Abstract
Microorganisms are cost-effective and eco-friendly alternative methods for removing heavy metals (HM) from contaminated agricultural soils. Therefore, this study aims to identify and characterize HM-tolerant (HMT) plant growth-promoting rhizobacteria (PGPR) isolated from industry-contaminated soils to determine their impact as bioremediators on HM-stressed pepper plants. Four isolates [Pseudomonas azotoformans (Pa), Serratia rubidaea (Sr), Paenibacillus pabuli (Pp) and Bacillus velezensis (Bv)] were identified based on their remarkable levels of HM tolerance in vitro. Field studies were conducted to evaluate the growth promotion and tolerance to HM toxicity of pepper plants grown in HM-polluted soils. Plants exposed to HM stress showed improved growth, physio-biochemistry, and antioxidant defense system components when treated with any of the individual isolates, in contrast to the control group that did not receive PGPR. The combined treatment of the tested HMT PGPR was, however, relatively superior to other treatments. Compared to no or single PGPR treatment, the consortia (Pa+Sr+Pp+Bv) increased the photosynthetic pigment contents, relative water content, and membrane stability index but lowered the electrolyte leakage and contents of malondialdehyde and hydrogen peroxide by suppressing the (non) enzymatic antioxidants in plant tissues. In pepper, Cd, Cu, Pb, and Ni contents decreased by 88.0-88.5, 63.8-66.5, 66.2-67.0, and 90.2-90.9% in leaves, and 87.2-88.1, 69.4-70.0%, 80.0-81.3, and 92.3%% in fruits, respectively. Thus, these PGPR are highly effective at immobilizing HM and reducing translocation in planta. These findings indicate that the application of HMT PGPR could be a promising “bioremediation” strategy to enhance growth and productivity of crops cultivated in soils contaminated with HM for sustainable agricultural practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pepper (Capsicum annuum L.) is an economically important crop that is cultivated for its nutritional values, bioactive compounds, antioxidant properties and natural colors (Santosh 2013). Like other vegetables in Egypt, pepper is mostly grown in soils originally polluted with heavy metals (HM) near industrial areas, or from disposal of wastes. These soils are less resilient and crops that grow in them exhibit reduced productivity (Gall et al. 2015; Alengebawy et al. 2021). Land degradation is also well-documented to have an impact on global food security and environmental quality (Alengebawy et al. 2021).
Although some essential HM, such as copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), silver (Ag), and zinc (Zn), play important roles in different biological systems, they are generally toxic to living organisms at high quantities (Rahman et al. 2022). On the other hand toxic, non-essential HM, including cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), and arsenic (As), can be harmful, even in small amounts (Rahman et al. 2022). Many pollution sources, such as chemical fertilizers, atmospheric precipitation, sewage sludge and agricultural industry, adversely increase HM concentrations in farmland soils and contribute to the accumulation of contaminants in edible plant parts (Kumari and Mishra 2021; Cheng et al. 2023). As a result, this affects not just plant productivity, but also human health and soil biodiversity (Manzoor et al. 2022).
In response to HM stress, cellular compartments activate signaling pathways and induce transcriptional programs to promote HM stress tolerance and establish homeostasis (Li et al. 2023). Oxidative stress is caused by the imbalance between production and accumulation of reactive oxygen species (ROS) in plants cells/tissues (Kohli et al. 2017; Sarkar et al. 2018). Depending on their concentration in plants, ROS play a crucial, dual role in plant biology (Hasanuzzaman et al. 2023). At high concentration, ROS cause damage to carbohydrates, proteins, lipids and DNA (Ahmad et al. 2009). At low/intermediate level, ROS act; however, as secondary messengers or signaling molecules to mediate plant responses to biotic and abiotic stresses and to remodel plant growth and development (Devireddy et al. 2021).
Therefore, plants employ a sophisticated antioxidant defense system to protect themselves from oxidative damage by scavenging ROS (Sofy et al. 2020). For instance, the plant enzymes, catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), superoxide dismutase (SOD), and glutathione reductase (GR) can catalyze reactions that break down ROS into less harmful molecules (Bhaduri and Fulekar 2012). The non-enzymatic antioxidants, such as ascorbic acid (AsA), carotenoids (Car), α-tocopherol (TOC), glutathione (GSH), and proline (Pro), play pivotal roles in plant acclimatization during abiotic stress i.e., HM (Bhaduri and Fulekar 2012).
In soil, HM reduce water potential and compete with essential nutrients, making them scarce for the uptake by root; thus, contributing to drought-like symptoms and osmotic stress (Ghori et al. 2019). In addition, high levels of HM in the apoplasm of leaves disrupt water balance in plants, causing cells to lose water (cell dehydration) (El-Saadony et al. 2021; Khan et al. 2021). HM stress is also linked with decreased chlorophyll (Chl) contents, stomata closure, and increased damage in photosynthetic attributes. Moreover, environmental risks of excessive HM accumulation in soils substantially impact microbial populations and their soil activities (Li et al. 2020).
Plant growth-promoting rhizobacteria (PGPR), play a pivotal role in mitigating HM accumulation in plants through multifaceted mechanisms (Desoky et al. 2020; Ashry et al. 2022; Patil et al. 2023). PGPR can solubilize insoluble HM in the rhizosphere, reducing their availability for plant absorption (Oubohssaine et al. 2022; Khoso et al. 2024). This process involves the secretion of organic acids, siderophores, and chelating agents that bind to HM, rendering them less toxic. Additionally, PGPR enables plants to acquire essential nutrients, mainly nitrogen (N), phosphorus (P), potassium (K), and Fe, while inhibiting the absorption of HM (Bhat et al. 2023).
Furthermore, these beneficial bacteria induce systemic resistance (ISR) in plants against HM stress by activating various defense mechanisms, including production of phytochelatins, and antioxidant enzymes (Yu et al. 2022). PGPR can also promote growth and extension of the root systems in plants to enhance their ability to exclude and detoxify HM (Mantelin et al. 2006). For example, PGPR can alleviate/reduce HM stress in plants by producing salicylic acid, exopolysaccharides (EPS), biosurfactants, Pro, and siderophores, and chelating different metal ions (El-Meihy et al. 2019; Nazli et al. 2021). Overall, the intricate interactions between PGPR and plants demonstrate their potential as environmentally friendly biotechnological tools for reducing HM accumulation and improving plant health in contaminated environments (Oubohssaine et al. 2022; Patil et al. 2023).
Microbial biostimulant technology (or bioremediation) has become a sustainable strategy to remove, degrade or immobilize toxic elements (i.e., HM) from contaminated soils (Desoky et al. (2020). Nitrosomonas, Mycobacterium, Flavobacterium, Bacillus, Pseudomonas, Xanthobacter and Penicillium are microorganisms that can degrade a variety of HM (Elnahal et al. 2022; Yin et al. 2023). Paenibacillus sp. is an endophyte isolated from Tridax procumbens roots that can grow in soils containing high concentrations of Zn, Pb, Cu, and Ar (Wu et al. 2021). Desoky et al. (2020) have also reported that the bacterial species, Bacillus, Paenibacillus and Pseudomonas, are considered potential bioremediators.
Several studies have demonstrated that PGPR can improve the defense mechanisms of plants growing under HM stress conditions (Eltahawy et al. 2022). As far as we know, no prior research has been performed to determine the effect of Pseudomonas azotoformans (Pa), Serratia rubidaea (Sr), Paenibacillus pabuli (Pp), or Bacillus velezensis (Bv), as bioremediators on pepper plants cultivated in HM-polluted soils.
We hypothesized that the combined application (Pa+Sr+Pp+Bv) would boost the growth and HM stress tolerance in peppers, compared to the single or double applications of PGPR. In the present investigation, we aimed to (i) isolate HM-tolerant (HMT) PGPR capable of tolerating HM from contaminated soils; and (ii) determine the response of pepper plants (growth and yield attributes) to soil inoculation with the most promising PGPR consortia of isolates in the field. In the current investigation we also evaluated the physio-biochemistry, components of antioxidant defense system, and HM accumulation in the tissues of pepper plants in response to PGPR.
Overall, this research can paved the way for developing new strategies to improve crop yields and HM bioaccumulation of pepper plants using Pa+Sr+Pp+Bv under field conditions.
Materials and methods
Collection of soil samples
In the current study, the HM contaminated soil samples were collected from the top 20 cm of six industrial regions in Abu-Zabal City, Qalyubia governorate, Egypt (30° 14′ 58″ N; 31° 21′ 16″ E) in sterile polythene bags. Soil samples were quickly transported to the Microbiology Laboratory, Faculty of Agriculture, Zagazig University, Zagazig, Egypt. The concentrations of HM in soil samples were determined by using AAnalyst 400 atomic absorption spectrophotometer (Perkin Elmer, Waltham, Massachusetts, USA).
Preliminary screening of HMT bacteria
Ten grams of homogenized soil samples were mixed into 90 mL sterilized peptone water (LB; Lab M Limited, Lancashire, UK). All flasks were kept on a gyratory shaker (Model G76, New Brunswick Scientific, NJ, USA) at 150 rpm at 30°C for 1 h. The flasks were left to settle for 30 more min and dilutions (10-2–10-7) were made using sterilized water.
The HM salt stock solution used in the current study is composed of HgCl2, CdCl2, CoCl2, CuCl2, MnCl2, K2Cr2O7, NiCl2, AgNO3, ZnSO4, and (CH3COO)2Pb (300 mg L-1 each). The HM salt stock solution was prepared in distilled water, filter-sterilized, and stored in sterile flasks in the dark at 4°C for 24 h (Kobya et al. 2005).
Aliquots (0.2 mL) from each dilution were spread onto Luria Bertani (LB; Lab M) agar plates supplemented with different concentrations of HM solutions (50, 100, 150, 250, and 300 mg L-1). Four plates were made for each dilution, and the plates were air dried and incubated for 3 days in the dark at 28±2°C. Colonies were counted and expressed as log10 colony forming units (cfu) g dry weight (DW) soil-1. All obtained bacteria were purified and stored on nutrient agar plates (Lab M). The purification and selection of colonies with a variety of morphologies was made possible through streaking on nutrient agar plates.
All the obtained HMT bacterial isolates were tentatively identified by colony morphology and by their cultural characteristics such as color, and the production of diffusible pigments. Biochemical tests were performed as described in Bergey’s Manual of Systematic of Bacteriology (Logan and De Vos 2009). Bacterial isolates were further identified using matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Bruker Daltonics, Bremen, Germany; Bille et al. 2012).
