Abstract
The aim of this study is to investigate the inhibition efficiency of 1,5-diaminonaphthalene (NDA), for mild steel corrosion in 1 M HCl solution. For this purpose, weight loss, electrochemical impedance spectroscopy and potentiodynamic measurements were realized. The effect of NDA on the mild steel corrosion was also studied by quantum chemical calculations. Increasing inhibitor concentration led to significant reduction in the corrosion rate of mild steel, with inhibitor efficiency value above 90%. The corrosion behavior of steel in 1 M HCl without and with the inhibitor at various concentrations was studied at the temperature range of 308–348 K. Potentiodynamic polarization showed that the inhibitor acts as mixed type. The Nyquist plots showed that increasing NDA concentration, polarization resistance increased and double-layer capacitance decreased, involving increased inhibition efficiency. The adsorption of NDA on the mild steel surface was well described by the Langmuir adsorption model. In addition, quantum chemical calculations based on density function theory and molecular dynamic simulations are done to support the accuracy of experimental results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Mild steel finds application in a wide spectrum of industries owing to its high mechanical strength, abundance and low cost. Corrosion, like an insidious cancer, deteriorates the surface of the metals and alloys in different environment [1,2,3]. In industries, acid solutions are more frequently used in boilers for descaling, pickling, etc. Mild steel suffers from severe corrosive attack in this acidic condition. Hence, the study on corrosion of mild steel in aggressive acidic condition assumes significance from industrial point of view [4, 5].
The use of chemical inhibitors is one of the most practiced methods for protecting against corrosion, especially in acid media, to prevent metal dissolution and acid consumption [6]. Various organic and non-organic compounds have been studied as inhibitors to protect metals from corrosion. Usually, organic compounds exert a significant influence on metal surface adsorption and therefore can be used as effective corrosion inhibitors. The efficiency of these organic corrosion inhibitors is related to the presence of polar functions containing S, O or N atoms which are centers for the establishment of the adsorption process [7,8,9,10,11,12].
Theoretical chemistry, including quantum chemical calculation and MD simulations have been proved to be a very powerful tool for studying the mechanism of corrosion inhibition [13,14,15]. This theoretical approach has been widely used to investigate a correlation between molecule structure and inhibition efficiency of an organic compound [16, 17].
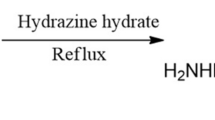
In the present work, the corrosion inhibition efficiency of naphthalene derivative (NDA) on mild steel in 1 M hydrochloric acid solution was studied using weight loss measurement, potentiodynamic polarization curves and electrochemical impedance spectroscopy (EIS). Effects of inhibitor concentration and temperature on inhibition action were investigated. The quantum chemical calculations and MD simulations were done to provide additional insights into the corrosion process. The chemical structure of inhibitor is given in Fig. 1.
2 Materials and Methods
2.1 Materials
The steel used in this study is a mild steel with a chemical composition (in wt%) of 0.09% P, 0.01% Al, 0.38% Si, 0.05% Mn, 0.21% C, 0.05% S and the remainder iron (Fe). The steel samples were pre-treated prior to the experiments by grinding with emery paper sic (220, 400, 800, 1000 and 1200); rinsed with distilled water, degreased in acetone, washed again with bidistilled water and then dried at room temperature before use.
2.2 Solutions
The aggressive solutions of 1 M HCl were prepared by dilution of analytical grade 37% HCl with distilled water. The concentration range of NDA used was 10−6 to 10−3 M.
2.3 Gravimetric Study
Gravimetric experiments were performed according to the standard methods [18], and the mild steel sheets of 1 × 1 × 0.1 cm3 were abraded with a series of emery papers SiC (120, 600 and 1200) and then washed with distilled water and acetone. After weighing accurately, the specimens were immersed in a 100-mL beaker containing 250 mL of 1 M HCl solution with and without addition of different concentrations of inhibitor. All the aggressive acid solutions were open to air. After 6 h of acid immersion, the specimens were taken out, washed, dried and weighed accurately. In order to get good reproducibility, all measurements were taken few times and average values were reported to obtain good reproducibility. The inhibition efficiency (\({\eta_{WL}}(\% )\)) and surface coverage (θ) were calculated as follows:
where \({W_{\text{b}}}\) and \({W_{\text{a}}}\) are the weight of the sample before and after immersion in the different solutions, \(C_{\text{R}}^{\circ}\) and \({C_{\text{R}}}\) are the corrosion rate without and with addition of inhibitor respectively, A is the area of the sample (cm2), and t is the time of exposure (h).
