Abstract
In the present study, detection of heavy metals was carried out in a textile industry soil by ICPOES method. Chromium was the most abundant heavy metal in the examined soil samples and its concentration (95–1180 ppm) exceed the EPA limit. Furthermore, six chromium-tolerant bacterial strains were isolated, most of which belong to Bacillus spp. The maximum tolerance limit of strains was 0.5 ppm for K2Cr2O7. Cr-tolerant strains in consortium were used for the inoculation in combination with biochar. The maximum increase in shoot and root length was (22–23.4%), and maximum increase in chlorophyll and SOD was (28–40%). In the similar way, the proline and sugar contents were improved up to 20.5% and 9.6%, respectively. Significant reduction in Cr uptake was recorded in dry biomass of wheat plants where Cr concentration was 0.28 ± 1.01 mg/kg as compared to control. Hence, according to the findings, PGPR and biochar are an important tool for amelioration of chromium and can be used as inoculums for better plant production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Wheat is considered as most important crop all over the world followed by coarse grains and rice. For last 8000 years, wheat is the basic staple food and one of the first domesticated food crops of the major civilizations (Maccaferri et al. 2009). Various abiotic stresses are responsible for poor wheat growth such as drought, saline stress poor soil fertility and heavy metal stress (Khan and Ashraf 2008; Rahaie et al. 2013).
Over the last century, urbanization and industrialization vigorously dumped various anthropogenic chemicals and toxins in the environment which pose notable increase in environmental pollution. Heavy metals are considered as severe threat for environment including agriculture (Wei and Yang 2010). Heavy metals are highly persistent in environment, and due to their inert and toxic nature, they can shift ecosystem diversity, function and structure. There are various heavy metals which are present in nature and occur in various forms, but most important includes chromium (Cr), arsenic (As), cadmium (Cd), nickel (Ni), copper (Cu), lead (Pb), cobalt (Co) and zinc (Zn) (Sandaa et al. 2001).
Hexavalent chromium is highly carcinogenic and mutagenic for various crops, as chromium drastically suppresses plant growth and development (Messer et al. 2006; Ahmad et al. 2012). Chromium disturbs the normal growth processes of plants, including germination, root and shoot length, stomatal conductance, chlorophyll contents and net photosynthesis (Dey et al. 2009). Chromium reduced the seed germination of Echinochloa colona by 25% (Rout et al. 2000), and sugar cane growth was highly effected up to 32–57% with 20–80 ppm chromium stress when applied in solution. There are various enzymes which are greatly affected due to chromium toxicity. As a result of elevated level of chromium, the protease and amylase activity increases which results in reduced transport of sugar to the embryo axis (Zeid 2001).
Plant growth-promoting rhizobacteria (PGPR) have a strong potential to improve plant growth in various abiotic stresses such as salt, drought and heavy metal stress previously documented by various studies (Mazhar et al. 2016; Saeed et al. 2016). Furthermore, the use of soil microbes (such as plant growth-promoting bacteria) also aids in the biodegradation of metals and could considerably stimulate plant growth in the presence of metals. The remediation of heavy metals with PGPR involves several mechanisms: rhizofiltration, phytostabilization, phytovolatilization and phytoextraction (Glick et al. 2007).
Inoculation of biochar with PGPR could improve the biochar efficiency, as biochar provides a substrate for inoculants, due to their high surface area and also provides nutrients which enables the inoculants survival in soil (Lehmann et al. 2011). High surface area and internal porosity are two important characters of biochar to act as inoculums source for PGPR (Abit et al. 2012; Herrmann and Lesueur 2013). Due to their surface heterogeneity, biochars have well-distributed pore network including macropores (> 50 nm) mesopores (2–50 nm), micropores (< 2 nm), in addition to have high surface area (Mukherjee et al. 2011; Kasozi et al. 2010). In the view of present scenario, the present study was conducted to isolate and test the inoculation potential of isolated strain for amelioration of chromium stress effect in wheat plants.
2 Materials and Methods
2.1 Collection of Soil Samples and Analysis
The soil samples were collected from root rhizosphere of plants grown near the main discharge point from industrial area in vicinity of Rawalpindi, Pakistan. Soil physical analysis was done for all the collected soil samples such as texture analysis, pH and EC according to Gee and Bauder (1979). Chemical analysis of soil such as organic contents (%), clay contents (%), moisture contents (%), humus (%) was carried out by following method of Olsen and Sommers (1982).