Determination of minimum inhibitory concentration (MIC) of bacterial isolates to HM
To determine the bacterial tolerance to HM, LB broth supplemented with different HM concentrations (0, 150, 100, 150, 250, and 300 mg L-1) was inoculated with 2 mL (108 cfu mL-1) of each bacterial isolate. The Erlenmeyer flasks were incubated for 4 days in the dark at 28°C on an orbital shaker incubator (New Brunswick Scientific) at 250 rpm. To determine the MIC, (the lowest concentration used to inhibit the growth of a bacterial culture) of HM, the optical density (OD600) of each bacterial culture was measured (Kowalska-Krochmal and Dudek-Wicher 2021).
Field experiments
Field experiments were carried out on a private farm with HM contaminated soil in Abu-Zabal City, Qalyubia governorate, Egypt in two growing seasons (March-July) using pepper in 2022 (SI) and 2023 (SII). Experimental conditions were as the following: Day/night length=12-13/11-12 h; temperature=25±3°C day, and 15±2°C night; precipitation=5-10 mm, and relative humidity=65.4-72.2%. Before each season, soil samples were randomly collected from the research sites and analyzed according to previous protocols (Black 1958; Jackson 1958). The physical and chemical properties of the tested soil in both seasons are presented in Table S1.
Pepper seeds (C. annuum cv. Top Star) obtained from Sacata Company (Cairo, Egypt) were used for the field experiment. Seeds were sown in growth trays, and seedlings were transplanted after 45 days to plots (3.0×3.50 m=10.5 m2), 60 cm-wide between the rows and 15–20 cm spaces between seedlings. Before planting, all plots were fertilized with a standardized fertilizer of 100 kg potassium sulfate (K2O, 50%), 200 kg calcium superphosphate (P2O5, 15.5%), and 250 kg ha-1 of ammonium nitrate (NH4NO3). The empirical design of our study was a completely randomized plot for 12 treatments, each with three replicates.
The following treatments were applied on pepper plants: (1) pepper plants cultivated in non-inoculated soil (control; C); (2) pepper plants cultivated in soil inoculated with the single HMT isolate Pseudomonas azotoformans (Pa); (3) pepper plants cultivated in soil inoculated with the single HMT isolate Serratia rubidaea (Sr); (4) pepper plants cultivated in soil inoculated with the single HMT isolate Paenibacillus pabuli (Pp); (5) pepper plants cultivated in soil inoculated with the single HMT isolate Bacillus velezensis (Bv); (6) pepper plants cultivated in soil inoculated with the double HMT isolates Pa+Sr; (7) pepper plants cultivated in soil inoculated with the double HMT isolates Pa+Pp; (8) pepper plants cultivated in soil inoculated with the double HMT isolates Pa+Bv; (9) pepper plants cultivated in soil inoculated with the double HMT isolates Sr+Pp; (10) pepper plants cultivated in soil inoculated with the double HMT isolates Sr+Bv, (11) pepper plants cultivated in soil inoculated with the double HMT isolates Pp+Bv; and (12) pepper plants cultivated in soil inoculated with the quadruple HMT isolates Pa+Sr+Pp+Bv.
Application of the selected HMT bacteria
The above bacterial isolates were cultivated on LB media to obtain a concentration of 109 cfu L-1 (Duxbury 1981). The selected bacteria (Pa, Sr, Pp and Bv) were applied to the soil in irrigation water at a rate of 10 L ha-1 in 3 equal doses at 20, 35, and 50 days after transplantation (DAT). The selected four bacteria were administered during the final 10 min of drip irrigation for each application.
Effect of bacteria on the growth and productivity of pepper plants
At 60 DAT, nine pepper plant samples were chosen randomly and plucked from the two outside rows of each experimental plot to measure stem length (SL; cm), shoot dry weight (SDW; g), number of leaves plant-1, and leaf area (LA; cm2 plant-1). At the marketable stage, 50 pepper fruits plot-1 were harvested to determine the number of fruits plant-1, and yield (tons ha-1).
Determination of the physicochemical properties of pepper plants
To evaluate the extent of photosynthetic pigments, leaf samples were extracted using acetone as described previously (Fadeel 1962). The OD was recorded at 480 nm (Chl a), 645 nm (Chl b), and 663 nm (Car) using a spectrophotometer (Beckman 640D, Brea, California, USA) to determine the contents of pigments [mg g-1 leaf fresh weight (FW)].
Leaf net photosynthetic rate (Pn), rate of transpiration (Tr), and stomatal conductance (gs) were measured using a portable photosynthesis system (LF6400XTR, LI-COR, Lincoln, NE, USA). To avoid potential stomatal closure around noon time, measurements were taken from the second wholly expanded leaf of the treated-plant at 9:00-11:00 AM.
We also estimated the relative water content (RWC) as previously described (Barrs and Weatherley 1962). The FW of a mixed sample of one leaf plant-1 was determined. Leaves of the same sample were soaked in distilled water for 3 h, and the turgid weight (TW) was recorded. Leaf tissues were dried at 80°C for 24 h to measure their dry weight (DW). RWC was calculated according to the following formula:
To calculate the membrane stability index (MSI), two sets of fresh leaf (0.2 g) were homogenized in 10-mL of double-distilled water: Group I was heated in a water bath at 40°C for 30 min and the electrical conductivity (EC) bridge (C1) was measured; while, Group II was heated at 100°C for 10 min in a boiling water bath to evaluate EC (C2) according to the method of Premachandra et al. (1990) and Oyenike et al. (2019). MSI was calculated using the following formula:
Twenty leaf discs (each 0.5 cm) were homogenized in ½ mL of distilled water to determine the electrolyte leakage (EL). To determine ECa, ECb and ECc, the solution was exposed to 25°C, 45-55°C for 30 min, and 100°C for 10 min, respectively. To calculate EL, the following formula was used (Hniličková et al. (2019):
Fresh leaf samples (100 mg) were homogenized in Na-phosphate buffer to evaluate the content of malondialdehyde (MDA; μmol g-1). The suspension was centrifuged at 20,000 × g for 25 min at 4°C (Heath and Packer 1968). The OD532 and OD600 of the supernatant were used to compensate the nonspecific turbidity.
H2O2 content (μmol g-1) was measured according to the method described by Liu et al. (2000). Extract of leaves was diluted with sulfuric acid 1M, followed by the addition of NH3 and titanium reagent. The OD at 415 nm was determined (Mukherjee and Choudhuri 1983). A standard curve was prepared using known concentrations of H2O2, and it was used for the calculations. The results were reported as l mol H2O2 g-1 FW.
The superoxide (O2•–) content was evaluated by immersing leaf pieces for 1 h in 10 mM K-phosphate buffer (pH 7.4), nitro blue tetrazolium chloride (NBT);(0.05%), and sodium azide (10 mM). The mixture was boiled at 85°C for 15 min and cooled; followed by recording OD readings at 580 nm (Kubiś 2008).
Determination of non-enzymatic antioxidant compounds of pepper plants
The amount of Pro (μmol g−1 of leaf DW) was determined using standard L-Pro calibration curve (Bates et al. 1973). Total soluble sugars (TSS) were extracted from leaves using 96% (v/v) ethanol. The extract was then mixed with a reagent containing 150 mL of sulfuric acid in 100 mL of 72% ethanol, boiled in a water bath for 10 min, and cooled. The mixture was quantified at 632 nm using spectrophotometer (Bausch and Lomb, Rochester, New York, USA) as recommended by Irigoyen et al. (1992).
The content of α-TOC (μmol g-1) in dried leaves was determined using the high-performance liquid chromatography (HPLC) system (Waters Corporation HPLC, Milford, MA, USA) combined with a UV detector (1.5 mL min-1 flow rate at 292 nm) and a mobile phase (94 mL methanol, and 6 mL water) (Konings et al. 1996; Ching and Mohamed 2001). About 20 mg of butylated hydroxyl toluene was dissolved to 900 mL of the extraction solvent (800 mL n-hexane + 100 mL of ethyl acetate). α-TOC (0.5 mg 100 mL-1 n-hexane) was used to generate the standard and dilution 0.02-0.2 mg mL-1 solutions.
Ascorbic acid (AsA), and GSH concentrations were measured using the methods previously described by Maruta and Ishikawa (2022) and Tsiasioti and Tzanavaras (2023), respectively.
Estimation of antioxidants activities of pepper plants
For enzyme extractions, 1 g FW of leaves were homogenized in 10 mL of phosphate buffer (pH 7.0) containing 0.5 mM ethylenediaminetetraacetic acid (EDTA), and urea. The homogenate was centrifuged at 15,000 × g for 15 min under chilling conditions to obtain a supernatant referred to as the enzyme extract.
CAT activity was measured according to He et al. (2020) with some modifications. The enzyme extract (0.1 mL) was added to 0.5 mL of H2O2, and 2.4 mL of phosphate buffer (pH 5.0). Using Bausch and Lomb spectrophotometer, the solution was measured trice (one min each) at OD240. The increase in absorbance of 0.01 U min-1 equals one enzymatic unit. The acquired values were estimated as U mL-1 min-1 DW using the following formula:
where, Af, final absorbance; As, initial absorbance; reaction volume=3; time interval=3; enzyme volume=0.1 mL.
The activity of POD was assessed at OD470 as recommended by Hussein et al. (2019). Leaves (1 g FW) was homogenized in 10 mL of phosphate buffer (pH 7.0), 0.5 mM EDTA, and polyvinyl pyrrolidone (PVP) for 10 min, and centrifuged at 15000 × g for 15 min at 4°C). The enzyme extract (0.1 mL) was added to 2.2 mL of guaiacol in phosphate buffer (3%), and incubated for 30 min, followed by 0.5 mL addition of H2O2. Using a spectrophotometer, absorbance at 470 nm was measured. The increase in absorbance of 0.01 min-1 equals one enzymatic unit. The obtained results (U mL-1 min-1 DW) were based on the calculations according to the following equation:
Where, Af, final absorbance; As, initial absorbance; reaction volume=3; time interval=3; enzyme volume=0.1 mL.
APX was tested colorimetrically according to Faize et al. (2013). The test was conducted at 25°C in a 1-cm light path cuvette containing a reaction mixture of 1500 μL phosphate buffer (pH 7.0), 20 μl EDTA, 1000 μL sodium ascorbate, and 20 μL enzyme extract. After mixing, 480 μL H2O2 was added to commence the reaction, and the decrease relative to the blank (water) at OD290 was continuously measured for 2 min.