2.4 Electrochemical Measurements
The electrochemical measurements were taken using Volta lab (Tacussel–Radiometer PGZ 100) potentiostat and controlled by Tacussel corrosion analysis software model (Voltamaster 4) under static condition. The corrosion cell used had three electrodes. The reference electrode was a saturated calomel electrode (SCE). A platinum electrode was used as auxiliary electrode of surface area of 1 cm2. The working electrode was mild steel of the surface 1.16 cm2. All potentials given in this study were referred to this reference electrode. The working electrode was immersed in test solution for 30 min to a establish steady-state open-circuit potential (Eocp). After measuring the Eocp, the electrochemical measurements were taken. All electrochemical tests have been performed in aerated solutions at 308 K. The EIS experiments were conducted in the frequency range with high limit of 100 kHz and different low limit 0.1 Hz at open-circuit potential, with 10 points per decade, at the rest potential, after 30 min of acid immersion, by applying 10 mV AC voltage peak to peak. Nyquist plots were made from these experiments. The best semicircle can be fit through the data points in the Nyquist plot using a nonlinear least square fit so as to give the intersections with the x-axis. The inhibition efficiency of the inhibitor was calculated from the charge transfer resistance values using the following equation:
where \({R_{\text{p(inh)}}}\) and \({R_{\text{p}}}\) are the polarization resistances with and without addition of inhibitor in 1 M HCl.
After AC impedance test, the potentiodynamic polarization measurements of mild steel substrate in inhibited and uninhibited solution were scanned from cathodic to the anodic direction, with a scan rate of 1 mV s−1. The potentiodynamic data were analyzed using the polarization VoltaMaster 4 software. The linear Tafel segments of anodic and cathodic curves were extrapolated to corrosion potential to obtain corrosion current densities (icorr).
The inhibition efficiency was evaluated from the measured icorr values using the following equation:
\(i_{\text{corr}}^\circ\) and \({i_{\text{corr}}}\) are the current density without and with addition of inhibitor in 0.5 M sulfuric acid.
2.5 Quantum Chemical Calculations
Complete geometry optimization of the inhibitor molecules were performed using density functional theory (DFT) with Beck’s three-parameter exchange functional along with Lee Yang–Parr non-local correlation functional (B3LYP) with 6-31G basis set using the Gaussian 09 program package [19,20,21]. The geometry optimizations were considered to be complete when the stationary point was located. The optimized structures were confirmed to be true minima by frequency analysis (no imaginary frequencies). Frontier molecular orbitals (HOMO and LUMO) were used to interpret the adsorption of inhibitor molecules on the metal surface. The electron affinity (EA) and ionization potential (IP) are deduced from EHOMO and ELUMO by the Eqs. (5) and (6) [22]:
Mulliken electronegativity (χ) and absolute hardness (η) can be approximated using [23, 24]:
The number of transferred electrons (ΔN) is calculated by application of the Pearson method using the equation [25]:
where \(\phi\) and χinh denote the work function and absolute electronegativity of iron and inhibitor molecule, respectively, ηFe and ηinh denote the absolute hardness of iron and the inhibitor molecule, respectively. A theoretical value ηFe=0 is frequently used (since for bulk metallic atoms I = A). The obtained DFT-derived \(\phi\) values for Fe (100), Fe (110) and Fe (111) surfaces are 3.91, 4.82 and 3.88 eV, respectively [26, 27]. In this study, we use only Fe (110) surface due to its higher stabilization energy and packed surface [28].
