Abstract
In this study, we developed Nb2O5-Ni composite coatings on carbon steel by DC sputtering technique. The effect of Nb2O5 particulate on the Ni coating properties was investigated. The weight percents of Nb2O5 in Ni coating were 0, 12, 15, and 30 wt%. The structural properties of the composite coatings were characterized using scanning electron microscope equipped with energy-dispersive spectrometer. The microhardness and thickness of composite coatings were measured, in addition to examine the corrosion properties in 3.5% NaCl using MLab 200 potentiostat/galvanostat with linear polarization technique. The results showed that hardness value increased to 164 HV for 30 wt% Niobia compared with 155 HV for Ni coating and the thickness increased slightly for Nb2O5-Ni composite coatings. The corrosion performance improved to obtain efficiencies better than Ni coating alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nickel coating is resistant to dry gases, oil, soaps, and petrol. Nickel coating increases resistance to many corrosion types in different media, but not to nitric acid and environments containing chloride. The life of nickel coatings may be further increased by a thin overlay of microcracked chromium because corrosion would not penetrate the nickel directly, but will spread laterally. Nickel is generally plated as a part of multilayer coating system. The coating system consisting of the dull nickel, bright nickel, and chromium layer is called the composite nickel coating. Nickel coatings are commonly applied to zinc, steel, and other metals to provide protection against corrosion. Incorporating other materials (metals or ceramics) with nickel coating can also be referred to as composite coating. On the other hand, applying ceramic coatings on metal surfaces gives protection against corrosion and erosion with some disadvantages. In this work, Nb2O5-Ni composite coatings were investigated. There are many works focused on Nb2O5 coating using different techniques [1,2,3,4,5,6,7,8,9,10].
2 Experimental Procedures
2.1 Materials Preparation and Co-deposition
Structural steel (AISI 1015) with the dimensions of (20 mm × 20 mm × 2 mm) were used as the substrate, its chemical composition (wt%) was as follows: C 0.161, Si 0.271, Mn 0.425, P 0.0073, S 0.0049, and balance of Fe. Before the coating test, the substrate was in turn ground with SiC abrasive papers of 400, 600, 800, and 1000 in water, rinsed with alcohol, dried in the air, and sandblasted with alumina grit.
DC sputtering technique was achieved in a low-pressure gas discharge device consisting of an evacuated chamber, a target (cathode), and anode disk of stainless steel. The cathode was placed opposite the anode and provided the gas discharge of electrical field with 4 KV DC power supply. The cathode electrode insulated with diameter of 14.5 cm, while the diameter of target electrode was 7.5 cm and the distance between them was 3.5 cm.
The gas source-flow controller system is responsible for supplying the feedstock at the desired flow rate and gas pressure to the plasma chamber. It is consists of dual-stage regulators, tubing, and fittings for the gas storage cylinders (Argon). Dual-stage needle valve regulated the flow pressure to the plasma chamber.
The vacuum system consists of a turbo pump (Variant, V-1000HT) assisted by a hydraulic rotary pump (60 m3/h, Balzer). The installation of penning (Edward CP25-K with controller 1102) and pirani gauges (LH-Thermovac with Combitron CM350) in the plasma chamber was required to monitor the actual pressure and the partial pressure of the discharge gases.
2.2 Characterization Methods
The morphologies and chemical compositions of the obtained coating were analyzed using scanning electron microscope (SEM) and its configured energy-dispersive spectrometer (EDS). This test was performed at University of Sanity Sheriff Tehran-Iran. The phases were analyzed by X-ray diffraction (XRD), type (Angstrom Advanced Inc model ADX 2700) at Ministry of Science and Technology, Iraq.
The coating roughness was measured using an image analysis from AFM (Model AA3000 Scanning Probe Microscope), at College of Science – University of Baghdad.
The electrochemical test was conducted using WINKINK MLab 200 Potentiostat/Galvanostat from Bank Company at 303 K. The classical three-electrode configuration was composed of platinum rod, saturated calomel electrode (SCE), and steel sample, which were used as the counter electrode, reference electrode, and working electrode, respectively. The working electrode with an area of 1 cm2 was immersed into the solution for 30 min to attain the stable state of open circuit potential (OCP); the potentiodynamic polarization test was conducted with scanning rate of 10 mV/s.