The SOD activity was assessed at 560 nm (He et al. (2020). The enzyme extract (0.1 mL) was added to 1 mL phosphate buffer (pH 5.0), 1 mL distilled water, 0.3 mL methionine (22 μM), and 0.1 mL NBT (20 μM). The vial was then exposed to UV-light for 15 min before 100 mL of 0.6 M riboflavin was added (as a substrate). One unit of SOD refers to the quantity of enzyme necessary to prevent the decrease of NBT by 50%. The OD at 470 nm was measured trice (one min each) using Bausch and Lomb spectrophotometer. The increase in absorbance of 0.01 U min-1 equals one enzymatic unit. The obtained results (U mL-1 min-1 DW) were calculated using this equation:
Where, Af, final absorbance; As, initial absorbance; reaction volume = 3; time interval = 3; enzyme volume = 0.1 mL.
Following the monitoring of NADPH oxidation, the activity of GR was evaluated by detecting three absorbance readings at 340 nm (Rao et al. 1996).
Determination of HM accumulation in leaves and fruits of pepper
The dried powdered leaf samples were weighed to estimate the HM content using the AAnalyst 400 atomic absorption spectrophotometer (Perkin Elmer) as recommended by Khan et al. (2015). Briefly, the upper completely extended leaves and fruits were burned at 500°C for 12 h and the ash was dissolved in HNO3 (3.3%, v/v). The levels of Cd, Cu, Pb, and Ni were measured using the inductively coupled plasma optical emission spectroscopy (ICP-OES, Varian, Inc. Belrose NSW, Australia).
All HM concentrations in plants were compared to the certified Cd, Cu, Pb, and Ni concentrations in various reference plant materials received from the National Institute of Standards and Technology (NIST; Gaithersburg, USA).
Statistical analysis
The data were analyzed using analysis of variance (ANOVA) in SAS Software version 9 (SAS Institute Inc., NC, USA). Mean values of treatments were subjected to pairwise comparisons using the Least Significant Difference (LSD) test at P<0.05 to determine the significant differences among treatments.
Results and discussions
Isolation and identification of HMT bacteria
Twenty bacterial strains were isolated from the HM-contaminated soils collected from Abu-Zabal city, Egypt, and were given a code according to their tolerance to HM (Table S2). These isolates were further screened in vitro for their tolerance to HM on LB medium supplemented with different concentrations of HM solutions (50, 100, 150, 250, and 300 mg L-1).
All obtained 20 isolates grew on LB media when supplemented with 50 or 100 mg L-1 of HM. The current study showed that only 13, and 8 isolates grew on media containing 150 and 250 mg L-1 of HM, respectively. Only four isolates (HMT21, HMT40, HMT52 and HMT75) tolerated the high concentration of 300 mg HM L-1 and were considered highly HMT isolates and were elected for identification, and subsequent experiments.
Although researchers are dependent on the molecular identification of bacterial isolates, others have routinely relied on MALDI-TOF MS using ribosomal proteins for the identification (Ashfaq et al. 2022). Based on their ribosomal protein mass signal, particular strain can be compared to a publicly bacterial reference library (Topić Popović et al. 2023).
Under the microscope, HMT21, and HMT75 were identified as Gram-positive, motile, long rod, and spore-forming bacteria, suggesting that both bacteria might belong to Bacillus species. Based on the biochemical tests in Beregy’s manual, isolate HMT21 was tentatively identified as Pp due to its high similarity to Paenibacillus pabuli DSM 3036T. Isolate HMT75, on the other hand, showed high similarity (99%) to Bacillus velezensis DSM 33864, and was tentatively identified as Bv.
The other two isolates (HMT40, and HMT52) were Gram-negative bacteria. Isolate HMT40 was a rod, motile bacterium with a single polar flagellum belonging to Pseudomonas species, whilst isolate HMT52 was red in color, rod-shaped, and non-spore-forming Serratia species. Isolates HMT40 (Pa), and HMT52 (Sr) were 99% similar to Pseudomonas azotoformans DSM 18862T, and Serratia rubidaea DSM 4480, respectively.
MIC of HMT bacterial isolates
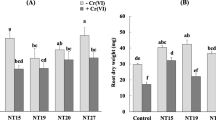
The efficacy of the four isolates measured by MIC was assessed. The HMT40 isolate (Pa) had MIC values of 13.34, 16.24, 11.89, 20.01, and 18.27 against Pb, Zn, Cd, Cu and Cr, respectively (Fig. 1), suggesting that Pa is highly tolerant to HM. The isolate HMT21 (Pp) had less MIC values than those in Pa., thus indicating that Pp is the second HMT isolate (Fig. 1).
Minimum inhibitory concentration of the tested bacterial isolates to HM. Data are means (n=9) ± standard error of the mean. Values of different letters indicate significant differences among isolates to the particular HM according to the least significant difference (LSD) test at P≤0.05. MIC, minimum inhibitory concentration; HM, heavy metal; Pb, lead; Zn, zinc; Cd, cadmium; Ni, nickel; Cu, copper; Ag, silver; Hg, mercury; Cr, chromium; HMT, heavy metal tolerant; HMT21, Paenibacillus pabuli (Pp); HMT40, Pseudomonas azotoformans (Pa); HMT52, Serratia rubidaea (Sr); HMT75, Bacillus velezensis (Bv)
HMT52 (Sr) and HMT75 (Bv) were mostly comparable in their MIC for Pb, Cd, Ni, Ag and Hg; however, Sr had higher MIC values than Bv for Zn, Cu and Cr (Fig. 1). Both isolates were less tolerant to HM than Pa or Pp (Fig. 1). Pa (HMT40) was the strongest HMT isolate among all other tested bacterial strains. This was followed by Pp, and finally Sr and Bv (Fig. 1).
Similarly, Desoky et al. (2020) have previously investigated the sensitivity of Bacillus species isolated from soil samples to determine the levels of HM-tolerance of Bacillus strains to Pb, Zn and Cu. The identified isolates were also examined for their plant growth-promoting (PGP) properties and bioremediation abilities. The method that measures MIC in the current investigation can be used to determine HM-tolerance in bacteria. For instance, Enterococcus mundtii has been identified due to its high tolerance to soils contaminated with HM using this method (Efe 2020).
Effect of soil inoculation with HMT bacteria on growth and productivity of pepper plants grown in HM-contaminated soil
Toxicant pollution is a major environmental hazard that has caused serious concerns to human health and agricultural productivity (Priyanka et al. 2021; Górski et al. 2023). In the current study, HM stress in the two growth seasons SI and SII significantly (P<0.05) lowered SL, number of leaves, LA, SDW, number of pepper fruits, and yield (Table 1). Our findings are consistent with others (Lamhamdi et al. 2013; Taie et al. 2019), who found that soil contaminated with HM led to reduced growth and biomass in wheat and spinach, respectively, and that plants were less resilient to HM stress. HM accumulation in shoots is likely a consequence of the root uptake from the soil, and subsequently translocated to shoots in excess quantities, leading to impaired physio-chemical processes, reduced growth and development, nutrient imbalance and increased cellular oxidative damage in plants (Tang et al. 2023). HM toxicity minimizes mitotic division of meristematic cells in roots and shoots, resulting in decreased length, dry mass, and yield (Rady et al. 2021).
In the current study we determined the effect of the soil inoculation of the four HMT bacterial strains on pepper plants grown in HM-contaminated soil in the field. Soil application with any of the HMT PGPR isolates either individually or in combination significantly (P<0.05) increased all parameters of growth and yield compared to control plants that were grown in non PGPR-inoculated soil (Table 1). On the other hand, the combined applications of any two tested bacterial strains to the soil significantly (P<0.05) increased all growth and yield parameters of pepper compared to control or individual application of isolates (Table 1).
The quadruple treatment (Pa+Sr+Pp+Bv) showed the highest increase in SL (94.9-98.1%), number of leaves (129-130%), LA (198.9-199.6%), SDW (184-185%), number of fruits (314-321%), and fruit yield (318-331%) compared to the control treatment during the two seasons. PGPR have the ability to enhance the productivity and HM tolerance of crop plants (Busnelli et al. 2021). Our findings were corroborated by Bhuyan et al. (2022), who found that PGPR can aid maize (Zea mays) plant growth while reducing HM levels in the soil. Furthermore, enhancement of plant growth and mitigation of HM stress by PGPR are associated with reducing the endogenous levels of HM in planta (Sun et al. 2023).
Impact of soil inoculation with HMT bacteria on photosynthetic pigment contents and photosynthetic efficiency of pepper cultivated in HM-contaminated soil
Under HM stress conditions, pepper plants grown in soils without the supplementation of any PGPR (control) showed decreased contents of Chl a and b, and Car, causing a reduction in Pn, Tr and gs (Table 2). Individual or dual applications of bacterial strains to the HM-polluted soil significantly (P<0.05) increased the levels of photosynthetic pigments and enhanced photosynthetic efficiency, compared to plants cultivated in non-PGPR inoculated soils (Table 2). The combined treatment of Pa+Sr+Pp+Bv on pepper plants showed the best results with an increment of 129, and 125% in Chl a, 75, and 78% in Chl b, 29, and 25% in Car, 86, and 85% in Pn, 88, and 86% in Tr, and 96, and 88% in gs in 2021/2022 and 2022/2023 seasons, respectively, compared to the control (Table 2). Similar trends were also found in rice plants inoculated with Burkholderia sp., which decreased Cd translocation in tissues and elevated the photosynthetic efficiency in plants (Dong et al. 2016). Furthermore, the contents of Chl and Car increased when rice plants were inoculated with Pseudomonas putida and Chollera vulgaris upon As treatment (Srivastava et al. 2018).
Our results were also in agreement with other studies indicating that plant photosynthesis is restrained when exposed to HM stress. This may occur directly by affecting the key enzymes of chlorophyll biosynthesis (Madhu and Sadagopan 2020), water-oxidizing complex of PSII (Dutta and Pal 2019), decreasing RuBPCase activity in leaves (Cuypers et al. 2023), causing damages to electron transfer and disturbance in photosynthesis (Chen et al. 2021), or preventing the absorption of essential elements from the soil for the formation of photosynthetic pigments (Guo et al. 2019). Deficiency of Fe, stomatal closure and reduction in gs (Chen et al. 2020), alteration cell size and stomatal density in the epidermis of leaves (ur Rehman et al. 2020) are among indirect causes of photosynthesis disruption. Normally, Fe deficiency in plants increases under HM stress conditions, leading to chlorosis (Burd et al. 1998). PGPR tend to bind to Fe and form siderophore complexes to provide plants with Fe.
PGPR can bind directly with HM to reduce their bioavailability and toxicity (Thiem et al. 2018; Patil et al. 2023). Moreover, these plant-associated bacteria may boost the plant defense system when exposed to HM stress. For example, PGPR may confer HM stress tolerance by mediating ISR and/or enhancing phytohormones (Yu et al. 2022; Patil et al. 2023). They can regulate metal transporter genes upon Cd exposure to reduce its accumulation in corn (Wang et al. 2016).
