AK, Hasan M, Mishra YK, Pandey AK, Tiwary BN, Kuhad RC, Gupta VK, Thakur VK (2019) Environmentally sound system for E-waste: biotechnological perspectives. Curr Res Biotechnol 1:58–64
Azevedo ÁBAD, Kieckbusch TG, Tashima AK, Mohamed RS, Mazzafera P, Melo SABVD (2008) Supercritical CO2 recovery of caffeine from green coffee oil: new experimental solubility data and modeling. Quim Nova 31:1319–1323
Baldé CP, Forti V, Gray V, Kuehr R, Stegmann P (2017) The global e-waste monitor 2017: quantities, flows and resources. United Nations University, International Telecommunication Union, and International Solid Waste Association
Becci A, Amato A, Fonti V, Karaj D, Beolchini F (2020) An innovative biotechnology for metal recovery from printed circuit boards. Resour Conserv Recycl 153:104549
Behnamfard A, Salarirad MM, Veglio F (2013) Process development for recovery of copper and precious metals from waste printed circuit boards with emphasize on palladium and gold leaching and precipitation. Waste management 33(11):2354-2363
Birloaga I, De Michelis I, Ferella F, Buzatu M, Vegliò F (2013) Study on the influence of various factors in the hydrometallurgical processing of waste printed circuit boards for copper and gold recovery. Waste Manage 33(4):935–941
Birloaga I, Vegliò F (2018) Hydrometallurgical processing of waste printed circuit boards. In: Waste electrical and electronic equipment recycling (pp. 95–113). Woodhead Publishing
Bosecker K (1997) Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev 20(3–4):591–604
Bougnoux P, Hajjaji N, Maheo K, Couet C, Chevalier S (2010) Fatty acids and breast cancer: sensitization to treatments and prevention of metastatic re-growth. Prog Lipid Res 49(1):76–86
Cakir O (2006) Copper etching with cupric chloride and regeneration of waste etchant. J Mater Process Technol 175(1–3):63–68
Castro FD, Bassin JP (2022) Electronic waste: environmental risks and opportunities. In: Hazardous waste management. Elsevier pp. 421–458
Chauhan G, Pant KK, Nigam KD (2012) Extraction of nickel from spent catalyst using biodegradable chelating agent EDDS. Ind Eng Chem Res 51(31):10354–10363
Chen J, Meng T, Leng E, Jiaqiang E (2022) Review on metal dissolution characteristics and harmful metals recovery from electronic wastes by supercritical water. J Hazard Mater 424:127693
Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: A review. J Hazard Mater 158(2-3):228-256
D’Adamo I, Ferella F, Gastaldi M, Maggiore F, Rosa P, Terzi S (2019) Towards sustainable recycling processes: Wasted printed circuit boards as a source of economic opportunities. Resour Conserv Recycl 149:455–467
Das N (2010) Recovery of precious metals through biosorption—a review. Hydrometallurgy 103(1–4):180–189
Elliott HA, Shastri NL (1999) Extractive decontamination of metal-polluted soils using oxalate. Water Air Soil Pollut 110:335–346
Elomaa H, Seisko S, Junnila T, Sirviö T, Wilson BP, Aromaa J, Lundström M (2017) The effect of the redox potential of aqua regia and temperature on the Au, Cu, and Fe dissolution from WPCBs. Recycling 2(3):14
Fan R, Xie F, Guan X, Zhang Q, Luo Z (2014) Selective adsorption and recovery of Au (III) from three kinds of acidic systems by persimmon residual based bio-sorbent: A method for gold recycling from e-wastes. Biores Technol 163:167–171
Faramarzi MA, Mogharabi-Manzari M, Brandl H (2020) Bioleaching of metals from wastes and low-grade sources by HCN-forming microorganisms. Hydrometallurgy 191:105228
Fayaz SM, Abdoli MA, Baghdadi M, Karbasi A (2021) Ag removal from e-waste using supercritical fluid: improving efficiency and selectivity. Int J Environ Stud 78(3):459–473
Ficeriová J, Baláž P, Gock E (2011) Leaching of gold, silver and accompanying metals from circuit boards (PCBs) waste. Acta Montanistica Slovaca 16(2):128
Ghosh B, Ghosh MK, Parhi P, Mukherjee PS, Mishra BK (2015) Waste printed circuit boards recycling: an extensive assessment of current status. J Clean Prod 94:5–19
Grčman H, Vodnik D, Velikonja-Bolta Š, Leštan D (2003) Ethylenediaminedissuccinate as a new chelate for environmentally safe enhanced lead phytoextraction. J Environ Qual 32(2):500–506
Gu W, Bai J, Dong B, Zhuang X, Zhao J, Zhang C, Wang J, Shih K (2017) Catalytic effect of graphene in bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 171:172–178
Gupta VK, Ali I (2000) Utilisation of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep Purif Technol 18(2):131–140
