Abstract
Asian soybean rust (ASR) is the most serious disease affecting soybean production in South America. Planting resistant genotypes is one way to control the disease; however, ASR populations in South America exhibit high pathogenic diversity. The soybean genotype No6-12–1 with three resistance genes (Rpp) to ASR has exhibited resistance to most of the South American ASR populations in laboratory trials. However, little is known about the resistance responses of No6-12-1 under field conditions. Here, we compared the resistance of six different genotypes of soybean to ASR under field conditions: (1) No6-12-1, a line with a pyramid of the Rpp2, Rpp4, and Rpp5; (2–4) lines with only Rpp2, Rpp4, or Rpp5, (5) PI 587880A, which harbors Rpp1-b, and (6) BRS 184, a susceptible genotype. Both fungicide-treated and untreated plots were grown in three cropping seasons, from 2014 to 2018, in the Brazilian state of Paraná. We evaluated disease severity, area under disease progress curve (AUDPC), the number of uredinia per lesion (NoU), and urediniospore production of the six genotypes. Both fungicide treatments and genotype affected disease severity and AUDPC, and genotype affected NoU. No6-12-1, the pyramided genotype, showed lower disease severity and AUDPC than the other genotypes that harbored only one resistance gene, except for sprayed plots of PI 459025 in the 2017/2018 crop season, and PI 587880A in the 2016/2017 and 2017/2018 crop seasons. NoU and urediniospore production were lower in No6-12-1 than in the other genotypes. These results indicate that the synergistic effects of Rpp-gene-pyramiding observed in laboratory assays also occur, especially in NoU, under field conditions in Brazil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asian soybean rust (ASR) is caused by Phakopsora pachyrhizi Syd. & P. Syd. and is one of the most devastating diseases of soybean [Glycine max (L.) Merr.]. Since the first record of ASR in 2001 in South America (Yorinori et al. 2005), yield losses and control costs have been substantial (Godoy et al. 2016; Savary et al. 2019; Wrather et al. 2010). Fungicide applications have been the main control measure against ASR; however, continuous and repeated use of fungicides causes reduction in their efficacy (Brent and Hollomon 2007). In fact, quinone outside inhibitor and demethylation inhibitor fungicides became less effective to ASR after a decade of use in Brazil (Dalla Lana et al. 2018; Godoy et al. 2016).
Planting resistant cultivars is another control measure against ASR and is cost-effective and environmental-friendly. Several resistance genes and alleles to P. pachyrhizi have been identified in seven resistance regions to P. pachyrhizi (Rpp) (Childs et al. 2018). However, there is a substantial pathogenic diversity in the P. pachyrhizi populations in South America (Akamatsu et al. 2013, 2017; Stewart et al. 2019), resulting in no single resistance gene being found to be effective against all races there. Cultivars expressing Rpp1-b and Rpp5 were relatively resistant to a wide range of rust populations collected in South America (Akamatsu et al. 2013, 2017; Kato 2017).
According to Flor’s gene for gene concept (Flor 1971), resistance is controlled by pairs of matching genes: a host producing a resistance gene is resistant toward a pathogen producing a corresponding avirulence gene. Therefore, host genotypes with pyramided resistance genes would resist pathogen genotypes that have recessive alleles in all the corresponding avirulence genes. However, No6-12-1, a line with a pyramid of the Rpp2, Rpp4, and Rpp5 resistance genes, was highly resistant to a P. pachyrhizi strain that was virulent to plant lines harboring each of these resistance genes individually under laboratory conditions, which suggests a synergistic effect of Rpp pyramiding (Lemos et al. 2011; Yamanaka et al. 2015). However, it remains unknown whether No6-12-1 is resistant to P. pachyrhizi under field conditions, where there is enhanced diversity in natural populations of this pathogen. Hence, in this study, we aimed to confirm whether the synergistic effects of Rpp-pyramiding that were reported in the laboratory would also be observed under field conditions.
Materials and methods
Plant materials
Five soybean genotypes harboring resistance genes to P. pachyrhizi were used in this experiment: No6-12-1, PI 230970, PI 459025, Shiranui (PI 200526), and PI 587880A. No6-12-1 harbors three resistance genes (Rpp2 + Rpp4 + Rpp5) (Lemos et al. 2011) and was developed by crossing with PI 230970 (Rpp2), PI 459025 (Rpp4), Kinoshita (Rpp5), and susceptible cultivars of BRS184 (Yamanaka et al. 2013b). PI 230,970 harbors the resistance gene Rpp2 (Hartwig and Bromfield 1983). PI 459025 harbors the resistance gene Rpp4 (Hartwig, 1986). Shiranui harbors a resistance gene at the Rpp5 locus (Garcia et al., 2008). PI 587880A harbors the resistance gene Rpp1-b inferred from the physiological position of Rpp1 region and resistance reactions to ASR isolates (Kim et al. 2012; Ray et al., 2009; Yamanaka et al. 2016) and was included in the experiment because it is resistant to most of the rust populations in South America (Akamatsu et al. 2013, 2017). BRS 184 was used as a susceptible control. No6-12-1 was obtained from Japan International Research Center for Agricultural Sciences, Japan, with an import permit (05,852). All the other genotypes were obtained from the soybean germplasm bank of Embrapa Soja, Londrina, Paraná state, Brazil, which were provided by the United States Department of Agriculture.