Impact of soil inoculation with HMT bacteria on pepper leaf integrity, oxidative stress indicators, and oxidative damage in HM-contaminated soil
PGPR colonizing the rhizosphere or internally inhabiting plant roots as endophytes enhance plant growth by solubilizing and assisting nutrient acquisition (El-Tarabily et al. 2021; Kaur and Pandove 2023), producing plant growth regulators (PGRs, El-Tarabily et al. 2020; Mathew et al. 2020; Shaffique et al. 2023), acting as biocontrol agents (Alwahshi et al. 2022; Nagrale et al. 2023), and producing 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase (El-Tarabily et al. 2019; Shahid et al. 2023). Under HM stress, PGPR with ACC-deaminase activity has the potential to lower ethylene production in plants by cleaving ACC into α-ketobutyrate and ammonia (Misra and Chauhan 2020). Bacillus anthracis PM21 enhanced growth of the legume tree, sesban (Sesbania sesban), by the production of ACC-deaminase, IAA and EPS under HM stress conditions (Ali et al. 2021). The application of Bacillus gibsonii PM11 and Bacillus xiamenensis PM14 to flax (Linum usitatissimum) resulted not only in increased tolerance to HM but also enhanced plant PGP traits (IAA, siderophores, EPS and ACC deaminase) (Sarkar et al. 2018; Zainab et al. 2020).
Production of organic acid or acidification by Zn-solubilizing PGPR is a major mechanism used to sequester Zn cations and decrease the pH of nearby soils (Alexander 1997; Singh et al. 2023). In addition, the anions can chelate Zn and enhance its solubility (Jones and Darrah 1994). Pseudomonas, Bacillus, and Serratia species have been reported as Zn-solubilizing PGPR to increase Zn uptake and nutrition in corn, soybean, wheat, and rice (Vaid et al. 2014; Pawar et al. 2015; Kamran et al. 2017). These reports confirm that PGPR isolates may have one or several mechanism(s) to alleviate HM toxicity, such as metal-detoxification, biosorption, bioaccumulation, bioleaching, bioexclusion, metal-solubilization, acidification, protonation, chelation, and metal-immobilization (Sorour et al. 2022).
In the current investigation, our objective was to figure out the ecophysiological mechanisms that underlie the interactions between plants and microorganisms in the presence of natural HM pollution. In general, HM toxicity in plants leads to notable damage to the cell membranes, resulting in decreased RWC, EL and MSI and elevated levels of H2O2, MDA, and O2•– in the leaves of pepper plants (Rady et al. 2023). These damages can be intensified as the concentrations of HM increase. Pepper plants (control) grown in soils contaminated with HM without the supplementation of any PGPR showed decreased RWC and MSI but increased levels of EL, MDA, H2O2, and O2•– (Table 3). Our results align with those reported by Rady et al. (2023) that the detrimental impacts could be attributed to ROS production. Thus, the harmful effect of ROS may interact with lipids, proteins and nucleic acids to trigger lipid peroxidation, impair membrane integrity, deactivate enzymes, disrupt the structure of membrane transporters, and consequently affect cell viability and function (Jung et al. 2021).
Application of the HMT PGPR showed completely opposite patterns in the abovementioned attributes. The combined application of Pa+Sr+Pp+Bv increased RWC in pepper plants by about 52%, and MSI by 39% (Table 3). The same treatment decreased EL by almost 55%, MDA by 72%, H2O2 by 71%, and O2•– by 56% compared to control plants in the two growing seasons of pepper. According to Rahman et al. (2022), our data showed that antioxidant defense mechanisms resulted from PGPR might have contributed to the mitigation of HM stress in plants.
The HM stress-induced EL is usually accompanied with ROS accumulation (Demidchik et al. 2014). The present study observed a significant increase (up to 51%) in membrane EL under HM stress in the control treatment (Table 3). The high levels of EL might be attributed to the increased production of ROS (Din et al. 2020). In radish plants, 18% EL was released after inoculation of PGPR under HM stress conditions (Ahmad et al. 2018). The findings of our study presented evidence that the PGPR strains under investigation are capable of coping with the effects of HM stress, presumably through the enhancement of MSI.
In the current investigation, inoculating soil containing pepper seedlings with individual or consortia of PGPR significantly reduced MDA content (Table 3) compared to their non-inoculated soils. Similarly, under HM stress conditions, Ahmad et al. (2018) showed that radish plants inoculated with PGPR had lower MDA levels. In HM-stressed plants, MDA production is often linked with membrane lipid peroxidation caused by oxidative stress (Biswas and Mano 2021). The MDA content reacts with amino groups, resulting in the degradation of proteins and macromolecules, as well as peroxidation of lipids, leading to necrosis (Islam et al. 2016).
Effect of soil inoculation with HMT bacteria on leaf osmo-protection and antioxidant activities in HM-stressed pepper plants
Pepper plants cultivated in soils lacking HMT bacteria exhibited higher levels of antioxidant enzymes and osmoprotectants than pepper plants grown in soils supplied with HMT bacteria (Table 4). The decreased profiles of oxidative stress and/or ROS-scavenging enzymes were previously remarked by Sapre et al. (2018), Alexander et al. (2020), and Shahid et al. (2021).
The osmo-protection and antioxidant activities in control pepper plats under HM toxicity without the supplementation of bacteria, significantly (P<0.05) increased in leaves (Table 4; Fig 2). In contrast, PGPR application significantly (P<0.05) decreased free Pro, TSS, α-TOC contents, and the non-enzymatic AsA and GSH contents (Table 4). Furthermore the application of PGPR significantly (P<0.05) decreased the enzymatic antioxidant activities (CAT, POD, APX, SOD and GR; Fig. 2) in HM-stressed-plants.
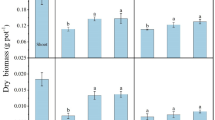
Effect of soil inoculation with HM-tolerant PGPR on antioxidant enzymes of pepper plants under HM stress inoculated with or without Pa, Sr, Pp and Bv in Season I and II. Data are means (n=3) ± SE. Values of different letters indicate significant differences among individual or consortia of isolates in a particular season according to the least significant difference (LSD) test at P≤0.05. HM, heavy metal; (a) catalase (CAT); (b) peroxidase (POD); (c) ascorbate peroxidase (APX); (d) superoxide dismutase (SOD); (e) glutathione reductase (GR), Pseudomonas azotoformans (Pa); Serratia rubidaea (Sr); Paenibacillus pabuli (Pp); and Bacillus velezensis (Bv)
This reduction in the current study was relatively superior in the combined treatment of the four tested PGPR. This was evident with the reduced levels of free Pro (32.4-32.7%), TSS (34.8-35.5%), α-Toc (52.1-52.9%), AsA (59-60%), and GSH (60.0-63.9%) in the consortia of the four isolates in both seasons (Table 4). CAT, POD, APX, SOD, and GR were also reduced by approximately 50, 70, 57, 54% and 67%, respectively, in pepper plants stressed with HM and cultivated in soils inoculated with the PGPR, compared to plants grown in non-PGPR inoculated soils (control; Fig. 2).
Our results are in accordance with Kang et al. (2014) who reported that the activities of CAT, POD, and polyphenol oxidase in cucumber (Cucumis sativus) grown in soils inoculated with Burkholdera cepacia SE4, Promicromonospora sp. SE188, and Acinetobacter calcoaceticus SE370 were found to be lower than cucumber grown in soils not inoculated with these bacteria in response to salt and osmotic stress. Similarly, our results are in agreement with Manaf and Zayed (2015) who also found that SOD activity and Pro content in cowpea plants treated with mycorrhizae or Pseudomonas fluorescence alone were lower than those in untreated plants. It was thought that the negative consequences of excessive HM caused plants to lose the ability to control their metabolites.
The reduction in Pro and antioxidant enzymes seen in maize plants treated with Bacillus in HM contaminated soil may have been brought about by the development of extracellular polysaccharides (EPS) and biofilm on the surface of the roots (Misra and Chauhan 2020). This prevented the plants from absorbing excessive levels of HM, hence reducing the negative impacts of hazardous ions on the plants. Furthermore, reduced levels of enzymatic and non-enzymatic antioxidants, as well as osmoregulators, were found in the oat tissues of Klebsiella treated plants, suggesting that they did not perceive stress as strongly as untreated plants under salinity stress conditions (Sapre et al. 2018). It was also reported that the increased levels of α-TOC in spinach plants grown in soils not inoculated with heavy metal–resistant bacteria can be attributed to its role as a component of the defense mechanisms to combat oxidative stress (Eltahawy et al. 2022).
In the current study, pepper plants cultivated in soils without HMT bacteria exhibited higher levels of AsA than pepper plants grown in soils supplied with HMT bacteria (Table 4). The incorporation of HMT PGPR in the HM polluted-soil inhibited the enzymatic activities in pepper plants (Table 4). It is well known that the endogenous production of AsA and GR increased the ROS-scavenging and HM-chelating capabilities in response to abiotic stresses (Desoky et al. 2019). In general, these components can help plants build complex antioxidant defense mechanisms against the oxidative stress generated by HM to attenuate and repair the ROS-induced damage (Rady et al. 2021). Moreover, AsA is a powerful antioxidant that helps in the protection from ROS damage (Kakan et al. 2021). APX uses AsA to oxidize MDA to dehydroascorbate (DHA); thus, reducing the effect of ROS (McClean and Davison 2022). AsA may also play a role as the "terminal antioxidant", leading to low redox potential of radicals (Rodríguez-Ruiz et al. 2019).
AsA synthesis from hexose phosphate and its effect on plant defense against the photo-oxidative stress suggest that there may be links between AsA and photosynthesis (Mukarram et al. 2021). In cucumber plants cultivated in HM-contaminated soils, the application of AsA increased plant tolerance to HM by improving their photosynthetic efficiency, growth, and antioxidant activities (Ghosh et al. 2022). This was also associated with reduced levels of HM in roots and leaves. AsA can maintain normal metabolic activities in stressed plants through osmotic modulation and cellular compatibility as suggested by Ghosh et al. (2022).
