Abstract
Purpose
Cancer detection is an important part of modern medical diagnosis and many strategies based on nanotechnology had been developed in recent years. Of which, silicon nanowire (SiNW) field-effect transistor (FET) biosensor via DNA aptamer capture is considered as an interesting and viable option. Hence, in this report, we had assembled a n-type triple SiNW FET biosensor for the detection of vascular endothelial growth factor (VEGF) via surface functionalized DNA aptamer for a proof-of-concept.
Method
The SiNW FET biosensor was fabricated via "top-down" approach and the physical nature of the nanowire assembly was examined via atomic force microscopy (AFM) as well as scanning electron microscopy (SEM). We had subsequently grafted VEGF specific DNA aptamer via conventional EDC/NHS chemistry and later measured the conductance of the nanowires in a four-point probe detector for VEGF detection.
Result
We had demonstrated that the detection of VEGF was possible even at a concentration of 2.59 nM which was highly comparable to the other common biosensors for detection of VEGF protein.
Conclusion
In addition to being scalable with complementary metal–oxide–semiconductor (CMOS) technology, the findings as demonstrated from our simple SiNW FET biosensor setup had helped to provide better insights towards optimizing the approach for the development of the next generation of miniature biosensors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Field-effect transistor (FET) biosensors has gained much popularity in recent years due to its high sensitivity and the wide range of customizable parameters compared to other conventional biosensors [1,2,3]. As such, newer FET biosensors had been developed over the years via nanotechnology-based miniaturization [4]. Among various types of nano-scaled design, nanowire FET is an attractive proposition for biosensing purposes. Due to the nano-sized configuration, nanowire FET is highly suitable for the detection and capture of most biological targets such as nucleic acids, proteins and viruses, and this renders them an excellent candidate for biosensing purposes [5, 6].
During the fabrication of nanowire FET, various materials such as metals (Ni, Au) [7], dielectrics (ZiO, TiO2) [8] and semiconductors (Si, GaN) [9] had been reported as transducers. Among these materials, silicon nanowire (SiNW) had been often advocated for biosensing applications due to their high sensitivity and potential for label-free real-time detection [10, 11]. Furthermore, the maturity and well-established silicon processing technologies provides the necessary platform for mass production and integration of various technologies, thereby making SiNW-based biosensor an excellent candidate for commercialization purposes.
SiNW can be generally described as a 1-dimensional (1D) nanostructure with a single crystalline silicon core externally coated with an amorphous silicon oxide layer [6, 12]. The assembly of one-dimensional structure permits more simpler manufacturing installations [13], and this help to reduce overall cost during the assembly process. In addition, surface chemical modification techniques can be easily performed to immobilize or covalently functionalized certain bioactive biomolecules directly onto the surface of SiNW for molecular capturing purposes. Thus in recent years, such FET biosensor assembly has been termed as silicon nanowire field-effect transistor (SiNW-FET) biosensors in literatures [11, 14]. SiNW-FET sensors have been reported in scientific literatures for various biosensing purposes such as through proteins [11, 15], nucleic acids [16, 17] and disease biomarkers [18, 19] capture. Generally, SiNW-FET biosensing devices were fabricated by commonly reported two strategies, namely the "top-down" method and the "bottom-up" method. "Top-down" SiNW-FETs biosensors are made with silicon-on-insulator (SOI) wafer via lithographic process combining with doping technique and electron-beam etching. This method utilizes the SiNW channels (light doping) and source/drain electrodes (heavy doping) to help define the nanostructure of SiNW-FET devices. On the other hand, "bottom-up" process is achieved with the growth of SiNWs via chemical vapor deposition (CVD) system and the vapor–liquid-solid (VLS) mechanism [20, 21]. The "bottom-up" method may offer a wider range of customization as it permits for the growth of nanowires of different lengths, cross-sectional profiles, materials and properties. Thus, the "bottom-up" method is a more attractive selection for the fabrication as the final result can be a high quality single crystal nanowire with a small diameter in the range of 3–5 nm [12].