2.1.1 ICPOES Analysis
Approximately 0.2 g of each soil samples was weighed into a PTFE beaker, and 2 ml of hydrochloric acid, 6 ml of nitric acid and hydrofluoric acid 2 ml in a combination have been used for the simultaneous extraction of a large number of metals in soils. The solution was digested by following procedure: heated to 120 °C in 8 min and holding 3 min; raising the temperature to 150 °C maintaining 5 min; increase the temperature to 190 °C keeping 35 min. After cooling, 2 ml of H2O2 was added to the digested mixture and transferred to heating block in 140 °C until the residue solution was left 1 ml. Finally, the solution was transported into 50 ml volumetric flasks, brought to volume with water and mixed fully. The detection of metals was done by ICP-OES with internal standard method and standard addition method (Mao et al. 2017).
2.2 Isolation of PGPR and 16S RRNA Characterization
Isolation of PGPR was done from a composite soil sample by serial dilution method. One gram of soil sample was mixed in 9 ml of sterile 1% saline distilled water (miliQ) and vortexed. Media such as trypticase soya agar (TSA), nutrient agar (NA) and Luria–Bertani Agar (LB agar) were used for isolation purpose. Colonies were monitored after every 24 h at 28 °C for 1 week. The DNA from each liquid culture was isolated by MOBIO KIT USA. The 16S rRNA sequencing was done by commercially available sources. For amplification of nucleotide universal primer, 800R (5′-TACCAGGGTATCTAATCC-3′) 518 F (5′- CCAGCAGCCGCGGTAATACG-3′) was used. The sequences obtained were analyzed in bioEDIT and were processed for BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for identification of all the strains at species level. All the obtained sequences were submitted in NCBI gene bank for accession numbers. For determination of available phosphorus Phospho-molybdate blue color method was used (Murphy and Riley 1962). Antifungal activity of the isolates was assayed against Fusarium as described by Petatan-Sagahon et al. (2011). IAA production by bacterial isolates was checked by using the method of Sarwar et al. (1992). Iron solubilization bacterial isolates were assayed for siderophore production on chrome azurol sulfonate (CAS) agar medium described by Schwyn and Neilands (1987). ACC deaminase activity plates containing minimal medium were supplemented with ammonium chloride (2 g l−1) (serving as control plates) or 3 mM 1-aminocyclopropane-1-carboxylic acid (ACC) (Glick et al. 1998) One plate was used as negative control, and all strains were streaked on respective plates and left for incubation at 28 °C for 1 week.
2.2.1 Determination of Heavy Metal Tolerance
The bacterial strains were streaked on various concentrations of PbNO3, NiCl2 and K2Cr2O7 (Sigma-Aldrich). Colonies were streaked from 0.5 to 20 mM plates amended with heavy metals salts and incubated at 28 °C for 48 h (Burd et al. 2000).
2.2.2 Heavy Metal Plant Assay
To evaluate the efficacy of biochar and isolated PGPR, a pot experiment was conducted in green house of PMAS Arid Agriculture University Rawalpindi, Pakistan. Pots with the maximum capacity of 5 kg were selected and filled with soil. Two wheat varieties (NARC 2009 and NARC 2011) were selected on the basis of their tolerance toward ionic stress. Seeds were surface sterilized with 0.1% NaOCl for 5 min and then washed (Abdul-Baki and Anderson 1973). A consortium of isolated well characterized strains was made; for inoculation of the plants, cultures were grown in LB broth at 30 ± 2 °C. Seeds were soaked overnight in 3-day-old cultures of PGPRs having OD 0.99 at 600 nm and 107 cfu/ml and were sown in soil. The 1% biochar was finely grounded and sieved before application. The solution of K2Cr2O7 2.5 mg/kg was applied in the pots along irrigational water. The desired concentration was obtained after the seeds were sown by application in solution forms serially (Table 1).
2.2.3 Morphological Parameters
Whole plants were uprooted for determining shoot length, root and leaf surface area by using area meter at tillering stage (CID, CI-202.).
2.2.4 Physiological Parameters
2.2.4.1 Relative Water Content
Fresh weight of each flag leaf was measured individually and dipped in 100 ml of distilled water for 24 h and reweight after 24 h. Weight after 24 h of dipping was considered as turgid weight. Leaves were placed in drying oven at 70 °C for 72 h, and dry weight was calculated. Formula used is as follows:
2.2.4.2 Membrane Stability Index (MSI)
Methods of Sairam (1994) were used for determination of leaf membrane stability index (MSI). Washed leaf disks (100 mg) were heated at 40 °C for half hour in water bath in 10 ml of double distilled water. EC meter was used to record electrical conductivity (C1). C2 was recorded after heating the same sample at 100 °C.