Gurgul A, Szczepaniak W, Zabłocka-Malicka M (2017) Incineration, pyrolysis and gasification of electronic waste. In E3S Web of conferences 22:00060, EDP Sciences
Gurung M, Adhikari BB, Kawakita H, Ohto K, Inoue K, Alam S (2013) Recovery of gold and silver from spent mobile phones by means of acidothiourea leaching followed by adsorption using biosorbent prepared from persimmon tannin. Hydrometallurgy 133:84–93
Ha VH, Lee JC, Jeong J, Hai HT, Jha MK (2010) Thiosulfate leaching of gold from waste mobile phones. J Hazard Mater 178(1–3):1115–1119
Ha VH, Lee JC, Huynh TH, Jeong J, Pandey BD (2014) Optimizing the thiosulfate leaching of gold from printed circuit boards of discarded mobile phone. Hydrometallurgy 149:118–126
Han J, Duan C, Lu Q, Jiang H, Fan X, Wen P, Ju Y (2019) Improvement of the crushing effect of waste printed circuit boards by co-heating swelling with organic solvent. J Clean Prod 214:70–78
Han J, Bai X, Yang Q, Wang B, Ma W, Li Y, Zhao Y (2023) Recovery and enrichment of platinum group metals from spent automotive catalysts by pyrometallurgy: a review. Rare Metal Technology 2023:61–72
Hong KJ, Tokunaga S, Kajiuchi T (2000) Extraction of heavy metals from MSW incinerator fly ashes by chelating agents. J Hazard Mater 75(1):57–73
Iannicelli-Zubiani EM, Giani MI, Recanati F, Dotelli G, Puricelli S, Cristiani C (2017) Environmental impacts of a hydrometallurgical process for electronic waste treatment: a life cycle assessment case study. J Clean Prod 140:1204–1216
Isıldar A, Rene ER, van Hullebusch ED, Lens PN (2018) Electronic waste as a secondary source of critical metals: management and recovery technologies. Resour Conserv Recycl 135:296–312
Islam A, Ahmed T, Awual MR, Rahman A, Sultana M, Abd Aziz A, Monir MU, Teo SH, Hasan M (2020) Advances in sustainable approaches to recover metals from e-waste-a review. J Clean Prod 244:118815
Jadhao P, Chauhan G, Pant KK, Nigam KDP (2016) Greener approach for the extraction of copper metal from electronic waste. Waste Manage 57:102–112
Jadhav U, Hocheng H (2015) Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci Rep 5(1):1–10
Joo SH, ju Shin D, Oh C, Wang JP, Senanayake G, Shin SM (2016) Selective extraction and separation of nickel from cobalt, manganese and lithium in pre-treated leach liquors of ternary cathode material of spent lithiumion batteries using synergism caused by Versatic 10 acid and LIX 84-I. Hydrometallurgy, 159:65-74.
Javed C, Singh J (2024) Process intensification for sustainable extraction of metals from e-waste: challenges and opportunities. Environ Sci Pollut Res 31(7):9886–9919
Jergensen GV (1999) Copper leaching, solvent extraction, and electrowinning technology. SME. https://doi.org/10.1016/j.mineng.2005.05.005
Jiang T, Xu S, Chen J (1993) Electrochemistry of gold leaching with thiosulfate. II. Cathodic behaviour and leaching mechanism of gold. J Central-South Inst Min Metal(china) (people’s Republic of China) 24(2):174–180
Jing-ying L, Xiu-Li X, Wen-quan L (2012) Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manage 32(6):1209–1212
Joda NN, Rashchi F (2012) Recovery of ultra fine grained silver and copper from PC board scraps. Sep Purif Technol 92:36–42
Kamberović Ž, Romhanji E, Filipović M, Korać M (2009) The recycling of high magnesium aluminum alloys estimation of the most reliable procedure. Metalurgija 15(3):189-200
Kamberović Ž, Korać M, Ranitović M (2011) Hydrometallurgical process for extraction of metals from electronic waste, part II: Development of the processes for the recovery of copper from printed circuit boards (PCB). Metalurgija 17(3):139–149
Katiyar PK, Randhawa NS (2020) A comprehensive review on recycling methods for cemented tungsten carbide scraps highlighting the electrochemical techniques. Int J Refract Hard Met 90:105251
Kaya M (2016a) Recovery of metals from electronic waste by physical and chemical recycling processes. Int J Chem Mole Eng 10(2):259–270
Kaya F (2016b) Recovery of metals and nonmetals from electronic waste by physical and chemical recycling processes. Waste Manage 57:64–90
Kaya M (2020) Waste printed circuit board (WPCB) recycling: conventional and emerging technology approach. Encycl Renew Sustain Mater 2020(4):677–694
Kellner D (2009) Recycling and recovery. In: Hester RE, Harrison RM (eds) Electronic waste management, design, analysis and application. RSC Publishing, Cambridge, pp 91–110.
