Abstract
This paper describes the concentrations of heavy metals in soils and in raisins (sultanas) cultivated upon the Gediz Plain (Manisa), western Turkey, which is cut by major roads from İzmir to İstanbul and İzmir to Ankara. A total of 212 samples of surface soil and 82 raisin samples were analysed. Soil samples have nearly same mineralogy, quartz, calcite, magnetite, pseudo-rutile and clay minerals. Dolomite is seen especially in areas close to Neogene sediments. Clay minerals are mainly mica (illite–muscovite), chlorite/kaolinite, smectite and mixed layers (Sm-Il). The concentrations of 21 elements (Ba, Ni, Mo, Cu, Pb, Zn, Co, Mn, As, U, Sr, Cd, Sb, Bi, Cr, B, W, Hg, Sn, Li and organic C) were determined in the surface soils. The degree of element enrichment in soil can be measured in many ways, the most common of which are the geoaccumulation index (Igeo), enrichment factor and the pollution index. Arsenic and Sb showed the highest Igeo values, corresponding to Igeo classes 3–4. Hence, the area is characterised as “being heavily contaminated to polluted” by As and Sb. Arsenic contamination has been reported from all over world. Arsenic-related pollutants enter the groundwater system by gradually moving with the flow of groundwater from rains and irrigation. Gediz Plain forms the main groundwater supply of İzmir city. The enrichment factor (EFarsenic) of the analysed soil samples is around 76, which corresponds to “extremely high enrichment”. The concentrations of 33 elements (Al, Sb, As, Ba, Be, Bi, B, Cd, Ca, Cr, Co, Cu, Fe, Pb, Li, Mg, Mn, Hg, Mo, Ni, K, Se, Ag, Na, Sr, Ta, Th, Sn, Ti, U, V, Zn and Zr) were determined in the raisin samples. The Pb and Cd contents of raisins are of great concern due to their toxicity. Pb contents ranged between 0.05 and 0.46 mg kg−1, and average Cd content was 0.04 mg kg−1. Only one sample contained high level of Cd, 0.23 mg kg−1. After cleaning the raisins, the heavy metal concentrations were low as in the European Community (EC) regulation No: 466/2001 for allowable levels of Pb (0.2 mg kg−1) and Cd (0.05 mg kg−1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Viticulture is an important component of agriculture in Turkey, where grape production (2008) is 3,918,442 tons with 4,827,887 decare of land planted with vines (Çelik et al. 1998; OIV 2007; TUIK 2008). As much as 38% of grape production was consumed for raisins. The Aegean region in Turkey is an important area for the production of sultana raisins, and constitutes 28% of the total land in Turkey planted with vines (Meyvacı et al. 2005). Sultana production is concentrated in western Turkey, in the Gediz Plain (Fig. 1), which is one of the most important agricultural areas of Turkey with highly fertile soils. The sultana is a seedless grape variety from which raisins are produced by dipping the grape in an alkaline solution (5% potassium carbonate + olive oil as surfactant) and then subjecting it to sun or oven drying. Annual raisin production in Turkey is 250,000–300,000 tons, almost one-third of the global production (Çalışır and Akman 2007). The characteristic golden colour of raisins is obtained after treatment with sulphur dioxide. Some producers also produce sun-dried raisins without first dipping the grapes into an alkaline solution (Çalışır and Akman 2007).
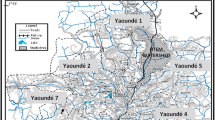
Location and geological map of the studied area (modified after Sözbilir 2000)
Historically, the Gediz Plain has been home to many civilizations and the scene of numerous wars. In the past 20 years, however, it has been the site of extensive industrial development that has resulted in the use of large quantities of groundwater and in the heavy pollution of Nif Creek and the Gediz River (Murray-Rust and Svendsen 2001).
The İzmir–İstanbul and İzmir–Ankara roads are the busiest roadways of western Turkey across the Gediz Plain, with a capacity of 40,000 cars/year (annual average daily traffic) in each direction. Consequently, various industries and housing developers prefer sites located near these roads. However, the cultivation and drying of grapes have continued in this area even as development has intensified. The Turgutlu and Salihli regions are also the largest production centres of tile and brick in Turkey, using as raw ingredients alluvium and Neogene clays (Çolak 2004).
The main sources of high concentrations of heavy metals in soils are metal-rich source rock, atmospheric pollution from motor vehicles, the combustion of fossil fuels, agricultural fertilisers and pesticides, organic manure, disposal of urban and industrial wastes, and mining and smelting (Alloway 1990). Many studies have investigated heavy metal concentrations in roadside soils (Al-Chalabi and Hawker 2000; Gratani et al. 1992; Turer 2005; Vissikirsky et al. 2008; Zupančič 1998). Clay minerals play a major role in heavy metal storage. Adsorption by clays is one of the most efficient methods of cleaning the environment of heavy metal pollutants (Lagaly 1995). Thus, clay content of soils is considerable in this study.