Table 4 shows that pepper plants cultivated in soils deficient in HMT bacteria had far greater levels of Pro than pepper plants grown in soils supplemented with HMT bacteria. Our results coincide with Alam et al. (2019) who repropted that the accumulation of Pro in tissues is one of the most important adaptations in plants to HM stress. Pro content restores the integrity of cell membranes and increases the enzymes present in AsA–GSH cycle in response to HM stress in tobacco (Khatun et al. 2020). As an efficient antioxidant defense component, Pro might also be involved in enzymatic and non-enzymatic antioxidant activities. It has been reported that Pro can decrease HM toxicity by eliminating toxins from ROS and boosting ASA and GSH levels, as well as enzymatic activities to induce gene expression machinery and antioxidant defense (Ghosh et al. 2022). In addition, Pro can react with Cd+2 ions in plants to form non-toxic compounds (Sharma and Devi 2010). It can also help protect plant cell membranes from damages caused by HM or EL (Aman Shamil 2022).
The increased enzymatic activity can alleviate the oxidative damage induced by HM (Desoky et al. 2019). ROS overproduction is a frequent response to various stimuli, including HM. In response to HM, the enzymatic (SOD, CAT, APX, POD, and GR) and the non-enzymatic antioxidants (Pro, α-TOC, and GSH) regulate ROS levels in plant tissues (Tripthi et al. 2020). The present investigation is the first study showing that HMT isolates of Pa, Sr, Pp and Bv had the ability to decrease enzymatic and non-enzymatic antioxidant activities in pepper plants under HM stress conditions.
Effect of soil inoculation with HMT bacteria on HM contaminants in plant tissues in response to HM stress
There was a significant (P>0.05) difference in the accumulation of Cd, Cu, Pb and Ni in the leaves and fruits of pepper plants cultivated in a soil contaminated with HM (Table 5). The rate of accumulation of these elements in the fruits was higher in control treatment (8.52-8.57, 47.0-47.8, 26.8-27.4, 12.5-12.7 mg kg-1 for Cd, Cu, Pb, and Ni, respectively) in both SI and SII seasons. Except of Cu, the concentrations of the other HM (Cd, Pb, and Ni) were higher than the accepted (0.8-1.0 mg kg-1; Luo et al. (2019). All single or combined treatments of PGPR significantly reduced HM concentrations relative to the control group (Table 5).
By far, the consortia of PGPR treatment performed the best among all other treatments, by decreasing Cd content (88.0-88.5% in leaves, and 87.2-88.1% in fruits), Cu (63.8-66.5% in leaves, and 69.4-70.0% in fruits), Pb (66.2-67.0% in leaves, and 80.0-81.3% in fruits), and Ni (90.2-90.9% in leaves, and 92.3% in fruits) (Table 5).
In response to HM stress, plant cells are unable to maintain lower levels of Cd+2 or Pb+2 through effective detoxification mechanisms; thus, resulting in oxidative damage to different cellular constituents (Desoky et al. 2019), and reduction in plant growth (Table 1). The plants employ adaptive mechanisms to reduce HM absorption and translocation towards their roots and stems in response to stressful conditions (Abdul 2010). In the current investigation, soil inoculation with HMT PGPR led to a decrease in HM accumulation in leaves and fruits of pepper (Table 5). Our results are in accordance with Rizvi and Khan (2018) who showed that inoculation of corn plants with Azotobacter chroococcum resulted in a reduction in Cu and Pb accumulation in plant tissues. This was most likely due to the release of different metabolites, protons, and exudates that act as metal chelators and limit Pb immobilization. Consistent with the findings of the present study, Bacillus megaterium and Exiguobacterium, which colonized the root surfaces of Vigna radiata, respectively, demonstrated decreased Ni and As translocation (Rajkumar et al. 2013; Pandey and Bhatt 2016).
At the molecular level, PGPR can induce biotransformation of As in wheat plants, through the upregulation of arsC, aioA, and arsM genes (Gu et al. 2017). In addition, metal homeostasis in plants is predominantly governed by transporters, which are bioavailable in relation to the metals (Noor et al. 2022). Although Cd contamination induced the expression of metal transporters in plant tissues, PGPR reduced the transcript levels (Chen et al. 2017). Metal chelators, which are typically composed of sulfhydryl (-SH) groups, immobilize metals through effective binding.
The current research also aligns with the investigations conducted by Mahmoud et al. (2021) regarding Scenedemus bijugatu-maize interaction under Cu stress. Furthermore, Cu accumulation regulates the levels of glutathione, thereby promoting metal binding and metal sequestration (Nagalakshmi and Prasad 2001). Inoculation with P. putida into rice plants exposed to As toxicity resulted in increased concentrations of glutathione, non-protein thiols, and phytochelatins (Awasthi et al. 2018), thus promoting the biosynthesis of metal-chelating compounds.
In general, PGPR-mediated resilience to interacting effects of HM stress in plants is increasingly acknowledged as a potent strategy for stress mitigation (Oubohssaine et al. 2022; Patil et al. 2023). At the top of our list of priorities is the investigation of potential future directions that focus on clarifying the mechanism that is responsible for reducing the toxicity of HM in pepper plants by the PGPR that was tested.
Conclusion
Land deterioration and metal pollution pose substantial problems to global food security. The current study demonstrated that bioremediation using PGPR was a viable method for reducing damage in pepper plants grown under HM stress conditions. This was associated with significant decreases in Cd, Cu, Pb, and Ni absorption and/or translocation to higher parts of plants. Our findings showed that inoculating soil containing pepper seedlings with bacterial strains (Pa, Sr, Pp, and Bv) promotes HM assimilation. The deployment of HMT PGPR consortia promotes food security and sustainable agricultural production. In this context, the application of stress tolerant PGRP in an appropriate manner for the purpose of remediating contaminated soils of HM could make a significant contribution to the achievement of this goal.
Data availability
Data will be made available on request.
References
Abdul G (2010) Effect of lead toxicity on growth, chlorophyll and lead (Pb+) contents of two varieties of maize (Zea mays L.). Pak J Nutr 9:887–891
Ahmad I, Akhtar MJ, Mehmood S, Akhter K, Tahir M, Saeed MF, Hussain MB, Hussain S (2018) Combined application of compost and Bacillus sp. CIK-512 ameliorated the lead toxicity in radish by regulating the homeostasis of antioxidants and lead. Ecotoxicol Environ Saf 148:805–812. https://doi.org/10.1016/j.ecoenv.2017.11.054
Ahmad P, Jaleel CA, Azooz MM, Nabi G (2009) Generation of ROS and non-enzymatic antioxidants during abiotic stress in plants. Bot Res Intern 2:11–20
Alam MZ, McGee R, Hoque MA, Ahammed GJ, Carpenter-Boggs L (2019) Effect of arbuscular mycorrhizal fungi, selenium and biochar on photosynthetic pigments and antioxidant enzyme activity under arsenic stress in mung bean (Vigna radiata). Front Physiol 10:193. https://doi.org/10.3389/fphys.2019.00193
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9:42. https://doi.org/10.3390/toxics9030042
Alexander A, Singh VK, Mishra A (2020) Halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front Microbiol 11:568289. https://doi.org/10.3389/fmicb.2020.568289
Alexander M (1997) Introduction to Soil Microbiology. John Wiley and Sons, New York, USA, p 467
Ali J, Ali F, Ahmad I, Rafique M, Munis MFH, Hassan SW, Sultan T, Iftikhar M, Chaudhary HJ (2021) Mechanistic elucidation of germination potential and growth of Sesbania sesban seedlings with Bacillus anthracis PM21 under heavy metals stress: An in vitro study. Ecotoxicol Environ Saf 208:111769. https://doi.org/10.1016/j.ecoenv.2020.111769
Alwahshi KJ, Purayil GP, Saeed EE, Abufarajallah HA, Aldhaheri SJ, AbuQamar SF, El-Tarabily KA (2022) The 1-aminocyclopropane-1-carboxylic acid deaminase-producing Streptomyces violaceoruber UAE1 can provide protection from sudden decline syndrome on date palm. Front Plant Sci 13:904166. https://doi.org/10.3389/fpls.2022.904166
Aman Shamil N (2022) Role of exogenous application of proline and glycine betaine in the salinity tolerance of Solanaceae family: a review. Acta Sci Agric 6:46–54. https://doi.org/10.31080/ASAG.2022.06.1179
Ashfaq MY, Da’na DA, Al-Ghouti MA (2022) Application of MALDI-TOF MS for identification of environmental bacteria: a review. J Environ Manag 305:114359. https://doi.org/10.1016/j.jenvman.2021.114359
Ashry NM, Alaidaroos BA, Mohamed SA, Badr OAM, El-Saadony MT, Esmael A (2022) Utilization of drought-tolerant bacterial strains isolated from harsh soils as a plant growth-promoting rhizobacteria (PGPR). Saudi J Biol Sci 29:1760–1769. https://doi.org/10.1016/j.sjbs.2021.10.054
Awasthi S, Chauhan R, Dwivedi S, Srivastava S, Srivastava S, Tripathi RD (2018) A consortium of alga (Chlorella vulgaris) and bacterium (Pseudomonas putida) for amelioration of arsenic toxicity in rice: A promising and feasible approach. Environ Exp Bot 150:115–126. https://doi.org/10.1016/j.envexpbot.2018.03.001
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428. https://doi.org/10.1071/BI9620413
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bhaduri AM, Fulekar MH (2012) Antioxidant enzyme responses of plants to heavy metal stress. Rev Environ Sci Biotechnol 11:55–69. https://doi.org/10.1007/s11157-011-9251-x
