Abstract
Lignocellulosic biomass (LB) despite its huge potential as a renewable bioenergy resource faces bottlenecks due to its recalcitrance and lack of appropriate pretreatment approaches. The current study evaluates the combinatorial application of alkali and acid pretreatment of pine needle biomass (PNB), for achieving high sugar release upon enzymatic saccharification. Pine needle accumulation poses a big threat to the forest soil fertility and overall ecosystem and environment. However, pine needle waste can be valorized after appropriate pretreatment and enzymatic saccharification for production of renewable energy, i.e. biofuel–ethanol. In combinatorial pretreatment strategy, first PNB was subjected to ammonium carbonate pretreatment, and parameters like ammonium carbonate concentration, incubation time and pretreatment temperature were optimized using design of experiment (DoE) approach. The relative influence of parameters on efficacy of pretreatment was established individually and in interactive terms. Based on DoE, sugar yield of 7.56 mg/g of PNB was obtained. Furthermore, DoE-based pretreated PNB was subjected to sulphuric acid pretreatment, followed by enzymatic saccharification. The sugar released during various steps was pooled (8.19 g/100 g), concentrated and subjected to ethanol fermentation with dual yeast cultures using Saccharomyces cerevisiae and Pichia stipitis. An ethanol yield of 8.8%, v/v (6.94% w/v), was obtained. This represents the process efficiency of 19.34% for bioethanol production from PNB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic biomass (LB) has emerged as the most reliable and sustainable energy solution for future in view of depleting fossil fuel reserves and increasing environmental apprehensions (Jin et al. 2015; Kim et al. 2016; Gao et al. 2017; Hou et al. 2017). LB, represented mainly by agricultural and forestry residues, is comprised of cellulose (35–50 wt %, dry basis), hemicellulose (15–30%), pectin (2–5%) and lignin (12–35%) (Kumar et al. 2016; Teramura et al. 2016). LB is a low-cost, eco-friendly and abundantly available raw material that may be exploited for production of bioethanol fuel and other value-added biorefined products (Phitsuwan et al. 2016; Oladi and Aita 2017; Sindhu et al. 2017). Bioethanol fuel generated from LB may potentially be utilized for the industrial and public transport sectors (He et al. 2015; Singh et al. 2016). Despite huge prospective, the LB ethanol technology faces several hurdles like high-cost and energy-intensive pretreatments, production of inhibitors, low sugar yield, unavailability of apt enzyme cocktails and fermenting organisms (Nargotra et al. 2016; Sharma and Bajaj 2017). Due to high recalcitrance, LB needs ample pretreatments prior to efficient enzymatic saccharification into simple fermentable sugars (Timung et al. 2015; Sindhu et al. 2017). Pretreatment of LB leads to disruption of the lignin structure and amorphization of the polysaccharides (holocellulose) which in turn leads to better enzymatic saccharification (Mohan et al. 2015; Timung et al. 2015).
Numerous pretreatment approaches like physical, chemical, biological and combination of these, have been used to decrystallize the LB polysaccharides (de Souza et al. 2014; Novy et al. 2015; Rabemanolontsoa and Saka 2016; Phitsuwan et al. 2016). Mild pretreatments are favourable to environment, but add less to the value of final product, while rigorous pretreatments release adequate sugars but are environmentally unfriendly, energy intensive and cause production of inhibitors, especially from hemicellulosic sugars (de Souza et al. 2014; Karcher et al. 2015). The inhibitors may hinder the fermentation process organism; thus, the detoxification of sugar hydrolysate may be necessary (Guo et al. 2013; Jonsson and Martin 2016).
In order to sustain the overall bioprocess economy and environmental safety, a right and balanced pretreatment approach must be employed which ensures the maximum sugar release from LB and minimal or no inhibitor production. Alkali pretreatment selectively removes lignin from LB, while the acid pretreatment hydrolyses hemicellulose and may produce non-sugary inhibitors like furfurals (Timung et al. 2015; Tian et al. 2017). A combination of both the pretreatments sequentially has been investigated for the decomposition of LB (Singh et al. 2016). Utilizing ammonia or ammonium salts like ammonium carbonate (NH4)2CO3 as a pretreatment agent is advantageous as they favour the limited inhibitor formation and enhance the sugar release (Kim et al. 2014; Oladi and Aita 2017). During pretreatment, (NH4)2CO3 is decomposed thermally into NH4 (aq.) and CO2, and NH4 (aq.) being an alkaline catalyst helps in deconstructing the lignin (Kim et al. 2014). Dilute sulphuric acid hydrolysis is a low-cost pretreatment which deconstructs the hemicellulose and exposes the cellulosic biomass for adequate enzymatic hydrolysis (Singh et al. 2016).