Field experiment
The experimental plots were arranged in a split-plot design with four repetitions in a field at the Embrapa Soja research station (51° 11′ W, 23° 11′ S) in Londrina. To compare resistance to ASR among six genotypes, fungicides were treated in the experiments. Fungicide applications divided the primary plots, and the six genotypes were the subplots in each run of the experiment. The plot contained a single row of 2 m with 0.70-m spacing, and the seed density was 20 seeds/m. Each experimental unit consisted of one plot row. Another susceptible soybean cultivar BRS 154 was sown on both sides of each main plot as a “spreader.” The subplots and the spreader rows were surrounded with one fallow row, and there was a 1–2-m spacing between the repetitions.
Sowing in each field experiment occurred at different seasons: (1) November 28, 2014 in field experiment-1 (FE-1); (2) November 28, 2016 in FE-2; and (3) November 30, 2017 in FE-3. A mixture of fungicides was sprayed on half of the primary plots using a back sprayer each year. FE-1 was sprayed on January 26 and February 21, 2015 with a mixture of epoxiconazole (25-g active ingredient (a.i.)/ha), pyraclostrobin (66.5 g a.i./ha), and mancozeb (1.125 kg a.i./ha). FE-2 was sprayed on January 11 and 26, 2017 with a mixture of azoxystrobin (60 g a.i./ha) and benzovindiflupyr (30 g a.i./ha), and then again on February 9, 2017 with a mixture of tebuconazole (60 g a.i./ha), picoxystrobin (100 g a.i./ha), cyproconazole (45 g a.i./ha), and difenoconazole (75 g a.i./ha). FE-3 was sprayed on January 15 and 31, 2018 with a mixture of trifloxystrobin (60 g a.i./ha) and prothioconazole (70 g a.i./ha), and then again on February 15 and March 1, 2018 with a mixture of picoxystrobin (60 g a.i./ha), cyproconazole (24 g a.i./ha), and mancozeb. The composition, amount, and spray timing of the fungicide mixtures were determined based on the observations of rust incidence and the recommendations of Embrapa Soja.
The experimental plots were not artificially inoculated with P. pachyrhizi. To evaluate disease severity, number of uredinia per lesion (NoU), and urediniospore production, ten soybean leaflets were collected from the mid-canopy of the plants of each experimental plot at multiple times each year. Leaflets from FE-1 were collected on February 9 and 20 and March 2, 12, and 23, 2015. Leaflets from FE-2 were collected on January 11 and 26; February 6 and 20; and March 6, 2017. Leaflets from FE-3 were collected on February 15 and 27 and on March 15, 2018. Insecticides were applied each year according to the standard practices of the Embrapa Soja region.
Disease severity, urediniospore production, and NoU
Ten leaflets collected from the experimental plots were evaluated for disease severity, urediniospore production, and NoU. Disease severity was assessed as the percentage of diseased leaf area of each leaflet using a standard diagram of disease severity (Godoy et al. 2006). Urediniospore production was assessed by observing lesions under a stereomicroscope. Sporulation per leaflet was recorded using a scale of sporulation level from 0 (none) to 3 (abundant) (Yamanaka et al. 2013a). Urediniospore production was assessed using samples collected on March 2 and 12, 2015 and on February 6 and 20, 2017. Leaflets from the third and fourth blocks were scored on March 2, 2015, while those from all the blocks on the other sampling dates. However, thirty-nine leaflets were examined in PI 230970 in sprayed plots on March 12, 2015. Ten leaflets per block were collected, totally 20 leaflets in the evaluation on March 2, 2015, and 40 leaflets on other sampling dates. Urediniospore production in a leaflet was rated using scores from 0 (none) to 3 (abundant) under a stereomicroscope. After the urediniospores were removed using a paintbrush, the NoU was counted under a stereomicroscope (Yamanaka et al. 2013a). The NoU on 50 lesions was counted by observing five lesions per leaflet. The NoU was evaluated in all samples except the leaflets collected on January 11 and 26, 2017, February 15, and March 15, 2018.