Korf N, Løvik AN, Figi R, Schreiner C, Kuntz C, Mählitz PM, Rösslein M, Wäger P, Rotter VS (2019) Multi-element chemical analysis of printed circuit boards–challenges and pitfalls. Waste Manage 92:124–136
Kumar A, Saini HS, Kumar S (2018) Bioleaching of gold and silver from waste printed circuit boards by Pseudomonas balearica SAE1 isolated from an e-waste recycling facility. Curr Microbiol 75:194–201
Lacoste-Bouchet P, Deschênes G, Ghali E (1998) Thiourea leaching of a copper-gold ore using statistical design. Hydrometallurgy 47(2–3):189–203
Li K, Xu Z (2019) A review of current progress of supercritical fluid technologies for e-waste treatment. J Clean Prod 227:794–809
Li J, Wen J, Guo Y, An N, Liang C, Ge Z (2020a) Bioleaching of gold from waste printed circuit boards by alkali-tolerant Pseudomonas fluorescens. Hydrometallurgy 194:105260
Li Y, Lin M, Ni Z, Yuan Z, Liu W, Ruan J, Tang Y, Qiu R (2020b) Ecological influences of the migration of micro resin particles from crushed waste printed circuit boards on the dumping soil. J Hazard Mater 386:121020
Li A, Oraby E, Eksteen J (2021) Cyanide consumption minimisation and concomitant toxic effluent minimisation during precious metals extraction from waste printed circuit boards. Waste Manage 125:87–97
Li W, Sun J, Ma DF, Liu XL, Li S, Bei JY, Chen T (2024) Dioxin control in the co-processing of waste printed circuit board and copper concentrate with an ausmelt furnace. Aerosol Air Quality Res 24(1):230126
Lin W, Zhang RW, Jang SS, Wong CP, Hong JI (2010) “Organic aqua regia”—powerful liquids for dissolving noble metals. Angew Chem Int Ed 49(43):7929–7932
Liu R, Li J, Ge Z (2016) Review on Chromobacterium violaceum for gold bioleaching from e-waste. Procedia Environ Sci 31:947–953
Liu K, Huang S, Jin Y, Ma L, Wang WX, Lam JCH (2022) A green slurry electrolysis to recover valuable metals from waste printed circuit board (WPCB) in recyclable pH-neutral ethylene glycol. J Hazard Mater 433:128702
Macaskie LE, Creamer NJ, Essa AMM, Brown NL (2007) A new approach for the recovery of precious metals from solution and from leachates derived from electronic scrap. Biotechnol Bioeng 96(4):631–639
Makovskaya OY, Shevchuk AP, Anikin YV (2020) Perspective method for regeneration of spent solutions from printed circuit boards etching. In: Materials science forum, Trans Tech Publications Ltd.