Exploration of novel LB feedstocks for biorefining has been a continuous practice. Pine needle biomass (PNB) may represent an underutilized and unexplored biomass resource. The pines belonging to family Pinaceae are coniferous trees which are native to northern hemisphere. Pine needles constitute the major litter fall in coniferous forests and pose numerous environmental hazards like unavailability of soil nutrients, delayed organic matter decomposition and mineralization, and potential risk of forest fires (Vats et al. 2013; Pandey and Negi 2015). However, PNB is comprised of about 65% of holocellulose which can be utilized for production of bioethanol and/or other valuable products (Ghosh and Ghosh 2011). Furthermore, utilization of PNB as a feedstock promises valorization of waste and obviation of problems associated with pine needle accumulation (Vaid and Bajaj 2017).
Optimization of process parameters is imperative for developing an efficient and economic bioprocess (Singh et al. 2016). Due to the limitations of traditional one variable at a time optimization approach, statistical design of experiments (DoE) has become very popular (Vaid et al. 2017). DoE approach involving response surface methodology (RSM) assists in designing and interpretation of response obtained after execution of designed experiments (Nargotra et al. 2016; Singh et al. 2016). RSM has been successfully used for optimization of parameters for development of an efficient process (Pandey and Negi 2015; Vaid et al. 2017).
Pretreatment of the LB is followed by enzymatic saccharification using cellulase/xylanase cocktail for generating fermentable sugars (Singh et al. 2016; Vaid and Bajaj 2017). Saccharomyces cerevisiae is the most commonly used fermenting organism for ethanol production, but it lacks the ability to ferment pentose sugars (Kang et al. 2014). To achieve complete utilization of sugars (pentoses and hexoses) from LB and high bioethanol yield, a pentose fermenting yeast Pichia stipitis is generally employed along with S. cerevisiae (Yadav et al. 2012). Varied pretreatment methods based upon acid and alkali, steam explosion, etc., have been used for a variety of LB feedstocks like rice straw (Kim et al. 2014), Pinus roxburghii fallen foliage (Vats et al. 2013; Singh et al. 2016), empty fruit bunches (Choi et al. 2013) and cane bagasse biomass (Oladi and Aita 2017) for achieving enhanced sugar yield.
The objective of the current study was to evaluate the combinatorial application of alkali (ammonium carbonate) and acid (sulphuric acid) for pretreatment of pine needle biomass, PNB. PNB was first pretreated with ammonium carbonate under DoE optimized process and then subjected to sulphuric acid pretreatment. The enzymatic saccharification of pretreated PNB was executed using cellulase and xylanase enzymes, and the sugar hydrolysate obtained was fermented by dual yeast cultures, i.e. Saccharomyces cerevisiae and Pichia stipitis to produce bioethanol.
2 Materials and methods
2.1 Chemicals and media
All the chemicals, media and media components used were of analytical grade obtained from Sigma-Aldrich Chemicals Ltd, St. Louis MO, USA; HiMedia Laboratories Ltd, Mumbai, India; Qualigens Fine Chemicals Ltd, Mumbai, India; and Merck and Co. Inc., White House Station, NJ, USA.
2.2 Preparation of pine needle biomass (PNB) and enzymatic saccharification
Pine needles were collected from Udhampur, Jammu, India. Pine needles were thoroughly washed and dried at 50 °C for 24 h to attain constant weight. The dried needles were grounded and sieved to obtain dry matter of a size of < 4 mm.