Area under disease progress curve (AUDPC)
AUDPC is a summary variable for disease severity. This variable is based on multiple assessments (Madden et al. 2007). It was calculated using the following formula:
where DSi is disease severity (%) on Dayi, and Dayi is the day (days after planting) at the ith observation.
Statistical analysis
The factors genotype, fungicide, and genotype × fungicide were analyzed as independent variables of fixed effect, block nested within fungicide as independent variables of random effect; and disease severity, AUDPC, and NoU as dependent variables. Disease severity, AUDPC, and NoU were analyzed using analysis of variance and Tukey’s HSD test. Urediniospore production was analyzed using ordinal logistic regression analysis, and the ranks of each genotype were obtained. Statistical analyses were conducted using JMP® 11 statistical software (SAS Institute Inc. Cary, NC, USA).
Results
Disease progression and AUDPC
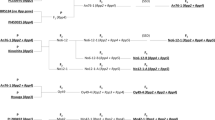
Disease progressions and AUDPC of ASR on the six genotypes unsprayed and sprayed with fungicides are shown in Fig. 1 and Table 1. Disease progression differed among genotypes and fungicide treatments (Fig. 1, Supplement 1). Disease progression in the pyramided line No6-12-1 (Rpp2 + Rpp4 + Rpp5) was slower than that in the genotypes that harbored only Rpp2 or Rpp5 and the susceptible cultivar BRS 184 (no Rpp gene) in all three seasons (Fig. 1). Comparing with PI 459025, the disease progression of No6-12-1 was slower in the unsprayed treatment in 2014/2015 and 2016/2017 and in the sprayed plot in 2016/2017, similar in the sprayed plot in 2014/2015, and faster in both plots in 2017/2018 (Fig. 1). Comparing with PI 587880A, the disease progression of No6-12-1 was slower in the unsprayed treatment in 2014/2015, similar in the sprayed plot in 2014/2015, and faster in both plots in 2016/2017 and 2017/2018. (Fig. 1). At the final assessment dates, the disease severity of No6-12-1 was significantly lower than that of PI 230970, Shiranui, and BRS 184 in all seasons and the fungicide treatments. Fungicide spraying reduced disease severity in all genotypes studied (Fig. 1 and Table 1). We observed an interaction between the factors genotype and fungicide in the 2014/2015 and 2016/2017 seasons (Table 1).
The effects of genotype and fungicide on AUDPC were similar to those on disease severity (Table 1). No6-12-1 had lower AUDPC than did PI 230970, Shiranui, and BRS 184 except in the 2017/2018 season, and lower or similar AUDPC than did PI 459025 in the all seasons. The AUDPC of PI 587880A was lower in the 2016/2017 season or higher in the 2014/2015 season than that of No6-12-1 in both of fungicide treatments. We observed an interaction between the factors genotype and fungicide in the 2014/2015 and 2017/2018 seasons. In comparison with the control genotype, fungicide treatments induced dramatic differences in AUDPC in PI 459025, BRS 184, and PI 587880A in 2014/2015 and PI 230970, Shiranui, and BRS 184 in 2017/2018, while a slighter difference was observed in No6-12-1.
NoU
The NoU of ASR on the six genotypes unsprayed and sprayed with fungicides was observed in the three seasons. The NoU of all sprayed and unsprayed genotypes increased gradually over time (Fig. 2, Supplement 2). The lowest and highest NoUs were observed in No6-12-1 and BRS 184, respectively, in all genotypes and treatments. Values of NoU in the last sampling date were 2.1—12.9-fold higher in genotypes harboring a single Rpp genes and 4.3—20.4-fold higher in BRA 184 than in No6-12-1. NoU differed among genotypes in the three seasons, and between fungicide treatments only in the 2016/2017 seasons (Table 2). We verified an interaction between the factors genotype and fungicide in the three seasons.
Urediniospore production
Urediniospore production of ASR on the six genotypes unsprayed and sprayed with fungicides was rated in the 2014/2015 and 2016/2017 seasons. Urediniospore production was affected by genotypes and fungicide treatments (Fig. 3, Supplement 3). Urediniospore production was highest in BRS 184 and lowest in No6-12-1. The other genotypes harboring a single resistance gene showed intermediate production of urediniospores.
