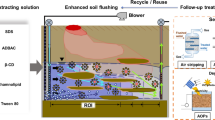
Abstract
Purpose of Review
This review is focused on the removal of chlorinated hydrocarbons (as representative of persistent pollutants) from soil by soil-washing techniques, paying special attention to the application of electrochemically assisted technologies for the treatment of the liquids and gases produced during this treatment. It considers the degree of maturity of the technologies and suggests challenges for future research.
Recent Findings
Electrochemical technologies can help to improve the overall efficiency of soil washing processes in the removal of chlorinated hydrocarbons, contributing to the depletion of these hazardous species from the soil washing liquid and gaseous effluents generated during the treatment of the soil.
Summary
Chlorinated hydrocarbons are a good example of persistent organic pollutants which can be found in very high concentrations in polluted soil, especially in industrial sites. Because of its fast action, soil washing can be efficient for preventing the spread of chlorinated hydrocarbons after accidental spills. Recent progress about fundamentals of this process and key parameters involved is discussed at the light of competing technologies, paying special attention to the liquid and gaseous wastes produced during this treatment, in the search of holistic approaches. Among the different alternatives proposed, electrochemical technologies are the focus of attention of many researchers and, because of that, recent progress in electrochemical technologies capable to deplete the pollutants is also discussed, within a comparison context with other competing technologies, indicating the technology readiness level of each electrochemical process and the challenges that must be overcome in order to reach full-scale applicability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is a very complex element, which is continuously interacting with the two other important components of the natural environment: water and air. Because of this interaction, when soil becomes polluted, contaminants may be propagated fastly, affecting ecosystems and human health. Among the large variety of compounds that can be found in soils as pollutants, it is worth to mention chlorinated hydrocarbons (CHCs), chemicals associated to important industrial products such as pesticides, solvents, thermal fluids, metal degreaser, and raw matters of the chemical industry [1]. Because of their anthropogenic nature and negative properties, such as high volatility and strong recalcitrance to degradation, the potentiality of damage that they can cause in the environment is extremely high. Their hazardousness is frequently related with the presence of chlorine atoms which favors the production of many chlorinated intermediates even more dangerous than the parental compound.
Recently, the development of treatment technologies related to the remediation of soils has gained lots of attention because society has started to be conscious of the importance of solving the problem of pollution into soils as soon as it is generated, preventing the spread of the pollutants to avoid low efficient remediations [2, 3•]. The success of technologies developed in the last century does not only depend on the technology itself but also on the origin and physical properties of the pollutants (such as water solubility and sorption characteristics) and the structure and soil composition.
Soil treatment technologies have been classified according to the location of the soil during the treatment in (1) in situ techniques that are applied directly in the soil placement where the pollution is originated without implying excavation and (2) ex situ techniques that require the excavation of the polluted soils to be treated in the same place (on-site) or another different place (off-site).
In situ treatments usually involve the movement of air or water (frequently including additives) throughout the polluted soil, which is favored by more permeable media. For soils with low permeability, these technologies are also useful, but applications of electric fields are required to mobilize pollutants. The application of these in situ technologies is recommended for long-term treatments, although some technologies can also be used to prevent the spread of the pollutants.
Ex situ treatments generally offer greater scope for managing conditions such as temperature, humidity, and stirring speed in order to optimize the treatment efficiency and to control fastly the potential spreading of pollutants when accidental spills of hazardous compounds happen. Normally, these processes treat less volume of soils with higher concentration, which normally reduces the overall costs concerning a soil in which pollution has been spread for a long time.
Generally, soil treatments can be classified according to the type of technology as thermal, biological, and physicochemical, although in many cases, not a single but a synergetic combination of the process may offer the most effective remediation strategy for a given case [4••]. Several examples of thermal remediation techniques were applied with heavy metals and organic compounds, but nowadays, these techniques have been applied to increase the volatilization rate of the volatile and semi-volatile target pollutants, promoting their extraction throughout wells, for later treatment of the gaseous polluted streams [5].
Regarding the biological process, most of them are focused on the degradation of organic pollutants under controlled conditions using different microbial communities adapted to each particular type of pollutant (such as petroleum, oil sludge, and CHCs) [6,7,8]. The long treatment times motivated for significant removals, motivate the necessity of coupling these technologies with other processes such as desorption extraction [9], adsorption onto activated carbon [10], or electrochemical processes [11].
Numerous studies were carried out with persistent organic pollutants (POPs) such as polycyclic aromatic hydrocarbons (PAH) [12] and pesticides such as lindane or 2,4, dichlorophenoxyacetic acid (2,4-D) [13•], although it is important to consider that the combination with other technologies obtained generally more efficient results than the application of the biological treatments alone [14••, 15].
However, biological treatments are not always successful. There is a large variety of potential anthropogenic pollutants, and it is not always possible to find microorganisms adapted to their degradation cost-effectively [16•]. In this line, physicochemical treatments can be applied to remove pollutants dragged with liquid or gaseous effluents, to transform the pollutants into less hazardous compounds, or to concentrate or isolate them for further treatment.
Thus, applications of in situ electrochemical techniques, such as electrokinetic remediation (EKR) alone [17] or combined with permeable reactive barriers (PRB), have been shown to behave as good alternatives. These technologies require less modification of the environment and less transport; however, the efficiency was sometimes limited, and long-term treatment is required [18, 19••, 20]. Soil properties, such as particle size, stratification, humidity, pH, and organic content, determine the viability of different soil remediation technologies, so a previous analysis would help to apply successful techniques [21]. Anyhow, they are not normally suitable for rapid contention of pollutants after an accidental spill.
In this context, to control main parameters of soil remediation and apply faster treatments to remove pollutants such as heavy metals and CHCs preventing their spread, ex situ, soil washing (SW) emerged as a technology capable of improving the removal of contaminants into soil, especially when accidental acute pollution happens, employing chemical-physical extraction and separation to remove or transfer pollutants to a liquid stream [22]. The study of this technology, their possible improvements, and couplings with other techniques is treated in the next section of this work.
Soil-Washing Remediation Techniques
The use of an extracting solution to recover soil pollutants could be materialized with an in situ process, known as soil flushing (SF), that consists of the movement of the groundwater and/or an aqueous solution with suitable chemicals throughout the soil to drag pollutants up to extraction wells where they are pumped out for further treatment and an ex situ process, known as soil washing (SW), that involves the excavation of the affected land before the treatment in an external extraction tank where the pollutants are transferred from the soil to a liquid solution that later should be further treated.
For the very first moments after acute discharges, ex situ treatments are rather preferred because of the shorter treatment time and the capability to easily combine with other technologies. SW employs chemical-physical extraction and separation process to remove or transfer organic and inorganic contaminants from the soil into a liquid stream, and it operates at a certain solid/liquid ratio frequently between 5 and 40% [23]. Then, after sedimentation, it is required the filtration of effluents to separate from the solid fraction a liquid stream to be further treated. Nowadays, within a context of increasing concern about the total sustainability of processes, it has also to be considered the gaseous fraction that is generated in these processes due to the potential presence of volatile and semi-volatile compounds in soils which are barely mentioned in other related reviews. Related with gaseous treatments, previous studies carried out mainly consider the emission of volatile or semi-volatile pollutants contained in soils, and it reported technologies as thermal desorption, [5], composting [24], advanced oxidation processes, and electrochemical technologies [25, 26], but there is very short information about the generation of gaseous pollutants during the treatment of polluted soil. In these systems, the capture of gaseous effluents must be carried out during the mixing with the extracting solution, so three phases of a very complex product have to be taken into account to understand completely the process in the design of a full remediation treatment (Fig. 1).