Ammonium carbonate (10 to 20%, w/v) and sulphuric acid (2%, v/v) pretreatment of PNB was performed at varied temperature (50–90 °C) and time (7–21 h). The pretreated biomass was filtered through Whatman No. 1 filter paper, and the filtrate was analysed for reducing sugars. Residual biomass was washed to attain neutrality, dried at 50 °C for 24 h to attain a constant weight and then subjected to enzymatic saccharification. Cellulase (Celluclast 1.5 L; from Trichoderma reesei ATCC 26921) and xylanase (from Thermomyces launginosus X2753), procured from Sigma-Aldrich Chemicals Ltd., St. Louis MO, USA, were used for saccharification of pretreated PNB biomass. Cellulase was used at 30 FPU/g biomass, and xylanase was used at 10 IU/g biomass for saccharification of sequential alkali and acid pretreated PNB. The enzymatic hydrolysis was executed at 50 °C for 24 h (100 mg residual biomass/2 ml enzyme in phosphate buffer, pH 7.0, 50 mM), and reducing sugar released was analysed spectrophotometrically (UV–Vis 1800, Shimadzu, Japan) using glucose as standard (Miller 1959). A total reducing sugar, TRS, yield (a sum total of reducing sugars obtained in different steps of pretreatment and saccharification individually) were considered as sugar hydrolysate for biofuel–ethanol production.
2.3 Optimization of ammonium carbonate pretreatment of PNB
Response surface methodology (RSM) generated central composite design (CCD, Design-Expert 7, Stat-Ease, Inc., Minneapolis, USA) was used for determining the optimum level of the pretreatment parameters for PNB. PNB was used at 1:10 solid to liquid loading. Three variables, i.e. ammonium carbonate concentration (A), temperature (B) and incubation time (C), were selected at minimum (10%, w/v; 50 °C and 7 h, respectively) and maximum values (20%, w/v; 90 °C and 21 h, respectively) to design the experiments (Table 1). TRS yield was considered as the response and denoted as ‘Y’. The maximum and minimum values are mentioned at five coded levels (− α, − 1, 0, + 1, + α) as presented in Table 2, and supported by these values overall 20 experiments were generated by the proposed model (Table 3). After each pretreatment run, pretreated biomass was filtered and the residual PNB was subjected to enzymatic saccharification using enzyme cocktail (cellulase and xylanase). The liquid hydrolysate obtained after pretreatment as well as after enzymatic saccharification was analysed for reducing sugar yield. The respective response values (Y, TRS yield obtained after pretreatment and enzymatic saccharification) were analysed and fed into the software for performing analysis of variance (ANOVA) and generating a polynomial equation.
Based on the ANOVA and regression equation, the quadratic model represented 3D response surface plots which were used to examine the interaction between the variables and the correlation between the response and experimental level of each variable. The validation of the model was done by executing an experiment based on optimal level of the each variable predicted by the point prediction tool of the model. The response values (Y) were measured as an average of triplicate experiments.
2.4 Acid pretreatment of ammonium carbonate pretreated PNB
DoE based ammonium carbonate pretreatment of PNB was followed by sulphuric acid pretreatment in order to examine the combinatorial influence of alkali and acid on enzymatic saccharification of PNB. A 100 g PNB was pretreated with ammonium carbonate under DoE optimized conditions, and the reaction mixture was filtered. Residual biomass was washed up to neutrality, dried and pretreated with sulphuric acid (2%, v/v) at 100 °C for 30 min and filtered. The biomass was washed, dried and hydrolysed with enzymes (cellulase 30 U/g biomass, xylanase 10 U/g biomass) at 50 °C for 24 h, and the contents were filtered. The filtrate obtained was examined for reducing sugar.
2.5 Detoxification of sugar hydrolysate for inhibitors
Detoxification of sequentially alkali and acid pretreated PNB filtrate was carried out with calcium hydroxide, till the pH of solution reached 10 with continuous stirring (Jonsson et al. 2013). The reaction contents were allowed to stand for 1 h with continuous stirring for adequate precipitation to take place. The slurry obtained was filtered to remove calcium-precipitated inhibitors, and the filtrate was obtained as sugar hydrolysate. All the liquid fractions obtained after sequential alkali and acid pretreatment and enzymatic hydrolysis were pooled, analysed for the reducing sugar content and used for ethanol fermentation.