Urediniospore production of Asian soybean rust in six genotypes unsprayed and sprayed with fungicides during the 2014/2015 and 2016/2017 seasons. Sampling date of leaflets are indicated at the top of graphs. The numbers above each graph indicate the rank of spore production with descending order using ordinal logistic analysis with the combination of sampling date and fungicide treatment. Resistance genes are indicated in parentheses under genotypes. Asterisk indicates that thirty-nine leaflets were examined (PI 230970 in sprayed plots on March 12, 2015)
Discussion
The main purpose of this study was to confirm the effect of pyramiding Rpp genes on resistance to ASR in the field by comparing No6-12-1 harboring three resistance genes Rpp2, Rpp4, and Rpp5, and genotypes harboring only one of these resistance genes. Disease severity of ARS was generally lower in the genotype No6-12-1 than in the genotypes PI 230970 and Shiranui, which expressed Rpp2 and Rpp5, respectively. PI 459025 harboring Rpp4 and No6-12-1 had lower or higher disease severity than each other depending on the season. The consistent lower disease severities in No6-12-1 than BRS 184, PI 230970, and Shiranui may be due to large difference in disease severity between No6-12-1 and others. The inconsistent progression in PI 459025 and PI 587880A, and No6-12-1 among seasons may be due to small difference in disease severities among them and large variations in disease severity.
The efficacy of the resistance genes depends on the pathogenic races of P. pachyrhizi in the field. Various pathogenic races of P. pachyrhizi were found within small areas in Japan (Yamaoka et al. 2014), and diversity in virulence phenotypes was observed in climatic zones in Nigeria (Twizeyimana et al. 2009). P. pachyrhizi populations collected in or close to the fields and greenhouses at Embrapa Soja in 2007/2008, 2008/2009, 2010/2011, 2012/2013, 2013/2014, and 2014/2015 caused compatible or intermediate reactions in differential genotypes harboring the Rpp2, Rpp4, or Rpp5 genes, indicating that P. pachyrhizi races defeating the Rpp genes were present around the field for a long time (Akamatsu et al. 2013, 2017). The field pathogen samples used in the above study were not single-spore pure lines. Therefore, it is possible that the non-purified samples contained a mixture of strains with pathogenic diversity. Purified P. pachyrhizi isolates BRP-2.5 and BRP-2.6, sourced from the greenhouses of Embrapa Soja, were complex races that induced susceptibility or slight resistance in three genotypes expressing Rpp2, Rpp4, and Rpp5 separately, while inducing resistance in No6-12-1 (Yamanaka et al. 2015). Their report suggested that there were complex races that were virulent to genotypes harboring each of the Rpp genes around the experiment fields. However, in this study, ASR progressed more slowly on No6-12-1 than on PI 230970 and Shiranui, even in the natural environment where various types of complex races might be present. This suggests a synergistic effect of the resistance genes Rpp2, Rpp4, and Rpp5. However, disease progression on PI 459025 occurred at a similar speed as that on No6-12-1. Hence, it is possible that the slower disease progression on No6-12-1 is explained only by the effect of the Rpp4.
The effect of treatments on AUDPC in genotypes was similar to those on disease severity. This is reasonable because AUDPC is based on disease severity. We expected that there would be evident interactions between the factors genotype and fungicide treatments with resistant genotypes exhibiting small differences in disease severity and AUDPC between unsprayed and sprayed plots, and less resistant genotypes exhibiting a larger difference between these two treatments. However, significant interactions were observed only in two of the three seasons and the efficacy of fungicide treatment was smaller than expected. No6-12-1 is not immune to P. pachyrhizi populations in South America causing resistant reactions. Since we used percent of diseased leaf area for the assessment of disease severity, considerable number of resistant lesions produced on No6-12-1 probably diminished the difference.
Pyramiding of the three resistance genes had a synergistic effect against NoU (Table 2). The pathogen also induced a relatively high NoU on PI 587880A. The reason of the high NoU on PI 587880A is not known. Fungicide treatment affected NoU only during the 2016/2017 season. The effect of fungicide on NoU was mild (Table 2). The fungicides used may have main protective targets at invasion and less at uredinial production. Interactions between genotypes and fungicide treatments were significant in the three seasons. The reasons for these interactions are unclear.
The low urediniospore production in No6-12–1 observed in the field (Fig. 3) was also verified in the laboratory (Yamanaka et al. 2013b, 2015). However, the difference between treatments was not as dramatic in the field as in the laboratory. This difference may be due to more diverse pathogen populations in the field and the age of lesions evaluated. Yamanaka et al. (2013a) evaluated sporulation 14 days after inoculation using single-lesion-purified isolates in the laboratory. In contrast to our study, soybeans were naturally infected in the above study and sporulation was evaluated as a general average on lesions of varying ages. The urediniospore production on BRS 184 declined early due to early senescence of lesions. The ability to produce urediniospores changes over the uredinial age, and urediniospores are produced 4 weeks after inoculation (Marchetti et al. 1975). Conversely, urediniospore production reached the highest at 2 or 3 days after uredinial eruption and decreased until 11 days in the laboratory (Twizeyimana and Hartman 2010).