2.6 Molecular Dynamics (MD) Simulations
Currently, molecular dynamics (MD) simulations have emerged as a very popular exploratory technique to studies of interaction between inhibitor and the concerned metal surfaces. In this present investigation, calculations were carried out using Materials Studio package [29] using the COMPASS force field and the Smart algorithm with NVT ensemble has been employed to study the interactions between the Fe (110) surface and the inhibitor molecules [29] with periodic boundary conditions to model a representative part of the interface devoid of any arbitrary boundary effects. The first step was to import the iron crystal, its surface cleaved along (1 1 0) plane with a thickness of 10.435 Å, and their lattice parameters are a = b = 24.82 Å, c = 10.13 Å. The Fe (110) surface was relaxed by minimizing its energy using smart minimizer method. The second step was to create a super cell (10 × 10) to increase the Fe (1 1 0) surface and change its periodicity. A vacuum slab with zero thickness was built. The third step, a supercell with a size of a = b = 25.72 Å, c = 26.38 Å, contains 491 H2O, 9Cl−, 9 H3O+, and one inhibitor molecule was created. Finally, the layer builder was used to create the entire model with a size of a = b = 25.72 Å, c = 37.83 Å. The simulation was carried out in a simulation box (25.72 × 25.72 × 37.83 Å3). Herein, Fe (110) surface has been preferred as it has a packed surface and high stability compared to other Fe surfaces [28]. The temperature was fixed at 303 K with a time step of 1 fs and simulation time of 2000 ps. An Andersen thermostat was used to maintain the system temperature at 303 K. The system was quenched every 5000 steps with the Fe (110) surface atoms constrained. Optimized structure of the inhibitor molecule was used for the simulation. More details of MD simulation are referenced from our published articles [30, 31].
3 Results and Discussion
3.1 Electrochemical Impedance Spectroscopic Study
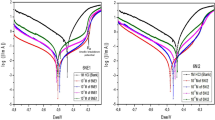
Nyquist plots of mild steel in 1 M HCl solution without and with different concentrations of NDA are shown in Fig. 2. It is seen that the Nyquist diagram shows a capacitive loop which suggest that the corrosion of mild steel in test solution is mainly controlled by charge transfer process [32, 33]. The diameter of the capacitive loop in the presence of inhibitor is bigger than that in the uninhibited solution and increases with the inhibitor concentrations. The impedance parameters such as polarization resistance (Rp), double-layer capacitance (Cdl), the frequency (fmax) and inhibition efficiency of NDA are listed in Table 1.
It is seen that the Rp value increases and the Cdl value decreases by the addition of inhibitor. A large polarization resistance (Rp) is associated with a slower corroding system. An increase in polarization resistance values could be ascribed to the adsorption of inhibitor at steel–acid interface, which effectively blocked the active sites on mild steel surface and hence enhances the corrosion resistance of mild steel in acidic medium. The decrease in Cdl values with an increase in the inhibitor concentration, suggesting that either thickness of protective layer increased, or local dielectric constant of film decreased, or both occurred simultaneously [34, 35]. The inhibition efficiency increases with increasing inhibitor concentration due to more coverage of mild steel surface with the inhibitor concentrations.
3.2 Potentiodynamic Polarization Study
Polarization measurements were taken to investigate the behavior of mild steel electrode in 1 M HCl in the absence and presence of inhibitor. The current–potential relationship for the mild steel electrode in various test solutions is shown in Fig. 3, while the electrochemical data obtained from the polarization curves are presented in Table 2.
Figure 3 shows that both anodic and cathodic current densities decreased in the presence of the investigated compound. This decrease is more pronounced with the growth in inhibitor concentration. This observation demonstrates that the inhibitor is adsorbed on the mild steel surface. Consequently, this adsorption reduces both anodic dissolution of iron at anodic sites and cathodic evolution of hydrogen at cathodic sites. As the inhibitor concentration rises, the extent of adsorption increases, leading to increased inhibition efficiency.
Comparing the samples without and with inhibitor, it is obvious that when NDA is used as inhibitor, the Ecorr values for mild steel in 1 M HCl shifts slightly toward more positive direction from that for the uninhibited sample (Fig. 3, Table 2) indicating the mixed mode of corrosion with predominately anodic effect.
