Abstract
The corrosion inhibition property of Cinnamomum verum leaf extract (CVE) for mild steel in hydrochloric and sulphuric acid media has been studied using weight loss technique, polarization method and impedance spectroscopy. The inhibition efficiency exhibited a linear relationship with inhibitor concentration up to an optimum concentration. While the temperature increase caused inhibition efficiency to drop down into lower values. Monitoring the inhibitive action of the major component eugenol, has also been a part of the study. The assumptions made on the basis of theoretical calculations for eugenol supported the experimental observations. The ΔE, EHOMO, ELUMO, and dipole moment values for eugenol and the protonated form of eugenol clearly suggests the electron-donating ability of the component which in turn stands for the inhibition potential of CVE. The adsorption of eugenol on Fe (1 1 0) surface has been simulated using Monte Carlo simulation studies. Adsorption studies revealed that the mode of adsorption obeys Langmuir isotherm. CVE exhibited better inhibition efficiency in hydrochloric acid compared to sulphuric acid medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Iron alloys are used in several engineering purposes and industrial environments which are susceptible to corrosion in different aggressive media [1]. In industries, hydrochloric and sulphuric acids in mild concentrations are used as cleaning agents for the metallic parts which again causes the destructive deterioration to happen. Sulphuric acid, in the concentrated form, although is the most produced reagent worldwide, corrosion by sulphuric acid has received little attention from researchers dedicated to the study of corrosion [2]. Corrosion protection in such aggressive media is mainly achieved using corrosion inhibitors. Organic compounds, particularly those containing polar functions with nitrogen, oxygen, and/or sulfur in a conjugated system have been widely used for this purpose [3]. These organic inhibitors get adsorbed on the metal surface by forming a coordinate covalent bond (chemical adsorption) or by the electrostatic interaction between the metal and inhibitor (physical adsorption) [4]. The adsorptive layer thus formed, isolates the metal surface from the aggressive environment consequently reducing the extent of corrosion [5]. The study and use of green inhibitors have now great acceptance worldwide. In these days, research in the field of green or eco-friendly corrosion inhibitors has been addressed towards the goal of using cheap, effective compounds at low or “zero” environmental impact [6,7,8,9]. Plants have been recognized as sources of naturally occurring compounds, some with rather complex molecular structures and having varying physical, chemical and biological properties [10, 11]. The inhibition activity of many plants against steel corrosion has been reported recently: Ginkgo leaves [12], garlic peel [13], and Salvia officinalis leaves [14]. As mentioned, only a few researchers attempted to study the corrosion of metals in sulphuric acid medium [9, 15]. One thing to be noted is that, more studies seem to focus on the corrosion inhibition performance of plant extracts, with interesting results; even though mechanistic insights lack [3]. The present work is an effort to study the corrosion inhibition activity of the leaf extract of Cinnamomum verum against mild steel corrosion in acid media. C. verum is an evergreen tree of tropical area and belongs to the family Lauraceae. Leaf extracts contain a number of phytochemical constituents and there may be occurring synergistic and/or antagonistic interactions among these components. It would be a very difficult task to understand which component or components particularly cause corrosion inhibition. Instead one or two major constituents in the extract may be investigated for their corrosion inhibition potential. The major component of C. verum was reported to be eugenol elsewhere [16]. Since the theoretical calculations suggested electron donating ability for eugenol (Fig. 1) it was also monitored experimentally for corrosion inhibition property.
2 Experimental
2.1 Inhibitor
The C. verum leaves were, washed, shade dried and powdered. A known weight of the powdered leaves was refluxed in ethanol for 3 h. It is then kept overnight and then filtered. The concentrated filtrate was then analysed for its corrosion inhibition property by making 1–4 V/V% solutions in 1 M HCl and 0.5 M H2SO4. A very low concentration range (0.03, 0.0, 0.10, and 0.13 V/V%) of eugenol were also investigated in these media for its corrosion inhibition potential.