2.2.4.3 Chlorophyll Content
Determination of total chlorophyll, chlorophyll a, chlorophyll b was done by using the method of Bruinsma (1963).
2.2.5 Biochemical Parameters
2.2.5.1 Proline Contents
Spectrophotometer method of Bates et al. (1973) was used for proline determination.
2.2.5.2 Soluble Sugar
Method of Dubois et al. (1951) was followed for analysis of soluble sugar in flag leaf
2.2.5.3 Super Oxide Dismutase Assay
SOD was determined by the method of Giannopolitis and Ries (1977).
2.2.6 Chromium Uptake in Wheat Plants
The accumulation of heavy metals in wheat plants was measured by using the method of Ouzounidou et al. (1992). Heavy metals in each sample were estimated individually by atomic absorption spectrophotometer.
2.3 Statistical Analysis
For statistical analysis, SPSS.20 version was applied to analyze the data. Analysis of variance method was used, and each treatment was with three replicates. The difference between mean was analyzed by the Duncan Multiple Range test (DMRT).
3 Results
3.1 Soil Analysis
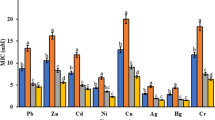
To access the soil quality, heavy metal contents and physiochemical characters of collected soil samples were performed (Tables 2, 3). The data obtained by using the ICPOES method are presented in Table 3. In general, heavy metal concentration was higher in the Group 1 soil samples than the Group 2 (as illustrated in Table 3). In Group 1, the higher concentrations of metals were detected in the four locations (first, third, fifth and seventh) and all these locations were near the main discharge point of the industrial effluents. The average amount of Fe, Zn and Cu was considerably higher, respectively, while in case of toxic heavy metals Cr, Ni and Pb were most abundant. Overall, the concentration of metals varies in following pattern (Fe > Cr > Cu > Zn > Ni > Pb > Co). The analysis for heavy metals has shown that the concentrations of most of the metals exceed the permissible limit of EPA standards. The concentration of chromium ranges between 95 and 1108 ppm in the analyzed soil samples. The highest concentration of chromium (1108 ppm) was observed in sample No 1 which was the closest to the main discharge point, followed by Sample No 3 (1016 ppm) and Sample No 7 in the range of (777 ppm). Hence, chromium concentration in all the collected soil samples surpasses the permissible limit of chromium in soil sludge and sediments (2–100 ppm).
3.2 Identification of Isolates by 16s rRNA
Based on the difference in morphological characters, total 6 strains were further characterized. The sequence results of six metal-tolerant strains were uploaded in Gene bank in NCBI, and the accession number is presented in Table 4. Phylogenetic analysis was done by Nie and Kumar (2000). Evolutionary analysis was conducted in MEGA7 (Kumar et al. 2016). The detail is presented in (Fig. 1).
3.3 Heavy Metal Analysis
The heavy metal tolerance of isolated strains was described as minimum inhibitory concentration. The majority of the isolates grew in the presence of NiCl2, PbNO3 and K2Cr2O7. However, most of the isolates were unable to grow when Cr was present in higher concentrations, e.g., 20 ppm. The maximum tolerance limit for PbNO3 and NiCl2 varies til 1 mM Ni and 5 mM Pb (Table 4). The strain G2 exhibits the multi-metal tolerance ability and also grew well in higher concentrations of Ni and Pb; furthermore, maximum tolerance ability of G22 varies accordingly. However, the other strains which grew well include G17, G18, G14.2. The detail is presented in Table 4.
3.4 Functional PGPR Characteristics
3.4.1 Phosphate Solubilization Assay and Others
All the isolated strains have shown variable PGPR characteristics; the detail is presented in Table 5.