Contaminants in foods may be present as a result of the various stages of its production, packaging, transport or holding. They might result also from environmental contamination. Çalışır and Akman (2007) studied the lead and copper levels of various types of Turkish raisins. The results of the study indicated a mean (range) of 0.056 (0.012–0.359) mg kg−1 Pb and 2.542 (0.770–4.706) mg kg−1 Cu.
The aims of this study were (a) to determine the mineralogy and grain size of soils and (b) to measure the concentrations of heavy metals in sultana-cultivation soils and sultana raisins, located at varying distances from the main roads on the Gediz Plain, Manisa, Turkey. The metal accumulation measurements from soil to roots, leaves and fruits were not studied.
Geology of the studied area
Western Turkey is located on seismically active crust that has been under approximately N–S extension since the Miocene (Dewey and Şengör 1979; Şengör 1987), leading to the development of normal faults in the upper crust and E–W trending grabens (Fig. 1). The youngest such grabens are the Gediz (Alaşehir) and Büyük Menderes. Recent studies on the Gediz Graben have revealed that the graben fill consists of four units separated by unconformities (Sözbilir 2000; Yılmaz et al. 2000). The rocks surrounding the Gediz Graben are mainly metamorphic rocks of the Menderes Massif (phyllites, mica schists, gneisses and marbles) and ophiolitic rocks that outcrop in the İzmir–Ankara Zone, overlying the Menderes Massif. The ophiolitic rocks consist of serpentinised ultrabasic rocks, cherts, interbedded sandstone and shale, and limestone. These units are unconformably overlain by Neogene sediments, including conglomerates, sandstones, claystones, marls and lacustrine limestones. In NW Manisa, volcanic rocks (andesite and dacite) are overlain by Neogene sediments. The youngest deposits in the study area are travertine and Quaternary alluvium derived from the basement rocks described above.
The Gediz River, which flows to the outer part of İzmir Bay, is the second-longest river (~300 km) along the eastern Aegean coast. The river is contaminated by industrial sources, sewage and agricultural runoff (Minareci et al. 2009). Some farmers use surface pumps to irrigate the land located close to the Gediz River.
Materials and methods
Sampling and sample preparation
A total of 212 soil samples were collected (0–5 cm depth) from across the Gediz Plain (Fig. 1), mainly from farmland areas. For each of these samples, the particle-size distribution, mineralogy, clay mineralogy, organic carbon and pH were measured.
The soil samples were air dried. Two kilogram of each sample was sieved to <2-mm grain size, and 0.5 kg of each sieved sample was ground for 45 s in a tungsten carbide mill to have 200-mesh grain sizes.
A total of 82 raisin samples were collected: 78 samples from drying sites, 2 samples (pre- and post-treatment) from Tariş (a processing company) and 2 samples (pre- and post-treatment) from Pagmat (a processing company) in August 2005. Sun-dried sultanas are delivered to a packinghouse, where they are subjected to inspection, de-clumping, sieving, aspiration, washing, destoning, cap-stemming, optical sorting, manual sorting, metal detection and packing.
Analytical methods
Subsequent treatment of soil samples was performed at ACME Analytical Laboratories Ltd. Vancouver, Canada (ISO 2002 Accredited Co). A total of 0.5 g of each sample was totally digested with 10 ml HClO4–HNO3–HCl–HF at 200°C to fuming and diluted to 10 ml with diluted aqua regia. The accuracy and analytical precision were determined using analyses of reference material (STD SO-18) and duplicate samples in each analytical set. Duplicate measurements were conducted for 10% of the analysed soil samples (precision 2–3%).
The heavy metal contents of the raisins were analysed by JR Laboratories Inc. (Canada) using samples of approximately 0.4 g accurately weighed (±0.001 g) into a 50-ml polypropylene digestion tube, to which 5 ml of environmental-grade nitric acid was added. The samples were digested in open test tubes in CEM MOS-2000 microwave digestion oven at 95°C for 30 min and at 115°C for a further 5 min. The samples were then cooled and the tubes filled to 40 ml with 18 M de-ionised water. The samples were then mixed to a homogeneous solution and filtered if necessary to produce a clear solution. The solution was then analysed using an Agilent 7500a Inductively Coupled Plasma Mass Spectrometer (ICP-MS). A standard, duplicate and blank are processed with each batch and at least every ten samples. The standard is a certified reference material, TORT-2, from The National Research Council Canada.
Soil pH was performed in duplicate according to the methods described by ISO 10390 (2005). Soil pH was determined by a pH meter using a WTW Multi 340i model in 1:5 (volume fraction) suspension of soil in 1 mol of KCl solution. The soil (1 part) and KCl (5 part) solution were mixed in polythene tubes. The tubes were capped and mixtures were shaken for 2 h on a mechanical shaker.