2.2 Materials and Methods
Mild steel specimens of composition (atom%): C (0.2%), Mn (1%), P (0.03%), S (0.02%), and Fe (98.75%) were the test specimens. The metal samples for weight loss studies were cut into 2 × 1.8 cm2 coupons. The electrochemical measurements were made by exposing 1 cm2 surface area of the metal samples in the corrosive medium. Prior to measurements, the samples were polished using different grades of emery papers and then subjected to the action of a buffing machine attached with a cotton wheel and a fibre wheel having buffing soap to ensure mirror bright finish, degreased by washing with acetone and finally washed with distilled water.
The corrosive media for the study were prepared from reagent grade HCl and H2SO4 (E. Merck) using double-distilled water. All tests were performed in aerated medium at different temperatures (303, 308 and 313 K).
2.3 UV–Visible Spectroscopy
UV–visible absorption spectra were measured with JASCO-V-550 spectrophotometer. The C. verum leaf extract (CVE) and eugenol (99%) were analysed using UV–visible absorption studies.
2.4 Theoretical Evaluation
Molecular structure and electronic characteristics of inhibitor molecules are the key factors in establishing the adsorption ability of inhibitors on metal surfaces [17]. Quantum chemical calculations being an effective method for determining the correlation between molecular structure and inhibition efficiency was also utilized to support the experimental results [18, 19]. A study of the mechanism of the action of corrosion inhibitors has relevance both from the point of view of the search for new inhibitors and also for their effective use. To investigate the influence of electronic structure on the corrosion inhibition property of eugenol, some parameters such as the energies of molecular orbital, EHOMO (highest occupied molecular orbital), ELUMO (lowest unoccupied molecular orbital energy), energy gap (ΔE) and the dipole moment (µ) were calculated at B3LYP/6-31G* level in gas phase. Using Gaussian 03 programme package. It has been stated that the experimental data can be correlated well with these quantum chemical parameters.
Monte Carlo simulation helps finding the stable adsorption sites on metal surfaces searching the low-energy adsorption sites on both periodic and non-periodic substrates [20]. Materials Studio 4.3, software from Accelrys, Inc. [21] has been used to simulate the adsorption of eugenol on Fe (110) plane. This plane has been selected for the simulation studies due to its stability compared to Fe (1 00) and Fe (111). Optimized structures of eugenol and the Fe surface were used for the simulation. As described on the Accelrys website, the Forcite module “is an advanced classical molecular mechanics tool that allows fast energy calculations and reliable geometry optimization of molecules and periodic systems.” COMPASS stands for condensed-phase optimised molecular potentials for atomistic simulation studies [22], which is used to optimise the Fe surface and inhibitor molecule. The molecular dynamic (MD) simulation of the interaction between eugenol molecule and Fe (1 1 0) surface was carried out in a simulation box (28.00 Å × 28.00 Å × 43.01 Å). Solvent and charge effects are neglected and the calculations are performed at the metal/vacuum interface. The binding energy between eugenol and Fe (1 1 0) surface was calculated using the following equation [23]:
\({E_{{\text{total}}}}\), \({E_{{\text{surface}}}}\), \({E_{{\text{eug}}}}\) correspond respectively to the total energies of the Fe (1 1 0) surface with adsorbed eugenol, Fe surface and eugenol, where a negative value of \({E_{{\text{binding}}}}\) corresponds to a stable adsorption structure.
2.5 Weight Loss Technique
The polished metal samples were weighed and then dipped in acid media without and with various concentrations of the inhibitors at 303 K. At different time intervals like 24, 48, 72 and 96 h, the weight loss values were noted. From this the corrosion rate and inhibition efficiencies of the inhibitors for mild steel corrosion were determined.
2.6 Electrochemical Impedance Spectroscopy (EIS)
Electrochemical impedance spectroscopy is a powerful technique which is used to determine the characteristics and kinetics of electrochemical processes occurring at the metal/aggressive media interfaces. A low amplitude alternating potential (or current) wave is imposed on top of a DC potential, with the input voltage by the output current furnishing the impedance. The variation in impedance (magnitude and phase angle) is used for the interpretation. The out-of-phase relationship between the input voltage and output current is analysed by the frequency response analyser. The circuit elements commonly used are thecapacitor, resistor and inductor.