2.6 Ethanol fermentation of sugar hydrolysate from PNB
Dual yeast cultures, i.e. Saccharomyces cerevisiae NCIM 3078 and Pichia stipitis NCIM 3497 (National Chemical Laboratory, Pune), were used as ethanol fermenting organisms. The ethanol production medium (EPM, pH 5.6) consisted of the sugar hydrolysate which was supplemented with yeast extract (1.5 g/L), peptone (1.0 g/L), ammonium sulphate (1.0 g/L), dipotassium phosphate (1.0 g/L) and magnesium sulphate (1.0 g/L) (Vaid and Bajaj 2017). Each of the freshly grown yeast culture of S. cerevisiae and P. stipitis (3:1) was inoculated into the EMP and incubated at 30 °C for ethanol fermentation to proceed under stationary conditions. Ethanol content was assayed after various time intervals (24–72 h) using acidified potassium dichromate (Gupta et al. 2012; Vaid and Bajaj 2017). Theoretical and experimental ethanol yield was calculated (Vogel et al. 2011; Vaid and Bajaj 2017) as per following Eqs. 1, 2 and 3:
All analytical experiments were conducted in triplicate, and data present the mean values.
3 Results and discussion
3.1 Optimization based on design of experiments for pretreatment of PNB
Ammonium carbonate pretreatment of PNB was DoE optimized for three different parameters, i.e. ammonium carbonate concentrations (A), pretreatment temperature (B) and pretreatment incubation time (C) by using RSM. The sugar released in each of the 20 experimental runs was measured (Table 4). The residual PNB was filtered and dried appropriately to constant weight. The enzymatic hydrolysis of pretreated PNB was executed at 50 °C for 24 h by using commercial cellulase and xylanase enzymes. The reducing sugar titre was estimated for each of the experimental run. The sugar hydrolysate obtained at different steps, i.e. DoE-based pretreatment and enzymatic saccharification, was pooled and represented as the response Y (total reducing sugar, TRS). The responses were fed into the design generated by the software (Table 5). The variable response (TRS yield) in various experimental runs signifies the adequate differential influence of process parameters on response.
The software presented a polynomial Eq. 4 after performing an analysis of variance (ANOVA) (Table 6). The coded terms in the equation symbolize the model variables, i.e. A—ammonium carbonate concentration, B—pretreatment temperature, and C—incubation time for pretreatment. The polynomial regression equation is represented below:
The model is significant with a model F value of 22.63 and indicates there is only 0.01% likelihood that this huge ‘model F value’ could occur due to noise. The probability figures > F < 0.05 represent the significant model terms. The F value for lack of fit is 2.90. There is 13.38% possibility that this large lack of fit value could be due to noise. The probability > F value of 0.1338 indicates the insignificant lack of fit relative to the pure error, and this points out the sturdiness of the model. The linear variables B and C, the squared terms B2 and C2 and the interaction BC showed considerable effect on the response as depicted by their significant p values (Table 6). Along with model summary, statistical parameters like high R2 value (0.9532) and a low PRESS (3.116E+006) are also useful in deciding the toughness of the quadratic model. The R2 value (0.9532) nearer to 1 denotes better correlation between the experimental and predicted values. The coefficient of variation (CV) provides information about the degree of precision with which the experiments were executed, and lower value of CV (3.63) denoted higher reliability of the experiments. The predicted R2 of 0.7098 is in accordance with the adjusted R2 of 0.9111. Adequate precision of 16.398 indicates an adequate signal for the present model. The result attained markedly implies the stoutness and good predictability of the quadratic model.
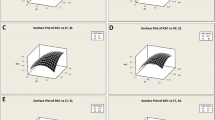
A response surface graph generated by the RSM software signifies the interaction between different independent variables on the response. The three dimensional response surface plots were generated to investigate the interaction between various variables (acid concentration A, pretreatment temperature B and incubation time C) and to visualize their combined effects on response, i.e. TRS (Fig. 1). The response in Fig. 1a depicts the non-significant interaction between ammonium carbonate concentration and pretreatment temperature (AB). With the increasing ammonium carbonate concentration with respect to increasing temperature, a higher reducing sugar yield was observed but effect was insignificant. In Fig. 1b the interaction between the ammonium carbonate concentration and incubation time (AC) is shown and an insignificant interactive effect of both the variables on the response was observed. A significant interaction between pretreatment temperature and incubation time (BC) was observed as shown in Fig. 1c, but the interaction was negatively significant as demonstrated by the polynomial equation. A decrease in reducing sugar yield was observed with respect to the designed space.