Different mechanisms affect disease progressions of different genotypes. Richardson et al. (2006) used latent period, infection efficiency, lesion size, and pustule density to evaluate resistance to barley stripe rust. We used disease severity, AUDPC, NoU, and urediniospore production to evaluate resistance of soybean genotypes to P. pachyrhizi. The ranks of Shiranui and No6-12-1 for sporulation (Fig. 3) were at the same ranks for NoU (Fig. 2). The correlation between NoU and sporulation was confirmed in the laboratory (Yamanaka et al. 2010). NoU and sporulation, however, did not correlate with disease progression on PI 587880A. ASR showed the slowest or second slowest progression (Fig. 1 and Table 1) and high number of NoU and intermediate urediniospore production on PI 587880A (Fig. 2, 4; and Table 2). These observations indicate that disease severity is not directly correlated to reproduction. Instead, disease severity seems to be closely related to infection efficiency, which is the proportion of successful infection per inoculum, and expansion rate of lesions. Since the disease severity of ASR increases mainly by the increase of the number of lesions, not by lesion expansion (Rupe and Sconyers 2008), the disease severity of ASR is more closely related to infection efficiency. PI 587880A harboring Rpp1-b also showed relatively lower disease severity. Other genotypes harboring Rpp1-b, such as PI 587905 and PI 594767A (Hossain et al. 2015), and PI 587855 (Yamanaka et al. 2016) were resistant to most of the P. pachyrhizi populations in South America (Akamatsu et al. 2013, 2017; Stewart et al. 2019), suggesting that the Rpp1-b gene also confers resistance to P. pachyrhizi as observed in PI 587880A.
Gene pyramiding provides a high level of resistance in plant diseases (Mundt 2014). Pair-wise pyramiding combinations of Rpp2, Rpp3, and Rpp4 genes had relatively lower disease severity and sporulation compared to the parents, suggesting complementary epistatic gene action for resistance to ASR in Uganda (Maphosa et al. 2012). Digenic and trigenic interactions of Rpp2, Rpp4, and Rpp5 affected NoU and sporulation of a Brazilian rust population in the laboratory (Lemos et al. 2011). Yamanaka and Hossain (2019) reported that pyramiding larger numbers of Rpp genes confers soybean a higher level of resistance to ASR pathogens. Pyramided genotypes of barley with three quantitative trait loci (QTL) for resistance to barley stripe rust (Richardson et al. 2006) and of rice with four QTL for resistance to rice blast (Fukuoka et al. 2015) presented enhanced resistance compared with the genotypes that had only one of the QTL. Pyramiding with two resistance genes delayed disease progression of Phytophthora infestans in the pathosystems of potato (Tan et al. 2010) and reduced the length of lesions in rice caused by Xanthomonas oryzae pv. oryzae (Zhang et al., 2006). Furthermore, some combinations of resistance genes to wheat stripe rust provided additive or epistatic effects on resistance (Liu et al. 2020). Therefore, elucidating the mechanisms of enhanced resistance in No6-12-1 and other pathosystems leads to more effective pyramiding of resistance genes.
Although yield loss is the most important variable affected by plant diseases, it was not evaluated in this experiment. One reason for this was that some of the genotypes matured very late in Brazil. The donor parents of Rpp2, Rpp4, and Rpp5 belong to maturity groups VII, VIII, and VIII, respectively (Walker et al. 2014), while BRS 184, developed for Brazil, belongs to maturity group 6.7 (Ribeiro et al. 2007). The late-season genotypes suffered from severe attacks by stink bugs. This occurred because most of the other soybean crops were harvested earlier, leading to a gathering of stink bugs on the late-season genotypes. This problem could have been solved by using genotypes that were adapted to the Brazilian environment. Near-isogenic lines for each resistance gene, with a genetic background adapted to Brazil, would have been a solution for the problem of late maturity. The recently developed near-isogenic lines with the genetic background of a Brazilian variety, BRS 184 (Kashiwa et al. 2020), are candidates.
Another reason yield loss could not be evaluated in the present study was that the potential yield level in the absence of rust could not be obtained. We selected fungicides based on the information of a fungicide network trial carried out in Brazil in the previous season (Godoy CV, personal communication). Even though the selected fungicide sprays decreased AUDPC in the sprayed genotypes, ASR was not controlled satisfactorily by the fungicide applications (Fig. 1). P. pachyrhizi populations in the surroundings of the experimental plots may have developed resistance to the fungicides used because the efficacy of the quinone outside inhibitor and demethylation inhibitor fungicides was reduced (Dalla Lana et al. 2018; Godoy et al. 2016), and the fungicides may not have been applied in a timely and effective manner. If the near-isogenic lines and the effective fungicides had been used, the effect of pyramiding of resistance genes on yield loss could have been evaluated. Another limitation of this study is that the AUDPC values observed in No6-12-1 may have been affected by the neighboring genotypes. It is likely that the urediniospores produced on the other genotypes infected No6-12-1 in small scale experiments.