But, normally, the impedance results for a solid electrode/electrolyte interface often reveal a frequency dispersion that cannot be described by these simple elements. And this frequency dispersion is attributed to a “capacitance dispersion” expressed in terms of a distributed electrical element called constant-phase element. Figure 2 represents the electrical equivalent circuit employed to analyse the impedance plots, where CPE is the constant-phase element, Rs is the solution resistance and Rct represents the charge transfer resistance. The origin of CPE behaviour at the interface or the frequency dispersion has been a subject of interest for many researchers [24, 25]. The impedance function of CPE is described by the following equation:
where Q is the CPE constant, ω is the angular frequency, J2 = − 1 is the imaginary number and n is the CPE exponent which gives details about the degree of surface inhomogeneity [26, 27]. Generally, a CPE cannot be described by a finite number of discrete elements such as an R, C and L with frequency-independent values. However, for some values of n, it simplifies to discrete elements: a capacitor for α = 1, a resistor for α = 0, and an inductor for α = − 1 [28, 29]. According to Kerner and Pajkossy [30], capacitance dispersion on solid electrodes is due to surface in homogeneity (i.e., heterogeneities on the atomic scale) rather than surface roughness (i.e., geometric irregularities much larger than those on the atomic scale).
2.7 Potentiodynamic Polarization Studies
Potentiodynamic polarization studies were also carried out with the aid of a three electrode cell assembly. The polarization measurements were conducted by scanning the metal within a potential range of − 250 to + 250 mV/min. By extrapolating the linear segments of cathodic and anodic polarization curves through Ecorr, the corresponding corrosion current density Icorr is obtained, using which the percentage inhibition efficiency IE (%) was calculated.
3 Results and Discussion
3.1 UV–Visible Spectroscopic Analysis
Figure 3 shows the absorption bands, in which ‘a’ represents the band corresponding to CVE and ‘b’ represents that of eugenol. The peak at 355 nm in ‘b’ stands for the absorption of eugenol. Curve ‘a’ that corresponds to CVE also shows an absorption maximum at this region (352 nm) suggesting the presence of eugenol in CVE. The presence of components other than eugenol in CVE contributes to the considerable difference in absorption maximum (≈ 2 nm). The wide range of absorption for CVE (370–640 nm) is also attributed to the presence of the phytochemicals other than eugenol.
3.2 Computational Calculations
Molecular structure and electronic characteristics are the key factors in establishing the adsorption ability of inhibitors on metal surface. The inhibiting action of an inhibitor may be ascribed to their interactions with the metal surface via adsorption process [17]. The optimized geometry obtained for eugenol is represented as Fig. 4a.
High values of EHOMO are likely to indicate a tendency of the molecule to donate electrons to appropriate acceptor molecules to the unoccupied d orbital of a metal. In the electronic configuration of Fe atom, 3d orbital is not fully filled, which could, therefore, bind with HOMO of the inhibitor [31], whereas the filled 4s orbital could donate the electron to LUMO of the inhibitor. Thus, it can be predicted that the adsorption of inhibitor on the mild steel surface may be ascribed to the interaction between 3d, 4s orbitals of Fe atom and the frontier molecular orbitals of the inhibitor [32]. On the other hand, the lower value of ELUMO, indicates that there is more probability of the molecule to accept electrons. From Table 1, the higher EHOMO and the smaller band gap (ΔE = ELUMO − EHOMO), 5.74700 eV of eugenol indicates its electron-donating ability which in turn points to the corrosion inhibition property. The HOMO and LUMO (Fig. 4b, c) of eugenol reflects that the orbital electron densities are distributed homogeneously throughout the molecule.
The quantum chemical parameters corresponding to the optimized geometry of the protonated form of eugenol are also summarised in Table 1. The very high dipole moment (5.2961 D) of the protonated form of eugenol accounts for the enhanced electron-donating ability of eugenol in the acid medium. Since the dipole moment of a molecule results from the non-uniform distribution of the electric charges, an increase in dipole moment probably increases the adsorption of a compound on the metallic surface. The energy of deformability gets increased with increasing dipole moment value making the adsorption of the molecule on the metal surface easier. Another factor is the increase in volume of the inhibitor associated with the increase in dipole moment. This increases the contact area between the molecule and metal surface leading to better inhibition ability [33].