Compared with the blank experiment, both the cathodic and anodic Tafel slope changed slightly which implied that the cathodic hydrogen evolution and anodic metal dissolved reaction had been compressed. The inhibition efficiencies calculated from potentiodynamic polarization showed the same trend as those obtained from EIS (Tables 1, 2).
3.3 Weight Loss Study
3.3.1 Effect of Inhibitor Concentration
Table 3 shows the results obtained from weight loss measurements for mild steel in 1 M HCl solutions in the absence and presence of different concentrations of NDA. It has been observed from the results that the IE (%) of NDA increases from 7 to 92% with the increase in inhibitor concentration from 10−6 to 10−3 M. Indeed, corrosion rate values of mild steel decreases from 0.56 to 0.04 mg cm−2 h−1 on the addition of 10−6–10−3 M of NDA. The results confirm that NDA is an efficient corrosion inhibitor, which gives efficiency values as high as 92% in room temperature. The inhibiting performance exhibited by the compound may be due to the presence of protonated form of nitrogen atoms of the compound which makes it adsorb quickly on the mild steel surface, thus forming an insoluble stable film on the surface of the mild steel. It is clear that NDA showed good inhibition for mild steel corrosion in 1 M HCl solution because the inhibitor molecule is made of planar aromatic and also contains N atoms and π-electrons (Table 3).
3.3.2 Adsorption Isotherm
The extent of adsorption of an inhibitor on the metal surface is usually influenced by the parameters such as the nature, chemical structure, distribution of charge on the molecule and charge on the metal. Basic information regarding the nature of interaction of the adsorbed inhibitor molecule and the mild steel surface can be elucidated using adsorption isotherm [36, 37]. The surface coverage θ can be obtained by using the well-known formula E%/100. The value of θ increased with the increase in inhibitor concentration, demonstrating the more pronounced adsorption of inhibitor on the metal surface. The θ value was fitted to various isotherms like Langmuir, Freundlich, Florry–Huggins and El-awady. Langmuir was found to give the best description on the adsorption of inhibitor. The equation corresponding to Langmuir adsorption isotherm is [38]:
where θ is the surface coverage degree, Cinh is the inhibitor concentration in the electrolyte, and Kads is the equilibrium constant of the adsorption process. A plot of Cinh versus Cinh/θ gave a straight line with a slope around unity (Fig. 4). This suggests monolayer adsorption of inhibitor on the surface of mild steel.
ΔGads calculated from Langmuir adsorption isotherm using the following equation:
where R is the universal gas constant, T the thermodynamic temperature, and the value of 55.5 is the concentration of water in the solution.
In Fig. 4, the intercept on the vertical axis is the value of 1/Kads, which is 3.20956 × 10−5 M. Generally, values of ΔGads are up to − 20 kJ mol−1, the types of adsorption were regarded as physisorption, the inhibition acts due to the electrostatic interactions between the charged molecules and the charged metal, while values around − 40 kJ mol−1 or smaller are associated with chemisorption as a result of sharing or transfer of electrons from organic molecules to the metal surface to form a coordinate type of bond (chemisorption) [39]. Then, according to Eq. (12), we calculated the ΔGads° = − 36, 78 kJ mol−1. Therefore, it can be concluded that the adsorption of the NDA on the mild steel surface is mainly the chemical adsorption inevitably accompanied by the physical adsorption [5].
3.3.3 Influence of Temperature
The loss in the weight of the steel samples in 1(M) HCl in the absence and presence of various concentrations of NDA at different temperatures was determined. The effect of temperature on the inhibition efficiency of the NDA is shown in Table 4. In all cases, an increase in NDA concentration leads to a decrease in the corrosion rate of samples indicating that the presence of NDA retards the general corrosion of samples in 1 M HCl. On the other hand, an increase in temperature from 308 to 348 K resulted in an increase in the corrosion rate for all the concentration of NDA, probably as a result of desorption of inhibitor molecules from the metal surface.