The ex situ SW process enhances the contact between the extracting agents and soil pollutants as the mixture can be energetically stirred. However, the transference of pollutants to a liquid solution sometimes requires the use of additives.
Hence, these reagents can reduce the time necessary to treat a polluted site, as compared to the use of water alone, while reducing the necessity of water, leading to more sustainable processes. Nevertheless, the formulation used must be of low ecotoxicity for the soil and, also, of high biodegradability [27].
Surfactants are the most important extractant agents used to improve the solubility of organic pollutants. They consist of amphiphilic molecules, composed of two main components, the hydrophobic tail group and the hydrophilic head group, and are characterized by their chemical structure, hydrophilic-lipophilic balance, and critical micellar concentration (CMC).
Synthetic surfactants are continuously being developed, and their selection is an important feature to reach the final success of SW technology. Among the different types of surfactants available in the market, it is worth to highlight anionics (such as sodium dodecyl sulfate (SDS) or sodium dodecyl benzenesulfonate (SDBS)), cationics (such as quaternary ammonium derivatives), amphoterics, and nonionics (such as Tween 80 or Triton 100) [28]. For ionic surfactants, a high concentration is required to overpower the electrostatic repulsion among ionic head groups, through micellization [29]. Additionally, it had developed new products with higher biodegradability and less affected by the precipitation or sorption onto the soil. Among these compounds, it is worth to mention the biosurfactants, which are amphiphilic compounds able to form micelles and have higher extraction efficiency. As examples, saponin or alkyl polyglycoside was used to enhance the remediation of soil polluted with o-dichlorobenzene (o-DCB) and p-dichlorobenzene (p-DCB) [30], or surfactin and rhamnolipid were applied to remove high quantities of crude oil accidentally spilled in soil [31]. The main advantages of biosurfactants include higher biodegradability, ecological safety, lower toxicity, and the possibility to be produced in situ [4, 32].
Other less common alternatives to be used as extracting agents are the humic acids, which have amphiphilic properties, and their carboxylic groups may bind with several hazardous metals after their deprotonation [33, 34], or the use of organic acids (such as oxalic, citric, and tartaric acids) coupled with and EDTA derivatives which have been proposed for the remediation of vanadium-contaminated soils [35].
Regarding the further treatment of the different SW solutions produced with extracting agents, Trellu et al. [36] summarized in a detailed review the wide variety of extracting agents (synthetic surfactants, biosurfactants, cyclodextrins, organic cosolvents, vegetable oils, and other acidic compounds or polymers), and then, the different options of advanced oxidation process (AOPs) suitable for the treatment of SW wastes generated, including the electrochemical ones.
Recently, the successful treatment evaluations carried out in lab-scale studies have encouraged to scale-up the technology, to develop in a pilot-scale system, and then, even go further in a commercial scale to treat a real portion of soil polluted. In real sample treatment, a pretreatment to remove large objects from the soil is required to obtain a homogeneous soil ready for the washing step. Removal is done through scalping, mechanical screening, jigging, and tabling. The oversized materials may be from construction wastes to large pieces of rock or gravel. These materials are usually not contaminated; however, if treatment is necessary, the size of these pieces must be reduced. Then, different soil-washing systems are designed considering the different remediation conditions as the presence of metallic pieces, larger solid masses, or sands. The most common SW process applied to remove persistent organic matter requires the use of hydrocyclons after control of the size of particles to ensure good mixing. In this step, it is proposed to collect the possible gaseous currents emitted from the SW systems to be further treated [37] which would help to attain a more sustainable process.
The size of industrial extractors for soil polluted depends on the needs of a specific remediation site. As a rule of thumb, the space requirement needed for a typical plant will range from approximately 100 × 200 to 125 × 250 ft for a plant that can process 25 or 50 t h−1, respectively [38]. This area contains the soil and contaminated piles and the equipment for the washing plant. Some treatment facilities that are installed in Glasgow (Scotland) [39] have a maximum capacity of 100 t h−1, and it also can recover the aggregates, sand, clay, and top soil to provide this material back into the construction/agriculture sector. An option to separate the washing solution (liquid phase) from the solid fraction might be the use of a screw decanter centrifuge. This device rotates at high speed (2000–6000 rpm) helping the solids settle further down by centrifugal force, and the solid fastly accumulates onto the inner surface of the tube. Then, an endless screw pushes the solid accumulated, which is moved, dewatered, and discharged by the bottom part. The washing fluid is converted into clarified liquid and discharged from the outlet on the side plate. The liquid obtained can be recovered to reuse in further SW processes, and the solid aggregates, depending on their size, can be used in road construction, building foundations, pavement sand, and as pipe bedding.
Recent Progress in the Ex Situ Soil Washing Treatment
The relevance of SW technologies for the treatment of soil contaminated with organic pollutants has enhanced the publication of relevant works in the last 5 years. It highlighted the use of anionic surfactants as sodium dodecyl sulfate (SDS), not only because of their lower sorption into the soil but also because of their low cost and higher extraction efficiency. In this line, Dos Santos et al. studied the removal of petroleum from low conductivity soils using SDS in a concentration ranged from 500 to 5000 mg L−1 with an efficiency higher than 92% [40••]. Removal of several refractory pesticides has been also evaluated, including pendimethalin [41•] or oxyfluorfen [42] aiming to produce SWWs with high organic load that enhances the performance of a further treatment to completely remove these contaminants. To enhance the soil washing process, other authors have proposed the use of different structures to remove organic compounds. Kim et al. [43] applied an ex situ SW process in soil contaminated with large quantities of petroleum using novel core-crosslinked amphiphilic polymer nanoparticles that have low sorption onto the soil particles as compared with the conventional non-ionic surfactants as Triton X-100 and Brij 30, obtaining the highest efficiency (96%) with these new nanoaggregates. To remove soil polluted with PAH such as acenaphthene (ACE), phenanthrene (PHE), fluoranthene (FLA), or pyrene (PYR), it was coupled the extraction effect of a non-ionic surfactant as Tween 80 with a CD as hydroxypropyl-beta-cyclodextrin showing extraction efficiency higher than 80% [44]. However, high loads of soil polluted with diesel were extracted using a different concentration of a single non-ionic surfactant (Tween 80) obtaining an efficiency close to 90%, which confirms the strong influence of physical properties of hazardous hydrocarbons in the viability of the process [45••]. In recent years, these surfactants have been applied in the treatment of heavy metal-polluted soils because of their potential risk to human health due to their detection in abandoned industrial soils. Eco-friendly washing agents are very in demand for practical applications of SW to remediate these contaminated sites. As an example, there are different artificial chelating agents, such as EDTA, which can bind multiple heavy metals to form soluble and stable complexes and generate large molecules with the pollutants that are easily further removed.
Recently, the combined utilization of multiple SW reagents has become a clear objective to improve the process. However, sometimes, it required different sequential extraction with alternative washing reagents that increase the total costs. Thereby, new formulations with the capacity of treating high concentrations of heavy metals and a reduction of toxicity and mobility were required. As an example, it mentioned the removal of Cu, Ni, and Zn from an industrial soil that was recovered with a low concentration of EDTA and three organic acids (citric, oxalic, and tartaric acid) with an efficiency higher than 80% [46].