Mineralogical and grain size studies
Mineral analyses were performed by X-ray diffraction (XRD) using CuKα radiation (30 kV, 40 mA) on a Philips X’Pert Pro housed at the Centre of Materials Research, Izmir Institute of Technology (Turkey). Data were collected from 2 to 70° 2θ with a step size of 1° and a counting interval of 0.6 s. Random powder samples were prepared for mineral identification; the size fraction <2 μm was separated and oriented clay aggregate samples were prepared after centrifuging (Gibbs 1965, 1968). Selected samples of the clay fraction were saturated with ethylene glycol for 12 h at 60°C and heated to 550°C before XRD analysis.
Grain sizes were analysed using a Malvern Mastersizer-X housed at the Marmara Research Centre of TUBITAK (Turkey).
Statistical testing
Linear correlation coefficients, maximum, minimum, mean values and STD statistical calculations were conducted using Excel for Windows. The values of the probability function (P) were calculated by Minitab Release 14 statistical software. The correlation coefficient is statistically significant at 95% significance level. Two-sided hypothesis was used in this study. Therefore, α/2 is 0.025.
The clustering analysis method is based on the algorithms that put similar objects into several clusters. It has great advantages, in that it does not need a mathematical mode and is suitable for dealing with complex and multi-score data; therefore, this method was used in this study. Cluster analysis was performed with the SPSS 15 software package. The cluster method of within-groups linkage and squared Euclidean distance interval was applied in this paper.
Results and discussion
Mineralogy and physicochemical properties of soil
The proportion of clay-sized soil particles (<2 μm) varies between 0 and 11.8% (average 3.58%). All the soil samples have similar mineralogy, and are dominated by quartz with lesser calcite, magnetite, pseudo-rutile and clay minerals. Dolomite occurs locally, especially in areas close to Neogene sediments. Almost all of the samples contain clay minerals, including mica (illite–muscovite), chlorite/kaolinite, smectite and mixed-layer clays. Semi-quantitative analyses reveal smectite, illite, chlorite, kaolinite and mixed-layer clays of the soil samples (Table 1). Illite is the dominant clay mineral, and forms up to 49% of the total clay fraction. Smectite, which has greatest absorption capacity among the clay minerals, forms up to 21% of the clay fraction on average, but makes up just 1% of the total soil samples.
The average organic carbon content in the soil samples is 0.96 wt%. The analysed soils have low clay-size fraction and low organic carbon content, which can result in low absorption capacity.
The pH of the soils is generally close to neutral or basic, with an average value of 8.16. In particular, the pH of sampled agricultural soils (6–7) is generally within the range suitable for plant growth. Accordingly, sulphur is added to the soils during treatment to lower the pH, but the concentration of heavy metals in the sulphur must be controlled.
Oxide and element concentration of soil
The average concentrations of major oxides in the 212 analysed soil samples are as follows: SiO2, 61.16%; Al2O3, 11.72%; Fe2O3, 4.45%; MgO, 2.51%; CaO, 5.44%; Na2O, 1.49%; K2O, 2.22%; TiO2, 0.69%; P2O5, 0.22%; MnO, 0.07%; Cr2O3, 0.03% (Table 2).
The main objective of this study was to assess the spatial trend in heavy metal concentrations in soil with increasing distance from two main roads. Linear correlation coefficients of all elements are not presented here, but Table 3 provides a correlation matrix of 20 heavy metals which are more toxic, <2 μm fraction and organic carbon. Size fraction is one of the most important factors that control the distribution and level of concentrations of trace element. Finer the grain sizes, usually higher are the concentrations (Zhang et al. 2002). There is no more correlation of metals with the <2 μm fraction size. Even if the correlation coefficients of the <2 μm fraction size are weak, the P values between the <2 μm fraction size and Ni (0.33), Pb (0.26), Mn (0.31), Sr (0.21), Cr (0.37) and B (0.17) are statistically significant in the positive direction. Organic carbon contents of soil show also weak positive correlation with Mo (0.51). However, the P values of organic carbon between and Cu (0.33), Pb (0.39), Zn (0.46), Mn (0.29), As (0.36), U (0.47), Sr (0.17), Cd (0.49), Sb (0.16), Bi (0.23), B (0.31), Sn (0.23), Li (0.17) and <2 μm fraction size (0.22) are statistically significant.
Nickel shows a positive correlation with Cr (0.86) and Mn (0.54) due to the occurrence of a Fe–Ni mine in ultrabasic rocks north of Turgutlu. Soils derived from these rocks contain high Ni and Cr. The P values of Ni are statistically important in the positive direction with Mo (0.20), Pb (0.19), As (0.19), Sr (0.20), Cd (0.21), Bi (0.20), B (0.17), Li (0.20) and <2 μm fraction size (0.33). Lead values show a positive relation with Zn (0.60), Mn (0.64) and Cd (0.64). Strontium also shows a positive relation with B (0.65), Li (0.64) and negative relation with W (−0.60). In this region, boron is derived mainly from geothermal waters and deep drill holes sunk to obtain water. Arsenic shows relationship with U (0.55), Bi (0.50), Cd (0.51) and Sb (0.54). However, the P values of As are also significant with Ba (0.40), Ni (0.19), Cu (0.42), Pb (0.34), Mn (0.43), Sr (0.25), Cr (0.21), B (0.34), Hg (0.31), Sn (0.24), Li (0.43) and ORG/C (0.36).