3.5 Chromium and its Amelioration by PGPR and Biochar (Pot Experiment)
3.5.1 Growth Attributes
Growth of wheat plants was affected drastically in the presence of chromium stress. Percent decrease in shoot and root length was 34% and 48% at 2.5 mg/kg K2Cr2O7 as compared to control. However, combined application of strain inoculation and biochar enhanced the shoot and root length by 22% and 23.4%, respectively (Figs. 2 and 4). In the similar way, there was a decrease in physiological attributes of wheat plant such as relative water contents, MSI and chlorophyll contents up to 10%, 37% and 48% as compared to control plants when exposed to chromium. Furthermore, combined application of PGPR and biochar improved all the physiological attributes up to 25.5% in MSI, and 28% in chlorophyll contents, respectively (Figs. 5, 6, 7). There was 13.9% and 8% increase in soluble sugar contents, and proline was observed with chromium treated plants as compared to control ones (Figs. 8, 9). Furthermore, biochar and rhizosphere inoculation facilitate the plants to maintain high soluble sugar contents and proline level up to 20.5% and 9.6% in 2.5 mM K2Cr2O7 stress as compared to plants without inoculation. Similarly, biochar and rhizosphere inoculation have shown a significant improvement in super oxide dismutase activity till 41% in the Cr presence, respectively (Fig. 10).
3.5.2 Detection of (Cr+) in Wheat Plants
In control plants, the Cr contents were detected up to 0.05 ± 0.1 mg/kg. There was a higher amount of Cr up to 0.31 ± 0.09 mg/kg detected in the wheat plants when exposed to 2.5 mg/kg K2Cr2O7. Furthermore, the combined application of biochar and PGPR significantly reduced the heavy metal concentration up to 0.28 ± 1.01 mg/kg inside the plant and ameliorated the stress effect (Table 6).
4 Discussion
4.1 Soil Analysis and Heavy Metals
Chromium is considered as a toxic metal for plants and animals; furthermore, chromium in soils is also harmful for soil bio-geo-chemical activities. Various sources are responsible for buildup of chromium in surface and agricultural soils such as tanneries, leather manufacturing and electroplating and municipal sewage sludge’s (Pacha et al. 2011). According to the results, the chromium was most abundant in soil samples as toxic heavy metals and its concentration exceeds the permissible limit of EPA (Table 2, 3). According to the present findings, strains isolated have shown variable tolerance toward Cr, Ni and Lead. As in one of the similar studies, seventeen bacterial strains were recovered from plants rhizosphere of metal-polluted area showed variable tolerance toward Cd, Cr, Ni and Cu (Azzam and Tawfik 2015; Mihdhir et al. 2016). Strains isolated from heavy metal-polluted soils have strong potential to tolerate metal stress (Ellis et al. 2003).
4.2 Heavy Metal Tolerance and PGPR Characteristics
4.2.1 Heavy Metal Tolerance Analysis and Minimum Inhibitory Concentration
The characterization of the six well identified bacterial isolates was done on the basis of their tolerance toward Pb, Ni, Cr, and all the six strains belong to bacillus sp. (Table 4). While all the isolates showed complete inhibition at 20 mM concentration for all the tested heavy metals. In accordance with previous findings, isolated strains such as Bacillus exhibited high level of Pb, Ni and Cr tolerance. Different bacterial strains pose different tolerance levels and the possible reason could be the different genetic material, growth media used and the nature of soil from where they have been isolated (Kumar and Nagendran 2009). Furthermore, there are several mechanisms adopted by bacterial strains to cope up with deleterious effect of heavy metals such as (1) efflux of metal ions outside the cell (2) accumulation and complexation of metal ions inside the cell (3) enzymatic degradation/solubilization of metals (Ayangbenro and Babalola 2017). Similar observation has been reported earlier (Fulekar et al. 2012; Sharma and Dubey 2005). According to the results, all the isolated strains possess PGPR characteristics (Table 5) and these bacteria identified as plant growth promoters (PGPR) (Bashan 1998; Compant et al. 2005). PGPRs have the strong potential to survive under stress conditions and can promote plant growth (Tica et al. 2011). There are various strategies exhibited by PGPR, to cope up with stress conditions, which includes synthesis of (ACC) deaminase, production of indole acetic acid (IAA) and secretion of metal binding chelators, i.e., siderophores (Glick et al. 2007). The application of PGPR with the potential of adsorbing heavy metals has the twin advantage of bioremediation and plant growth promotion when isolated from that field contaminated with heavy metals (Table 5).