Also, a new and interesting concept called “technology readiness level” (TRL) firstly applied by NASA in 1974 has appeared to evaluate the level of maturity that a technology has reached. Thus, during the last quarter of the last century, the TRL applied to soil washing technology for the remediation of soils polluted with hazardous organic compounds increased from very low values (3–4) to almost levels 7–9, with all elements of the required value chain in full operation for the simpler technologies and, even, companies that are selling key-on-hand solutions to real problems. However, there is still a lot of work to be done regarding the optimization in the combination of processes and the effect of the formulation of the washing fluids, with many studies whose TRL that still need to be largely improved [47•] to reduce the big gap in their development and scale-up which has to be considered in future research.
Ex Situ Soil Washing Treatment for the Removal of CHCs
Many technologies have been developed to remediate polluted soils with CHCs using SW techniques, being differentiated in terms of the different strategies faced and/or the combination with other processes. Thus, the identification of the type of surfactants and the most useful concentration according to the target pollutants has been studied. Laha et al. [48] described the selection of the appropriate surfactant concentration with different CHCs and the influence of surfactant sorption onto soils that appears with the increasing surfactant concentration until the onset of micellization. It was provided a discussion of equilibrium partitioning theory to account for the distribution of CHCs between soil, aqueous phase, sorbed surfactant, and micellar surfactant phases. In this line, more studies were reported using different surfactants, types of soils, and pollutants. Zhang et al. [49] applied a coupled process with non-ionic surfactant (Triton X-100) and powdered activated carbon (PAC) to remove chlorine pesticides such as chlordene, chlordane, and mirex, with the aim to extract the maximum quantity from polluted soil and later retain in the PAC for a further treatment.
Among typical surfactants used, anionic surfactants, such as sodium dodecyl sulfate (SDS), or non-ionic, such as Tween 80 and Triton X-100, are less likely to be adsorbed onto the soil, and initially, they were used in works to confirm the efficiency to extract the pollutants from soils [50••]. CHCs such as lindane [51], clopyralid [52], and trichloroethylene [53] have been removed from low permeability soils with good results in a concentration that ranged from 1 to 100 mg kg−1 soil. However, later, it was necessary to enhance the treatment to remove contaminants from the soil washing wastes (SWW) generated. The aim of these studies was not only the study of the remediation of soils but also the study of properties of the resulting SWWs, such as their regeneration capacity to create an environmentally friendly process, the degree of sorption onto soil, and the evaluation of biological parameters of washing solutions as biodegradability, enzyme activity, or toxicity to avoid the use of large amount of surfactants that can cause possible harms to the soil ecosystem.
Thus, many technologies are still being developed to extract pollutants in a cost-efficient way. In this line, the coupling of SW with other processes and technologies seems to be the most valuable alternative to face the problem of spiked soils, not only with CHCs compounds but also with other non-chlorinated hazardous hydrocarbons and other toxic inorganic compounds such as heavy metals. Therefore, new perspectives of the ex situ treatment of spiked soils would be focused on the following:
-
1)
The improvements of extracting agents applied to be more environmentally friendly for soils, to increase their final recovery and
-
2)
To determine an efficient coupling with Electrochemical Advanced Oxidation Processes (EAOPs) technologies to remove the more refractory and hazardous compounds.
The alternatives could be biological processes which would be focused on the reduction of their final toxicity and the removal of a biodegradable fraction or with processes that consist of a separation step that enhance a previous recovery of the extracting agent and further complete removal of pollutants, but these processes should be applied as a coupled treatment. Table 1 summarizes most relevant works published in the last 5years and, with informative purposes, the approximate TRL of these works.
Treatment of Liquid Soil-Washing Wastes by Improved Degradation Processes
Oxidation technologies are very important for the removal of CHCs from liquid wastes. Several recent reviews show that these technologies produce outstanding results [54••, 55], although the operating conditions must be carefully evaluated to obtain high efficiencies and to avoid the formation of byproducts that can be even more dangerous than the original pesticides, from a viewpoint of potential toxicity, mutagenicity, and carcinogenicity.
Technologies that are related with the generation of hydroxyl radicals are called advanced oxidation processes (AOPs), and they can be classified depending on the mechanism that promotes the formation of these radicals. Thus, technologies based on the production of hydrogen peroxide [56••] or other species have attracted considerable attention due to their simplicity, high efficiency, and easy application. As an example, Fenton process was used to treat high load of non-aqueous liquids produced in the treatment of soils polluted with lindane and other CHCs using novel formulations of surfactants as E-mulse 3 (a mixture of non-ionic surfactants and citrus terpenes) [57]. To increase efficiency, instead of using AOPs as a single treatment, it is preferred to combine them with other processes. In this line, the light irradiation coupled with the addition of a photocatalyst increases the efficiency in the production of hydroxyl radicals, and many works have reviewed the application of this process for the treatment of hazardous organic compounds in effluents [58]. Titanium dioxide was the most used semiconductor photocatalyst, due to its good properties as cost-effectiveness, inert nature, photostability, and the efficiency of this process, which mainly depends on the adsorption capacity of target pollutants onto the photocatalyst because their oxidation is promoted by hydroxyl radicals formed on the surface of the photocatalyst. Thus, higher doses of surfactants may generate the production of micelles that cannot react or adhere at the surface of catalysts, pointing out a paramount influence on the process of the concentration and type of extracting agent used [59].
To treat SW effluents, technologies that use Fenton reaction have been widely reviewed. This process consists of the catalytic decomposition of H2O2 in acidic media to generate the hydroxyl radicals using ferrous salts. It can be enhanced by its combination with heterogeneous photocatalysis [60], dehalogenation processes [61, 62], or irradiation using sono- [63] and photoenergies [64, 65]. These approaches can be used to achieve complete or almost complete removal of organics in the treatment of polluted solutions.
Some of the drawbacks of chemical Fenton processes, in which the H2O2 and the iron salts are added externally, arise from the cost, storage, transport, and environmental impact of decentralized H2O2 production, as well as the use of high amounts of iron salts with further sludge formation [66••].
Electrochemical Advanced Oxidation Processes (EAOPs)
In last decades, electrochemical advanced oxidation processes have emerged as a new class of AOPs to treat polluted effluents [55, 67]. The main feature of those processes is the use of the electron as an efficient, versatile, cost-effective, and clean reagent. However, today the market share of EAOPs for the treatment of polluted effluents on an industrial scale is relatively small, and most of their applications remain on a bench/pilot plant scale [68•]. Some of the main drawbacks that prevent the widespread application of those technologies are the electrode lifetime, the costs associated to energy supply [69•], or the reaction rate limited by the heterogeneous nature of charge transference processes.
The simplest and, probably, most popular EAOP is anodic oxidation (AO). In the 1970s, studies demonstrated that the reactions occurring during AO could be used for the degradation of organic pollutants in wastewater [70, 71]. During an AO treatment, pollutants can be removed by two different mechanisms, direct and mediated oxidation explained in many research works [68•]. Innovations over the last decades lead to the discovery of more efficient and stable coatings, such as boron-doped diamond [72] and sub-stoichiometric titanium oxide [73], which paved the way for the development of robust AO reactors for wastewater treatment [74, 75, 76•]. Particularly, for the treatment of SWW, the high carbon content (extracting agent, target pollutant, and soil organic matter) leads to a competition for the oxidation of pollutants and extracting agents, and the behavior and efficiencies depend on the nature of all these organics, pollutants, and extracting agents used.