Environmental risk
Various methods have been proposed for describing the degree of element enrichment in soil, with the most commonly employed being the geoaccumulation index (Igeo), enrichment factor and the pollution index.
The geoaccumulation index (Igeo) was originally defined by Müller (1969) for a quantitative measure of the metal pollution in aquatic sediments. However, numerous studies, such as Shacklette and Boerngen (1984), Loska et al. (2004) and Gemici and Tarcan (2007), have employed it to assess the contamination of soils. Igeo formula is as follows:
where C n is the metal content in tested soil, and B n is the background metal content, which is the average metal content in the Earth’s crust (Shacklette and Boerngen 1984).
According to the Igeo scheme, soils are classified as follows (Müller 1969):
- Igeo < 0:
-
practically uncontaminated
- 0 < Igeo < 1:
-
uncontaminated to moderately contaminated
- 1 < Igeo < 2:
-
moderately contaminated
- 2 < Igeo < 3:
-
moderately to heavily contaminated
- 3 < Igeo < 4:
-
heavily contaminated
- 4 < Igeo < 5:
-
heavily to extremely contaminated
- Igeo > 5:
-
extremely contaminated.
The enrichment factor (EF) is also used to assess the degree of soil contamination by metals (Buat-Menard 1979). Aluminium is used as a reference element (83,000 mg/kg in the Earth’s crust). Aluminium is often used as an indicator of crustal composition, because other crustal metals (e.g. Fe and Mn) may be contaminated by anthropogenic sources. Local background elemental values were not used due to various mineral deposits in the region. EF is defined as follows:
where C n is the content of the examined element in the examined environment, C ref is the content of the examined element in the reference environment, B n is the content of the reference element in the examined environment (Lide 2003) and B ref is the content of the reference element in the reference environment (Turekion and Wedepohl 1961).
Sutherland (2000) proposed five categories of contamination according to the degree of enrichment:
- EF < 2:
-
depletion to minimal enrichment
- EF = 2–5:
-
moderate enrichment
- EF = 5–20:
-
significant enrichment
- EF = 20–40:
-
very high enrichment
- EF > 40:
-
extremely high enrichment
The pollution index (PI) has also been proposed to assess the degree of pollution in soils (Nishida et al. 1982). PI values have been used in many studies to estimate the degree of pollution by multiple elements (Nishida et al. 1982; Sponza and Karaoğlu 2002; Gemici and Tarcan 2007; Gemici 2008). The PI is computed by averaging the ratios of the total concentrations of the elements to tolerable levels. In this study, PI values were calculated for 13 elements:
Tolerable levels were taken from Sponza and Karaoğlu (2002) for Cu, Pb, Fe, Zn, Ni, Cr, Ti and Mn, and from Gemici (2008) for the other elements. PI values above 1.0 indicate that the soils are possibly polluted by anthropogenic input.
The PI values of the soils analysed in the present study are between 0.14 and 3.77 (average 0.57). This low average value indicates that the soils are overall unpolluted; however, the area north of Manisa is affected by anthropogenic pollution. High PI values obtained for the area north of Turgutlu reflect high Ni values derived from the proximity of the Çaldağ Fe–Ni mine (Fig. 2).
The highest Pb concentrations in soil were observed between the Manisa and Turgutlu areas near Nif Creek (28.83–56.11 mg kg−1; irrigation water is taken from Nif Creek), central Manisa (29.97 mg kg−1), and alongside the road between Sart and Salihli (23.30 mg kg−1). The Pb concentrations of soils decrease away from roads, but elevated Pb levels are found near city centres, especially in industrial areas. The Pb levels in all soils sampled from this region were well below the 600 mg kg−1 Australian Ecological Investigation Limit (EIL) (NEPC 1999). The relatively high Pb concentrations in these regions may have resulted from atmospheric deposition from mixed anthropogenic sources, including motor vehicle emissions, the combustion of fossil fuels and other industrial emissions.
The highest Cd concentration in soil was found near central Manisa along the Istanbul road (0.46 mg kg−1); this sample also yielded a high EF value. Sample collected from along the Istanbul road, contained 0.22 mg kg−1 Cd, although the EF value was 42, corresponding to “extremely high enrichment”. Only two samples collected close to roads in the Manisa and Salihli regions contained Cd concentrations exceeding 3 mg kg−1 EIL (NEPC 1999).