4.3 Effect of Chromium on Wheat Growth and Amelioration by PGPR and Biochar
According to our results, growth parameters of wheat plants effected greatly due to the toxic effect of chromium at 2.5 mg/L (Figs. 2, 3, 4). Chromium at concentration of 5 mg/L can affect the plant growth and at higher concentrations such as (20 and 40 mg/L) the growth of many crop plants could be inhibited completely (Sundaramoorthy et al. 2010). Lopez-Luna et al. 2009 also documented the similar trend as higher concentration of Cr could inhibit the growth of wheat and oat crop (Lopez-Luna et al. 2009). However, in our findings, application of PGPR and biochar ameliorated the toxic effect of chromium at 2.5 mg/L and significantly improved the growth condition of wheat plants. Plant growth-promoting bacteria can help to improve the growth of plants by several direct as well as indirect mechanisms (Glick et al. 1998). Furthermore, biochar also contains micro- and nano-pores with aromatic structure, which make it strong adsorbent for various contaminants, including heavy metals (Harvey et al. 2011; Chen et al. 2014).
Similarly, the physiological and biochemical attributes such as chlorophyll contents and MSI were reduced with the increase in chromium concentration upto 47.8–37% (Figs. 5, 6, 7, 8, 9). Plant at higher metal concentrations may experience oxidative stress that results in DNA damage, membrane disturbance and lipid peroxidation (Hossain et al. 2012). Furthermore, protein contents and pigment production also decreased due to Cr-induced oxidative stress in plants (Dey et al. 2009). Biochar and PGPR improved the pigment concentration with the maximum increase by 9.5% by limiting the bioavailability of heavy metals and made it less available to roots directly (Park et al. 2008). Certain reports have recognized the particular procedure(s) of PGPR for stimulating plant growth in the presence of metal for example the production of IAA, ACC deaminase and siderophores. IAA-producing bacteria can stimulate the growth of plants. ACC deaminase inhibits ethylene production (Glick et al. 2007), and siderophores help the plants to obtain enough iron under heavy metal stress (Burd et al. 2000). In the similar way, biochar application can also reduce the leaching of metals through its effect of redox reactions of metals (Choppala et al. 2012).
According to our results, chromium stress resulted in an increase in the superoxide dismutase activity in wheat, but at higher level of chromium there was a decline in SOD activity was observed (Fig. 10). Plants have developed various antioxidant mechanisms to cope up with stress conditions, but when exposed to high concentration of contaminants such as heavy metal they are unable to maintain homeostasis inside their cells (Wang and Zang 2014; Wang et al. 2007). The decrease in SOD activity might be due to denaturation of the protein structure of enzyme which leads to the reduction in its activity. Numerous studies have proven that microbial isolates that help in bioremediation have the ability to synthesize biosurfactants, solubilize phosphorus and scavenge free radicals thereby facilitating the growth in the presence of metals (Sheng and Xia 2006). Moreover, the physical properties of biochars such as mineral contents, surface area and pore structure (Lehmann et al. 2011) might increase the microbial diversity of metal contaminated soil to cope up with toxic effects of metals (Liu et al. 2008).
4.3.1 Cr Uptake by PGPR and Biochar in Wheat
According to the present results, PGPR and biochar application significantly reduced the metal uptake in wheat plants (Table 6). The application of PGPR with the potential of adsorbing heavy metals has the twin advantage of bioremediation and plant growth promotion when isolated from the field contaminated with heavy metals. The decreased uptake in inoculated plants might also be due to high surface area and high aromaticity of biochar (Tong et al. 2011). As in last few years, several studies documented the role of biochar addition for phytoremediation and plant growth promotion (Lu et al. 2014).
5 Conclusion
The present study was designed to detect the heavy metals in soil samples from an industrial area. Several heavy metals were detected by ICPOES analysis; according to the results, the concentration of Cr was the highest in all soil samples. Furthermore, six Bacillus strains were isolated on the basis of their metal tolerance; similarly, these strains possess strong PGPR characteristics. To ameliorate the Cr effect in wheat crop, PGPR strains were inoculated along with 1% biochar to increase the efficacy of inoculation. Hence, heavy metal-tolerant PGPR along with biochar has strong potential to ameliorate stress effect and has the ability to improve plant growth attributes.