To remove persistent hydrocarbons from SW effluents using EAOPs, the coupling of electrooxidation was carried out with alternative processes to overcome one of the significant drawbacks of this technology, which are related to the mass transfer limitations of pollutants to the electrode surface where most of the generated hydroxyls are concentrated (they have a low lifetime to be transported to the bulk). In this line, the coupling with ultrasounds or UV-light irradiation, trying to promote the production of large amounts of oxidants and free radicals in the bulk, favors the removal of organic pollutants by mediated oxidation mechanisms and reduces the competition effect of soil organic matter and target pollutants [77, 78•].
Synthetic SWWs polluted with pendimethalin were treated, using sono- and photoelectrolysis processes and SDS as a surfactant, obtaining removal percentages higher than 75% [41•]. Likewise, effluents polluted with phenanthrene were studied using different coupled processes as the addition of persulfate to the photoelectrolysis using active electrodes as mixed metal oxide anodes [79] or also with the addition of an easily biodegradable complex as Fe (III) -EDDS (ethylenediamine-N-N-disuccinic acid) using simulated solar light irradiation [80]. In all studies evaluated, the aim was the development of coupled processes to improve the EAOPs and to reduce the reagents and energy consumption.
CHCs are another specific group of hydrocarbons that should be considered in the development of efficient EAOPs technologies to remove these refractory compounds. BDD electrooxidation is a well-known technology that promotes suitable reactive conditions to remove these compounds and also their intermediates, but it is necessary for the transport of CHCs to a liquid phase. In the case of an extended herbicide as atrazine that has polluted many groundwaters, it was studied that their removal from a SW fluid using a single electrooxidation process with different electrode material as BDD, mixed metal oxide with Ir and Ru and carbon felt to confirm the full removal only using BDD [81] 2,4-dichlorophenoxyacetic acid was removed from aqueous solutions using a hybrid process based on the combination of electrooxidation and ozone for the integration of hydroxyl and sulfate radicals. 2,4-D was removed in 90 min, and 68.9% of total organic matter was removed within 3 h [82]. Table 2 summarizes some of the most relevant treatments carried out in SWW polluted with CHCs using EAOPs technologies.
Treatment of Gaseous Emissions from Soil-Washing Wastes
To end up, it should be considered the development of novel technologies related with the efficient treatments of gaseous compounds generated during soil treatments. In this context, recent studies of VOCs using advanced oxidation process coupled with other technologies have reached relevant results.
Processes such as thermal oxidation and photocatalytic oxidation (PCO) are promising technologies after VOCs are captured because the pollutants can be oxidized to H2O and CO2. However, thermally catalytic oxidation requires temperatures higher than 200 °C and a relatively high concentration of pollutants for an efficient operation, and, hence, it is not always economically feasible when combined with soil washing [83]. Thus, integrated removal processes have been proposed which combine the mediated electrochemical oxidation (MEO) and an absorption column into an electrochemically assisted scrubbing process [84••]. In these processes, the pollutants are oxidized through the mediation of some electrochemically generated redox reagents (Eqs. 1 and 2), which act as mediators for electron transfer between the electrode and organics [85]. These mediators can be metallic redox couples, such as Ag(II/I), Ce(IV/III), Co(III/II), Fe(III/II), and Mn(III/II), or strong oxidizing chemicals, such as active chlorine species, ozone, hydrogen peroxide, persulfate, percarbonate, and perphosphate. The optimum temperature and pH depend on the metallic redox pair used (M(n+)/M(n)). Additionally, the role of efficient TiO2 or BDD electrodes and electrolytes is key to promote the production of these mediators from precursors.
Thus, Muthuraman et al. studied the removal of some gaseous pollutants as carbon tetrafluoride [86•] and nitrous oxide [87], both very recalcitrant greenhouse gases, using wet scrubbing methods with an in situ electrogenerated Co and Ni mediators in a highly alkaline medium.
Lately, novel studies applied coupled technologies with EAOPs also to treat VOCs. In this line, Chen et al. [88] removed gaseous pollutants, such as ethyl acetate or toluene, using a novel continuous system integrating UV-assisted photo-electrochemical catalysis with a microbial fuel cell. In the cathode, it was used as a metallic catalyst of CeO2 and TiO2 loaded on the activated carbon fiber felt substrate, obtaining high elimination capacities for both pollutants.
All these previous studies were related to the direct treatment of gases, but it is worth to take in mind that very few studies about gaseous compounds emitted during SW processes were carried out. Most of them aimed to quantify the gaseous emissions, but they are not focused on the capture and treatment of these pollutants. These processes are characterized by a low TRL, commonly in level 2 or 3 with manuscripts related to the technology formulation or experimental proofs of concepts. Chao et al. [89] reported in 2006 the high influence of parameters as soil organic matter, water solubility, and surfactant concentration in the volatilization of VOCs with low solubility after applying SW technologies.
Then, because of their particularity, chlorinated VOCs have been considered as a specific group of toxic gaseous compounds that are removed using catalytic oxidation with temperatures ranged from 200 to 500 °C. In these processes, it is more important, the complete oxidation pathways without generating byproducts than the oxidation efficiency of the target compound. In a very recent document, Lin et al. [90•] explained some critical aspects related to the formation routes of chlorinated byproducts in gaseous treatments to achieve their complete destruction including some strategies focused on the inhibition of chlorinated intermediates and the improvements of further oxidation to final products. Regarding the treatment of specific gaseous compounds produced from CHCs, some recent works removed perchloroethylene in gaseous streams to evaluate the capacity of different electro-absorbers to remove it obtaining a surprising reactivity with different reaction pathways depending of the system and experimental conditions applied [91•, 92]. Likewise, carbon tetrachloride and trichloroethylene [93, 94••] were removed from a synthetic gas current using in situ electrogenerated Co mediators from an electrode of Co(OH)2 in a divided cell using an electrolyzer. Other efficient redox pairs are Ce(IV/III) that were applied to remove CHCs such as chlordane, Ambush, and 2,4 D with an efficiency higher than 85% [95, 96]. Some examples with the main parameters of the removal of chlorinated VOCs are summarized in Table 3, but the authors have not found any real studies of these compounds with high TRL produced during the SW treatments yet. Thus, there is still slot for the improvement to be carried out in terms of the capture and treatment of VOCs generated during treatment processes using electrooxidation. Therefore, future works related with a whole treatment of polluted soil have to be focused on developing a cost-efficient technology to combine the recovery and removal of all gaseous currents generated in SW processes and the treatment of solid and liquid effluents generated.
Conclusions
This review identifies the key aspects carried out in the soil treatments using ex situ techniques coupled with electrochemical processes and remarks a very important treatment technology for the remediation of soils, especially when this treatment is faced immediately after an acute discharge of pollutants occurs because it may help to prevent diffusion of the pollutants. A suitable formulation of the soil-washing fluid has a paramount influence to obtain an efficient transfer of the pollutants from the soil to the selected washing fluid, which can be further treated by different efficient technologies including very efficient electrochemically assisted processes. This type of process can also be used for the treatment of the gases emitted during the soil washing and the treatment of the liquid SWFs, being especially important the use of homogenous catalyst salts to promote the efficiency in the removal of the volatile and semi-volatile pollutants. In this work, it has reviewed the most recent and important works in all these topics, allowing to shed light on the future perspectives of application of all these technologies.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mrema EJ, Colosio C, Rubino FM. Pesticide residues: organochlorines. Encycl Food Saf. 2014:23–30. https://doi.org/10.1016/B978-0-12-378612-8.00238-9.