The detection and quantification of many bioactive markers are particularly important for clinical diagnosis and biomedical researches. One example of protein biomarker is the vascular endothelial growth factor (VEGF) as an indicator for cancer prognosis [22, 23]. VEGF is an heparin-binding homodimeric glycoprotein and is considered as a key regulator of physiological vascular development during embryogenesis as well as blood-vessel formation in adult tissues [24]. Furthermore, VEGF plays a critical role as a signaling protein in the pathological angiogenesis observed in many tumor types and other conditions [25, 26]. Hence the detection of VEGF in serum is often used as a screening biomarker and many novel biosensor systems had been developed for its detection [27, 28]. Of the many types of capturing moieties, DNA aptamer for VEGF had also been gaining popularity due to its long shelf-life and excellent sensitivity [29,30,31]. Hence, the tagging of DNA aptamer for VEGF on SiNW biosensors was seen as an obvious development of these biosensor types over time.
In this report, we functionalized a VEGF specific DNA aptamer on customized n-type SiNW biosensors as a proof of concept. The physical characteristics of the biosensor had been examined via scanning electron microscopy (SEM) as well as atomic force microscopy (AFM). The process chemistry is documented in this article while the sensitivity of these biosensors is characterized in turn to demonstrate the versatility of our design.
2 Materials and Methods
In this work, the structural and photomask design of the SiNW FET biosensor were performed with the L-Edit software version 8.30 in Windows 7. Low-resistance P-type (111) silicon (0.001–0.005 Ω cm) wafer was acquired from Semiconductor Wafer, Inc. (SWI) and all surface reactions were performed on the surface other otherwise specified. 3-Aminopropyl was purchased from Sigma-Aldrich and used without purification, while all solvents and consumables were also acquired from Sigma-Aldrich unless specified otherwise.
2.1 Nanowire Production
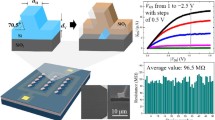
The SiNW FET device was entirely implemented at the National Nano Device Laboratory (NDL) in Hsin-Chu, Taiwan. In brief, the n-channel silicon biosensor device was fabricated using the "top-down" method which makes it possible to manufacture aligned nanowires that are compatible with standard complementary metal-oxide semiconductor (CMOS) technology [32]. A 6-inch silicon on insulator (SOI) wafer was used for the design of SiNW FET. Firstly, the topmost silicon layer was oxidized to form a thick silicon dioxide (SiO2) layer which was grown on the wafer by etching the SiO2 with BOE (buffered oxide etching). This was then followed by the growth of polysilicon over the surface. A layer of titanium nitride (TiN), which has excellent conductivity [33], was subsequently deposited on the contact area of FET source and drain electrodes in order to pattern the device and avoid the impedance effect of the polysilicon during the measurement. The top view graphical presentation of the triple nanowire was as shown in Fig. 1a, while the top process of our device was as shown in Fig. 1b. The triplicate nanowire was selected in our design so as to help amplify the necessary signals from solution that may in turn increase the sensitivity. The photoresist of the coating was cleaned and the SiO2 was removed by etching using the reactive ion etching (RIE) process. Afterwards, the titanium nitride layer was deposited on the exposed area of the polysilicon. In order to prevent any possibility of short circuit during detection, a layer of SiO2 is deposited on the device's surface using the chemical vapor deposition (CVD) technique. This can protect the contact areas and the exposed nanowires are as shown in Fig. 1a.
2.2 Surface Chemical Modification on the SiNW FET
The SiNW was firstly cleaned with a mix solution of sulfuric acid (H2SO4) and hydrogen peroxide (H2O2), typically known as piranha solution, at a concentration ratio of 3:1. As piranha solution may affect the nanowires, the surface was subsequently rinsed with copious amount of deionized water to remove the residual of the solution from the surface of SiNW. The device is then immersed in 10 ml toluene solution at a concentration of 50 mM 3-aminopropyl-triethoxysilane (ATPES) for 2 h at room temperature [34]. Subsequently, the SiNW surfaces were washed and sonicated in sequential order of methanol, ethanol, chloroform, and deionized water for 1 min each. Next, a buffer solution of 100 mM 2-(N-morpholino) ethane sulfonic acid (MES) and 500 mM sodium chloride (NaCl) was prepared prior to immobilize the DNA aptamer to the surface of SiNW.