Figure 5 obtained for the Monte Carlo simulation represents the cross-section of the lowest energy adsorption configuration for a single eugenol molecule on Fe (1 1 0) surface. The eugenol molecule seems well adsorbed on the Fe surface and this adsorption mode may be attributed to the strong interaction between Eugenol and the metal surface.
Different energy forms obtained in Monte Carlo simulation are summarized in Table 2. The total energy is the sum of the energies of the adsorbate components, the rigid adsorption energy and the deformation energy. The substrate energy (energy of iron surface) is taken as zero. Adsorption energy in kJ/mol reports the energy released (or required) when the relaxed adsorbate component gets adsorbed on the substrate. The adsorption energy is the sum of the rigid adsorption energy and the deformation energy for the adsorbate. The rigid adsorption energy reports the energy, in kJ/mol, released (or required) when the unrelaxed adsorbate component (i.e., before the geometry optimization step) is adsorbed on the substrate. The deformation energy reports the energy, in kJ/mol, released when the adsorbed adsorbate component is relaxed on the substrate surface. In addition, dEads/dNi reports the energy, in kJ/mol, of substrate–adsorbate configurations where the adsorbate has been removed. According to Eq. (1) the binding energy was obtained as − 51.8601 kJ/mol. The negative sign and considerable magnitude, suggests a stable adsorption structure of eugenol on Fe surface.
3.3 Weight Loss Study
Weight loss study is not a mere accounting of the inhibitive capacity of the inhibitor; instead it is a method by which an assessment of the duration of the protective layer formed by the inhibitor molecules on the metallic surface can be done. It was observed that, as the inhibitor concentration in the corrosive medium increased, the efficiency also got increased. The IE (%) was calculated using the equation:
where Wcorr and W0 are the corrosion rates of steel with and without the inhibitor, respectively. The efficiencies obtained in different acid media at different exposure periods are given in Table 3. The short-term methods such as electrochemical techniques are carried out immediately after the immersion of the specimen in the electrolyte and yields the instantaneous corrosion rates which may significantly differ from those obtained from long-term measurements such as weight loss as the later corresponds to a steady state [34].
The variation in inhibition efficiency (corresponding to the highest inhibitor concentration) with increasing exposure time is depicted in Fig. 6. In hydrochloric acid, CVE exhibits appreciable inhibition efficiency consistently. Eugenol also shows a similar trend. But in sulphuric acid medium, the inhibitors showed varying inhibition behaviour. At the longest exposure period in sulphuric acid, the efficiency of both CVE and eugenol were noticeably lowered compared to that in HCl. This may be attributed to various factors such as an increase in the cathodic or reduction rate or increase in the ferrous ion concentration [35].
3.4 Electrochemical Impedance Spectroscopy
The impedance responses were recorded as Nyquist plots, and those ones obtained for the corrosion process of mild steel in 1 M HCl and 0.5 M H2SO4 containing the highest inhibitor concentrations studied, are represented in Figs. 7 and 8. The semi-circular appearance of Nyquist plot shows that the charge transfer process takes place during dissolution [36]. Tables 4 and 5 summarises the values of Rct, Cdl and IE (%) corresponding to mild steel corrosion in both the acid media at different temperatures in the absence and presence of CVE and eugenol, respectively. The extract CVE and eugenol showed their maximum corrosion inhibition potential at the highest inhibitor concentrations. The IE (%) was obtained as:
where \(R_{{{\text{ct}}}}^{{\text{*}}}\) and \({R_{{\text{ct}}}}\) are the charge transfer resistances in the presence and absence of the inhibitor, respectively. Cdl shows an opposite trend [37] to that of \({R_{{\text{ct}}}}\). The decrease in Cdl values is due to the displacement of water molecules by the adsorbed inhibitor molecule at the metal solution interface which led to the formation of a protective layer on the mild steel surface and then it retarded the extent of the dissolution reaction [38]. The decrease in Cdl may be attributed to the decrease in dielectric constant and increase in the thickness of the electrical double layer [39].