2.3 Microhardness and Thickness
Microhardness values were calculated for coated specimens with load of 9.8 g for 15 s. The average readings were taken for three measurements. Optical method for determining film thickness is commonly used because it refers to both opaque and transparent film and typically yields high-precision thickness value. These tests were performed at the Department of Material Engineering-University of Technology, Iraq.
3 Results and Discussion
3.1 Morphological Studies
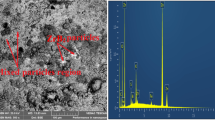
Figure 1 shows the morphological structure of Nb2O5-Ni composite coatings 0, 12, 15, and 30 wt% of total weight of target, from this Figure can be seen crystallite particles growth distributed on the steel surface within nickel layer. Also, it is clearly showing a perfect incorporation of Nb2O5 within the nickel-rich region. The incorporation of Nb2O5 particles increased with increasing wt% of Nb2O5 addition due to microstructural bonding and increasing nucleation process in Ni metal as carrier for Nb2O5.
EDS pattern of deposited Nb2O5-Ni layers shows combined elemental composition of the deposited composite coating with indication of Ni, Nb, and O present as major components.
Figure 2 shows the AFM images. These images indicate a smooth surface for uncoated surface with very small roughness equal to (2.97 nm) due to atmospheric corrosion with random distribution of particles with highest average diameter (148.06 nm). Ni-coated surface shows some summits and valleys due to deposition of Ni particles and nickel oxide with roughness average (9.52 nm) and more ordered distribution of particles with average diameters equal to (81.14).
The Nb2O5-Ni composites-coated surfaces gave smoother surface and the smoothness increases with increasing the weight percent of Nb2O5 in composite as shown in Table 1. The decrease in surface roughness can be attributed to the Nb2O5-incorporated Ni film which enhanced grain growth to obtain a larger particle size as shown in Fig. 3.
3.2 Microhardness and Coating Thickness
The microhardness measurement was done for coated specimens and the results are shown in Fig. 4. These data show the higher hardness for Ni-coated surface (155 HV) compared with uncoated surface (132 H.V). Adding Nb2O5 with 12 and 15 wt% to Ni coating decreased the hardness to (139) and (148), respectively, because of the presence of some pores within the coating. While adding the higher weight percent (30 wt%) of Nb2O5 led to increasing the hardness to (164 HV) due to filling of the pores within Ni layer.
The incorporation of Nb2O5 within Ni coating led to slight increases in thickness of coating by ≈ 1 μm because of filling of the gaps as shown in Fig. 4.
3.3 Corrosion Characteristics
The results of the corrosion progression in 3.5 wt% NaCl at 303 K from the linear potentiodynamic polarization study are shown in Fig. 5 and Table 2. The Tafel data were extrapolated to obtain Ecorr, icorr, and Tafel slopes. From the polarization curves of the Nb2O5-Ni composite coatings on carbon steel, it can be seen that corrosion potential (Ecorr) was shifted toward more noble value due to attaining passivity. The corrosion current density (icorr) was decreased with increasing wt% of Nb2O5 in Ni coating confirming the role of protection of applied coatings. Tafel slopes (bc & ba) deceased referring to reducing the reactions at cathodic and anodic sites.
Polarization resistance (Rp) can be calculated by the following formula [11]:
The data of Rp as listed in Table 3 indicate that the polarization resistance increased with increasing Nb2O5 percent in Ni coating because of filling of the pores within the Ni layer.