Song P, Huang G, An C, Xin X, Zhang P, Chen X, et al. Exploring the decentralized treatment of sulfamethoxazole-contained poultry wastewater through vertical-flow multi-soil-layering systems in rural communities. Water Res. 2021;188:116480. https://doi.org/10.1016/j.watres.2020.116480.
• Carboneras Contreras MB, Villaseñor Camacho J, Fernández-Morales FJ, Cañizares PC, Rodrigo Rodrigo MA. Biodegradability improvement and toxicity reduction of soil washing effluents polluted with atrazine by means of electrochemical pre-treatment: influence of the anode material. J Environ Manag. 2020;255:109895. https://doi.org/10.1016/j.jenvman.2019.109895This article explains the influence of anode material in the removal pretreatment of atrazine from soil washing effluents using biological technologies.
•• Gan S, Lau EV, Ng HK. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater. 2009;172:532–49. https://doi.org/10.1016/j.jhazmat.2009.07.118This review evaluates the different methodologies to remove PAH from soil polluted considering biological, chemical, physico-chemical and thermal processes.
Vidonish JE, Zygourakis K, Masiello CA, Sabadell G, Alvarez PJJ. Thermal treatment of hydrocarbon-impacted soils: a review of technology innovation for sustainable remediation. Engineering. 2016;2(4):426–37. https://doi.org/10.1016/j.eng.2016.04.005.
Marin JA, Hernandez T, Garcia C. Bioremediation of oil refinery sludge by landfarming in semiarid conditions: influence on soil microbial activity. Environ Res. 2005;98(2):185–95. https://doi.org/10.1016/j.envres.2004.06.005.
Benyahia F, Abdulkarim M, Zekri A, Chaalal O, Hasanain H. Bioremediation of crude oil contaminated soils: a black art or an engineering challenge? Process Saf Environ Prot. 2005;83(4):364–70. https://doi.org/10.1205/psep.04388.
Trindade PVO, Sobral LG, Rizzo ACL, Leite SGF, Soriano AU. Bioremediation of a weathered and a recently oil-contaminated soils from Brazil: a comparison study. Chemosphere. 2005;58(4):515–22. https://doi.org/10.1016/j.chemosphere.2004.09.021.
Posada-Baquero R, Martín ML, Ortega-Calvo J-J. Implementing standardized desorption extraction into bioavailability-oriented bioremediation of PAH-polluted soils. Sci Total Environ. 2019;696:134011. https://doi.org/10.1016/j.scitotenv.2019.134011.
Igun OT, Meynet P, Davenport RJ, Werner D. Impacts of activated carbon amendments, added from the start or after five months, on the microbiology and outcomes of crude oil bioremediation in soil. Int Biodeterior Biodegradation. 2019;142:1–10. https://doi.org/10.1016/j.ibiod.2019.04.008.
Barba S, Carvela M, Villaseñor J, Rodrigo MA, Cañizares P. Improvement of the electro-bioremediation process of a non-polar herbicide-polluted soil by means of surfactant addition. Sci Total Environ. 2019;650:1961–8. https://doi.org/10.1016/j.scitotenv.2018.09.338.
Bianco F, Race M, Papirio S, Esposito G. Removal of polycyclic aromatic hydrocarbons during anaerobic biostimulation of marine sediments. Sci Total Environ. 2020;709:136141. https://doi.org/10.1016/j.scitotenv.2019.136141.
• Yang Z, Xu X, Dai M, Wang L, Shi X, Guo R. Combination of bioaugmentation and biostimulation for remediation of paddy soil contaminated with 2,4-dichlorophenoxyacetic acid. J Hazard Mater. 2018;353:490–5. https://doi.org/10.1016/j.jhazmat.2018.04.052This study showed a desorption extraction method to assess the bioavalilability of PAHS that are in contaminanted soils.
•• Müller JB, Ramos DT, Larose C, Fernandes M, HSC L, Vogel TM, et al. Combined iron and sulfate reduction biostimulation as a novel approach to enhance BTEX and PAH source-zone biodegradation in biodiesel blend-contaminated groundwater. J Hazard Mater. 2017;326:229–36. https://doi.org/10.1016/j.jhazmat.2016.12.005This article analyzed the potential of biodiesel because of the combined biostimulation of bacterias to enhance BTEX and PAH biodegradation mainly using polluted groundwater.
Raimondo EE, Saez JM, Aparicio JD, Fuentes MS, Benimeli CS. Coupling of bioaugmentation and biostimulation to improve lindane removal from different soil types. Chemosphere. 2020;238:124512. https://doi.org/10.1016/j.chemosphere.2019.124512.
• Kim S-S, Kim J-H, Han S-J. Application of the electrokinetic-Fenton process for the remediation of kaolinite contaminated with phenanthrene. J Hazard Mater. 2005;118(1):121–31. https://doi.org/10.1016/j.jhazmat.2004.10.005This article describes an in situ method as electrokinetic-Fenton to remediate a soil polluted with phenanthrene.
Reddy KR, Chinthamreddy S. Electrokinetic remediation of heavy metal-contaminated soils under reducing environments. Waste Manag. 1999;19(4):269–82. https://doi.org/10.1016/S0956-053X(99)00085-9.
Risco C, Rubí-Juárez H, Rodrigo S, López Vizcaíno R, Saez C, Cañizares P, et al. Removal of oxyfluorfen from spiked soils using electrokinetic fences. Sep Purif Technol. 2016;167(Supplement C):55–62. https://doi.org/10.1016/j.seppur.2016.04.050.
•• López-Vizcaíno R, Risco C, Isidro J, Rodrigo S, Saez C, Cañizares P, et al. Scale-up of the electrokinetic fence technology for the removal of pesticides. Part I: some notes about the transport of inorganic species. Chemosphere. 2017;166:540–8. https://doi.org/10.1016/j.chemosphere.2016.09.113This article evaluated the use of different surfactants improving the treatment of PAH contaminated soils.
Virkutyte J, Sillanpää M, Latostenmaa P. Electrokinetic soil remediation — critical overview. Sci Total Environ. 2002;289(1–3):97–121. https://doi.org/10.1016/S0048-9697(01)01027-0.
López-Vizcaíno R, Sáez C, Cañizares P, Rodrigo MA. The use of a combined process of surfactant-aided soil washing and coagulation for PAH-contaminated soils treatment. Sep Purif Technol. 2012;88(Supplement C):46–51. https://doi.org/10.1016/j.seppur.2011.11.038.
Yang K, Zhu L, Xing B. Enhanced soil washing of phenanthrene by mixed solutions of TX100 and SDBS. Environ Sci Technol. 2006;40(13):4274–80. https://doi.org/10.1021/es060122c.
Mousset E, Oturan M, van Hullebusch E, Guibaud G, Esposito G. Soil washing/flushing treatments of organic pollutants enhanced by cyclodextrins and integrated treatments: state of the art. 2014.