The sequence selected for the arrestment of the VEGF was 5′ATACCAGTCTATTCAATTGGGCCCGTCCGTATGGTGGGTGTGCTGGCCAGATAGTATGTGCAATCA 3′ as reported previously by Kaur et al. [35]. To functionalize the DNA aptamer to the surface, 0.5 μM of DNA aptamer was first introduced into the MES solution containing 200 mM of EDC/NHS solution for a period of 2 h. After that, the surfaces were washed copiously with ethanol followed by water and stored under vacuum prior to usage.
2.3 Atomic Force Microscopy
To form a visual analysis of the SiNW-FET biosensor, atomic force microscopy (SPM) scan was performed at Feng Chia University (Taichung, Taiwan). The results were acquired via the Digital Instrument NS4/D3100CL/Multimode Scanning Probe Microscope software that controls the in-build AFM tapping mode with cantilever tuned at the frequency 150 kHz with a force 5 N/m. Scan area on the surfaces of 80 μm × 80 μm was selected and the scan speed was set at 0.6 Hz with the integral and proportional gain set at automatic mode to reduce surface noise by observing the trace and retrace scans.
2.4 Scanning Electron Microscopy
Scanning electron microscopy (SEM) was conducted to visualize the surface of the SiNW FET biosensor. A field emission scanning electron microscope (FE-SEM) Model S-4800 (Hitachi Systems, Japan) scanned the samples at an accelerating voltage of 15 kV with a cold field emission electron gun equipped with a 3-stage electromagnetic lens. The scanning distance was set at 15.4 mm and a high image magnification mode was adopted to achieve the best results. The experiment was conducted at Feng Chia University (Taichung, Taiwan).
3 Results and Discussion
When designing the biosensor, various options were considered as candidates for the biomolecule capture and we had finally decided on the use of DNA aptamer for several reasons. Firstly, DNA aptamers do have a longer shelf-life as compared with the conventional antibodies. Secondly, as shown in Fig. 2a, it is perceivable that short oligonucleotides would have a shorter Debye length that can help to improve on the registered signature from the nanowire surface [36]. Hence, in order to graft aptamers to silicon nanowire, we have adopted the commonly used approach through APTES reaction to the silicon surface and subsequent EDC/NHS coupling. The reaction model is as shown in Fig. 2b. It is once again important to state that VEGF is considered an important candidate for detection due to its clinical importance as an essential cancer marker during many diagnostic procedures.
A four-point probe setup as shown in Fig. 3a was used to measure the I–V curve characteristics of the fabricated SiNW FET (as demonstrated in Fig. 3b). In order to examine the characteristics of the SiNW FET device, the I–V curve was analyzed to confirm the transistor characteristics of the fabricated FET. As illustrated in Fig. 3c, Vg was the voltage between the gate and the source to control the current flowing between the drain and the source electrodes, while Vds represents the voltage between the drain and the source. Please note that the gate voltage was applied at the bottom of the fabricated FET as the top side was the nanowire for target detection. With an increasing in the applied voltage Vg, the width of n channel for semiconductor carriers in the p-type material would also be increased from the bottom. Because the drain and source are more heavily doped to be of n type in the wafer, the conductance can be predicted to be lower with increasing Vg as more n-type carriers having been attracted to the bottom. The results shown in Fig. 3c had verified that the derived I-V characteristics was indeed of the n-channel FET. This would inherently affect our detection outcome which would be further elaborated in the subsequent section.
Visualization of the device was also performed via atomic force microscopy (AFM), as shown in Fig. 4a, and via scanning electron microscopy (SEM) in Fig. 4b and c. From these figures, we are able to clearly discern the triple nanowire that spanned across both source and drain. The visualization of the implemented device was an important aspect of our device fabrication as any breakage on the surface would adherently affect the detection outcome and thus resulting in inconsistency in our studies. As shown from both the AFM and SEM, the nanowire was connected across a region approximately 40 μm long and this region served as our detection window for the incoming VEGF protein in solution. The nanowires were 250 nm in width from measurement and this would help to desensitize certain effects from solution pH as reported previously by Elfstrom et al. [37]. The exposed triple nanowire system as designed in our setup was mean to help improved on sensitivity of the detection unlike single nanowire system. It was also in this window region that the reaction with APTES and the subsequently coupling of the DNA aptamer occurred.