Other parameters can be calculated from corrosion data such as protection efficiency (PE%) from corrosion current density of coated sample (icorr,coated) and corrosion current density of uncoated sample (icorr,uncoated) as follows [12]:
The protection efficiency increases with increasing wt% of Nb2O5 in coating composite to get the best efficiency equal to 80.826% for 30% Nb2O5-Ni mixture. The porosity percentage (PP%) can be calculated from the polarization resistance of uncoated (Rp,uncoated) and coated (Rp,coated) sample using the following equation [13]:
The porosity percentage increases with increasing wt% of Nb2O5 in nickel layer to get the best value of 0.433% for 30% Nb2O5-Ni composite coating due to incorporation of Nb2O5 with Ni layer.
4 Conclusions
-
Nb2O5-Ni composite coatings were successfully fabricated using DC sputtering.
-
The structural properties indicate the presence of Nb2O5 within Ni coating by SEM/EDS and AFM.
-
Good corrosion resistance was attained for all deposited coatings as against the as-received specimen.
-
The significant performance improvement in the protection efficiency and porosity percentage was found as a result of physical barriers produced by Nb2O5 particles by filling of the pores within the Ni layer.
References
Huang Y, Zhang Y, Hu X (2003) Structural morphological and electrochromic properties of Nb2O5 films deposited by reactive sputtering. Solar Energy Mater Solar Cells 77:155–162
Serényi M, Lohner T, Petrik P, Zolnai Z, Horváth ZE, Khánh NQ (2008) Characterization of sputtered and annealed niobium oxide films using spectroscopic ellipsometry, Rutherford backscattering spectrometry and X-ray diffraction. Mater Sci Thin Solid Films 516:8096–8100
Olivares-Navarrete R, Olaya JJ, Ramírez C, Rodil SE (2011) Biocompatibility of niobium coatings. Coatings 1:72–87
Rojas PN, Rodil SE (2012) Corrosion behaviour of amorphous niobium oxide coatings. Int J ElectrochemSci 7:1443–1458
Rodrigues PRP, Terada M, Junior ORA, Lopes AC, Costa I, Banczek EP (2014) Niobium pentoxide coating replacing zinc phosphate coating. RevistaMatéria 19(02):105–116
Chen H, Wang Q, Dong H, Xi L, Lin X, Pan F, Ma Z (2015) Electroless plating of Ni-P-W coatings containing scattered Nb2O5 on sintered NdFeB substrate. Mater Res 18(5):1089–1096
Joshi P, Gesawat AA, Singh K (2015) Development and characterization of Ti-Nb-N coatings on stainless steel using reactive DC magnetron sputtering. ChemSci Rev Lett 4(16):1059–1068
Mohan Reddy R, Praveen BM, Praveen Kumar CM (2017) Corrosion behavior and characterization of Ni–Nb2O5 composites prepared by pulse electro deposition. Surf EngApplElectrochem 53(2):179–185
Oluyori T, Olorunniwo OE, Fayomi OSI, Atanda PO, Popola API (2017) Performance evaluation effect of Nb2O5 particulate on the microstructural, wear and anti-corrosion resistance of Zn–Nb2O5 coatings on mild steel for marine application. J Bio TriboCorros 3(51):2–6
Shi K, Zhang Y, Zhang J, Xie Z (2019) Electrochemical properties of niobium coating for biomedical application. Coatings 9:546
Yaqo EA, Anaee RA, Abdulmajeed MH, Tomi IHR, Kadhim MM (2019) Aminotriazole derivative as anti-corrosion material for Iraqi kerosene tanks: electrochemical, computational and the surface study. ChemistrySelect 4:9883–9892
Anaee RA, Abd Al-Majeed MH, Naser SA, Kathem MM, Ahmed OA (2019) Antibacterial inhibitor as an expired metoclopramide in 0.5M phosphoric acid. Al-Khwarizmi Eng J 15(1):71–81
Anaee RA (2015) Properties of functionally graded coating of Al2O3/ZrO2/HAP on SS 316L. Intl J SciEng Res 6(5):953–957
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anaee, R.A., Abdulkarim, A.A., Mathew, M.T. et al. The Effect of Nb2O5-Ni Coatings on the Microstructural and Corrosion Behavior on Carbon Steel for Marine Application. J Bio Tribo Corros 7, 1 (2021). https://doi.org/10.1007/s40735-020-00440-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00440-0