Tradler SB, Mayr S, Himmelsbach M, Priewasser R, Baumgartner W, Stadler AT. Hydrothermal carbonization as an all-inclusive process for food-waste conversion. Bioresour Technol Rep. 2018;2:77–83. https://doi.org/10.1016/j.biteb.2018.04.009.
Rodrigo MA, Oturan N, Oturan MA. Electrochemically assisted remediation of pesticides in soils and water: a review. Chem Rev. 2014;114(17):8720–45. https://doi.org/10.1021/cr500077e.
Chang J-H, Cheng S-F. The remediation performance of a specific electrokinetics integrated with zero-valent metals for perchloroethylene contaminated soils. J Hazard Mater. 2006;131(1):153–62. https://doi.org/10.1016/j.jhazmat.2005.09.026.
Mulligan CN, Yong RN, Gibbs BF. Surfactant-enhanced remediation of contaminated soil: a review. Eng Geol. 2001;60(1):371–80. https://doi.org/10.1016/S0013-7952(00)00117-4.
López-Vizcaíno R, Sáez C, Cañizares P, Navarro V, Rodrigo M. Influence of the type of surfactant on the mobility of flushing fluids for electro-remediation processes. Sep Sci Technol. 2011;46:2148–56. https://doi.org/10.1080/01496395.2011.594477.
Amirianshoja T, Junin R, Kamal Idris A, Rahmani O. A comparative study of surfactant adsorption by clay minerals. J Pet Sci Eng. 2013;101:21–7. https://doi.org/10.1016/j.petrol.2012.10.002.
Pei G, Zhu Y, Cai X, Shi W, Li H. Surfactant flushing remediation of o-dichlorobenzene and p-dichlorobenzene contaminated soil. Chemosphere. 2017;185:1112–21. https://doi.org/10.1016/j.chemosphere.2017.07.098.
Fanaei F, Moussavi G, Shekoohiyan S. Enhanced treatment of the oil-contaminated soil using biosurfactant-assisted washing operation combined with H2O2-stimulated biotreatment of the effluent. J Environ Manag. 2020;271:110941. https://doi.org/10.1016/j.jenvman.2020.110941.
Lv Y, Sun J, Yu G, Wang W, Song Z, Zhao X, et al. Hydrophobic design of adsorbent for VOC removal in humid environment and quick regeneration by microwave. Microporous Mesoporous Mater. 2020;294:109869. https://doi.org/10.1016/j.micromeso.2019.109869.
Meng F, Yuan G, Wei J, Bi D, Ok YS, Wang H. Humic substances as a washing agent for Cd-contaminated soils. Chemosphere. 2017;181:461–7. https://doi.org/10.1016/j.chemosphere.2017.04.127.
Yang T, Hodson ME. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil. Sci Total Environ. 2019;647:290–300. https://doi.org/10.1016/j.scitotenv.2018.07.457.
Jiang J, Yang M, Gao Y, Wang J, Li D, Li T. Removal of toxic metals from vanadium-contaminated soils using a washing method: reagent selection and parameter optimization. Chemosphere. 2017;180:295–301. https://doi.org/10.1016/j.chemosphere.2017.03.116.
Trellu C, Mousset E, Pechaud Y, Huguenot D, van Hullebusch ED, Esposito G, et al. Removal of hydrophobic organic pollutants from soil washing/flushing solutions: a critical review. J Hazard Mater. 2016;306:149–74. https://doi.org/10.1016/j.jhazmat.2015.12.008.
Contaminated land: applications in real environments (CL:AIRE. Understanding Soil Washing: TB13; September 2007.
Hari D. Sharma KRR. Geoenvironmental engineering: site remediation, waste containment, and emerging waste management technologies. Wiley; 2004.
Soil Treatment Systems Ltd. https://www.soiltreatmentsystems.co.uk/soil-washing/ Accessed 19/08/2020.
•• dos Santos EV, Sáez C, Cañizares P, da Silva DR, Martínez-Huitle CA, Rodrigo MA. Treatment of ex-situ soil-washing fluids polluted with petroleum by anodic oxidation, photolysis, sonolysis and combined approaches. Chem Eng J. 2017;310(Part 2):581–8. https://doi.org/10.1016/j.cej.2016.05.015This article describes the removal of a non-polar compound from surfactant-aided soil washing effluents.
• Almazán-Sánchez PT, Cotillas S, Sáez C, Solache-Ríos MJ, Martínez-Miranda V, Cañizares P, et al. Removal of pendimethalin from soil washing effluents using electrolytic and electro-irradiated technologies based on diamond anodes. Appl Catal B: Environ. 2017;(213):190–7. https://doi.org/10.1016/j.apcatb.2017.05.008The article explains the way of using anodic oxidation and other advance oxidation processes to remove soil polluted with petroleum.
Dos Santos E, Saez C, Canizares P, Martinez-Huitle CA, Rodrigo MA. Treating soil-washing fluids polluted with oxyfluorfen by sono-electrolysis with diamond anodes. Ultrason Sonochem. 2017;34:115–22. https://doi.org/10.1016/j.ultsonch.2016.05.029.
Kim N, Kwon K, Park J, Kim J, Choi J-W. Ex situ soil washing of highly contaminated silt loam soil using core-crosslinked amphiphilic polymer nanoparticles. Chemosphere. 2019;224:212–9. https://doi.org/10.1016/j.chemosphere.2019.02.144.
Mousset E, Huguenot D, van Hullebusch ED, Oturan N, Guibaud G, Esposito G, et al. Impact of electrochemical treatment of soil washing solution on PAH degradation efficiency and soil respirometry. Environ Pollut. 2016;211:354–62. https://doi.org/10.1016/j.envpol.2016.01.021.
•• Liu F, Oturan N, Zhang H, Oturan MA. Soil washing in combination with electrochemical advanced oxidation for the remediation of synthetic soil heavily contaminated with diesel. Chemosphere. 2020;249:126176. https://doi.org/10.1016/j.chemosphere.2020.126176This article described the efficiencies of soil washing with high quantities of diesel coupling processes to remove the pollution.
Cheng S, Lin Q, Wang Y, Luo H, Huang Z, Fu H, et al. The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids. Arab J Chem. 2020;13(4):5160–70. https://doi.org/10.1016/j.arabjc.2020.02.015.
• Lacasa E, Cotillas S, Saez C, Lobato J, Canizares P, Rodrigo MA. Environmental applications of electrochemical technology. What is needed to enable full-scale applications? Curr Opinion Electrochem. 2019;16:149–56. https://doi.org/10.1016/j.coelec.2019.07.002This article evaluated the key parameters required to develop full scale applications.
Laha S, Tansel B, Ussawarujikulchai A. Surfactant–soil interactions during surfactant-amended remediation of contaminated soils by hydrophobic organic compounds: a review. J Environ Manag. 2009;90(1):95–100. https://doi.org/10.1016/j.jenvman.2008.08.006.
Zhang S, He Y, Wu L, Wan J, Ye M, Long T, et al. Remediation of organochlorine pesticide-contaminated soils by surfactant-enhanced washing combined with activated carbon selective adsorption. Pedosphere. 2019;29(3):400–8. https://doi.org/10.1016/S1002-0160(17)60328-X.