Three-dimensional response surface plots showing interaction between the pretreatment variables for reducing sugar yield. a–c represent the interaction between ammonium carbonate ‘A’ and temperature ‘B’, ammonium carbonate ‘A’ and incubation time ‘C’, and temperature ‘B’ and incubation time ‘C’, respectively; d shows the perturbation plot which indicates the effect of individual variables with respect to reference point
It was inferred from the 3-D response surface plots that the interactive influence of pretreatment temperature and incubation time (BC) influences the reducing sugar yield maximally followed by that of ammonium carbonate concentration and incubation time (AC). The interactive effect of ammonium carbonate concentration and pretreatment temperature (AB) was the least. The perturbation plot (Fig. 1d) depicts the change in response as each factor deviates from the chosen reference point by maintaining the constancy of all the other factors at the reference value. Pretreatment temperature (B) as an independent variable appeared to be the most imperative variable that affects the response as compared to the other factors with respect to the reference point.
The statistical model was validated using point prediction tool of RSM by conducting an experiment based on optimum value of all the three variables, i.e. ammonium carbonate concentration (A), pretreatment temperature (B) and incubation time (C). The strength of the model was indicated by the defined familiarity between the observed response (7.596 mg/g) obtained after shake flask experiments and the predicted response (7.471 mg/g) generated by the RSM software. Ammonium carbonate pretreatment results showed that it is effective for pretreatment of PNB, however, at higher concentration of 20%.
DoE approach has widely been used for alkali and acid-based pretreatment of various LB feedstocks. Ammonium carbonate pretreatment is effective for disrupting the lignin sheath of LB by breaking the ester bonds between lignin, hemicellulose and cellulose, thereby providing an open access for enzymatic hydrolysis of remaining polysaccharides (Kim et al. 2014). Ammonium carbonate (20% concentration) pretreatment of rice straw increased the external surface area, thus exposing its amorphous structure for efficient enzymatic digestion (Kim et al. 2014). RSM-based optimization was done for pretreatment and enzymatic hydrolysis of P. roxburghii fallen foliage. A reducing sugar yield of 334 mg/g was obtained by hydrolysis of steam exploded foliage with enzyme cocktail of cellulase, xylanase and laccase (Vats et al. 2013). The pretreatment parameters like NaOH concentration, temperature and time were optimized for empty fruit bunch biomass using response surface methodology (Choi et al. 2013). High glucan (93%) and xylan (78%) yields were achieved at optimized conditions, i.e. 3% NaOH concentration, 160 °C pretreatment temperature and a pretreatment time of 11 min 20 s (Choi et al. 2013).
Singh et al. (2016) optimized various physicochemical variables for pretreatment of pine needle biomass followed by enzymatic saccharification for 48 h. It was reported that maximum sugar yield (286 mg/g PNB) was obtained at optimized conditions, i.e. 0.25% sulphuric acid concentration, 65 °C temperature and pretreatment time of 5 min. In another study (Oladi and Aita 2017), pretreatment conditions for cane bagasse biomass were optimized (208 °C temperature, 36-min incubation time and 0.4:1.0 of ammonium hydroxide-to-biomass ratio) to achieve a maximum glucose yield of 30.77 g/100 g biomass. A xylose yield of 9.10 g/100 g was obtained at 160 °C for 60 min at an ammonium hydroxide-to-biomass ratio of 0.31:1.0, under optimized pretreatment conditions.
3.2 Acid pretreatment ammonium carbonate-pretreated PNB
Ammonium carbonate pretreated biomass was subjected to acid (sulphuric acid) pretreatment. Ammonium carbonate pretreatment of PNB (100 g) was carried out under optimized conditions, i.e. ammonium carbonate concentration (20%), pretreatment temperature (50 °C) and incubation time (21 h). The amount of reducing sugar equivalent obtained after optimized pretreatment in the filtrate was 0.432 g/100 g. The ammonium carbonate pretreated PNB was neutralized and pretreated with sulphuric acid (2%) at 100 °C for 30 min. The contents were filtered and examined for sugar yield. The sugar yield in the filtrate was 6.318 g/100 g. This pretreated biomass after neutralization was hydrolysed with cellulase and xylanase (3:1). The sugar yield in the hydrolysate was 1.440 g/100 g. Thus, the overall TRS yield obtained after sequential ammonium carbonate and sulphuric acid pretreatment and enzyme hydrolysis of PNB was 8.19 g/100 g.