Manikkampatti Palanisamy M, Myneni VR, Gudeta B, Komarabathina S (2022) Toxic metal recovery from waste printed circuit boards: a review of advanced approaches for sustainable treatment methodology. Adv Mater Sci Eng 2022:1-9
Martinez-Ballesteros G, Valenzuela-García JL, Gómez-Alvarez A, Encinas-Romero MA, Mejía-Zamudio FA, Rosas-Durazo ADJ, Valenzuela-Frisby R (2021) Recovery of ag, au, and pt from printed circuit boards by pressure leaching. Recycling 6(4):67
Moses LB, Petersen FW (2000) Flotation as a separation technique in the coal gold agglomeration process. Miner Eng 13(3):255–264
Murthy DSR, Prasad PM (1996) Leaching of gold and silver from Miller Process dross through non-cyanide leachants. Hydrometallurgy 42(1):27–33
Nayaka GP, Zhang Y, Dong P, Wang D, Zhou Z, Duan J, Li X, Lin Y, Meng Q, Pai KV, Santhosh G (2019) An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J Environ Chem Eng 7(1):102854
Nguyen TH, Sonu CH, Lee MS (2015) aration of platinum (IV) and palladium (II) from concentrated hydrochloric acid solutions by mixtures of amines with neutral extractants. J Ind Eng Chem 32:238–245
Oguchi M, Murakami S, Sakanakura H, Kida A, Kameya T (2011) A preliminary categorization of end-of-life electrical and electronic equipment as secondary metal resources. Waste Manage 31(9–10):2150–2160
Ogunniyi IO, Vermaak MKG, Groot DR (2009) Chemical composition and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manage 29(7):2140–2146
Pant D, Joshi D, Upreti MK, Kotnala RK (2012) Chemical and biological extraction of metals present in E waste: a hybrid technology. Waste Manage 32(5):979–990
Petter PMH, Veit HM, Bernardes AM (2014) Evaluation of gold and silver leaching from printed circuit board of cellphones. Waste Manage 34(2):475–482
Qi Y, Yi X, Zhang Y, Meng F, Shu J, Xiu F, Sun Z, Sun S, Chen M (2019) Effect of ionic liquid [MIm] HSO 4 on WPCB metal-enriched scraps refined by slurry electrolysis. Environ Sci Pollut Res 26:33260–33268
Qin B, Lin M, Yao Z, Zhu J, Ruan J, Tang Y, Qiu R (2020) A novel approach of accurately rationing adsorbent for capturing pollutants via chemistry calculation: rationing the mass of CaCO3 to capture Br-containing substances in the pyrolysis of nonmetallic particles of waste printed circuit boards. J Hazard Mater 393:122410
Qiu R, Lin M, Ruan J, Fu Y, Hu J, Deng M, Tang Y, Qiu R (2020) Recovering full metallic resources from waste printed circuit boards: arefined review. J Clean Prod 244:118690
Quanyin TAN, Chao DENG, Jinhui LI (2017) Effects of mechanical activation on the kinetics of terbium leaching from waste phosphors using hydrochloric acid. J Rare Earths 35(4):398–405
Safarzadeh MS, Bafghi MS, Moradkhani D, Ilkhchi MO (2007) A review on hydrometallurgical extraction and recovery of cadmium from various resources. Miner Eng 20(3):211–220
Sakunda P (2013) Strategy of e-waste management. Handbook of solid waste management, 1523–1557
Senophiyah-Mary J, Loganath R, Meenambal T (2018) A novel method for the removal of epoxy coating from waste printed circuit board. Waste Manage Res 36(7):645–652
Sethurajan M, van Hullebusch ED, Fontana D, Akcil A, Deveci H, Batinic B, Leal JP, Gasche TA, Kucuker MA, Kuchta K, Neto IFF, Soares HMVM, Chmielarz A (2019) Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes-a review. Crit Rev Environ Sci Technol 49(3):212–275
Shen S, Guishen L, Pan T, He J, Guo Z (2011) Selective adsorption of Pt ions from chloride solutions obtained by leaching chlorinated spent automotive catalysts on ion exchange resin Diaion WA21J. J Colloid Interface Sci 364(2):482–489
Sikander A, Kelly S, Kuchta K, Sievers A, Willner T, Hursthouse AS (2022) Chemical and microbial leaching of valuable metals from PCBs and tantalum capacitors of spent mobile phones. Int J Environ Res Public Health 19(16):10006
Somasundaram M, Saravanathamizhan R, Basha CA, Nandakumar V, Begum SN, Kannadasan T (2014) Recovery of copper from scrap printed circuit board: modelling and optimization using response surface methodology. Powder Technol 266:1–6