We calculated Igeo values for Ni, Cu, Pb, Zn, Co, As, Cd, Sb, Cr, Hg and B (although boron is not a heavy metal, its concentration in soil is important for plant life). Only arsenic concentrations corresponded to “heavily to extremely contaminated” (Fig. 3). The average Igeo values for all samples corresponded to “heavily contaminated” (Table 3). Arsenic values were highest near central Salihli (4.7–83 mg kg−1) due to the operation of a tannery. Arsenic concentrations in most of the soils in this region exceeded the NEPC (1999) EIL of 20 mg kg−1. High EF values were obtained for most of the samples (221–250), especially east of Sart, corresponding to “extremely high enrichment” (Fig. 4). The average EFarsenic value for all of the soil samples collected in this region is 76, corresponding to “extremely high enrichment” (Table 4).
Igeo values calculated for antimony in soils around Salihli, north of Turgutlu and east of Manisa, correspond to “moderately to heavily contaminated” (Fig. 5). All of the studied area yields values that are close to “moderately contaminated”. The EF values for antimony are highest in soils of the Salihli and Alaşehir regions (Fig. 6). The highest concentration of antimony in the analysed soils is around 2 mg kg−1. The EIL for Sb in urban areas is set to 20 mg kg−1. All the studied soils fell below this limit; however, the EF calculations reveal “extremely high enrichment” of antimony in soils from the Salihli and Alaşehir regions. The EF of Sb is also very highly enriched in soils with 35.27 averages. Arsenic and Sb show large SD values due to wide intervals of the results. This can show local As and Sb contaminations (Table 4).
One soil sample from the Alaşehir roadside contained Hg concentrations of around 330 μg kg−1. Similar Hg concentrations were obtained for soil samples from Salihli, north of Turgutlu, near the Urganlı geothermal area, and the Kemalpaşa industrial area. EF values calculated for soil samples around Alaşehir show “very high enrichment”. Gemici (2008) determined the extent of contamination by Hg and heavy metals in the Alaşehir area related to a mercury mine that ceased production in the early 1990s. The mine is located 3-km south of Alaşehir town, at an altitude of 550 m on the northern side of the Alaşehir Mountains, which rise steeply from the Alaşehir Plain. The mine site is drained by a stream that flows down to the plain. Samples of stream sediment contain anomalously high values of metals, especially Hg, As, Ni and Cr. Hg concentrations in the stream sediments vary between 0.37 and 76.94 mg kg−1, and levels of arsenic are as high as 999 mg kg−1.
Antimony levels range from 2 to 246 mg kg−1. This small-scale mine may have contributed to the contamination of soils in the Alaşehir area.
Mercury concentrations are very low in soils of the Manisa and Akhisar regions.
Nickel concentrations in the analysed soils varied between 14.6 and 255.1 mg kg−1, with the highest levels measured around the Çaldağ Fe–Ni mine, reflecting the composition of the neighbourhood lithologies.
Boron is not a heavy metal, but it is necessary in very small quantities for normal plant growth, although higher concentrations are toxic. As a general guideline, boron concentrations in groundwater exceeding 1 mg/L are harmful to plants (Richards 1954). Boron concentrations in the analysed soils ranged between 2 and 29 mg kg−1, with the highest values obtained for soil samples from the area NE of central Manisa and near Salihli. The EF values indicate that the samples from along the Alaşehir road and north of Muradiye present “very high enrichment” boron levels, due mainly to deep drill holes sunk in this area to obtain water.
Element concentrations in raisin
A total of 82 raisin samples were analysed at JR Laboratories Inc. (Canada). Sultana seedless grapes are grown in the Aegean region of western Turkey, especially in the Gediz Plain. There are two types of raisins supplied to the retail market: one with a golden colour that is produced by the grape being dipped in an alkaline solution (5% potassium carbonate + olive oil as a surfactant) and then subjected to sun or oven drying (Fig. 7); the other type is a natural, sun-dried raisin produced without dipping in an alkaline solution. Two samples (golden type, pre- and post-treatment) were collected from Tariş (a processing company) and two samples (natural, pre- and post-treatment) were collected from Pagmat (a processing company).
European Community (EC) regulation No. 466/2001 stipulates that the concentrations of lead and cadmium in soft fruits such as strawberry and cherry should not exceed 0.2 and 0.05 mg kg−1, respectively, in their fresh forms. The level of heavy metals permitted in raisins varies by country.
Angelova et al. (1999) studied grapes grown in Pladiv (Bulgaria), close to a highly industrialised area, revealing that the seeds contained up to 2.3 mg kg−1 of Pb. The concentration of Pb on the peel decreased after washing. A number of heavy metals, especially Pb and Cd, are toxic to people of all age groups, whereas other heavy metals (e.g. Cu, Mo, Ni and Zn) are beneficial to certain age groups in low amounts. Therefore, the Pb and Cd contents of raisins are of great importance.
Table 5 lists the maximum and minimum results of chemical analyses of raisins sampled from vineyards in the present study.