•• Trellu C, Ganzenko O, Papirio S, Pechaud Y, Oturan N, Huguenot D, et al. Combination of anodic oxidation and biological treatment for the removal of phenanthrene and Tween 80 from soil washing solution. Chem Eng J. 2016;306:588–96. https://doi.org/10.1016/j.cej.2016.07.108This review described coupled processes of biological and electrochemical method to remove a soil washing solution.
Muñoz-Morales M, Braojos M, Sáez C, Cañizares P, Rodrigo MA. Remediation of soils polluted with lindane using surfactant-aided soil washing and electrochemical oxidation. J Hazard Mater. 2017;339:232–8. https://doi.org/10.1016/j.jhazmat.2017.06.021.
Martín de Vidales MJ, Castro MP, Sáez C, Cañizares P, Rodrigo MA. Radiation-assisted electrochemical processes in semi-pilot scale for the removal of clopyralid from soil washing wastes. Sep Purif Technol. 2019;208:100–9. https://doi.org/10.1016/j.seppur.2018.04.074.
Tian H, Liang Y, Zhu T, Zeng X, Sun Y. Surfactant-enhanced PEG-4000-NZVI for remediating trichloroethylene-contaminated soil. Chemosphere. 2018;195:585–93. https://doi.org/10.1016/j.chemosphere.2017.12.070.
•• Oturan MA, Aaron J-J. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit Rev Environ Sci Technol. 2014;44(23):2577–641. https://doi.org/10.1080/10643389.2013.829765This review explained the main studies and their applications in wastewater treatment carried out until 2014.
Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M. Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res. 2014;21(14):8336–67. https://doi.org/10.1007/s11356-014-2783-1.
•• Brillas E, Boye B, Sirés I, Garrido JA, Rodriguez RM, Arias C, et al. Electrochemical destruction of chlorophenoxy herbicides by anodic oxidation and electro-Fenton using a boron-doped diamond electrode. Electrochim Acta. 2004;49(25):4487–96. https://doi.org/10.1016/j.electacta.2004.05.006This article showed the synergies of coupling the anodic oxidation with electro-Fenton to remove very refractory herbicides.
Dominguez CM, Romero A, Santos A. Selective removal of chlorinated organic compounds from lindane wastes by combination of nonionic surfactant soil flushing and Fenton oxidation. Chem Eng J. 2019;376:120009. https://doi.org/10.1016/j.cej.2018.09.170.
Liu JW, Han R, Wang HT, Zhao Y, Chu Z, Wu HY. Photoassisted degradation of pentachlorophenol in a simulated soil washing system containing nonionic surfactant Triton X-100 with La–B codoped TiO2 under visible and solar light irradiation. Appl Catal B Environ. 2011;103(3):470–8. https://doi.org/10.1016/j.apcatb.2011.02.013.
Fabbri D, Prevot AB, Pramauro E. Effect of surfactant microstructures on photocatalytic degradation of phenol and chlorophenols. Appl Catal B Environ. 2006;62(1):21–7. https://doi.org/10.1016/j.apcatb.2005.06.011.
Jho EH, Singhal N, Turner S. Fenton degradation of tetrachloroethene and hexachloroethane in Fe(II) catalyzed systems. J Hazard Mater. 2010;184(1):234–40. https://doi.org/10.1016/j.jhazmat.2010.08.027.
Minella M, Bertinetti S, Hanna K, Minero C, Vione D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Environ Res. 2019;179:108750. https://doi.org/10.1016/j.envres.2019.108750.
Minella M, Sappa E, Hanna K, Barsotti F, Maurino V, Minero C, et al. Considerable Fenton and photo-Fenton reactivity of passivated zero-valent iron. RSC Adv. 2016;6(89):86752–61. https://doi.org/10.1039/C6RA17515E.
Panda D, Manickam S. Heterogeneous Sono-Fenton treatment of decabromodiphenyl ether (BDE-209): debromination mechanism and transformation pathways. Sep Purif Technol. 2019;209:914–20. https://doi.org/10.1016/j.seppur.2018.06.069.
Liu X, Zhou Y, Zhang J, Luo L, Yang Y, Huang H, et al. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: mechanism study and research gaps. Chem Eng J. 2018;347:379–97. https://doi.org/10.1016/j.cej.2018.04.142.
Nitoi I, Oncescu T, Oancea P. Mechanism and kinetic study for the degradation of lindane by photo-Fenton process. J Ind Eng Chem. 2013;19(1):305–9. https://doi.org/10.1016/j.jiec.2012.08.016.
•• Moreira FCRB, Brillas E, Vilar VJP. Electrochemical advanced oxidation processes: a review on their application to synthetic and real wastewaters. Appl Catal B: Environ. 2017;202:217–61 This review presented the treatment of various synthetic and real wastewaters by five key EAOPs, i.e., anodic oxidation (AO), anodic oxidation with electrogenerated H2O2 (AO-H2O2), electro-Fenton (EF), photoelectro-Fenton (PEF) and solar photoelectro-Fenton (SPEF).
Chen G. Electrochemical technologies in wastewater treatment. Sep Purif Technol. 2004;38(1):11–41. https://doi.org/10.1016/j.seppur.2003.10.006.
• Panizza M, Cerisola G. Direct and mediated anodic oxidation of organic pollutants. Chem Rev. 2009:109. https://doi.org/10.1021/cr9001319This review describes the differences between active and non-active electrodes and describes the different oxidants produced in the electrochemical treatments.
• Cañizares P, Paz R, Sáez C, Rodrigo MA. Costs of the electrochemical oxidation of wastewaters: a comparison with ozonation and Fenton oxidation processes. J Environ Manag. 2009;90(1):410–20. https://doi.org/10.1016/j.jenvman.2007.10.010This article presented an estimation of treatment costs of wastewater using electrochemical technologies and advance oxidation processes.
Poon CP, Brueckner TG. Physicochemical treatment of wastewater-seawater mixture by electrolysis. J Water Pollut Control Fed. 1975;47(1):66–78.
Weinberg NL, Brown EA. The anodic oxidation of organic compounds. I. The electrochemical methoxylation of 2,6-dimethoxypyridine and N-methylpyrrole. J Organic Chem. 1966;31(12):4054–8. https://doi.org/10.1021/jo01350a040.
Cañizares P, Lobato J, Paz R, Rodrigo MA, Sáez C. Electrochemical oxidation of phenolic wastes with boron-doped diamond anodes. Water Res. 2005;39:2687–703. https://doi.org/10.1016/j.watres.2005.04.042.
Ganiyu SO, Oturan N, Raffy S, Cretin M, Esmilaire R, van Hullebusch E, et al. Sub-stoichiometric titanium oxide (Ti4O7) as a suitable ceramic anode for electrooxidation of organic pollutants: a case study of kinetics, mineralization and toxicity assessment of amoxicillin. Water Res. 2016;106:171–82. https://doi.org/10.1016/j.watres.2016.09.056.
Cañizares P, García-Gómez J, Lobato J, Rodrigo MA. Modeling of wastewater electro-oxidation processes part I. General description and application to inactive electrodes. Ind Eng Chem Res. 2004;43:1915–22. https://doi.org/10.1021/ie0341294.
Cañizares P, García-Gómez J, Lobato J, Rodrigo MA. Modeling of wastewater electro-oxidation processes part II. Application to active electrodes. Ind Eng Chem Res. 2004;43:1923–31. https://doi.org/10.1021/ie0341303.