To evaluate the performance of the device, three control groups were used. As shown in Fig. 5, “Aptamer” represents the nanowire surface grafted with the DNA aptamer while “APTES” denotes surfaces without the aptamer but passivated through silanization of APTES while the unmodified surfaces served as our control (designated as “Control”). A final concentration 25.91 nM VEGF was added into the surface under a pH of 7 (deionized water). Herein, it is important to state that at a pH of 7, VEGF is positively charged as its isoelectric point is at 8.5 [38, 39]. This was an important aspect in our study as a positively charge protein would raise the conductance of n-type silicon nanowire as previously described by Lee et al. [38].
In the experiments, the gate voltage (Vg) was fixed to be 0.5 V while the drain-source voltage (Vds) ranged from 0 to 5 V. As shown in Fig. 5a, it can be observed that in the absence of any protein target, the overall current flow Id is relatively low (\(<0.2\times {10}^{10}\) Amperes) for the three conditions regardless of surface modification. On the other hand, when VEGF at a concentration of 25.91 nM was added to the device surface, an increase of the current flow could be detected. This result was in corroboration with the studies performed on n-type silicon nanowires by Lee et al. as they described the contribution from the positively charged VEGF helped to contributed to the overall conductance of the n-type nanowire and hence raising the current across the system [38]. While our work may have shown parallel similarities with Lee et al. at this junction, what that had set this work apart from their report is that we have used triple nanowire instead of a single one and that could help to increase the detection sensitivity. This wouls be shown in the subsequent section highlighting the detection sensitivity as a function against concentration.
To determine the concentration dependency and sensitivity of our device, two concentrations were selected for this work. As shown in Fig. 6, the current value was recorded around 200 pA for a VEGF at a concentration of 25.91 nM, while it was registered near 140 pA for a VEGF at a concentration of 2.59 nM. The sensitivity in detection was relatively comparable to more recent reports on VEGF biosensing [40, 41] and this was highly promising for our current setup.
As mentioned earlier, one of major concerns with FET had always been the issue of the detection moiety being positioned to far above the Debye screening length (λD) that would compromise the detection sensitivity of the device as previously described by Stern et al. [42]. Our implementation of silane chemistry using APTES may seemingly have a deleterious effect as it was previously found that the indiscriminate passivation of both the nanowire as well as the underlying oxide film may outcompete for binding to the bioactive target [11]. However, we suspected that, as a result of triple nanowire configuration, this effect could be somewhatovercomed as demonstrated by our sensitivity. Furthermore, our surface modification was carefully limited to a small confined region as shown in our SEM as well as AFM images. At the pH of 7 by which all detections were performed, the amine group at the distal end from surface unmodified by the subsequent DNA coupling may still be inherently positive [43]. When this was placed in prospective with the positively charged VEGF, the absence of any capturing aptamer may actually help to discourage non-specific binding and the overall condition during detection was in our favor. Concerns regarding the conductance of SiNW passivated with APTES had been covered in the past and was found to only have direct correlation to the both pH and size [11, 37] and under our experimental setup, these positively charged APTES may in fact help improved on the conductivity of our n-type SiNW.
4 Conclusion
In this study, we had relied on some of the intrinsic properties of our material to design a straightforward SiNW-based FET biosensor for the detection of VEGF and while the design was seemingly simplistic in nature, this was in actuality a deliberation from our part for future commercial considerations. Firstly, we had designed a triple nanowire-based system to help increase the sensitivity of the nanowire. Secondly, we had selected well-established APTES chemistry that did not compromise with our overall detection regime in terms of surface charge etc. The choice of using VEGF was also deliberate due to its isoelectric point, the protein remains positively charged and this would help elevate the conductance of our n-type SiNW. While certain reports so far in literature for VEGF had reach to femtomolar level [44, 45], clinical setting may not require such sensitivity and often many of these systems are extremely complicated to reproduce in an industrial setting. On the other hand, our device relies on solely the fabrication as well as two simple and commonplace surface functionalization steps and our detection regime were recorded to be fairly close sub-nano/pico levels and for point-of-care (POC) systems, such simplicity would suffice in our opinion. Furthermore, our choice of DNA aptamer over antibody had given our system an advantage in terms of shelf-life as well as overall biological stability.