In hydrochloric acid, CVE exhibited appreciably good inhibition efficiency which reached up to a maximum of 97%. In sulphuric acid, the highest efficiency reached only up to 93%. The component eugenol showed no noticeable changes in the inhibition mechanism compared to CVE as it can be assured from Figs. 7b and 8b. At higher temperatures, the increase in the charge transfer resistance values was not very good as it was at 303 K (Tables 4, 5). This may be attributed to the increased rate of metal dissolution and probably to the partial desorption of the inhibitor at higher temperatures [40].
3.5 Potentiodynamic Polarization Studies
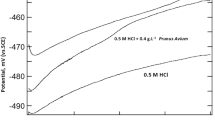
Polarization measurements were carried out to get knowledge concerning the kinetics of the cathodic and anodic regions. In potentiodynamic polarization technique, the rate of anodic or cathodic reactions are represented by the current. The polarization curves for mild steel corresponding to the highest inhibitor concentration studied, in the corrosive media at different temperatures are represented in Figs. 9 and 10. The extrapolation of the linear portion of the cathodic and anodic polarization curve through the Ecorr value gives the corresponding Icorr value. The inhibitory action of CVE and eugenol reflects in the corrosion current values as it is clear from Tables 6 and 7. The shift in Icorr after the addition of the inhibitors clearly indicates that there occurs an inhibition to the process of corrosion in the presence of CVE and eugenol.
The parallel cathodic Tafel curves obtained in HCl medium suggest that hydrogen evolution is activation controlled and the reduction mechanism is not affected by the presence of the inhibitor. The region between the linear part of cathodic and anodic branch of polarization curves becomes wider as the inhibitor is added to the acid solution. Similar results were found in the literature [41]. The overall action of the inhibitor suggests mixed-type behaviour. The Ecorr values and inhibition efficiencies are summarised in Tables 6 and 7. The percentage inhibition efficiencies were obtained as:
where \({I_{{\text{corr}}}}\) and \(I_{{{\text{corr}}}}^{{\text{*}}}\) are the corrosion current densities in the absence and presence of the inhibitor.
The IE (%)’s obtained for CVE and eugenol were comparable with that obtained using EIS technique. The corrosion rate (CR) was found decreasing with increasing concentration of CVE and eugenol, predominantly affecting the cathodic part of the iron corrosion reaction.
3.6 Adsorption studies
The inhibitor forms a protective layer on the metal surface. This leads to a decrease in the metal oxidation process. It could be seen that as the temperature increases the surface coverage ‘θ’ (IE% divided by 100) gets decreased suggesting a lower stability of the corrosion products at higher temperatures. This observation may be explained to occur as a result of either of the two factors such as a fast-formed aggregate of corrosion products (Rhomboclase, Goelhiteetc) on the surface, which disturbs the protective layer of the inhibitor. This is due to the fact that the kinetic oxidation reaction may get promoted as the temperature is increased. The second factor is that the desorption velocity of the inhibitor on the metal surface is faster than its adsorption while temperature increases. The stability of the inhibitor layer keeps a close relation with the electrical charge on the metal surface which increases with temperature. As a result, an inverse relation appears between θ and temperature [42].
The inhibitor molecules when adsorbed on the metal surface, the active adsorption sites are blocked thereby avoiding the availability of metal surface (1 − θ) [43]. The inhibitor molecules get adsorbed on the metal surface when the inhibitor–metal surface interaction is higher than that between the metal surface and water molecules.
To find the adsorption pattern, the most frequently used isotherms are Langmuir, Frumkin, Flory–Huggins. The best fit of all was obtained for Langmuir isotherm which is a plot of Cinh/θ vs. Cinh.
The Langmuir adsorption isotherm can be described as:
where Cinh is the inhibitor concentration, Kads is the adsorption equilibrium constant and \(\theta\) is the surface coverage. The Langmuir isotherms for CVE and eugenol are represented in Fig. 11.
Though the linearity of Langmuir plot may be taken to suggest that the adsorption of inhibitor follows the Langmuir adsorption isotherm, considerable deviation of the slope from unity indicated that the isotherm could not be strictly applied. Langmuir says that there is no interaction among the adsorbent molecules. This may not be true always as in the case of large molecules having polar atoms or groups. In addition, the deviation of the slope from unity may be interpreted due to the changes in adsorption of heat with increasing surface coverage which has also been ignored in the derivation of Langmuir isotherm [44].