Thermodynamic parameters are important to understand the inhibition mechanism. The thermodynamic functions for dissolution of mild steel without and with the addition of optimum concentration of NDA at various temperatures were calculated from the logarithm of corrosion rate (w) of metal in acidic HCl solution by using the Arrhenius equation:
where w is the corrosion rate, Ea is the apparent activation energy, and A is the pre-exponential factor. The Arrhenius plots of Ln W versus 1/T for the blank and optimum concentration of NDA give a straight line and a slope equal to—Ea/R shown in Fig. 5, from which the values of Ea for the inhibited corrosion reaction of mild steel have been calculated and are recorded in Table 5.
In 1 M HCl solution, the addition of NDA leads to an increase in the apparent activation energy to value greater than that of the uninhibited solution, suggesting that the energy barrier of the corrosion reaction increases, meaning that the dissolution of the steel is difficult [40].
In order to calculate activation parameters for the corrosion process, an alternative formulation of Arrhenius (14) was used:
where W is the corrosion rate, h is the Plank’s constant, N is the Avogadro’s number, ∆Ha is the enthalpy of activation , and ∆Sa is the entropy of activation. Figure 6 shows a representative plot for the transition state in 1 M HCl solutions without and with 10−3 M of NDA. Activation parameters obtained from these graphs are given in Table 5.
The thermodynamic parameters (∆Ha and ∆Sa) of the dissolution reaction of steel in 1 M HCl in the presence of NDA are higher than that in the absence of inhibitor (blank). The positive signs of the enthalpies ∆Ha reflect the endothermic nature of the steel dissolution process and mean that the dissolution of steel is difficult [41]. In the presence of NDA, the increase in ∆Sa reveals that an increase in disordering takes place on going from reactants to the activated complex [42].
3.4 Quantum Chemical Studies
Figure 7 represents the optimized geometry, HOMO and LUMO orbitals of the inhibitor molecule, while the computed quantum chemical parameters are given in Table 6. It is well known that HOMO is often associated with the electron-donating ability of a molecule, whereas LUMO indicates its ability to accept electrons [41]. Reportedly, excellent corrosion inhibitors are usually those organic compounds which not only offer electrons to unoccupied orbital of the metal, but also accept free electrons from the metal [41]. Figure 7 shows that the HOMO location is mainly distributed on the NDA molecule and LUMO location is distributed on the two-ring benzene. This indicates that electrons are transferred and accepted from the entire molecule of NDA.
The value of highest occupied molecular orbital EHOMO indicates the tendency of the molecule to donate electrons to acceptor molecule with empty and low-energy orbital. Therefore, the energy of the lowest unoccupied molecular orbital ELUMO indicates the tendency of the molecule to accept electrons [28]. The energy gap ∆E is an important parameter which is related to reactivity of the inhibitor molecule toward the metal surface. In the present study, the energy gap value reflects the higher inhibition efficiency of NDA because the lower ΔE causes the improvement on the reactivity of the molecule, which facilitates adsorption [43, 44]. The number of transferred electrons (∆N) means also electron-donating ability, which gives us information of the ability of the inhibitor to donate electrons (if ∆N > 1), and the positive value of this parameter is related to the ability of tested compound to donate electron to metal surface [4, 45].
3.5 MD Simulations
The MD simulations were carried out to better understand the interaction between the NDA and Fe (110) surface. The simulations were performed in the system containing 491 water molecules, 9Cl−, 9H3O+ and one molecule of the NDA. After 2000 ps, the system reaches the equilibrium which explains that both the temperature and energy reach a balance [46]. By inspection of Fig. 8, it could be observed that the NDA adsorbs nearly to Fe (110) surface, where chemical interactions can possibly occur through reactive sites in the molecule as interpreted in experimental and theoretical study. In this case, the inhibitor molecule protects the steel surface from the aggressive medium by adsorbing on the Fe (110) surface in the possible nearby planar form, reducing the MS dissolution [47, 48]. The interactions and binding energies are calculated using Eqs. (14) and (15) [48]:
where \({E_{\text{total}}}\) is the total energy of the entire system \({E_{\text{surface + solution}}}\) referred to the total energy of Fe (110) surface and solution without the inhibitor and \({E_{\text{inhibitor}}}\) represents the total energy of inhibitor. The corresponding interaction energy value is − 265.9 kJ mol−1 indicating that NDA can more tightly adsorb on iron surface and achieve a better corrosion inhibition effectiveness [5, 49].