Bhat MA, Mishra AK, Jan S, Bhat MA, Kamal MA, Rahman S, Shah AA, Jan AT (2023) Plant growth promoting rhizobacteria in plant health: A perspective study of the underground interaction. Plants 12:629. https://doi.org/10.3390/plants12030629
Bhuyan B, Kotoky R, Maheshwari DK, Pandey P (2022) Rhizoremediation of Cd-contaminated soil using Zea mays Sturt, with heavy metal resistant rhizobacteria that alleviate Cd-induced stress in plant. Environ Sustain 5:375–387. https://doi.org/10.1007/s42398-022-00241-w
Bille E, Dauphin B, Leto J, Bougnoux ME, Beretti JL, Lotz A, Suarez S, Meyer J, Lambert OJ, Descamps P, Grall N, Mory F, Dubreuil L, Berche P, Nassif X, Ferroni A (2012) MALDI-TOF MS Andromas strategy for the routine identification of bacteria, mycobacteria, yeasts, Aspergillus spp. and positive blood cultures. Clin Microbiol Infect 18:1117–1125. https://doi.org/10.1111/j.1469-0691.2011.03688.x
Biswas MS, Mano J (2021) Lipid peroxide-derived reactive carbonyl species as mediators of oxidative stress and signaling. Front Plant Sci 12:720867. https://doi.org/10.3389/fpls.2021.720867
Black CA (1958) Soil-plant relationships. Soil Sci 85:175. https://doi.org/10.1097/00010694-195803000-00023
Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64:3663–3668. https://doi.org/10.1128/AEM.64.10.3663-3668.1998
Busnelli MP, Lazzarini Behrmann IC, Ferreira ML, Candal RJ, Ramirez SA, Vullo DL (2021) Metal- Pseudomonas veronii 2E interactions as strategies for innovative process developments in environmental biotechnology. Front Microbiol 12:622600. https://doi.org/10.3389/fmicb.2021.622600
Chen LL, Wang HY, Gong XC, Zeng ZH, Xue XZ, Hu YG (2021) Transcriptome analysis reveals effects of red and blue light-emitting diodes (LEDs) on the growth, chlorophyll fluorescence and endogenous plant hormones of potato (Solanum tuberosum L.) plantlets cultured in vitro. J Integr Agric 20:2914–2931. https://doi.org/10.1016/S2095-3119(20)63393-7
Chen XX, Liu YM, Zhao QY, Cao WQ, Chen XP, Zou CQ (2020) Health risk assessment associated with heavy metal accumulation in wheat after long-term phosphorus fertilizer application. Environ Pollut 262:114348. https://doi.org/10.1016/j.envpol.2020.114348
Chen Y, Hua CY, Jia MR, Fu JW, Liu X, Han YH, Liu Y, Rathinasabapathi B, Cao Y, Ma LQ (2017) Heterologous expression of Pteris vittata arsenite antiporter PvACR3; 1 reduces arsenic accumulation in plant shoots. Environ Sci Technol 51:10387–10395. https://doi.org/10.1021/acs.est.7b03369
Cheng B, Wang Z, Yan X, Yu Y, Liu L, Gao Y, Zhang H, Yang X (2023) Characteristics and pollution risks of Cu, Ni, Cd, Pb, Hg and As in farmland soil near coal mines. SEH. 1:100035. https://doi.org/10.1016/j.seh.2023.100035
Ching LS, Mohamed S (2001) Alpha-tocopherol content in 62 edible tropical plants. J Agric Food Chem 49:3101–3105. https://doi.org/10.1021/jf000891u
Cuypers A, Vanbuel I, Iven V, Kunnen K, Vandionant S, Huybrechts M, Hendrix S (2023) Cadmium-induced oxidative stress responses and acclimation in plants require fine-tuning of redox biology at subcellular level. Free Radic Biol Med 199:81–96. https://doi.org/10.1016/j.freeradbiomed.2023.02.010
Demidchik V, Straltsova D, Medvedev SS, Pozhvanov GA, Sokolik A, Yurin V (2014) Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J Exp Bot 65:1259–1270. https://doi.org/10.1093/jxb/eru004
Desoky E-SM, Elrys AS, Rady MM (2019) Integrative moringa and licorice extracts application improves Capsicum annuum fruit yield and declines its contaminant contents on a heavy metals-contaminated saline soil. Ecotoxicol Environ Saf 169:50–60. https://doi.org/10.1016/j.ecoenv.2018.10.117
Desoky E-SM, Merwad AM, Semida WM, Ibrahim SA, El-Saadony MT, Rady MM (2020) Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol Environ Saf 198:110685. https://doi.org/10.1016/j.ecoenv.2020.110685
Devireddy AR, Zandalinas SI, Fichman Y, Mittler R (2021) Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J 105:459–476. https://doi.org/10.1111/tpj.15010
Din BU, Amna RM, Javed MT, Kamran MA, Mehmood S, Khan M, Sultan T, Hussain Munis MF, Chaudhary HJ (2020) Assisted phytoremediation of chromium spiked soils by Sesbania sesban in association with Bacillus xiamenensis PM14: a biochemical analysis. Plant Physiol Biochem 146:249–258. https://doi.org/10.1016/j.plaphy.2019.11.010
Dong MF, Feng R, Wang R, Sun Y, Ding YZ, Xu YM, Fan Z, Guo J (2016) Inoculation of Fe/Mn-oxidizing bacteria enhances Fe/Mn plaque formation and reduces Cd and As accumulation in rice plant tissues. Plant Soil 404:75–83. https://doi.org/10.1007/s11104-016-2829-x
Dutta D, Pal AK (2019) Physiological basis of cadmium tolerance in groundnut (Arachis hypogaea L.). J Pharmacogn Phytochem 8:548–552
Duxbury T (1981) Toxicity of heavy metals to soil bacteria. FEMS Microbiol Lett 11:217–220. https://doi.org/10.1111/j.1574-6968.1981.tb06967.x
Efe D (2020) Potential plant growth-promoting bacteria with heavy metal resistance. Curr Microbiol 77:3861–3868. https://doi.org/10.1007/s00284-020-02208-8
El-Meihy RM, Abou-Aly HE, Youssef AM, Tewfike TA, El-Alkshar EA (2019) Efficiency of heavy metals-tolerant plant growth promoting bacteria for alleviating heavy metals toxicity on sorghum. Environ Exp Bot 162:295–301. https://doi.org/10.1016/j.envexpbot.2019.03.005
Elnahal AS, El-Saadony MT, Saad AM, Desoky E-SM, El-Tahan AM, Rady MM, AbuQamar SF, El-Tarabily KA (2022) The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Eur J Plant Pathol 162:759–792. https://doi.org/10.1007/s10658-021-02393-7
El-Saadony MT, Desoky E-SM, Saad AM, Eid RSM, Selem E, Elrys AS (2021) Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J Environ Sci 106:1–14. https://doi.org/10.1016/j.jes.2021.01.012
Eltahawy AMAE, Awad EAM, Ibrahim AH, Merwad AMA, Desoky E-SM (2022) Integrative application of heavy metal–resistant bacteria, moringa extracts, and nano-silicon improves spinach yield and declines its contaminant contents on a heavy metal–contaminated soil. Front Plant Sci 13:1019014. https://doi.org/10.3389/fpls.2022.1019014
El-Tarabily KA, AlKhajeh AS, Ayyash MM, Alnuaimi LH, Sham A, ElBaghdady KZ, Saeed T, AbuQamar SF (2019) Growth promotion of Salicornia bigelovii by Micromonospora chalcea UAE1, an endophytic 1-aminocyclopropane-1-carboxylic acid deaminase-producing actinobacterial isolate. Front Microbiol 10:1694. https://doi.org/10.3389/fmicb.2019.01694
El-Tarabily KA, ElBaghdady KZ, AlKhajeh AS, Ayyash M, Aljneibi RS, El-Keblawy A, AbuQamar SF (2020) Polyamine-producing actinobacteria enhance biomass production and seed yield in Salicornia bigelovii. Biol Fertil Soils 56:499–519. https://doi.org/10.1007/s00374-020-01450-3
El-Tarabily KA, Sham A, Elbadawi AA, Hassan AH, Alhosani BKK, El-Esawi MA, AlKhajeh AS, AbuQamar SF (2021) A consortium of rhizosphere-competent actinobacteria exhibiting multiple plant growth-promoting traits improves the growth of Avicennia marina in the United Arab Emirates. Front Mar Sci 8:715123. https://doi.org/10.3389/fmars.2021.715123
Fadeel AA (1962) Location and properties of chloroplasts and pigment determination in roots. Physiol Plant 15:130–146. https://doi.org/10.1111/j.1399-3054.1962.tb07994.x
Faize M, Faize L, Petri C, Barba-Espin G, Diaz-Vivancos P, Clemente-Moreno MJ, Koussa T, Rifai LA, Burgos L, Hernandez JA (2013) Cu/Zn superoxide dismutase and ascorbate peroxidase enhance in vitro shoot multiplication in transgenic plum. J Plant Physiol 170:625–632. https://doi.org/10.1016/j.jplph.2012.12.016
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187:201. https://doi.org/10.1007/s10661-015-4436-3
Ghori N-H, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16:1807–1828. https://doi.org/10.1007/s13762-019-02215-8
Ghosh UK, Islam MN, Siddiqui MN, Cao X, Khan MAR (2022) Proline, a multifaceted signalling molecule in plant responses to abiotic stress: understanding the physiological mechanisms. Plant Biol 24:227–239. https://doi.org/10.1111/plb.13363
Górski F, Gerotti GM, Gonçalves JE, Gazim ZC, Magalhães HM (2023) Methyl jasmonate and copper activate volatiles and antioxidant mechanisms in 'Grecco a Palla' basil produced in vitro. J Crop Sci Biotechnol 26:615. https://doi.org/10.1007/s12892-023-00206-3
Gu CS, Liu LQ, Deng YM, Zhang YX, Wang ZQ, Yuan HY, Huang SZ (2017) De novo characterization of the Iris lactea var. chinensis transcriptome and an analysis of genes under cadmium or lead exposure. Ecotoxicol Environ Saf 144:507–513. https://doi.org/10.1016/j.ecoenv.2017.06.071
Guo J, Qin S, Rengel Z, Gao W, Nie Z, Liu H, Li C, Zhao P (2019) Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol Environ Saf 172:380–387. https://doi.org/10.1016/j.ecoenv.2019.01.069
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2023) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
He F, Zhao L, Zheng X, Abdelhai MH, Boateng NS, Zhang X, Zhang H (2020) Investigating the effect of methyl jasmonate on the biocontrol activity of Meyerozyma guilliermondii against blue mold decay of apples and the possible mechanisms involved. Physiol Mol Plant Pathol 109:101454. https://doi.org/10.1016/j.pmpp.2019.101454
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Hniličková H, Hnilička F, Orsák M, Hejnák V (2019) Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ 65:90–96. https://doi.org/10.17221/620/2018-PSE
Hussein H-AA, Darwesh OM, Mekki BB, El-Hallouty SM (2019) Evaluation of cytotoxicity, biochemical profile and yield components of groundnut plants treated with nano-selenium. Biotechnol Rep 24:e00377. https://doi.org/10.1016/j.btre.2019.e00377
Irigoyen J, Einerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Islam F, Yasmeen T, Ali Q, Mubin M, Ali S, Arif MS, Hussain S, Riaz M, Abbas F (2016) Copper-resistant bacteria reduces oxidative stress and uptake of copper in lentil plants: potential for bacterial bioremediation. Environ Sci Pollut Res Int 23:220–233. https://doi.org/10.1007/s11356-015-5354-1