Alkali pretreatment breaks the ester bonds between lignin, hemicellulose and cellulose but doesn’t promote cellulose/hemicellulose decrystallization (Singh et al. 2016). Pretreatment using sulphuric acid encourages amorphization of cellulose, dissolution of hemicelluloses that results in high monomer recovery in the liquid fraction, and also helps in cellulose exposure to enzymatic hydrolysis (Alvira et al. 2010). The effect of sulphuric acid at varied concentrations (0–2.5%) was studied on the three different lignocellulosic feedstocks, i.e. pinewood, timothy grass and wheat straw at 121 °C for 1 h and pinewood resulted in maximum fermentable sugar release of 68.5 g/L at 2% sulphuric acid followed by enzymatic hydrolysis (Nanda et al. 2014).
3.3 Detoxification of sugar hydrolysate with calcium hydroxide
The acid pretreatment of biomass at high temperature (100 °C) causes formation of inhibitors which may reduce the sugar yield and potentially inhibit the fermentation process organism (Jonsson and Martin 2016; Singh et al. 2016). Therefore, the pretreated biomass was treated with Ca(OH)2 for detoxification. The calcium-precipitated inhibitors formed light brown sediments which settled at bottom and were removed by filtration to get a clear solution. Detoxification of lignocellulosic hydrolysate is needed to achieve efficient bioethanol production. Guo et al. (2013) investigated various types of detoxification methods for inhibitor removal and concluded that detoxification methods efficiently decrease the concentrations of inhibitors.
3.4 Ethanol fermentation of the sugar hydrolysate from PNB
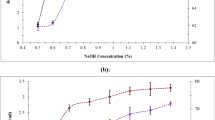
Ethanol production from sugar hydrolysate was done by using dual yeast cultures, i.e. Saccharomyces cerevisiae and Pichia stipitis. The sugar obtained at various steps of DoE-based ammonium carbonate pretreatment followed by sulphuric acid pretreatment, and finally enzymatic hydrolysis of residual PNB was pooled, and the resultant sugar hydrolysate was subjected to ethanol fermentation. The samples were collected at an interval of 24 h for 3 consecutive days and were analysed for the ethanol content (Fig. 2). An ethanol yield of 8.8%, v/v (6.94% w/v) was obtained after 72 h of fermentation. The process had an efficiency of 19.34% for bioethanol production from PNB. The estimation of residual sugar during ethanol fermentation showed successive decrease over the period of fermentation. Nanda et al. (2014) studied the H2SO4 pretreatment and enzymatic hydrolysis of three different feedstocks, i.e. pinewood, timothy grass and wheat straw. It was observed that pine wood sugar hydrolysate yielded maximum ethanol (24.1 g/L) upon fermentation with S. cerevisiae. Similarly, analysis of ethanol fermentation of pine wood, rice straw and oak wood hydrolysates, showed that pine wood yielded high ethanol (412 ml ethanol/kg biomass) that represented a theoretical yield 85.0% (Wi et al. 2015). Aqueous ammonia-pretreated rice straw under simultaneous saccharification and fermentation using monoculture of Saccharomyces cerevisiae yielded 22 g/L ethanol. However, application of co-culture of Saccharomyces cerevisiae and Candida tropicalis yielded 25 g/L ethanol (Phitsuwan et al. 2016). Thus, various LB feedstocks yield varied amount of sugars under different pretreatment regimes.
4 Conclusions
The study concludes that the combinatorial pretreatment involving (sequential) ammonium carbonate followed by sulphuric acid may be an efficient and effective strategy for delignification and amorphization of pine needles for efficient enzymatic saccharification. A sugar yield of 8.19 g/100 g biomass was obtained after combinatorial pretreatment and enzymatic saccharification. The sugar hydrolysate was concentrated and fermented to ethanol by dual yeast cultures using Saccharomyces cerevisiae and Pichia stipitis. The process had an efficiency of 19.34% for bioethanol production from PNB. Further studies on optimization of additional variables like enzyme cocktail/enzyme dose, biomass loading, temperature/time must be accomplished to get enhanced sugar yield from PNB. Thus, valorization of the pine needle biomass may not only help combating hazardous environmental effects but promises production of valuable industrial commodities like bioethanol fuel.