SiNW-based FET biosensor remains an important proposition due to the fact of the wide availability of the silicon source material that enables potential for quick commercialization. Yet interestingly, aptamer-based biosensors remain largely at the periphery of the developments of nanowire biosensors despite the many obvious advantages. Herein, in this work, we have fabricated a triple nanowire aptamer-based detection FET biosensor. Our SiNW FET biosensor was designed accordingly to the "top-down" method and our results showed that detection was achievable at a concentration of 2.59 nM thus making our biosensor very comparable to current nanowire biosensors for the detection of VEGF protein. The results of our SiNW FET biosensor, along with being scalable with CMOS technology, have also provided a better understanding of the approach to optimize the development of the future generation of biosensors for direct, label-free, real time and ultra-sensitive detection of various biomarkers. The mechanism as described in this report will be further improved and adapted to detect other types of biomarkers and ultimately realizing the aims of producing POC devices in the long run.
References
Anand, S., Singh, A., Amin, S. I., & Thool, A. S. (2019). Design and performance analysis of dielectrically modulated doping-less tunnel FET-based label free biosensor. IEEE Sensors Journal, 19(12), 4369–4374.
Lin, C. H., Hung, C. H., Hsiao, C. Y., Lin, H. C., Ko, F. H., & Yang, Y. S. (2009). Poly-silicon nanowire field-effect transistor for ultrasensitive and label-free detection of pathogenic avian influenza DNA. Biosensors and Bioelectronics, 24(10), 3019–3024.
Tian, M., Xu, S.C., Zhang, J.Y., Wang, X.X., Li, Z. H., Liu, H. L., et al. (2018). RNA Detection based on graphene field-effect transistor biosensor. Advances in Condensed Matter Physics, 2018, 8146765.
Majd, S. M., Salimi, A., & Ghasemi, F. (2018). An ultrasensitive detection of miRNA-155 in breast cancer via direct hybridization assay using two-dimensional molybdenum disulfide field-effect transistor biosensor. Biosensors and Bioelectronics, 105, 6–13.
Rahong, S., Yasui, T., Kaji, N., & Baba, Y. (2016). Recent developments in nanowires for bio-applications from molecular to cellular levels. Lab on a Chip, 16(7), 1126–1138.
Puppo, F., Doucey, M. A., Delaloye, J. F., Moh, T., Pandraud, G., Sarro, P. M., et al. (2016). SiNW-FET in-air biosensors for high sensitive and specific detection in breast tumor extract. IEEE Sensors Journal, 16(10), 3374–3381.
Lin, Y. R., Tsai, W. T., Wu, Y. C., & Lin, Y. H. (2017). Ultra thin poly-Si nanosheet junctionless field-effect transistor with nickel silicide contact. Materials, 10(11), 1276.
Vieira, N. C. S., Figueiredo, A., Faceto, A. D., de Queiroz, A. A. A., Zucolotto, V., & Guimaraes, F. E. G. (2012). Dendrimers/TiO2 nanoparticles layer-by-layer films as extended gate FET for pH detection. Sensors and Actuators B-Chemical, 169, 397–400.
Lin, Y. H., Lin, W. S., Wong, J. C., Hsu, W. C., Peng, Y. S., & Chen, C. L. (2017). Bottom-up assembly of silicon nanowire conductometric sensors for the detection of apolipoprotein A1, a biomarker for bladder cancer. Microchimica Acta, 184(7), 2419–2428.
Gao, A. R., Lu, N., Dai, P. F., Li, T., Pei, H., Gao, X. L., et al. (2011). Silicon-nanowire-based CMOS-compatible field-effect transistor nanosensors for ultrasensitive electrical detection of nucleic acids. Nano Letters, 11(9), 3974–3978.
Cui, Y., Wei, Q., Park, H., & Lieber, C. M. (2001). Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science, 293(5533), 1289–1292.
Syedmoradi, L., Ahmadi, A., Norton, M. L., & Omidfar, K. (2019). A review on nanomaterial-based field effect transistor technology for biomarker detection. Microchimica Acta, 186(11), 739.
Cui, Y., & Lieber, C. M. (2001). Functional nanoscale electronic devices assembled using silicon nanowire building blocks. Science, 291(5505), 851–853.
Gao, A. R., Chen, S. X., Wang, Y. L., & Li, T. (2018). Silicon nanowire field-effect-transistor-based biosensor for biomedical applications. Sensors and Materials, 30(8), 1619–1628.
Patolsky, F., Zheng, G., & Lieber, C. M. (2006). Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nature Protocols, 1(4), 1711–1724.