Jackson M (1958) Soil chemical analysis. Prentice-Hall, Inc., Englewood Cliffs, NJ, p 498
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257. https://doi.org/10.1007/BF00008338
Jung HI, Lee TG, Lee J, Chae MJ, Lee EJ, Kim MS, Jung GB, Emmanuel A, Jeon S, Lee BR (2021) Foliar-applied glutathione mitigates cadmium-induced oxidative stress by modulating antioxidant-scavenging, redox-regulating, and hormone-balancing systems in Brassica napus. Front Plant Sci 12:700413. https://doi.org/10.3389/fpls.2021.700413
Kakan X, Yu Y, Li S, Li X, Huang R, Wang J (2021) Ascorbic acid modulation by ABI4 transcriptional repression of VTC2 in the salt tolerance of Arabidopsis. BMC Plant Biol 21:112. https://doi.org/10.1186/s12870-021-02882-1
Kamran MA, Bibi S, Xu RK, Hussain S, Mehmood K, Chaudhary HJ (2017) Phyto-extraction of chromium and influence of plant growth promoting bacteria to enhance plant growth. J Geochem Explor 182:269–274. https://doi.org/10.1016/j.gexplo.2016.09.005
Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, Hamayun M, Lee IJ (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9:673–682. https://doi.org/10.1080/17429145.2014.894587
Kaur J, Pandove G (2023) Understanding the beneficial interaction of plant growth promoting rhizobacteria and endophytic bacteria for sustainable agriculture: a bio-revolution approach. J Plant Nutr 202:3569–3597. https://doi.org/10.1080/01904167.2023.2206425
Khan MIR, Jahan B, AlAjmi MF, Rehman MT, Iqbal N, Irfan M, Sehar Z, Khan NA (2021) Crosstalk of plant growth regulators protects photosynthetic performance from arsenic damage by modulating defense systems in rice. Ecotoxicol Environ Saf 222:112535. https://doi.org/10.1016/j.ecoenv.2021.112535
Khan N, Ryu KY, Choi JY, Nho EY, Habte G, Choi H, Kim MH, Park KS, Kim KS (2015) Determination of toxic heavy metals and speciation of arsenic in seaweeds from South Korea. Food Chem 169:464–470. https://doi.org/10.1016/j.foodchem.2014.08.020
Khatun M, Matsushima D, Rhaman MS, Okuma E, Nakamura T, Nakamura Y, Munemasa S, Murata Y (2020) Exogenous proline enhances antioxidant enzyme activities but does not mitigate growth inhibition by selenate stress in tobacco BY-2 cells. Biosci Biotechnol Biochem 84:2281–2292. https://doi.org/10.1080/09168451.2020.1799747
Khoso MA, Wagan S, Alam I, Hussain A, Ali Q, Saha S, Poudel TR, Manghwar H, Liu F (2024) Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress 11:100341. https://doi.org/10.1016/j.stress.2023.100341
Kobya M, Demirbas E, Senturk E, Ince M (2005) Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresour Technol 96:1518–1521. https://doi.org/10.1016/j.biortech.2004.12.005
Kohli SK, Handa N, Gautam V, Bali S, Sharma A, Khanna K, Arora S, Thukral AK, Ohri P, Karpets YV, Kolupaev YE, Bhardwaj R (2017) ROS signaling in plants under heavy metal stress. In: Khan M, Khan N (eds) Reactive oxygen species and antioxidant systems in plants: Role and regulation under abiotic stress. Springer, Singapore, pp 185–214. https://doi.org/10.1007/978-981-10-5254-5_8
Konings EJ, Roomans HH, Beljaars PR (1996) Liquid chromatographic determination of tocopherols and tocotrienols in margarine, infant foods, and vegetables. J AOAC Int 79:902–906. https://doi.org/10.1093/jaoac/79.4.902
Kowalska-Krochmal B, Dudek-Wicher R (2021) The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 10:165. https://doi.org/10.3390/pathogens10020165
Kubiś J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406. https://doi.org/10.1016/j.jplph.2007.02.005
Kumari S, Mishra A (2021) Heavy metal contamination. In: Larramendy ML, Soloneski S (eds) Soil contamination-threats and sustainable solutions. IntechOpenLimited, London, United Kingdom. https://doi.org/10.5772/intechopen.93412
Lamhamdi M, El Galiou O, Bakrim A, Novoa-Munoz JC, Arias-Estevez M, Aarab A, Lafont R (2013) Effect of lead stress on mineral content and growth of wheat (Triticum aestivum) and spinach (Spinacia oleracea) seedlings. Saudi J Biol Sci 20:29–36. https://doi.org/10.1016/j.sjbs.2012.09.001
Li S, Zhao B, Jin M, Hu L, Zhong H, He Z (2020) A comprehensive survey on the horizontal and vertical distribution of heavy metals and microorganisms in soils of a Pb/Zn smelter. J Hazard Mater 400:123255. https://doi.org/10.1016/j.jhazmat.2020.123255
Li Y, Ye Z, Yu Y, Li Y, Jiang J, Wang L, Wang G, Zhang H, Li N, Xie X, Cheng X, Liu K, Liu M (2023) A combined method for human health risk area identification of heavy metals in urban environments. J Hazard Mater 449:131067. https://doi.org/10.1016/j.jhazmat.2023.131067
Liu J, Lu B, Xun AL (2000) An improved method for the determination of hydrogen peroxide in leaves. Prog Biochem Biophys 27:548–551
Logan NA, De Vos P (2009) Genus I. Bacillus Cohn 1872, 174AL. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (eds) Bergey's Manual of Systematic Bacteriology, vol 3, 2nd edn. Springer, New York, pp 21–128
Luo X, Bing H, Luo Z, Wang Y, Jin L (2019) Impacts of atmospheric particulate matter pollution on environmental biogeochemistry of trace metals in soil-plant system: a review. Environ Pollut 255:113138. https://doi.org/10.1016/j.envpol.2019.113138
Madhu PM, Sadagopan RS (2020) Effect of heavy metals on growth and development of cultivated plants with reference to cadmium, chromium and lead–a review. J Stress Physiol Biochem 16:84–102
Mahmoud A, AbdElgawad H, Hamed BA, Beemster GTS, El-Shafey NM (2021) Differences in cadmium accumulation, detoxification and antioxidant defenses between contrasting maize cultivars implicate a role of superoxide dismutase in Cd tolerance. Antioxidants 10:1812. https://doi.org/10.3390/antiox10111812
Manaf HH, Zayed MS (2015) Productivity of cowpea as affected by salt stress in presence of endomycorrhizae and Pseudomonas fluorescens. Ann Agric Sci 60:219–226. https://doi.org/10.1016/j.aoas.2015.10.013
Mantelin S, Desbrosses G, Larcher M, Tranbarger TJ, Cleyet-Marel JC, Touraine B (2006) Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta 223:591–603
Manzoor N, Ali L, Ahmed T, Noman M, Adrees M, Shahid MS, Ogunyemi SO, Radwan KSA, Wang G, Zaki HEM (2022) Recent advancements and development in nano-enabled agriculture for improving abiotic stress tolerance in plants. Front Plant Sci 13:951752. https://doi.org/10.3389/fpls.2022.951752
Maruta T, Ishikawa T (2022) Analysis of ascorbate metabolism in Arabidopsis under high-light stress. Methods Mol Biol 2526:15–24. https://doi.org/10.1007/978-1-0716-2469-2_2
Mathew BT, Torky Y, Amin A, Mourad A-HI, Ayyash MM, El-Keblawy A, Hilal-Alnaqbi A, AbuQamar SF, El-Tarabily KA (2020) Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front Microbiol 11:552. https://doi.org/10.3389/fmicb.2020.00552
McClean C, Davison GW (2022) Circadian clocks, redox homeostasis, and exercise: Time to connect the dots? Antioxidants. 11:256. https://doi.org/10.3390/antiox11020256
Misra S, Chauhan PS (2020) ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 10:119. https://doi.org/10.1007/s13205-020-2104-y
Mukarram M, Choudhary S, Kurjak D, Petek A, Khan MMA (2021) Drought: sensing, signalling, effects and tolerance in higher plants. Physiol Plant 172:1291–1300. https://doi.org/10.1111/ppl.13423
MuKherjee SP, Choudhuri MA (1983) Implication of water stress—induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedling. Physiol Plant 58:166–170. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x
Nagalakshmi N, Prasad MN (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299. https://doi.org/10.1016/s0168-9452(00)00392-7
Nagrale DT, Chaurasia A, Kumar S, Gawande SP, Hiremani NS, Shankar R, Gokte-Narkhedkar N, Renu PYG (2023) PGPR: the treasure of multifarious beneficial microorganisms for nutrient mobilization, pest biocontrol and plant growth promotion in field crops. World J Microbiol Biotechnol 39:100. https://doi.org/10.1007/s11274-023-03536-0
Nazli F, Wang X, Ahmad M, Hussain A, Bushra DA, Nasim M, Jamil M, Panpluem N, Mustafa A (2021) Efficacy of indole acetic acid and exopolysaccharides-producing Bacillus safensis strain FN13 for inducing Cd-stress tolerance and plant growth promotion in Brassica juncea (L.). Appl Sci 11:4160. https://doi.org/10.3390/app11094160
Noor I, Sohail H, Sun J, Nawaz MA, Li G, Hasanuzzaman M, Liu J (2022) Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 303:135196. https://doi.org/10.1016/j.chemosphere.2022.135196
Oubohssaine M, Sbabou L, Aurag J (2022) Native heavy metal-tolerant plant growth promoting rhizobacteria improves Sulla spinosissima (L.) growth in post-mining contaminated soils. Microorganisms 10:838. https://doi.org/10.3390/microorganisms10050838
Oyenike MA, Akpan HB, Otulana OJ, Adefule AK, Adedokun KA, Oluogun WA, Muhibi MA, Ojokuku HO (2019) In vitro anti-sickling and membrane stability potentials of Mishenland polyherbal extract on sickle red blood cells. Egy J Haematol 44:65–71. https://doi.org/10.4103/ejh.ejh_33_18
Pandey N, Bhatt R (2016) Role of soil associated Exiguobacterium in reducing arsenic toxicity and promoting plant growth in Vigna radiata. Eur J Soil Biol 75:142–150. https://doi.org/10.1016/j.ejsobi.2016.05.007
Patil S, Ansari A, Sarje A, Bankar A (2023) Heavy Metals Pollution and Role of Soil PGPR: A Mitigation Approach. In: Parray JA (ed) Climate Change and Microbiome Dynamics. Climate Change Management. Springer, Cham, pp 349–371. https://doi.org/10.1007/978-3-031-21079-2_18
Pawar A, Ismail S, Mundhe S, Patil VD (2015) Solubilization of insoluble zinc compounds by different microbial isolates in vitro condition. Int J Trop Agric 33:865–869