• Cotillas S, Llanos J, Castro-Ríos K, Taborda-Ocampo G, Rodrigo MA, Cañizares P. Synergistic integration of sonochemical and electrochemical disinfection with DSA anodes. Chemosphere. 2016;163:562–8. https://doi.org/10.1016/j.chemosphere.2016.08.034This paper describes the integration of advanced oxidation process in the electrochemical treatment of urban wastewater and their synergistic effects.
Yaqub A, Ajab H. Applications of sonoelectrochemistry in wastewater treatment system. Rev Chem Eng. 2013;29(2):123–30. https://doi.org/10.1515/revce-2012-0017.
• Cotillas S, Lacasa E, Herraiz-Carboné M, Sáez C, Cañizares P, Rodrigo MA. Innovative photoelectrochemical cell for the removal of CHCs from soil washing wastes. Sep Purif Technol. 2020;230:115876. https://doi.org/10.1016/j.seppur.2019.115876This paper shows novel photoelectrochemical cell concept for the treatment of soil washing effluents polluted with chlorinated hydrocarbons (CHCs), using the synergistic effects of anodic oxidation and UV light.
Tao Y, Huang H, Zhang H. Remediation of Cu-phenanthrene co-contaminated soil by soil washing and subsequent photoelectrochemical process in presence of persulfate. J Hazard Mater. 2020;123111:123111. https://doi.org/10.1016/j.jhazmat.2020.123111.
Tao Y, Brigante M, Zhang H, Mailhot G. Phenanthrene degradation using Fe(III)-EDDS photoactivation under simulated solar light: a model for soil washing effluent treatment. Chemosphere. 2019;236:124366. https://doi.org/10.1016/j.chemosphere.2019.124366.
Komtchou S, Dirany A, Drogui P, Robert D, Lafrance P. Removal of atrazine and its by-products from water using electrochemical advanced oxidation processes. Water Res. 2017;125:91–103. https://doi.org/10.1016/j.watres.2017.08.036.
Jaafarzadeh N, Ghanbari F, Zahedi A. Coupling electrooxidation and oxone for degradation of 2,4-dichlorophenoxyacetic acid (2,4-D) from aqueous solutions. J Water Process Eng. 2018;22:203–9. https://doi.org/10.1016/j.jwpe.2018.01.020.
Zang M, Zhao C, Wang Y, Chen S. A review of recent advances in catalytic combustion of VOCs on perovskite-type catalysts. J Saudi Chem Soc. 2019;23(6):645–54. https://doi.org/10.1016/j.jscs.2019.01.004.
•• Muthuraman G, Moon I-S. A review on an electrochemically assisted-scrubbing process for environmental harmful pollutant’s destruction. J Ind Eng Chem. 2012;18(5):1540–50. https://doi.org/10.1016/j.jiec.2012.03.021This review focuses on the development and applications of the MEO-assisted scrubbing processes for the treatment of environmental air pollution paying special attention to metal iron as mediators.
Panizza M, Cerisola G. Removal of colour and COD from wastewater containing acid blue 22 by electrochemical oxidation. J Hazard Mater. 2008;153(1):83–8. https://doi.org/10.1016/j.jhazmat.2007.08.023.
• Muthuraman G, Moon IS. Innovative reductive remediation of carbon tetrafluoride at room temperature by using electrogenerated Co1+. J Hazard Mater. 2017;325:157–62. https://doi.org/10.1016/j.jhazmat.2016.12.002This research manuscript describes the removal of carbon tetrafluoride using a non-combustion electroscrubbing method with an electrogenerated Co1+ mediator in a highly alkaline medium.
Muthuraman G, Ramu AG, McAdam E, Moon IS. Sustainable removal of N2O by mediated electrocatalytic reduction at ambient temperature electro-scrubbing using electrogenerated Ni(I) electron mediator. J Hazard Mater. 2019;378:120765. https://doi.org/10.1016/j.jhazmat.2019.120765.
Chen Q, Liu L, Liu L, Zhang Y. A novel UV-assisted PEC-MFC system with CeO2/TiO2/ACF catalytic cathode for gas phase VOCs treatment. Chemosphere. 2020;255:126930. https://doi.org/10.1016/j.chemosphere.2020.126930.
Chao H-P, Lee J-F, Juang L-C, Kuo C-H, Gurusamy A. Volatile organic compounds emission from contaminated soil during surfactant washing. Environ Eng Sci. 2006;23:923–32. https://doi.org/10.1089/ees.2006.23.923.
• Lin F, Zhang Z, Li N, Yan B, He C, Hao Z, et al. How to achieve complete elimination of Cl-VOCs: a critical review on byproducts formation and inhibition strategies during catalytic oxidation. Chem Eng J. 2021;404:126534. https://doi.org/10.1016/j.cej.2020.126534This review explains the different pathways to determine the byproduct distribution during the removal of chlorinated volatile hydrocarbons using catalytic oxidation.
• Castañeda-Juárez M, Muñoz-Morales M, Souza FL, Sáez C, Cañizares P, Almazán-Sánchez PT, et al. Electro-absorbers: a comparison on their performance with jet-absorbers and absorption columns. Catalysts. 2020;10(6):653 This paper describes the differences in the electroabsortion process using a packed column reactor or a jet-venturi using perchloroethylene as a model compound.
González-Pérez O, Muñoz-Morales M, Souza FL, Saez C, Cañizares P, Rodrigo MA. Jet electro-absorbers for the treatment of gaseous perchloroethylene wastes. Chem Eng J. 2020;125096:125096. https://doi.org/10.1016/j.cej.2020.125096.
Muthuraman G, Moon IS. Sustainable generation of a homogeneous Ni(I) catalyst in the cathodic compartment of a divided flow electrolytic cell for the degradation of gaseous carbon tetrachloride by electroscrubbing. ACS Sustain Chem Eng. 2016;4(3):1364–72. https://doi.org/10.1021/acssuschemeng.5b01383.
•• Muthuraman G, Ramu AG, Moon IS. Gaseous trichloroethylene removal using an electrochemically generated homogeneous low-valent ligand-free Co(I) electrocatalyst by electro-scrubbing. J Hazard Mater. 2016;311:210–7. https://doi.org/10.1016/j.jhazmat.2016.03.011This article is focused on a ligand-free homogeneous electrocatalyst for the degradation of gaseous trichloroethylene in NaOH in a divided electrolytic cell using the pair Co(II)/Co(I).
Varela JA, Oberg SG, Neustedter TM, Nelson N. Non-thermal organic waste destruction: characterization of the CerOx system 4. Environ Prog. 2001;20(4):261–71. https://doi.org/10.1002/ep.670200415.
Nelson N. Electrochemical destruction of organic hazardous wastes. Platin Met Rev. 2002;46(1):18–23.
Funding
Financial support from the Spanish Agencia Estatal de Investigación and European Union through project PID2019-107271RB-I00 (AEI/FEDER, UE) and the Spanish Government (Grant N° FPU16/00067) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Land Pollution
Rights and permissions
About this article
Cite this article
Muñoz-Morales, M., Sáez, C., Cañizares, P. et al. Electrochemically Assisted Soil Washing for the Remediation of Non-polar and Volatile Pollutants. Curr Pollution Rep 7, 180–193 (2021). https://doi.org/10.1007/s40726-021-00179-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40726-021-00179-3