Lead contents ranged between 0.05 and 0.46 mg kg−1, and showed an increasing trend towards the roads between Manisa and Turgutlu, and between Manisa and Saruhanlı.
Cadmium levels are around 0.01 mg kg−1 or below the detection limit, with only one sample (K40; between Manisa and Turgutlu) containing higher levels of 0.23 mg kg−1.
Boron concentrations in raisins (21.4–94.9 mg kg−1) are higher than those in the soil, with the highest levels recorded in the Salihli and Alaşehir areas.
Arsenic concentrations in soils are high around the Salihli region, whereas raisins from this area contain low amounts of As. Arsenic levels in the analysed raisins ranged between 0.04 and 0.7 mg kg−1. High concentrations were obtained for areas between Manisa and Muradiye, which is a highly industrialised region.
The copper levels in raisins ranged between 2.03 and 13.99 mg kg−1. Çalışır and Akman (2007) reported a weak correlation between total lead and copper in raisins (correlation coefficient = 0.23, 46 samples from Turkey, including 11 samples from the Aegean region).
Çalışır and Akman (2007) reported a lead content of 0.012–0.359 mg kg−1 in raisins produced mainly in 2005, much less than that reported by Dabeka et al. (2002) for raisins produced between 1993 and 1995, which ranged between 0.056 and 3.1 mg kg−1. The Ministry of Agriculture, Fisheries and Foods in the UK, and the US Food and Drug Administration declared high lead levels in sultanas in 1998 and 2003, respectively, with measured values ranging from 0.83 to 0.98 mg kg−1 Çalışır and Akman (2007).
Lead concentrations of raisins in this study (ranged between 0.05 and 0.46 mg kg−1) were in accordance with those of Çalışır and Akman (2007).
We compared the heavy metal concentrations in raisins subjected to different drying processes [i.e. golden colour (dipped in alkaline solution) versus natural (without dipping)] at the same production facility. The two samples yielded similar concentrations, indicating that different drying processes have no effect in terms of heavy metal concentrations.
A linear correlation coefficient is presented in Table 6. The Pb content of raisins shows no more strong relation to the contents of other elements. The P values of Pb are statistically significant with Al (0.39), As (0.36), Ba (0.38), Cd (0.40), Ca (0.44), Cr (0.34), Co (0.36), Fe (0.43), Mn (0.35), Ni (0.29) and Ti (0.25). Arsenic concentrations show weak positive correlations with Fe (0.57), U (0.71) and V (0.73), as also observed in the soil samples. The P values of As with Sb (0.31), Ba (0.40), B (0.25), Cr (0.44), Pb (0.36), Li (0.27), Mg (0.41), Mn (0.36), Mo (0.36), Ni (0.39), Se (0.25) and Na (0.34) are significant. The most pronounced positive relations were observed between Al and As (0.50), Ba (0.71), Fe (0.99), Cr (0.90), Co (0.94), Ni (0.84), Ti (0.91) and V (0.94). Positive correlation was seen also between Ba and Fe (0.73), Ba and Ti (0.74), Cd and Se (0.64), Cr and Co (0.97), Cr and Fe (0.89), Cr and Ni (0.94), Cr and Ti (0.70), Cr and V (0.81), Co and Fe (0.93), Co and Mn (0.69), Co and Ni (0.93), Co and Ti (0.78), Co and V (0.85), Fe and Ni (0.82), Fe and Ti (0.91), Fe and V(0.95), Mo and V (0.95) and Ti and V (0.88).
Raisins prepared for retail sale by the two processing companies considered here are subjected to careful processing prior to distribution. Table 7 lists the results of chemical analyses of these raisins. The concentrations of Al, As, Ba, Cr, Co, Fe, Pb, Li, Ni, Sn and Ti show a decrease during processing, whereas levels of Cu and K remain unchanged. The contents of B, Sn and Zn show a decrease in Pagmat (K79), but no change in the other, due mainly to the type of processing and/or processing periods.
Hierarchical cluster analysis classified two main groups after the processing (Fig. 8). The K80 and K82 are the main groups after processing. The natural dried sample (K79) is close to the K80 and K82 groups. The cleaning processes to which the raisins are subjected act to reduce the chemical content and dust pollution on the raisins.
The high Pb concentrations in Turkish raisins reported by Dabeka et al. (2002), the Ministry of Agriculture, Fisheries and Foods in the UK (1998) and US Food and Drug Administration (2003) have been reduced in later crops due to improved processing. The post-cleaning lead and cadmium contents of the raisins which are most important in terms of human health are below those stipulated by EC regulation No. 466/2001, which states the maximum allowable levels for lead (0.2 mg kg−1) and Cd (0.05 mg kg−1).
Potential sources of contamination are pesticides and fertilisers. Elemental analysis results after cleaning procedure show that the elemental enrichment of raisins result from the dust which adhered on.