The pyramided genotype, No6-12-1, developed a lower NoU per lesion and urediniospores compared with the other genotypes. This is supported by laboratory assessments of P. pachyrhizi isolates that produced almost no uredinia and urediniospores on No6-12-1, compared with genotypes with only single resistance genes or other pyramided genotypes (Rpp2 + Rpp4) (Yamanaka et al. 2015). Rust lesions persist for a longer period than the period of 14 days used in laboratory assessments, and to an extent, produce urediniospores for more than 14 days. The NoU increases and reaches 8–14 uredinia until up to 7 weeks after inoculation (Melching et al. 1979). Marchetti et al. (1975) reported that one uredinium produced urediniospores for about 3 weeks. Sporulation is another good indicator of resistance because it is directly related to the intrinsic multiplication rate of the pathogen. The pyramided line showed the lowest sporulation among the genotypes, which might be one of the reasons for the slow increase in disease progression in this genotype. Sporulation under field conditions, however, is influenced by environmental conditions, such as wash-out by rain, dispersal by wind, and microclimates around the leaves. Therefore, the scores of urediniospore production may have been underestimated.
Disease severity was compared among genotypes planted side-by-side in this experiment. Therefore, lesions observed on the pyramided genotype may have been produced from urediniospores on neighboring genotypes. In future studies, the pyramided genotype should be planted on a larger field, so that rust population growth can be retarded by less urediniospore production, leading to slower disease progression than that observed in this study. Given that sporulation of ASR correlates with NoU (Yamanaka et al. 2010), genotypes with a lower NoU and urediniospore production should be another breeding target.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Akamatsu H, Yamanaka N, Yamaoka Y, Soares RM, Morel W, Ivancovich AJG, Bogado AN, Kato M, Yorinori JT, Suenaga K (2013) Pathogenic diversity of soybean rust in Argentina, Brazil, and Paraguay. Journal of General Plant Pathology 79:28–40
Akamatsu H, Yamanaka N, Soares RM, Ivancovich AJG, Lavilla MA, Bogado AN, Morel G, Scholz R, Yamaoka Y, Kato M (2017) Pathogenic variation of South American Phakopsora pachyrhizi populations isolated from soybeans from 2010 to 2015. Japan Agricultural Research Quarterly 51:221–232
Brent KJ, and Hollomon DW (2007) Fungicide resistance in crop pathogens: how can it be managed? FRAC Monograph No. 1 (2nd, revised edition) Fungicide Resistance Action Committee, Brussels
Childs SP, King ZR, Walker DR, Harris DK, Pedley KF, Buck JW, Boernma HR, Li Z (2018) Discovery of a seventh Rpp soybean rust resistance locus in soybean accession PI 605823. Theoretical Applied Genetics 131:27–41
Dalla Lana F, Paul PA, Godoy CV, Utiamada CM, da Silva LHCP, Siqueri FV, Forcelini CA, Jaccoud-Filho DdS, Miguel-Wruck DS, Borges EP, Juliatti FC, Campos HD, Nunes J Jr, Carneiro LC, Canteri MG, Ito MF, Meyer MC, Martins MC, Balardin RS, Furlan SH, Carlin VJ, Del Ponte EM (2018) Meta-analytic modeling of the decline in performance of fungicides for managing soybean rust after a decade of use in Brazil. Plant Disease 102:807–817
Flor HH (1971) Current status of the gen-for-gene concept. Annual Review of Phytopathology 9:275–296
Fukuoka S, Saka N, Mizukami Y, Koga H, Yamanouchi U, Yoshioka Y, Hayashi N, Ebana K, Mizobuchi R, Yano M (2015) Gene pyramiding enhances durable blast disease resistance in rice. Scientific Reports 5:7773
Garcia A, Calvo ES, de Souza Kiihl RA, Harada A, Hiromoto DM, Vieira LGE (2008) Molecular mapping of soybean rust (Phakopsora pachyrhizi) resistance genes: discovery of a novel locus and alleles. Theoretical and Applied Genetics 117:545–553
Godoy CV, Koga LJ, Canteri MG (2006) Diagrammatic scale for assessment of soybean rust severity. Fitopatologia Brasileira 31:63–68