4 Conclusion
All the measurements showed that the NDA has excellent inhibition properties against the mild steel corrosion in hydrochloric acid solution. Inhibition efficiency of this inhibitor decreases with the increase in temperature, and further it leads to an increase in activation energy. The inhibitor follows the Langmuir adsorption isotherm in the process of adsorption. EIS measurements also indicate that the inhibitor performance increases due to the adsorption of molecule on the metal surface. Potentiodynamic polarization measurements showed that the inhibitor acts as mixed type of inhibitor. The density distributions of the frontier molecular orbitals (HOMO, highest occupied molecular orbital and LUMO, lowest unoccupied molecular orbital) indicate that the studied inhibitor adsorbs through the active centers N and O atoms and π electrons. MD simulations study showed that the NDA adsorbed on the Fe (110) surface in a near flat orientation and the value of interaction energy of the equilibrium configuration support the experimental inhibition efficiency of the compound. The inhibition efficiencies determined by EIS, potentiodynamic polarization and weight loss studies are in good agreement.
References
Perumal S, Muthumanickam S, Elangovan A et al (2017) Bauhinia tomentosa leaves extract as green corrosion inhibitor for mild steel in 1 M HCl medium. J Bio Tribo Corros 3:13. https://doi.org/10.1007/s40735-017-0072-5
Fouda AE-AS, Etaiw SH, Hammouda M (2017) Corrosion inhibition of aluminum in 1 M H2SO4 by Tecoma non-aqueous extract. J Bio Tribo Corros 3:29. https://doi.org/10.1007/s40735-017-0090-3
Fouda AS, Mohamed FS, El-Sherbeni MW (2016) Corrosion inhibition of aluminum–silicon alloy in hydrochloric acid solutions using carbamidic thioanhydride derivatives. J Bio Tribo Corros 2:11. https://doi.org/10.1007/s40735-016-0039-y
Messali M, Lgaz H, Dassanayake R et al (2017) Guar gum as efficient non-toxic inhibitor of carbon steel corrosion in phosphoric acid medium: electrochemical, surface, DFT and MD simulations studies. J Mol Struct 1145:43–54. https://doi.org/10.1016/j.molstruc.2017.05.081
Lgaz H, Salghi R, Subrahmanya Bhat K et al (2017) Correlated experimental and theoretical study on inhibition behavior of novel quinoline derivatives for the corrosion of mild steel in hydrochloric acid solution. J Mol Liq 244:154–168. https://doi.org/10.1016/j.molliq.2017.08.121
Musa AY, Kadhum AAH, Mohamad AB et al (2010) Electrochemical and quantum chemical calculations on 4, 4-dimethyloxazolidine-2-thione as inhibitor for mild steel corrosion in hydrochloric acid. J Mol Struct 969:233–237
Özcan M, Karadağ F, Dehri I (2008) Investigation of adsorption characteristics of methionine at mild steel/sulfuric acid interface: an experimental and theoretical study. Colloids Surf Physicochem Eng Asp 316:55–61
Fouda AS, Megahed HE, Fouad N, Elbahrawi NM (2016) Corrosion inhibition of carbon steel in 1 M hydrochloric acid solution by aqueous extract of Thevetia peruviana. J Bio Tribo Corros 2:16. https://doi.org/10.1007/s40735-016-0046-z
Tezeghdenti M, Dhouibi L, Etteyeb N (2015) Corrosion inhibition of carbon steel in 1 M sulphuric acid solution by extract of Eucalyptus globulus leaves cultivated in Tunisia arid zones. J Bio Tribo Corros 1:16. https://doi.org/10.1007/s40735-015-0016-x
Paul S, Koley I (2016) Corrosion inhibition of carbon steel in acidic environment by papaya seed as green inhibitor. J Bio Tribo Corros 2:6. https://doi.org/10.1007/s40735-016-0035-2