Conclusions
The Gediz Graben is filled by sediment derived from the surrounding rocks, mainly metamorphic rocks such as gneisses, schists and marbles. This region is one of the most important agricultural areas in Turkey, including for the cultivation of seedless sultana raisins. The region is experiencing ongoing population growth and industrialisation. Highways have been constructed across agricultural areas due to the low construction costs, but this has led to the contamination of agricultural soils, for which the cost of remediation will exceed the amount saved by construction in regions away from populated areas. In this context, the present study analysed the mineralogy, heavy metal contents, particle size and pH of these soils.
The main minerals in the soils are quartz, calcite and mica. Semi-quantitative analysis shows that the clay fraction in the soils contains smectite, illite, chlorite, kaolinite and mixed-layer clays. The average content of clay minerals (particle size below 2 μm) in the soil samples is 4. This result demonstrates that the soils contain a low proportion of clay minerals, and they are classified as sandy silt loam. The pH values of soils are close to neutral, and they contain low levels of organic carbon. It is questionable whether heavy metal concentrations in soils are higher at roadside sites, as only minor Pb contamination occurs as a consequence of motor vehicle emissions. Arsenic in the analysed soils is derived mainly from pesticides, and arsenic is more of a concern than Pb. The average Igeo value for As in the analysed soils corresponds to “heavily contaminated”. Enrichment factor (EF) values show that the soils are classified as “extremely high enrichment” in terms of arsenic. Considering the entire study area, the pollution index (PI) of soils is below 1, indicating they are unpolluted.
The present results reveal that after long-term sultana cultivation in western Turkey, there is no accumulation of heavy metals in sultana grapes, although arsenic levels are strongly elevated in surface soils. It is concluded that As and Sb are the most harmful elements from an environmental perspective. Nevertheless, Ni and Cr enrichment in soils in northern Turgutlu can be attributed in part to natural sources, specifically the presence of ultramafic ophiolites of the Çaldağ Fe–Ni mine.
Grapes that are dried near roadways contain high concentrations of heavy metals; however, the heavy metal content of raisins sent to the market is low, meeting EC standards (after cleaning of the raisins). The drying of grapes on roadsides and close to petrol stations should be avoided. In addition, authorities should not allow residential or industrial developments on agricultural land.
References
Al-Chalabi AS, Hawker D (2000) Distribution of vehicular lead in roadside soils of major roads of Brisbane, Australia. Water Air Soil Pollut 118:299–310
Alloway BJ (1990) Heavy metals in soils. Blackie and Son Ltd., Glasgow and London
Angelova VR, Ivanov AS, Braikov DM (1999) Heavy metals (Pb, Cu, Zn and Cd) in the system soil–grapevine–grape. J Sci Food Agric 79:713–721
Buat-Menard P (1979) Influnce de la retombée atmospherique sur la chimie des metaux en trace dans la matière en suspension de l’Atlantique Nord. Thesis, University of Paris
Çalışır F, Akman S (2007) Survey of lead and copper in Turkish raisins. Food Addit Contam 24(9):960–968
Çelik H, Ağaoğlu YS, Fidan Y, Marasalı B, Söylemezoğlu G, Boz Y, Özer C, Atak A (1998) Genel Bağcılık, Sunfidan A.Ş. Mesleki Kitaplar Serisi 1 (in Turkish)
Çolak M (2004) Firing transformations of Turgutlu (Manisa–Turkey) brick-tile raw material used in the manufacturing of ceramic bodies. Ind Ceram 1:15–19
Dabeka RW, MacKenzie AD, Pepper K (2002) Lead contamination of raisins sold in Canada. Food Addit Contam 19:47–54
Dewey JF, Şengör AMC (1979) Aegean and surrounding regions: complex multiplate and continuum tectonics in convergent zone. Geol Soc Am Bull 90:84–92
Food Safety Standards Group (1998) Ministry of Agriculture, Fisheries and Food (MAFF UK). Lead in dried fruit. Food Surveillance Information Sheet No. 155, August 1998. HM Stationary Office, London. http://archieve.food.gov.uk/maff/archieve/food/infsheet/1998/no155/155dried.htm. Accessed 23 September 2009
Gemici Ü (2008) Evaluation of the water quality related to the acid mine drainage of an abandoned mercury mine (Alaşehir, Turkey). Environ Monit Assess 147:93–106
Gemici Ü, Tarcan G (2007) Assessment of the pollutants in farming soils and waters around untreated abandoned Türkönü Mercury Mine (Turkey). B Environ Contam Tox 79:20–24
Gibbs RS (1965) Error due to segregation in quantitative clay mineral X-ray diffraction mounting techniques. Am Mineral 50:741–751
Gibbs RS (1968) Clay mineral mounting techniques for X-ray diffraction analysis: a discussion. J Sediment Petrol 38:242–244