Godoy CV, Seixas CDS, Soares RM, Marcelino-Guimarães FC, Meyer MC, Costamilan LM (2016) Asian soybean rust in Brazil: past, present, and future. Pesquisa Agropecuária Brasileira 51:407–421
Hartwig EE (1986) Identification of a fourth major gene conferring resistance to soybean rust. Crop Science 26:1135–1136
Hartwig EE, Bromfield KR (1983) Relationships among three genes conferring specific resistance to rust in soybeans. Crop Science 23:237–239
Hossain MdM, Akamatsu H, Morishita M, Mori T, Yamaoka Y, Suenaga K, Soares RM, Bogado AN, Ivancovich AJG, Yamanaka N (2015) Molecular mapping of Asian soybean rust resistance in soybean landraces PI 594767A, PI 587905 and PI 416764. Plant Pathology 64:147–156
Kashiwa T, Muraki Y, Yamanaka N (2020) Near-isogenic soybean lines carrying Asian soybean rust resistance genes for practical pathogenicity validation. Scientific Reports 10:13270
Kato M (2017) Effectiveness of resistance genes to the soybean rust pathogen Phakopsora pachyrhizi. Japan Agricultural Research Quarterly 51:199–207
Kim KS, Unfried JR, Hyten DL, Frederick RD, Hartman GL, Nelson RL, Song Q, Diers BW (2012) Molecular mapping of soybean rust resistance in soybean accession PI 561356 and SNP haplotype analysis of the Rpp1 region in diverse germplasm. Theoretical and Applied Genetics 125:1339–1352
Lemos NG, de Lucca e Braccini A, Abdelnoor RV, de Oliveira MCN, Suenaga K, Yamanaka N (2011) Characterization of genes Rpp2, Rpp4, and Rpp5 for resistance to soybean rust. Euphytica 182:53–64
Liu R, Lu J, Zhou M, Zheng S, Liu Z, Zhang C, Du M, Wang M, Li Y, Wu Y, Zhang L (2020) Developing stripe rust resistant wheat (Triticum aestivum L.) lines with gene pyramiding strategy and marker-assisted selection. Genetic Resources and Crop Evolution 67:381–391
Madden LV, Hughes G, Van Den Bosch F (2007) The study of plant disease epidemics. APS Press St, Paul
Maphosa M, Talwana H, Tukamuhabwa P (2012) Enhancing soybean rust resistance through Rpp2, Rpp3 and Rpp4 pair wise gene pyramiding. African Journal of Agricultural Research 7:4271–4277
Marchetti MA, Uecher FA, Bromfiled KR (1975) Uredial development of Phakopsora pachyrhizi in soybeans. Phytopathology 65:822–823
Melching JS, Bromfield KR, Kingsolver CH (1979) Infection, colonization, and urediniospores production on Wayne soybean by four cultures of Phakopsora pachyrhizi, the cause of soybean rust. Phytopathology 69:1262–1265
Mueller TA, Miles MR, Morel W, Marois JJ, Wright DL, Kemerait RC, Levy C, Hartman GL (2009) Effect of fungicide and timing of application on soybean rust severity and yield. Plant Disease 93:243–248
Mundt CC (2014) Durable resistance: a key to sustainable management of pathogens and pests. Infection, Genetics and Evolution 27:446–455
Ray JD, Morel W, Smith JR, Frederick RD, Miles MR (2009) Genetics and mapping of adult plant rust resistance in soybean PI 587886 and PI 587880A. Theoretical and Applied Genetics 119:271–280
Richardson KL, Vales MI, Kling JG, Mundt CC, Hayes PM (2006) Pyramiding and dissecting disease resistance QTL to barley stripe rust. Theoretical and Applied Genetics 113:485–495
Ribeiro AS, Moreira JUV, Pierozzi PHB, Rachid BF, de Toledo JFF, Arias CAA, Soares RM, Godoy CV (2007) Genetic control of Asian rust in soybean. Euphytica 157:15–25
Rupe J, Sconyers L (2008) Soybean rust. The Plant Health Instructor. https://doi.org/10.1094/PHI-I-2008-0401-01
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019) The global burden of pathogens and pests on major food crops. Nature Ecology & Evolution 3:430–439
Stewart S, Rodríguez M, Yamanaka N (2019) Pathogenic variation of Phakopsora pachyrhizi isolates from Uruguay. Tropical Plant Pathology 44:309–317
Tan MYA, Hutten RCB, Visser RGF, van Eck HJ (2010) The effect of pyramiding Phytophthora infestans resistance genes R Pi-mcd1 and R Pi-ber in potato. Theoretical and Applied Genetics 121:117–125
Twizeyimana M, Hartman GL (2010) Culturing Phakopsora pachyrhizi on detached leaves and urediniospore survival at different temperatures and relative humidities. Plant Disease 94:1453–1460