References
Alvira P, Tomas-Pejo E, Ballesteros M, Negro MJ (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Choi WI, Park JY, Lee JP, Oh YK, Park YC, Kim JS, Park JM, Kim CH, Lee JS (2013) Optimization of NaOH-catalyzed steam pretreatment of empty fruit bunch. Biotechnol Biofuel 6:170
de Souza ROMA, Mirandaa LSM, Luque R (2014) Bio(chemo)technological strategies for biomass conversion into bioethanol and key carboxylic acids. Green Chem 16:2386
Gao W, Tabil LG, Dumonceaux T, Ríos SE, Zhao R (2017) Optimization of biological pretreatment to enhance the quality of wheat straw pellets. Biomass Bioenergy 97:77–89
Ghosh MK, Ghosh UK (2011) Utilization of pine needles as bed material in solid state fermentation for production of lactic acid by lactobacillus strains. BioResources 6:1556–1575
Guo X, Cavka A, Jonsson LJ, Hong F (2013) Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb Cell Fact 12:1
Gupta P, Samant K, Sahu A (2012) Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int J Microbiol. https://doi.org/10.1155/2012/578925
He YC, Liu F, Gong L, Lu T, Ding Y, Zhang Dan-Ping, Qing Q, Zhang Y (2015) Improving enzymatic hydrolysis of corn stover pretreated by ethylene glycol-perchloric acid-water mixture. Appl Biochem Biotechnol 175:1306–1317
Hou Q, Ju M, Li W, Liu L, Chen Y, Yang Q (2017) Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems. Molecules 22:490
Jin S, Zhang G, Zhang P, Fan S, Li F (2015) High-pressure homogenization pretreatment of four different lignocellulosic biomass for enhancing enzymatic digestibility. Bioresour Technol 181:270–274
Jonsson LJ, Martin C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112
Jonsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuel 6:1. https://doi.org/10.1186/1754-6834-6-16
Kang Q, Appels L, Tan T, Dewil R (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. Sci World J. https://doi.org/10.1155/2014/298153
Karcher MA, Iqbal Y, Lewandowski T (2015) Comparing the performance of Miscanthus giganteus and wheat straw biomass in sulfuric acid based pretreatment. Bioresour Technol 180:360–364
Kim I, Lee B, Song D, Han JI (2014) Effects of ammonium carbonate pretreatment on the enzymatic digestibility and structural features of rice straw. Bioresour Technol 166:353–357
Kim JS, Lee YY, Kim TH (2016) A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour Technol 199:42–48
Kumar R, Tabatabaei M, Karimi K, Sárvári Horváth I (2016) Recent updates on lignocellulosic biomass derived ethanol—a review. Biofuel Res J 9:347–356
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mohan M, Banerjee T, Goud VV (2015) Hydrolysis of bamboo biomass by subcritical water treatment. Bioresour Technol 191:244–252
Nanda S, Dalai AK, Kozinski JA (2014) Butanol and ethanol production from lignocellulosic feedstock: biomass pretreatment and bioconversion. Energy Sci Eng 2:138–148
Nargotra P, Vaid S, Bajaj BK (2016) Cellulase production from Bacillus subtilis SV1 and its application potential for saccharification of ionic liquid pretreated pine needle biomass under one pot consolidated bioprocess. Fermentation 2:19
Novy V, Longus K, Nidetzky B (2015) From wheat straw to bioethanol: integrative analysis of a separate hydrolysis and co-fermentation process with implemented enzyme production. Biotechnol Biofuel 8:46
Oladi S, Aita GM (2017) Optimization of liquid ammonia pretreatment variables for maximum enzymatic hydrolysis yield of energy cane bagasse. Ind Crops Product 103:122–132
Pandey AK, Negi S (2015) Impact of surfactant assisted acid and alkali pretreatment on lignocellulosic structure of pine foliage and optimization of its saccharification parameters using response surface methodology. Bioresour Technol 192:115–125
Phitsuwan P, Permsriburasuk C, Waeonkul R, Pason P, Tachaapaikoon C, Ratankhanokchai K (2016) Evaluation of fuel ethanol production from aqueous ammonia-treated rice straw via simultaneous saccharification and fermentation. Biomass Bioenergy 150:150–157