Gratani L, Taglioni S, Crescente MF (1992) The accumulation of lead in agricultural soil and vegetation along a highway. Chemosphere 24:941–949
ISO 10390 (2005) Soil quality—Determination of pH. International Organization for Standardization, Geneva
Lagaly G (1995) Surface and interlayer reactions: bentonites as adsorbents. In: Churchman, GJ, Fitzpatrick, RW, Eggleton RA (eds) Clays: Controlling the Environment, Proceedings of the 10th International Clay Conference, Adelaide, Australia, 1993. CSIRO Publishing, Melbourne, pp 137–144
Lide DR (2003) Handbook of chemistry and physics, 84th edn. CRC Press, London
Loska K, Wiechula D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30:159–165
Meyvacı KB, Altındişli A, Aksoy U, Eltem R, Turgut H, Arasiler Z, Kartal N (2005) Ochratoxin A in sultanas from Turkey I: Survey of unprocessed sultanas from vineyards and packing-houses. Food Addit Contam 22:1138–1143
Minareci O, Öztürk M, Egemen Ö, Minareci E (2009) Detergent and phosphate pollution in Gediz River, Turkey. Afr J Biotechnol 8(15):3568–3575
Müller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geojournal 2:108–118
Murray-Rust D, Svendsen M (2001) Performance of locally managed irrigation in Turkey: Gediz case study. Irrig Drain 15:373–388
NEPC (National Environment Protection Council) (1999) National environmental protection (assessment of site contamination) measures. National Environment Protection Council, Adelaide
Nishida H, Miyai F, Tada F, Suzuk F (1982) Computations of the index of pollution caused by heavy metals in river sediment. Environ Pollut Ser B 4:241–248
OIV (2007) Office Internationale de la Vigne et du Vin. http://news.reseau_conceot.net/images/oiv_UK/Client/Stat_2004-definitif_EN.pdf. Accessed 9 October 2009
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agriculture Handbook No. 60. U.S. Department Agriculture, Washington DC
Şengör AMC (1987) Cross-faults and differential stretching of hanging walls in regions of low-angle normal faulting: examples from western Turkey. In: Coward MP, Dewey JF, Hancock P (eds) Continental extensional tectonics. J Geol Soc London, Special Publication 28, pp 575–589
Shacklette HT, Boerngen JG (1984) Element concentrations in soils and other surficial materials of the conterminous US. USGS Professional Paper 1270, p 105
Sözbilir H (2000) Active faults and potential active faults from West Anatolia. In Proceedings of Seismicity of West Anatolian Symposium, pp 133–142 (in Turkish)
Sponza D, Karaoğlu N (2002) Environmental geochemistry and pollution studies of Aliağa metal industry district. Environ Int 27:541–553
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environ Geol 39:611–627
TUIK (2008) Türkiye İstatistik Kurumu. http//www.turkstat.gov.tr/PreIstatistikTablo.do?istab|_id=65. Accessed 23 September 2009
Turekion KK, Wedepohl KH (1961) Distribution of the elements in some major units of the Earth’s crust. Geol Soc Am Bull 72:175–192
Turer D (2005) Effect of non-vehicular sources on heavy metal concentrations of roadside soils. Water Air Soil Pollut 166:251–264
US Food and Drug Administration (2003) http://www.accessdata.fda.gov/ImportAlerts/ora_import_ia2003.html. Reported in January and February, 1996. Accessed 23 September 2009
Vissikirsky VA, Stepashko SV, Kalavrouziotis IK, Varnavas SP (2008) The road pollution impact on Zea mays: Inductive modelling and qualitative assessment. Water Air Soil Pollut 195:301–310
Yılmaz Y, Genç ŞC, Gürer F, Bozcu M, Yılmaz K, Karacık Z, Altunkaynak Ş, Elmas A (2000) When did the western Anatolian grabens begin to develop? In: Bozkurt E, Winchester JA, Piper JAD (eds) Tectonics and magmatism in Turkey and the surrounding area, J Geol Soc London, Special Publication 173, pp 131–162
Zhang C, Wang L, Li G, Dong S, Yang J, Wang X (2002) Grain size effect on multi-element concentrations in sediments from the intertidal flats of Bohai Bay, China. Appl Geochem 17:59–68
Zupančič N (1998) Lead contamination in the roadside soils of Slovenia. Environ Geochem Hlth 21:37–50
Acknowledgments
The author acknowledges the financial support of the TUBITAK (The Scientific and Technical Research Council of Turkey) Research Fund (TUB 104Y285). My thanks are also due to the TARİŞ and PAGMAT companies for supplying raisin samples for analysis. I thank reviewers for their reviews and constructive criticism of the manuscript. I am also grateful to Dr. Cem Kıncal for MapInfo and to Dr. Umay Koçer for Minitab statistical studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Çolak, M. Heavy metal concentrations in sultana-cultivation soils and sultana raisins from Manisa (Turkey). Environ Earth Sci 67, 695–712 (2012). https://doi.org/10.1007/s12665-011-1511-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1511-0