References
Abdul-Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria 1. Crop Sci 13(6):630–633
Abit SM, Bolster CH, Cai P, Walker SL (2012) Influence of feedstock and pyrolysis temperature of biochar amendments on transport of Escherichia coli in saturated and unsaturated soil. Environ Sci Technol 46(15):8097–8105
Ahmad I, Akhtar MJ, Zahir ZA, Jamil A (2012) Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak J Bot 44(5):1569–1574
Ayangbenro A, Babalola O (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94
Azzam AM, Tawfik A (2015) Removal of heavy metals using bacterial bio-flocculants of Bacillus sp. and Pseudomonas sp. J Environ Eng Land Manag 23(4):288–294
Bashan Y (1998) Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol Adv 16(4):729–770
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Bruinsma J (1963) Absorption of light by chlorophyll a and b in plant extracts. Photochem Photobiol 2:241–249
Burd GI, Dixon DG, Glick BR (2000) Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can J Microbiol 46(3):237–245
Chen S, Rotaru AE, Liu F, Philips J, Woodard TL, Nevin KP, Lovley DR (2014) Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour Technol 173:82–86
Choppala GK, Bolan N, Megharaj M, Chen Z, Naidu R (2012) The influence of biochar and black carbon on reduction and bioavailability of chromate in soils. J Environ Qual 41(4):1175–1184
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959
Dey SK, Jena PP, Kundu S (2009) Antioxidative efficiency of Triticum aestivum L exposed to chromium stress. J Environ Biol 30(4):539–544
Dubois M, Gilles K, Hamilton J, Rebers P, Smith F (1951) A colorimetric method for the determination of sugars. Nature 168(4265):167
Ellis RJ, Morgan P, Weightman AJ, Fry JC (2003) Cultivation-dependent and-independent approaches for determining bacterial diversity in heavy-metal-contaminated soil. Appl Environ Microbiol 69(6):3223–3230
Fulekar M, Sharma J, Tendulkar A (2012) Bioremediation of heavy metals using biostimulation in laboratory bioreactor. Environ Monit Assess 184(12):7299–7307
Gee G, Bauder J (1979) Particle size analysis by hydrometer: a simplified method for routine textural analysis and a sensitivity test of measurement parameters 1. Soil Sci Society Am J 43(5):1004–1007
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59(2):309–314
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 90(1):63–68
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. In: Bakker PAHM, Raaijmakers JM, Bloemberg G, Höfte M, Lemanceau P, Cooke BM (eds) New perspectives and approaches in plant growth-promoting Rhizobacteria research. Springer, Dordrecht, pp 329–339
Harvey DJ, Gange AC, Hawes CJ, Rink M (2011) Bionomics and distribution of the stag beetle, Lucanus cervus (L.) across Europe. Insect Conserv Divers 4(1):23–38
Herrmann L, Lesueur D (2013) Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol 97(20):8859–8873
Hossain M, Hanafi M, Saleh G, Foroughi M, Behmaram R, Noori Z (2012) Growth, photosynthesis and biomass allocation of different kenaf (‘Hibiscus cannabinus’ L) accessions grown on sandy soil. Aust J Crop Sci 6(3):480
Kasozi GN, Zimmerman AR, Nkedi-Kizza P, Gao B (2010) Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ Sci Technol 44(16):6189–6195
Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp Bot 63(1–3):224–231
Kumar RN, Nagendran R (2009) Fractionation behavior of heavy metals in soil during bioleaching with Acidithiobacillus thiooxidans. J Hazard Mater 169(1–3):1119–1126
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota–a review. Soil Biol Biochem 43(9):1812–1836
Liu H, Zhang J, Christie P, Zhang F (2008) Influence of iron plaque on uptake and accumulation of Cd by rice (Oryza sativa L.) seedlings grown in soil. Sci Total Environ 394(2–3):361–368
Lopez-Luna J, Gonzalez-Chavez M, Esparza-Garcia F, Rodriguez-Vazquez R (2009) Toxicity assessment of soil amended with tannery sludge, trivalent chromium and hexavalent chromium, using wheat, oat and sorghum plants. J Hazard Mater 163(2–3):829–834
Lu H, Li Z, Fu S, Mendez A, Gasco G, Paz-Ferreiro J (2014) Can biochar and phytoextractors be jointly used for cadmium remediation? PLoS ONE 9(4):e95218
Maccaferri M, Sanguineti MC, Giuliani S, Tuberosa R (2009) Genomics of tolerance to abiotic stress in the Triticeae. In: Muehlbauer G, Feuillet C (eds) Genetics and genomics of the triticeae. Springer, New York, pp 481–558