Song Q, Xia Q, Yuan X, Xu Z (2023) Multi-metal electrochemical response mechanism for direct copper recovery from waste printed circuit boards via sulfate-and chloride-system electrolysis. Resour Conserv Recycl 190:106804
Sun L, Qiu K (2012) Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manage 32(8):1575–1582
Tanısalı E, Özer M, Burat F (2021) Precious metals recovery from waste printed circuit boards by gravity separation and leaching. Miner Process Extr Metall Rev 42(1):24–37
Torres R, Lapidus GT (2016) Copper leaching from electronic waste for the improvement of gold recycling. Waste Manage 57:131–139
Trivedi A, Hait S (2020) Bioleaching of selected metals from e-waste using pure and mixed cultures of Aspergillus species. Meas, Anal Remediat Environ Pollut, 271–280
Tuncuk A, Stazi V, Akcil A, Yazici EY, Deveci H (2012) Aqueous metal recovery techniques from e-scrap: hydrometallurgy in recycling. Miner Eng 25(1):28–37
Udayakumar S, Abd Razak MIB, Ismail S (2022) Recovering valuable metals from Waste Printed Circuit Boards (WPCB): a short review. Mater Today: Proc 66:3062–3070
Vats MC, Singh SK (2015) Assessment of gold and silver in assorted mobile phone printed circuit boards (PCBs). Waste Manage 45:280–288
Verma A, Trivedi A, Hait S (2020) Extraction of selected metals from high-grade waste printed circuit board using diethylene triamine penta-acetic acid. Urban Mining and Sustainable Waste Management, 49–57
Vuyyuru KR, Pant KK, Krishnan VV, Nigam KD (2010) Recovery of nickel from spent industrial catalysts using chelating agents. Ind Eng Chem Res 49(5):2014–2024
Wan J, Sun J, Zhao XL, Le AS, Ren PB, Zhan MX, Chen T (2023) Emission of brominated pollutants from waste printed circuit boards during thermal treatment: a review. Aerosol Air Qual Res 23(12):230135
Wang J, Xu Z (2015) Disposing and recycling waste printed circuit boards: disconnecting, resource recovery, and pollution control. Environ Sci Technol 49(2):721–733
Wang J, Zeng B, Lv J, Lu Y, Chen H (2020a) Environmentally friendly technology for separating gold from waste printed circuit boards: a combination of suspension electrolysis and a chlorination process. ACS Sustain Chem Eng 8(45):16952–16959
Wang Q, Zhang B, Yu S, Xiong J, Yao Z, Hu B, Yan J (2020b) Waste-printed circuit board recycling: focusing on preparing polymer composites and geopolymers. ACS Omega 5(29):17850–17856
Wang R, Zhang C, Zhao Y, Zhou Y, Ma E, Bai J, Wang J (2021) Recycling gold from printed circuit boards gold-plated layer of waste mobile phones in “mild aqua regia” system. J Clean Prod 278:123597
Won SW, Kotte P, Wei W, Lim A, Yun YS (2014) Biosorbents for recovery of precious metals. Biores Technol 160:203–212
Wu J, Qiu LJ, Chen L, Chen DH (2009) Gold and silver selectively leaching from printed circuit boards scrap with acid thiourea solution. Nonferrous Metals 61:90–93
Wu Y, Zhang Q, Zuo T (2019) Selective recovery of Y and Eu from rare-earth tricolored phosphorescent powders waste via a combined acid-leaching and photo-reduction process. J Clean Prod 226:858–865
Xia M, Bao P, Liu A, Wang M, Shen L, Yu R, Liu Y, Chen M, Li J, Wu X, Qiu G, Zeng W (2018) Bioleaching of low-grade waste printed circuit boards by mixed fungal culture and its community structure analysis. Resour, Conserv Recycl 136:267–275
Xie F, Cai T, Ma Y, Li H, Li C, Huang Z, Yuan G (2009) Recovery of Cu and Fe from printed circuit board waste sludge by ultrasound: evaluation of industrial application. J Clean Prod 17(16):1494–1498
Xie X, Luo J, Guan L, Zhong W, Jing C, Wang Y (2021) Cadmium isotope fractionation during leaching with nitrilotriacetic acid. Chem Geol 584:120523
Xiong J, Yu S, Wu D, Lü X, Tang J, Wu W, Yao Z (2020) Pyrolysis treatment of nonmetal fraction of waste printed circuit boards: focusing on the fate of bromine. Waste Manage Res 38(11):1251–1258
Xiu FR, Qi Y, Zhang FS (2015) Leaching of Au, Ag, and Pd from waste printed circuit boards of mobile phone by iodide lixiviant after supercritical water pre-treatment. Waste Manage 41:134–141
Xu XL, Li JY (2011) Experimental study of thiourea leaching gold and silver from waste circuit boards. J Qingdao Univ(Engineering & Technology Edition) 26(2):69–73