3.7 SEM
The micrograph of the polished mild steel surface is shown in Fig. 12a. Figure 12b, c represents that of mild steel exposed to 1 M HCl and 0.5 M H2SO4, respectively. There it can be seen an extensively damaged metal surface with large number of cracks on it due to the attack of aggressive media. Figure 12d, e represent the micrographs of mild steel in 1 M HCl containing optimum concentration of CVE and eugenol, respectively. Figure 12f, g shows the image of mild steel in 0.5 M H2SO4 containing CVE and eugenol, respectively. The micrographs representing the mild steel surface in the acid media containing CVE, clearly show the protective layer formed on the surface. If eugenol containing acid media are considered, there is no such uniform coating observed. But the metal surface seems protected damage free. This evidently shows that eugenol individually can also act against corrosion. Thus, the corrosion inhibition property of CVE is further confirmed.
3.8 Mechanism of Corrosion Inhibition
The major reason for the process of corrosion of mild steel in hydrochloric acid is the adsorption of Cl− ions onto the metallic surface sites. The mobility of Cl− is higher than the inhibitor molecules, therefore, they cannot compete with Cl− ions. But the inhibitor molecules can prevent Cl− ions to reach the steel surface sites by steric effect. When an inhibited solution contains adsorbable anions, such as halide ions, these adsorb on the metal surface by creating oriented dipoles and consequently increase the adsorption of the organic cations on the dipoles. In such cases, a positive synergistic effect arises; therefore, the degree of inhibition in the presence of both the moieties (anions and organic cations) is higher than the sum of the individual effects [45]. This explains the higher inhibition efficiency of various organic inhibitors in hydrochloric acid medium compared to sulphuric acid. CVE also exhibits a dominating corrosion inhibition property in HCl than in H2SO4 medium.
4 Conclusions
-
The results of weight loss studies and electrochemical chemical measurements reveal that the leaf extract of C. verum (CVE) has appreciable inhibition efficiency against the corrosion of mild steel in 1 M HCl and 0.5 M H2SO4. The efficiency found decreasing with the rise in temperature.
-
The inhibition potential of CVE was better in HCl than in H2SO4.
-
The corrosion inhibition potential of CVE has been confirmed from the experimental studies carried out with eugenol, the major component of the leaves.
-
Potentiodynamic polarization studies reveal the mixed-type corrosion inhibition behaviour of the extract CVE.
-
The results obtained from both electrochemical measurements are in good agreement which in turn is supported by the theoretical calculations made for eugenol.
-
Monte Carlo simulation study suggests a stable adsorptive interaction of eugenol and iron surface.
-
Adsorption and surface morphological studies reveal protection offered by CVE and eugenol to the mild steel surface.