Rabemanolontsoa H, Saka S (2016) Various pretreatments of lignocellulosics. Bioresour Technol 199:83–91
Sharma M, Bajaj BK (2017) Optimization of bioprocess variables for production of a thermostable and wide range pH stable carboxymethyl cellulase from Bacillus subtilis MS 54 under solid state fermentation. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.12557
Sindhu R, Binod P, Mathew AK, Abraham A, Gnansounou E, Ummalyma SB, Thomas L, Pandey A (2017) Development of a novel ultrasound-assisted alkali pretreatment strategy for the production of bioethanol and xylanases from chili post harvest residue. Bioresour Technol. https://doi.org/10.1016/j.biortech.2017.03.001
Singh S, Anu, Vaid S, Singh P, Bajaj BK (2016) Physicochemical pretreatment of pine needle biomass by design of experiments approach for efficient enzymatic saccharification. J Mater Environ Sci 7:2034–2041
Teramura H, Sasaki K, Oshima T, Matsuda F, Okamoto M, Shirai T, Kawaguchi H, Ogino C, Hirano K, Sazuka T, Kitano H (2016) Organosolv pretreatment of sorghum bagasse using a low concentration of hydrophobic solvents such as 1-butanol or 1-pentanol. Biotechnol Biofuel 9:27
Tian D, Chandra RP, Lee JS, Lu C, Saddler JN (2017) A comparison of various lignin-extraction methods to enhance the accessibility and ease of enzymatic hydrolysis of the cellulosic component of steam-pretreated poplar. Biotechnol Biofuel 10:157
Timung R, Mohan M, Chilukoti B, Sasmal S, Banerjee T, Goud VV (2015) Optimization of dilute acid and hot water pretreatment of different lignocellulosic biomass: a comparative study. Biomass Bioenerg 81:9–18
Vaid S, Bajaj BK (2017) Production of ionic liquid tolerant cellulase from Bacillus subtilis G2 using agroindustrial residues with application potential for saccharification of biomass under one pot consolidated bioprocess. Waste Biomass Valor 8:949–964
Vaid S, Nargotra P, Bajaj BK (2017) Consolidated bioprocessing for biofuel-ethanol production from pine needle biomass. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.12691
Vats S, Maurya DP, Jain A, Mall V, Negi S (2013) Mathematical model-based optimization of physico-enzymatic hydrolysis of Pinus roxburghii needles for the production of reducing sugars. Indian J Exp Biol 51:944–953
Vogel KP, Dien BS, Jung HG, Casler MD, Masterson SD, Mitchell RB (2011) Quantifying actual and theoretical ethanol yields for switchgrass strains using NIRS analyses. Bioenergy Res 4:96–110
Wi SG, Cho EJ, Lee DS, Lee SJ, Lee YJ, Bae HJ (2015) Lignocellulose conversion for biofuel: a new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol Biofuel 8:228
Yadav SK, Naseeruddin S, Prashanthi SG, Sateesh L, Rao VL (2012) Bioethanol fermentation of concentrated rice straw hydrolysate using co-culture of Saccharomyces cerevisiae and Pichia stipitis. Bioresour Technol 102:6473–6478
Acknowledgements
Dr. Bijender Kumar (Bajaj) gratefully acknowledges the Institute of Advanced Study, Durham University, UK, for providing COFUND International Senior Research Fellowship for ‘Research Stay’ at Department of Biosciences, Durham University, Durham, UK; Department of Science and Technology (Govt. of India) is acknowledged for financial support (Research Project Ref. SR/SO/BB-66/2007), and Commonwealth Scholarship Commission, UK, for providing Commonwealth Fellowship (INCF-2013-45) for ‘Research Stay’ at Institute of Biological, Environmental and Rural Sciences (IBERS), Aberystwyth University, Aberystwyth, UK. Authors thank the Director, School of Biotechnology, University of Jammu, Jammu, for necessary laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vaid, S., Bhat, N., Nargotra, P. et al. Combinatorial application of ammonium carbonate and sulphuric acid pretreatment to achieve enhanced sugar yield from pine needle biomass for potential biofuel–ethanol production. Energ. Ecol. Environ. 3, 126–135 (2018). https://doi.org/10.1007/s40974-018-0083-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40974-018-0083-1