Lin, C. H., Chu, C. J., Teng, K. N., Su, Y., Jr., Chen, C. D., Tsai, L. C., et al. (2012). Recovery based nanowire field-effect transistor detection of pathogenic avian influenza DNA. Japanese Journal of Applied Physics, 51(2), 02BL02.
Yang, F., & Zhang, G.-J. (2014). Silicon nanowire-transistor biosensor for study of molecule-molecule interactions. Reviews in Analytical Chemistry, 33(2), 95–110.
Zhou, F., Li, Z. Y., Bao, Z. T., Feng, K., Zhang, Y., & Wang, T. (2015). Highly sensitive, label-free and real-time detection of alpha-fetoprotein using a silicon nanowire biosensor. Scandinavian Journal of Clinical and Laboratory Investigation, 75(7), 578–584.
Yang, C. Y., Chiang, H. C., Kuo, C. J., Hsu, C. W., Chan, S. F., Lin, Z. Y., et al. (2018). Hepatocellular carcinoma diagnosis by detecting alpha-fucosidase with a silicon nanowire field-effect transistor biosensor. ECS Journal of Solid State Science and Technology, 7(7), Q3153–Q3158.
Hobbs, R. G., Petkov, N., & Holmes, J. D. (2012). Semiconductor nanowire fabrication by bottom-up and top-down paradigms. Chemistry of Materials, 24(11), 1975–1991.
Zhang, A., & Lieber, C. M. (2016). Nano-bioelectronics. Chemical Reviews, 116(1), 215–257.
Kakeji, Y., Koga, T., Sumiyoshi, Y., Shibahara, K., Oda, S., Maehara, Y., et al. (2002). Clinical significance of vascular endothelial growth factor expression in gastric cancer. Journal of Experimental and Clinical Cancer Research, 21(1), 125–129.
Gasparini, G. (2000). Prognostic value of vascular endothelial growth factor in breast cancer. The Oncologist, 5(S1), 37–44.
Holmes, D. I. R., & Zachary, I. (2005). The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biology, 6(2), 209.
Kluger, H. M., Siddiqui, S. F., Angeletti, C., Sznol, M., Kelly, W. K., Molinaro, A. M., et al. (2008). Classification of renal cell carcinoma based on expression of VEGF and VEGF receptors in both tumor cells and endothelial cells. Laboratory Investigation, 88(9), 962–972.
Yukata, K., Matsui, Y., Goto, T., Kubo, T., & Yasui, N. (2005). Differential expression of VEGF isoforms and VEGF receptors in cartilaginous tumors. Anticancer Research, 25(2A), 955–957.
Teke, M., Sayikli, C., Canbaz, C., & Sezginturk, M. K. (2014). A novel biosensing system using biological receptor for analysis of vascular endothelial growth factor. International Journal of Peptide Research and Therapeutics, 20(2), 221–230.
Li, J., Sun, K., Chen, Z., Shi, J., Zhou, D., & Xie, G. (2016). A fluorescence biosensor for VEGF detection based on DNA assembly structure switching and isothermal amplification. Biosensors and Bioelectronics, 89, 964–969.
Pan, L. H., Kuo, S. H., Lin, T. Y., Lin, C. W., Fang, P. Y., & Yang, H. W. (2017). An electrochemical biosensor to simultaneously detect VEGF and PSA for early prostate cancer diagnosis based on graphene oxide/ssDNA/PLLA nanoparticles. Biosensors and Bioelectronics, 89, 598–605.
Lin, C. W., Wei, K. C., Liao, S. S., Huang, C. Y., Sun, C. L., Wu, P. J., et al. (2015). A reusable magnetic graphene oxide-modified biosensor for vascular endothelial growth factor detection in cancer diagnosis. Biosensensors and Bioelectronics, 67, 431–437.
Ni, S., Shen, Z., Zhang, P., & Liu, G. (2020). Enhanced performance of an electrochemical aptasensor for real-time detection of vascular endothelial growth factor (VEGF) by nanofabrication and ratiometric measurement. Analytica Chimica Acta, 1121, 74.
Ambhorkar, P., Wang, D. Z., Ko, H., Lee, S., Koo, K. I., Kim, K., et al. (2018). Nanowire-based biosensors: From growth to applications. Micromachines, 9, 679.