References
Farsak M, Keles H, Keles M (2015) A new corrosion inhibitor for protection of low carbon steel in HCl solution. Corros Sci 98:223–232
Panossian Z, de Almeida NL, de Sousa RM, de Souza Pimenta G, Marques LB (2012) Corrosion of carbon steel pipes and tanks by concentrated sulfuric acid: a review. Corros Sci 58:1–11
Oguzie EE, Adindu CB, Enenebeaku CK, Ogukwe CE, Chidiebere MA, Oguzie KL (2012) Natural products for materials protection: mechanism of corrosion inhibition of mild steel by acid extracts of Piper guineense. J Phys Chem C 116:13603–13615
Goulart CM, Esteves-Souza A, Martinez-Huitle CA, Rodrigues CJF, Maciel MAM, Echevarria A (2013) Experimental and theoretical evaluation of semicarbazones and thiosemicarbazones as organic corrosion inhibitors. Corros Sci 67:281–291
Avci G (2008) Inhibitor effect of N, N0-methylenediacrylamide on corrosion behaviour of mild steel in 0.5 M HCl. Mater Chem Phy 112:234–238
Lahhit N, Bouyanzer A, Desjobert JM, Hammouti B, Salghi R, Costa J, Jama C, Bentiss F, Majidi L (2011) Fennel (Foeniculum vulgare) essential oil as green corrosion inhibitor of carbonsteel in hydrochloric acid solution. Port Electrochim Acta 29:127–138
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2SO4 solutions. Corros Sci 55:407–415
Li X, Deng S, Fu H (2012) Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract. Corros Sci 62:163–175
Lebrini M, Robert F, Lecante A, Roos C (2011) Corrosion inhibition of C38 steel in 1M hydrochloric acid medium by alkaloids extract from Oxandraasbeckii plant. Corros Sci 53:687–695
Mukherjee D, Berchman J, Rajsekkar A, Sundarsanan N, Mahalingam R, Maruthamuthu S, Thiruchelvam T, Karaikudi D (1997) Plant-based alkaloids inhibit corrosion of marine alloys. Anti-Corros Method Mater 44:186–194
Philip JNY, Buchweishaija J, Mkayula L (2011) Cashew nut shell liquid as an alternative corrosion inhibitor for carbon steel. Tanzania J Sci 27:9–19
Deng S, Li X (2012) Inhibition by Ginkgo leaves extract of the corrosion of steel in HCl and H2 SO4 solutions. Corros Sci 55:407–415
Pereira SSA, Pegas MM, Fernandez TL, Magalhaes M, Schontag TG, Lago DC, de Senna LF, D’Elia E (2012) Inhibitory action of aqueous garlic peel extract on the corrosion of carbon steel in HCl solution. Corros Sci 65:360–366
Soltani N, Tavakkoli N, Khayatkashani M, Jalali MR, Mosavizade A (2012) Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros Sci 62:122–135
Kamal C, Sethuraman MG, Caulerpin (2012) A bis-indole alkaloid as a green inhibitor for the corrosion of mild steel in 1 M HCl solution from the marine alga Caulerpa racemosa. Ind Eng Chem Res 51:10399–10407
Patel K, Ali S, Sotheeswaran S, Dufour JP (2007) Composition of the leaf essential oil of Cinnamomum verum (Lauraceae) from Fiji Islands. J Essent Oil Bearing Plants 10:374–377
Fiori-Bimbi MV, Alvarez PE, Vaca H, Gervasi CA (2015) Corrosion inhibition of mild steel in HCl solution by pectin. Corros Sci 92:192–199
Resit, Yıldız (2015) An electrochemical and theoretical evaluation of 4, 6-diamino-2-pyrimidinethiol as a corrosion inhibitor for mild steel in HCl solutions. Corros Sci 90:544–553
Obot IB, Gasem ZM (2014) Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives. Corros Sci 83:359–366
Khaled KF (2009) Monte Carlo simulations of corrosion inhibition of mild steel in 0.5 M sulphuric acid by some green corrosion inhibitors. J Solid State Electrochem 13:1743–1756
Barriga J, Coto B, Fernandez B (2007) Molecular dynamics study of optimal packing structure of OTS self-assembled monolayers on SiO2 surfaces. Tribol Int 40:960–966
Sun H, Ren P, Fried JR (1998) The COMPASS forcefield: parameterization and validation for polyphosphazenes. Comput Theor Polym Sci 8:229–24611
Khaled K (2008) Molecular simulation, quantum chemical calculations and electrochemical studies for inhibition of mild steel by triazoles. Electrochim Acta 53:3484–3492
Macdonald JR (ed) (1987) Impedance spectroscopy, emphasizing solid materials and systems. Wiley, New York