Twizeyimana M, Ojiambo PS, Sonder K, Ikotun T, Hartman GL, Bandyopadhyay R (2009) Pathogenic variation of Phakopsora pachyrhizi infecting soybean in Nigeria. Phytopathology 99:353–361
Walker RD, Harris DK, King ZR, Li Z, Boerma HR, Buckley JB, Weaver DB, Sikora EJ, Shipe ER, Mueller JD, Buck JW, Schneider RW, Marois JJ, Wright DL, Nelson RL (2014) Evaluation of soybean germplasm accessions for resistance to Phakopsora pachyrhizi populations in the southeastern United States, 2009–2012. Crop Science 54:1673–1689
Wrather A, Shannon G, Balardin R, Carregal L, Escobar R, Gupta GK, Ma Z, Morel W, Ploper D, Tenuta A (2010) Effect of diseases on soybean yield in the top eight producing countries in 2006. Online. Plant Health Progress https://doi.org/10.1094/PHP-2010-0125-01-RS
Yamanaka N, Yamaoka Y, Kato M, Lemos NG, Passianotto ALDEL, dos Santos JVM, Benitez ER, Abdelnoor RV, Soares RM, Suenaga K (2010) Development of classification criteria for resistance to soybean rust and differences in virulence among Japanese and Brazilian rust populations. Tropical Plant Pathology 35:153–162
Yamanaka N, Akamatsu H, Yamaoka Y (2013a) Laboratory manual for studies on soybean rust resistance. JIRCAs website. https://www.jircas. go.jp/english/manual/soybean_rust/soybean_rust.html. Accessed on 30 January 2015. Now the latest version is available at: https://www.jircas.go.jp/ja/publication/manual_gudeline/manual_guideline-_-_62.pdf. Last accessed on June 15, 2020
Yamanaka N, Lemos NG, Uno M, Akamatsu H, Yamaoka Y, Abdelnoor RV, e Braccini AL, Suenaga K (2013) Resistance to Asian soybean rust in soybean lines with the pyramided three Rpp genes. Crop Breeding and Applied Biotechnology 13:75–82
Yamanaka N, Morishita M, Mori T, Lemos NG, Hossain MdM, Akamatsu H, Kato M, Yamaoka Y (2015) Multiple Rpp-gene pyramiding confers resistance to Asian soybean rust isolates that are virulent on each of the pyramided genes. Tropical Plant Pathology 40:283–290
Yamanaka N, Morishita M, Mori T, Muraki Y, Hasegawa M, Hossain MdM, Yamaoka Y, Kato M (2016) The locus for resistance to Asian soybean rust in PI 587855. Plant Breeding 135:621–626
Yamanaka N, Hossain MdM (2019) Pyramiding three rust-resistance genes confers a high level of resistance in soybean (Glycine max). Plant Breeding 138:686–695
Yamaoka Y, Yamanaka N, Akamatsu H, Suenaga K (2014) Pathogenic races of soybean rust Phakopsora pachyrhizi collected in Tsukuba and vicinity in Ibaraki, Japan. Journal of General Plant Pathology 80:184–188
Yorinori JT, Paiva WM, Frederick RD, Costamilan LM, Bertagnolli PF, Hartman GE, Godoy CV, Nunes J Jr (2005) Epidemics of soybean rust (Phakopsora pachyrhizi) in Brazil and Paraguay from 2001 to 2003. Plant Disease 89:675–677
Zhang J, li X, Jiang G, Xu Y, He Y (2006) Pyramiding of Xa7 and Xa21 for the improvement of disease resistance to bacterial blight in hybrid rice. Plant Breeding 125:600–605
Acknowledgements
We thank Embrapa Soja for providing soybean genotypes, the staff of the Phytopathology group in Embrapa Soja for their fieldwork; Dr. Koji Yamamura, National Agriculture, and Food Research Organization, for suggestions for statistical analysis; and Dr. Naoki Yamanaka and Dr. Takeshi Kashiwa for their advice on the draft of the manuscript.
Funding
This study was supported by the projects “Development of breeding technologies toward improved production and stable supply of upland crops” and “Development of technologies for the control of migratory plant pests and transboundary diseases” of Japan International research Center for Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. MK and RMS planned the experiment, collected the data, and prepared the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kato, M., Soares, R.M. Field trials of a Rpp-pyramided line confirm the synergistic effect of multiple gene resistance to Asian soybean rust (Phakopsora pachyrhizi). Trop. plant pathol. 47, 222–232 (2022). https://doi.org/10.1007/s40858-021-00471-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-021-00471-z