Lengauer, W., Binder, S., Aigner, K., Ettmayer, P., Guillou, A., Debuigne, J., et al. (1995). Solid-state properties of group IVB carbonitrides. Journal of Alloys and Compounds, 217, 137–147.
Khung, Y. L., Graney, S. D., & Voelcker, N. H. (2006). Micropatterning of porous silicon films by direct laser writing. Biotechnology Progress, 22(5), 1388–1393.
Kaur, H., & Yung, L. Y. L. (2012). Probing high affinity sequences of DNA aptamer against VEGF165. PLoS ONE, 7(2), e31196.
Khung, Y. L., & Narducci, D. (2013). Synergizing nucleic acid aptamers with 1-dimensional nanostructures as label-free field-effect transistor biosensors. Biosensors and Bioelectronics, 50, 278–293.
Elfstrom, N., Juhasz, R., Sychugov, I., Engfeldt, T., Karlstrom, A. E., & Linnros, J. (2007). Surface charge sensitivity of silicon nanowires: Size dependence. Nano Letters, 7(9), 2608–2612.
Lee, H. S., Kim, K. S., Kim, C. J., Hahn, S. K., & Jo, M. H. (2009). Electrical detection of VEGFs for cancer diagnoses using anti-vascular endotherial growth factor aptamer-modified Si nanowire FETs. Biosensors and Bioelectronics, 24(6), 1801–1805.
Houck, K. A., Leung, D. W., Rowland, A. M., Winer, J., & Ferrara, N. (1992). Dual regulation of vascular enfothelial growth factor bioavailability by genetic and proteolytic mechanisms. Journal of Biological Chemistry, 267(36), 26031–26037.
Mita, C., Abe, K., Fukaya, T., & Ikebukuro, K. (2014). Vascular endothelial growth factor (VEGF) detection using an aptamer and PNA-based bound/free separation system. Materials, 7(2), 1046–1054.
Lv, Z., Wang, K., & Zhang, X. (2014). A new electrochemical aptasensor for the analysis of the vascular endothelial growth factor. Journal of Immunoassay and Immunochemistry, 35(3), 233–240.
Stern, E., Wagner, R., Sigworth, F. J., Breaker, R., Fahmy, T. M., & Reed, M. A. (2007). Importance of the debye screening length on nanowire field effect transistor sensors. Nano Letters, 7(11), 3405–3409.
Kim, K. M., Kim, H. M., Lee, W. J., Lee, C. W., Kim, T. I., Lee, J. K., et al. (2014). Surface treatment of silica nanoparticles for stable and charge-controlled colloidal silica. International Journal of Nanomedicine, 9, 29–40.
Liu, Y. L., Da, H. M., Chai, Y. Q., Yuan, R., & Liu, H. Y. (2019). Photoelectrochemical aptamer-based sensing of the vascular endothelial growth factor by adjusting the light harvesting efficiency of g-C3N4 via porous carbon spheres. Microchimica Acta, 186(5), 275.
Gao, A. R., Yang, X., Tong, J., Zhou, L., Wang, Y. L., Zhao, J. L., et al. (2017). Multiplexed detection of lung cancer biomarkers in patients serum with CMOS-compatible silicon nanowire arrays. Biosensors and Bioelectronics, 91, 482–488.
Acknowledgements
The authors would to express their gratitude to the NARLabs for their assistance in the fabrication of the nanodevices.
Funding
The work was carried out with funding under Ministry of Science and Technology in Taiwan (MOST 108-2221-E-035-032, MOST 107-2113-M-039-002-MY2, 106-2221-E-039-005-MY2 and 109-2221-E-039-013-MY2).
Author information
Authors and Affiliations
Contributions
CCY had performed the surface modifications as well as the detection studies, while SIZ had characterized the surface via AFM and SEM and had written half of the manuscript. YDL had provided all the funding for surface detection studies as well as examining the data while YLK had conceived the project and had written a large fraction of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zida, S.I., Yang, CC., Khung, Y.L. et al. Fabrication and Characterization of an Aptamer-Based N-type Silicon Nanowire FET Biosensor for VEGF Detection. J. Med. Biol. Eng. 40, 601–609 (2020). https://doi.org/10.1007/s40846-020-00552-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40846-020-00552-5