DeLevie R (1965) On impedance measurements: the determination of the double layer capacitance in the presence of an electrode reaction. Electrochim Acta 10:395–402
Pajkossy T, Wandlowski T, Kolb DM (1996) Impedance aspects of the anion adsorption on gold single crystal electrodes. J Electroanal Chem 414:209–220
Pavithra MK, Venkatesha TV, Vathsala K, Nayana KO (2010) Synergistic effect of halide ions on improving corrosion inhibition behaviour of benzisothiozole-3-piperizine hydrochloride on mild steel in 0.5 M H2SO4medium. Corros Sci 52:3811–3819
Ping Song ShuShen, Li C-C, Guo X-Y, Wen Y, Hai-Feng Yang (2015) Insight in layer-by-layer assembly of cysteamine and l-cysteine on the copper surface by electrochemistry and Raman spectroscopy. Appl Surf Sci 328:86–94
Kim CH, Pyun SI, Kim JH (2003) An investigation of the capacitance dispersion on the fractal carbon electrode with edge and basal orientations. Electrochim Acta 48:3455–3463
Kerner Z, Pajkossy T (1998) Impedance of rough capacitive electrodes—the role of surface disorder. J Electroanal Chem 448:139–142
Zhao P, Li Y, Liang Q (2005) Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/l HCl. Appl Surf Sci 252:1596–1607
Bentiss F, Traisnel M, Vezin H, Hildebr HF, Lagrenee M (2004) 2,5-Bis(4 dimethylaminophenyl)-1,3,4-oxadiazole and 2,5-bis(4-dimethylaminophenyl)-1,3,4-thiadiazole as corrosion inhibitors for mild steel in acidic media. Corros Sci 46:2781–2792
Li X, Deng S, Fu H, Li T (2009) Adsorption and inhibition effect of 6-benzylaminopurine oncold rolled steel in 1.0 M HCl. ElectrochimActa 54:4089–4098
Shi Z, Liu M, Atrens A (2010) Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros Sci 52:579–588
Schmitt G (1984) Application of inhibitors for acid Media. Br Corros J 19:165–176
Muralidharan S, Azim SS, Berchmans LJ, Iyer SV (1997) Synergistic influence of iodide ions on the inhibition of corrosion of mild steel in sulphuric acid by hexyl amine. Anti-Corros Methods Mater 44:30–36
Bentiss F, Lagrence M, Traisnel M, Hornez JC (1999) The corrosion inhibition of mild steel in acidic media by a new triazole derivative. Corros Sci 41:789–803
Bentiss F, Traisnel M, Lagrenee M (2000) The substituted 1,3,4-oxadiazoles: a new class of corrosion inhibitor of mild steel in acidic media. Corros Sci 42:127–146
Ibrahim TH, Habbab M (2011) Corrosion inhibition of mild steel in 2M HCl using aqueous extract of eggplant peel. Int J Electrochem Sci 6:5357–5371
Popova A, Christov M, Vasilev A (2015) Mono- and dicationicbenzothiazolic quaternary ammonium bromides as mild steel corrosion inhibitors. Part III: influence of the temperature on the inhibition process. Corros Sci 94:70–78
Morad MS, Kamal El-Dean AM (2006) 2,2-Dithiobis(3-cyano-4, 6-dimethyl pyridine): a new class of acid corrosion inhibitors for mild steel. Corros Sci 48:3398–3412
Chen W, Luo HQ, Li NB (2011) Inhibition effects of 2,5-dimercapto-1,3,4-thiadiazole on the corrosion of mild steel in sulphuric acid solution. Corros Sci 53:3356–3365
Döner A, Solmaz R, Özcan M, Gülfeza G (2011) Experimental and theoretical studies of thiazoles as corrosion inhibitors for mild steel in sulphuric acid solution. Corros Sci 53:2902–2913
Oguzie EE, Okolue BN, Ebenso EE, Onuoha GN, Onuchukwu AI (2004) Evaluation of the inhibitory effect of methylene blue dye on the corrosion of aluminium in hydrochloric acid. Mater Chem Phys 87:394–401
Oguzie EE (2004) Influence of halide ions on the inhibitive effect of Congo red dye on the corrosion of mild steel in sulphuric acid solution. Mater Chem Phys 87:212–217
Acknowledgements
One of the authors (Anupama KK) gratefully acknowledges the financial support obtained from the University Grant Commission for conducting the investigations in the form of senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anupama, K.K., Joseph, A. Experimental and Theoretical Studies on Cinnamomum verum Leaf Extract and One of Its Major Components, Eugenol as Environmentally Benign Corrosion Inhibitors for Mild Steel in Acid Media. J Bio Tribo Corros 4, 30 (2018). https://doi.org/10.1007/s40735-018-0146-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0146-z