Elgahawi H, Gobara M, Baraka A, Elthalabawy W (2017) Eco-friendly corrosion inhibition of AA2024 in 3.5% NaCl using the extract of Linum usitatissimum seeds. J Bio Tribo Corros 3:55. https://doi.org/10.1007/s40735-017-0116-x
Jafari H, Akbarzade K (2017) Effect of concentration and temperature on carbon steel corrosion inhibition. J Bio Tribo Corros 3:8. https://doi.org/10.1007/s40735-016-0067-7
Kandemirli F, Sagdinc S (2007) Theoretical study of corrosion inhibition of amides and thiosemicarbazones. Corros Sci 49:2118–2130
Xie S-W, Liu Z, Han G-C et al (2015) Molecular dynamics simulation of inhibition mechanism of 3,5-dibromo salicylaldehyde Schiff’s base. Comput Theor Chem 1063:50–62. https://doi.org/10.1016/j.comptc.2015.04.003
Zhang Z, Tian N, Zhang L, Wu L (2015) Inhibition of the corrosion of carbon steel in HCl solution by methionine and its derivatives. Corros Sci 98:438–449. https://doi.org/10.1016/j.corsci.2015.05.048
El Aoufir Y, El Bakri Y, Lgaz H et al (2017) Understanding the adsorption of benzimidazole derivative as corrosion inhibitor for carbon steel in 1 M HCl: experimental and theoretical studies. J Mater Environ Sci 8:3290–3302
Azzaoui K, Mejdoubi E, Jodeh S et al (2017) Eco friendly green inhibitor Gum Arabic (GA) for the corrosion control of mild steel in hydrochloric acid medium. Corros Sci 129:70–81
ASTM Committee G-1 on Corrosion of Metals (2011) Standard practice for preparing, cleaning, and evaluating corrosion test specimens. ASTM International, West Conshohocken
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Frisch M, Trucks G, Schlegel H et al (2009) 01. Gaussian, Inc, Wallingford
Gholami M, Danaee I, Maddahy MH, RashvandAvei M (2013) Correlated ab initio and electroanalytical study on inhibition behavior of 2-mercaptobenzothiazole and its thiole–thione tautomerism effect for the corrosion of steel (API 5L X52) in sulphuric acid solution. Ind Eng Chem Res 52:14875–14889
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Sastri V, Perumareddi J (1997) Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 53:617–622
Martinez S (2003) Inhibitory mechanism of mimosa tannin using molecular modeling and substitutional adsorption isotherms. Mater Chem Phys 77:97–102
Cao Z, Tang Y, Cang H et al (2014) Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: theoretical studies. Corros Sci 83:292–298
Kokalj A (2012) On the HSAB based estimate of charge transfer between adsorbates and metal surfaces. Chem Phys 393:1–12
Saha SK, Murmu M, Murmu NC, Banerjee P (2016) Evaluating electronic structure of quinazolinone and pyrimidinone molecules for its corrosion inhibition effectiveness on target specific mild steel in the acidic medium: a combined DFT and MD simulation study. J Mol Liq 224:629–638
Bunte SW, Sun H (2000) Molecular modeling of energetic materials: the parameterization and validation of nitrate esters in the COMPASS force field. J Phys Chem B 104:2477–2489
Kaya S, Tüzün B, Kaya C, Obot IB (2016) Determination of corrosion inhibition effects of amino acids: quantum chemical and molecular dynamic simulation study. J Taiwan Inst Chem Eng 58:528–535. https://doi.org/10.1016/j.jtice.2015.06.009
Khaled K, El-Maghraby A (2014) Experimental, Monte Carlo and molecular dynamics simulations to investigate corrosion inhibition of mild steel in hydrochloric acid solutions. Arab J Chem 7:319–326
Verma C, Quraishi MA, Singh A (2015) 2-Amino-5-nitro-4,6-diarylcyclohex-1-ene-1,3,3-tricarbonitriles as new and effective corrosion inhibitors for mild steel in 1 M HCl: experimental and theoretical studies. J Mol Liq 212:804–812. https://doi.org/10.1016/j.molliq.2015.10.026