Mao J, Liu X, Chen B, Luo F, Wu X, Jiang D, Luo Z (2017) Determination of heavy metals in soil by inductively coupled plasma mass spectrometry (ICP-MS) with internal standard method. Electrn Sci Technol Appl 4(1):1–6
Mazhar R, Ilyas N, Saeed M, Bibi F, Batool N (2016) Biocontrol and salinity tolerance potential of Azospirillum lipoferum and its inoculation effect in wheat crop. Int J Agric Biol 18(3):494–500
Messer J, Reynolds M, Stoddard L, Zhitkovich A (2006) Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Rad Bio Med 40(11):1981–1992
Mihdhir A, Assaeedi A, Abulreesh H, Osman G (2016) Detection, identification and characterization of some heavy metals tolerant bacteria. J Microbiol Biochem Technol 8:226–230
Mukherjee A, Zimmerman A, Harris W (2011) Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163(3–4):247–255
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, Oxford
Olsen S, Sommers L (1982) Phosphorus. In: Page AL et al (eds) Methods of soil analysis. Part 2. Agron. monogr., vol 9. ASA and SSSA, Madison, pp 403–430
Ouzounidou G, Eleftheriou E, Karataglis S (1992) Ecophysical and ultrastructural effects of copper in Thlaspi ochroleucum (Cruciferae). Can J Bot 70(5):947–957
Pacha C, Baumann T, Georgakos G, Kamgaing AUT (2011) Circuit arrangement with a test circuit and a reference circuit and corresponding method: Google Patents
Park D, Lim SR, Yun YS, Park JM (2008) Development of a new Cr(VI)-biosorbent from agricultural biowaste. Biores Technol 99(18):8810–8818
Petatan-Sagahon I, Anducho-Reyes MA, Silva-Rojas HV, Arana-Cuenca A, Tellez-Jurado A, Cárdenas-Álvarez IO, Mercado-Flores Y (2011) Isolation of bacteria with antifungal activity against the phytopathogenic fungi Stenocarpella maydis and Stenocarpella macrospora. Int J Mol Sci 12(9):5522–5537
Rahaie M, Xue GP, Schenk PM (2013) The role of transcription factors in wheat under different abiotic stresses abiotic stress-plant responses and applications in agriculture. InTech, Rijeka
Rout GR, Samantaray S, Das P (2000) Effects of chromium and nickel on germination and growth in tolerant and non-tolerant populations of Echinochloa colona (L.) Link. Chemosphere 40(8):855–859
Saeed M, Ilyas N, Mazhar R, Bibi F, Batool N (2016) Drought mitigation potential of Azospirillum inoculation in canola (Brassica napus). J Appl Bot Food Qual 89:26–32
Sairam R (1994) Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Ind J Exp Biol 32:594
Sandaa RA, Torsvik V, Enger O (2001) Influence of long-term heavy-metal contamination on microbial communities in soil. Soil Biol Biochem 33(3):287–295
Sarwar M, Arshad M, Martens DA, Frankenberger W (1992) Tryptophan-dependent biosynthesis of auxins in soil. Plant Soil 147(2):207–215
Schwyn B, Neilands J (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Pathol 17(1):35–52
Sheng XF, Xia JJ (2006) Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 64(6):1036–1042
Sundaramoorthy P, Chidambaram A, Ganesh KS, Unnikannan P, Baskaran L (2010) Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. CR Biol 333(8):597–607
Tica D, Udovic M, Lestan D (2011) Immobilization of potentially toxic metals using different soil amendments. Chemosphere 85(4):577–583
Tong XJ, Li JY, Yuan JH, Xu RK (2011) Adsorption of Cu (II) by biochars generated from three crop straws. Chem Eng J 172(2–3):828–834
Wang X, Zang S (2014) Distribution characteristics and ecological risk assessment of toxic heavy metals and metalloid in surface water of lakes in Daqing Heilongjiang Province, China. Ecotoxicology 23(4):609–617
Wang Y, Shi J, Wang H, Lin Q, Chen X, Chen Y (2007) The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol Environ Saf 67(1):75–81
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94(2):99–107
Zeid I (2001) Responses of Phaseolus vulgaris chromium and cobalt treatments. Biol Plant 44(1):111–115
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mazhar, R., Ilyas, N., Arshad, M. et al. Isolation of Heavy Metal-Tolerant PGPR Strains and Amelioration of Chromium Effect in Wheat in Combination with Biochar. Iran J Sci Technol Trans Sci 44, 1–12 (2020). https://doi.org/10.1007/s40995-019-00800-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-019-00800-7