Premachandra GS, Saneoka H, Fujita K, Ogata S (1990) Cell membrane stability and leaf water relations as affected by phosphorus nutrition under water stress in maize. Soil Sci Plant Nutr 36:661–666. https://doi.org/10.1080/00380768.1990.10416803
Priyanka N, Geetha N, Manish T, Sahi S, Venkatachalam P (2021) Zinc oxide nanocatalyst mediates cadmium and lead toxicity tolerance mechanism by differential regulation of photosynthetic machinery and antioxidant enzymes level in cotton seedlings. Toxicol Rep 8:295–302. https://doi.org/10.1016/j.toxrep.2021.01.016
Rady MM, Alshallash KS, Desoky E-SM, Taie HA, Mohamed IA, El-Badri AM, Howladar SM, AbdelKhalik A (2023) Synergistic effect of trans-zeatin and silymarin on mitigation of cadmium stress in chili pepper through modulating the activity of antioxidant enzymes and gene expressions. J Appl Res Med 35:100498. https://doi.org/10.1016/j.jarmap.2023.100498
Rady MM, EL-Yazal MA, Taie HA, Ahmed SM (2021) Physiological and biochemical responses of wheat (Triticum aestivum L.) plants to polyamines under lead stress. Innovare J Agric Sci 9:1–10. https://doi.org/10.22159/ijags.2021.v9i1.40687
Rahman SU, Nawaz MF, Gul S, Yasin G, Hussain B, Li Y, Cheng H (2022) State-of-the-art OMICS strategies against toxic effects of heavy metals in plants: a review. Ecotoxicol Environ Saf 242:113952. https://doi.org/10.1016/j.ecoenv.2022.113952
Rajkumar M, Ma Y, Freitas H (2013) Improvement of Ni phytostabilization by inoculation of Ni resistant Bacillus megaterium SR28C. J Environ Manag 128:973–980. https://doi.org/10.1016/j.jenvman.2013.07.001
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136. https://doi.org/10.1104/pp.110.1.125
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20. https://doi.org/10.1016/j.ecoenv.2018.03.063
Rodríguez-Ruiz M, González-Gordo S, Cañas A, Campos MJ, Paradela A, Corpas FJ, Palma JM (2019) Sweet pepper (Capsicum annuum L.) fruits contain an atypical peroxisomal catalase that is modulated by reactive oxygen and nitrogen species. Antioxidants 8:374. https://doi.org/10.3390/antiox8090374
Santosh K (2013) Genetic variability studies in bell pepper (Capsicum annuum L.). Asian J Hort 8:280–284
Sapre S, Gontia-Mishra I, Tiwari S (2018) Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol Res 206:25–32. https://doi.org/10.1016/j.micres.2017.09.009
Sarkar A, Ghosh PK, Pramanik K, Mitra S, Soren T, Pandey S, Mondal MH, Maiti TK (2018) A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res Microbiol 169:20–32. https://doi.org/10.1016/j.resmic.2017.08.005
Shaffique S, Khan MA, Alomrani SO, Injamum-Ul-Hoque M, Peter O, Imran M, Kang SM, Lee I (2023) Unlocking the potential of newly isolated phytohormone-producing bacterial strains for enhanced plant growth and stress tolerance. Plant Stress 10:100260. https://doi.org/10.1016/j.stress.2023.100260
Shahid M, Ameen F, Maheshwari HS, Ahmed B, AlNadhari S, Khan MS (2021) Colonization of Vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, stressor metabolites, antioxidant status and yield under NaCl stress. Appl Soil Ecol 158:103809. https://doi.org/10.1016/j.apsoil.2020.103809
Shahid M, Singh UB, Khan MS, Singh P, Kumar R, Singh RN, Kumar A, Singh HV (2023) Bacterial ACC deaminase: Insights into enzymology, biochemistry, genetics, and potential role in amelioration of environmental stress in crop plants. Front Microbiol 14:1132770. https://doi.org/10.3389/fmicb.2023.1132770
Sharma RK, Devi S, dan Dhyani PP (2010) Comparative assessment of the toxic effects of copper and cypermethrin using seeds of Spinacia Oleracea L. plants. Trop Ecol 51:375–387.
Singh A, Mishra S, Choudhary M, Chandra P, Rai AK, Yadav RK, Sharma PC (2023) Rhizobacteria improve rice zinc nutrition in deficient soils. Rhizosphere 25:100646. https://doi.org/10.1016/j.rhisph.2022.100646
Sofy AR, Dawoud RA, Sofy MR, Mohamed HI, Hmed AA, El-Dougdoug NK (2020) Improving regulation of enzymatic and non-enzymatic antioxidants and stress-related gene stimulation in cucumber mosaic cucumovirus-infected cucumber plants treated with glycine betaine, chitosan and combination. Molecules. 25:2341. https://doi.org/10.3390/molecules25102341
Sorour AA, Khairy H, Zaghloul EH, Zaghloul HAH (2022) Microbe-plant interaction as a sustainable tool for mopping up heavy metal contaminated sites. BMC Microbiol 22:174. https://doi.org/10.1186/s12866-022-02587-x
Srivastava S, Srivastava S, Bist V, Awasthi S, Chauhan R, Chaudhry V, Singh PC, Dwivedi S, Niranjan A, Agrawal L, Chauhan PS, Tripathi RD, Nautiyal CS (2018) Chlorella vulgaris and Pseudomonas putida interaction modulates phosphate trafficking for reduced arsenic uptake in rice (Oryza sativa L.). J Hazard Mater 351:177–187. https://doi.org/10.1016/j.jhazmat.2018.02.039
Sun S, Xu X, Jiang X, Yue Y, Dai Y, Yang X, Xiu Q, Duan L, Zhao S (2023) Unveiling the neglected roles of chloride and sulfate in the removal of nitro compounds by sulfidated zero-valent iron/ferrous ion systems. ACS EST Water 3:1212–1222. https://doi.org/10.1021/acsestwater.2c00661.s001
Taie HAA, Seif El-Yazal MA, Ahmed SMA, Rady MM (2019) Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ Sci Pollut Res 26:22338–22350. https://doi.org/10.1007/s11356-019-05555-7
Tang Z, Wang HQ, Chen J, Chang JD, Zhao FJ (2023) Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J Integr Plant Biol 65:570–593. https://doi.org/10.1111/jipb.13440
Thiem D, Złoch M, Gadzała-Kopciuch R, Szymańska S, Baum C, Hrynkiewicz K (2018) Cadmium-induced changes in the production of siderophores by a plant growth promoting strain of Pseudomonas fulva. J Basic Microbiol 58:623–632. https://doi.org/10.1002/jobm.201800034
Topić Popović N, Kazazić SP, Bojanić K, Strunjak-Perović I, Čož-Rakovac R (2023) Sample preparation and culture condition effects on MALDI-TOF MS identification of bacteria: a review. Mass Spectrom Rev 42:1589–1603. https://doi.org/10.1002/mas.21739
Tripthi DK, Varma RK, Singh S, Sachan M, Guerriero G, Kushwaha BK, Bhardwaj S, Ramawat N, Sharma S, Singh VP, Prasad SM (2020) Silicon tackles butachlor toxicity in rice seedlings by regulating anatomical characteristics, ascorbate-glutathione cycle, proline metabolism and levels of nutrients. Sci Rep 10:14078. https://doi.org/10.1038/s41598-020-65124-8
Tsiasioti A, Tzanavaras PD (2023) Determination of glutathione and glutathione disulfide using liquid chromatography: A review on recent applications. Microchem J 193:109157. https://doi.org/10.1016/j.microc.2023.109157
Ur Rehman MZU, Zafar M, Waris AA, Rizwan M, Ali S, Sabir M, Usman M, Ayub MA, Ahmad Z (2020) Residual effects of frequently available organic amendments on cadmium bioavailability and accumulation in wheat. Chemosphere 244:125548. https://doi.org/10.1016/j.chemosphere.2019.125548
Vaid SK, Kumar B, Sharma A, Shukla AK, Srivastava PC (2014) Effect of zinc solubilizing bacteria on growth promotion and zinc nutrition of rice. J Soil Sci Plant Nutr 14:889–910. https://doi.org/10.4067/S0718-95162014005000071
Wang Q, Chen L, He LY, Sheng XF (2016) Increased biomass and reduced heavy metal accumulation of edible tissues of vegetable crops in the presence of plant growth-promoting Neorhizobium huautlense T1-17 and biochar. Agric Ecosyst Environ 228:9–18. https://doi.org/10.1016/j.agee.2016.05.006
Wu W, Chen W, Liu S, Wu J, Zhu Y, Qin L, Zhu B (2021) Beneficial relationships between endophytic bacteria and medicinal plants. Front Plant Sci 12:646146. https://doi.org/10.3389/fpls.2021.646146
Yin Y, Wang X, Hu Y, Li F, Cheng H (2023) Soil bacterial community structure in the habitats with different levels of heavy metal pollution at an abandoned polymetallic mine. J Hazard Mater 442:130063. https://doi.org/10.1016/j.jhazmat.2022.130063
Yu Y, Gui Y, Li Z, Jiang C, Guo J, Niu D (2022) Induced systemic resistance for improving plant immunity by beneficial microbes. Plants 11:386. https://doi.org/10.3390/plants11030386
Zainab N, Amna DBU, Javed MT, Afridi MS, Mukhtar T, Kamran MA, Ain QU, Khan AA, Ali J, Jatoi WN, Hussain Munis MF, Chaudhary HJ (2020) Deciphering metal toxicity responses of flax (Linum usitatissimum L.) with exopolysaccharide and ACC-deaminase producing bacteria in industrially contaminated soils. Plant Physiol Biochem 152:90–99. https://doi.org/10.1016/j.plaphy.2020.04.039
Funding
This project was supported by Khalifa Center for Biotechnology and Genetic Engineering-UAEU (Grant number: 31R286), Abu Dhabi Award for Research Excellence-Department of Education and Knowledge (Grant number: 21S105), and UAEU program of Advanced Research (Grant number: 12S169).
Author information
Authors and Affiliations
Contributions
ME-S: Conceptualization, data curation, investigation, methodology, and writing – original draft; E-SD: Data curation, and methodology; KE-T: Conceptualization, design, funding acquisition, investigation, methodology, writing – original draft, and writing – review & editing; SAQ: Conceptualization, data curation, funding acquisition, investigation, methodology, writing – original draft, and writing – review & editing; AS: Conceptualization, design, and formal analysis. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to for publication
Not applicable.
Competing interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 93 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Saadony, M.T., Desoky, ES.M., El-Tarabily, K.A. et al. Exploiting the role of plant growth promoting rhizobacteria in reducing heavy metal toxicity of pepper (Capsicum annuum L.). Environ Sci Pollut Res 31, 27465–27484 (2024). https://doi.org/10.1007/s11356-024-32874-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32874-1