Yadav M, Kumar S, Sinha RR et al (2015) New pyrimidine derivatives as efficient organic inhibitors on mild steel corrosion in acidic medium: electrochemical, SEM, EDX, AFM and DFT studies. J Mol Liq 211:135–145. https://doi.org/10.1016/j.molliq.2015.06.063
Yadav M, Gope L, Kumari N, Yadav P (2016) Corrosion inhibition performance of pyranopyrazole derivatives for mild steel in HCl solution: gravimetric, electrochemical and DFT studies. J Mol Liq 216:78–86. https://doi.org/10.1016/j.molliq.2015.12.106
Verma C, Ebenso EE, Bahadur I et al (2015) 5-(Phenylthio)-3H-pyrrole-4-carbonitriles as effective corrosion inhibitors for mild steel in 1 M HCl: experimental and theoretical investigation. J Mol Liq 212:209–218. https://doi.org/10.1016/j.molliq.2015.09.009
El-Rehim SA, Ibrahim MA, Khaled K (1999) 4-Aminoantipyrine as an inhibitor of mild steel corrosion in HCl solution. J Appl Electrochem 29:593–599
Mu G, Zhao T, Liu M, Gu T (1996) Effect of metallic cations on corrosion inhibition of an anionic surfactant for mild steel. Corrosion 52:853–856
Salghi R, Jodeh S, Ebenso EE et al (2017) Inhibition of C-steel corrosion by green tea extract in hydrochloric solution. Int J Electrochem Sci 12:3283–3295. https://doi.org/10.20964/2017.04.46
Singh P, Srivastava V, Quraishi MA (2016) Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: electrochemical, SEM, AFM, and XPS studies. J Mol Liq 216:164–173. https://doi.org/10.1016/j.molliq.2015.12.086
Gomma GK, Wahdan MH (1995) Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater Chem Phys 39:209–213
Zhang F, Tang Y, Cao Z et al (2012) Performance and theoretical study on corrosion inhibition of 2-(4-pyridyl)-benzimidazole for mild steel in hydrochloric acid. Corros Sci 61:1–9
Zhao P, Liang Q, Li Y (2005) Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/l HCl. Appl Surf Sci 252:1596–1607
Lgaz H, Subrahmanya Bhat K, Salghi R et al (2017) Insights into corrosion inhibition behavior of three chalcone derivatives for mild steel in hydrochloric acid solution. J Mol Liq 238:71–83. https://doi.org/10.1016/j.molliq.2017.04.124
Kumar R, Chahal S, Kumar S et al (2017) Corrosion inhibition performance of chromone-3-acrylic acid derivatives for low alloy steel with theoretical modeling and experimental aspects. J Mol Liq 243:439–450. https://doi.org/10.1016/j.molliq.2017.08.048
Lgaz H, Salghi R, Jodeh S, Hammouti B (2017) Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J Mol Liq 225:271–280. https://doi.org/10.1016/j.molliq.2016.11.039
Fouda A, Elewady G, Shalabi K, El-Aziz HA (2015) Alcamines as corrosion inhibitors for reinforced steel and their effect on cement based materials and mortar performance. RSC Adv 5:36957–36968
Oguzie E, Chidiebere M, Oguzie K et al (2014) Biomass extracts for materials protection: corrosion inhibition of mild steel in acidic media by Terminalia chebula extracts. Chem Eng Commun 201:790–803
Zhang Z, Tian NC, Huang XD et al (2016) Synergistic inhibition of carbon steel corrosion in 0.5 M HCl solution by indigo carmine and some cationic organic compounds: experimental and theoretical studies. RSC Adv 6:22250–22268. https://doi.org/10.1039/C5RA25359D
El Aoufir Y, Sebhaoui J, Lgaz H et al (2017) Corrosion inhibition of carbon steel in 1 M HCl by 1,5-benzodiazepine derivative: experimental and molecular modeling studies. J Mater Environ Sci 8:2161–2173
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Titi, A., Mechbal, N., El Guerraf, A. et al. Experimental and Theoretical Studies on Inhibition of Carbon Steel Corrosion by 1,5-Diaminonaphthalene. J Bio Tribo Corros 4, 22 (2018). https://doi.org/10.1007/s40735-018-0140-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0140-5