Xu Q, Chen DH, Chen L, Huang MH (2010) Gold leaching from waste printed circuit board by iodine process. Nonferrous Metals 62(3):88–90
Xu Y, Li J, Liu L (2016) Current status and future perspective of recycling copper by hydrometallurgy from waste printed circuit boards. Procedia Environ Sci 31:162–170
Yang X, Moats MS, Miller JD, Wang X, Shi X, Xu H (2011) Thiourea–thiocyanate leaching system for gold. Hydrometallurgy 106(1–2):58–63
Yao Z, Ling TC, Sarker PK, Su W, Liu J, Wu W, Tang J (2018) Recycling difficult-to-treat e-waste cathode-ray-tube glass as construction and building materials: a critical review. Renew Sustain Energy Rev 81:595–604
Yao Z, Xiong J, Yu S, Su W, Wu W, Tang J, Wu D (2020) Kinetic study on the slow pyrolysis of nonmetal fraction of waste printed circuit boards (NMF-WPCBs). Waste Manage Res 38(8):903–910
Yazici EY, Deveci HACI (2014) Ferric sulphate leaching of metals from waste printed circuit boards. Int J Miner Process 133:39–45
Yazici EY, Deveci HACI (2015) Cupric chloride leaching (HCl–CuCl2–NaCl) of metals from waste printed circuit boards (WPCBs). Int J Miner Process 134:89–96
Yu ZX, Zhou BN, Lu ZH (1999) Hydro-electro metallurgical process for recovering scrap from cuprous chloride solution. Shanghai Nonferrous Metals 2:24–28
Yu S, Su W, Wu D, Yao Z, Liu J, Tang J, Wu W (2019) Thermal treatment of flame retardant plastics: a case study on a waste TV plastic shell sample. Sci Total Environ 675:651–657
Zhang X, Chen L, Fang Z (2009) Review on gold leaching from PCB with non-cyanide leach reagents. Nonferrous Metals 61(1):72–72
Zhang Y, Liu S, Xie H, Zeng X, Li J (2012) Current status on leaching precious metals from waste printed circuit boards. Procedia Environ Sci 16:560–568
Zhang S, Li Y, Wang R, Xu Z, Wang B, Chen S, Chen M (2017) Superfine copper powders recycled from concentrated metal scraps of waste printed circuit boards by slurry electrolysis. J Clean Prod 152:1–6
Zhao S, Jia L, Duo L (2016) Combining nitrilotriacetic acid and permeable barriers for enhanced phytoextraction of heavy metals from municipal solid waste compost by lolium perenne and reduced metal leaching. J Environ Qual 45(3):933–939
Zhihui WANG, Shuqi YUAN, Jinghao LIU, Yufeng WU, Jiamei YU (2021) Research progress of hydrometallurgy technology for leaching precious metals in waste printed circuit board. Environ Chem 3:886–895
Zhong F, Li D, Wei J (2006) Experimental study on leaching gold in printed circuit boards scrap with thiourea. Non-Ferrous Metals Recycling and Utilization 6:25–27
Zhou W, Chen Y, Cheng H, Liu X, Tian Z, Zhang L, Zhou H (2020) A novel process for the biological detoxification of non-metal residue from waste copper clad laminate treatment: from lab to pilot scale. J Clean Prod 255:120116
Zhou QFZW, Zhu W (2003) Recovery of gold from waste computer and its accessories. China Resour Compr Util 7:31–35
Zhu P, Chen Y, Wang LY, Zhou M, Zhou J (2013) The separation of waste printed circuit board by dissolving bromine epoxy resin using organic solvent. Waste Manage 33(2):484–488
Zhu P, Tang J, Tao Q, Wang Y, Wang J, Li Z, Cao Z, Qian G, Theiss F, Frost RL (2019) The kinetics study of dissolving SnPb solder by hydrometallurgy. Environ Eng Sci 36(9):1236–1243
Zou L, Chen J, Pan X (1998) Solvent extraction of rhodium from aqueous solution of Rh (III)–Sn (II)–Cl− system by TBP. Hydrometallurgy 50(3):193–203
Acknowledgements
This work was supported by the Ministry of Science and Higher Education of the Russian Federation, South Ural State University, Chelyabinsk, Russian Federation.
Funding
No funding information.
Author information
Authors and Affiliations
Contributions
Shanmugavel Sudarsan: Conceptualization, Methodology, Writing- Original draft preparation, Investigation. Mariappan Anandkumar: Reviewing and Editing. E. A. Trofimov: Reviewing and Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sudarsan, S., Anandkumar, M. & Trofimov, E.A. Survey of diverse hydrometallurgy techniques for recovering and extracting valuable metals from PCB waste: an overview. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05755-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05755-w