Abstract
Purpose of Review
The potential of nanotechnology and nanocomposites in various sectors of research and application is promising and attracting increasing investment. The purpose of this paper is to provide a review of the preparation of nanomaterials from different cellulosic sources including wood. The transformation of cellulose down to the nanoscale endow these nanomaterials with new properties that give cellulose many new industrial applications in different fields, and an overview of the sound markets that can be impacted by cellulose nanomaterials is provided.
Recent Findings
Unexpected and attractive properties can be observed when decreasing the size of a material down to the nanoscale. Cellulose is no exception to the rule. Cellulose nanomaterials exhibit specific outstanding properties and are potentially useful for a large number of industrial applications. Now, after intensive research, several initiatives have emerged in the perspective of producing cellulose nanomaterials at large scale. A number of organizations have announced cellulose nanomaterial demonstration plants.
Summary
Despite being the most available natural polymer on earth, it is only quite recently that cellulose has gained prominence as a nanostructured material. Different forms of cellulose nanomaterials, resulting from a top-down deconstructing strategy (cellulose nanocrystals-CNCs, cellulose nanofibrils-CNFs) or bottom-up strategy (bacterial cellulose-BC) can be prepared. Multiple mechanical shearing actions applied to cellulosic fibers release more or less individually the nanofibrils. A controlled strong acid hydrolysis treatment can be applied to cellulosic fibers allowing dissolution of amorphous domains. The mechanical modulus of crystalline cellulose is the basis of many potential applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is a linear macromolecule composed of β-l,4-linked D-glucopyranose rings and it is the most abundant polymer on Earth. It is considered one of the most important structural elements in plants and other living species serving to maintain their structure. Each of these living species, from tree to bacteria, produces cellulose day-by-day, e.g., a tree produces about 10 g of cellulose per day and the global annual production of cellulose is estimated at 1.5. × 1012 tons [1••]. It has been used for centuries in highly diverse applications. More recently, the recognition that, by suitable chemical and mechanical treatments, it is possible to produce fibrous materials with one or two dimensions in the nanometer range from any naturally occurring sources of cellulose has been emphasized and opens the door to new applications. The initial concept of the chemical extraction of cellulose nanomaterials through an acid hydrolysis process was pioneered in 1947 [2]. The first report on the mechanical destructuration of cellulose fibers was published latter in 1983 [3, 4].
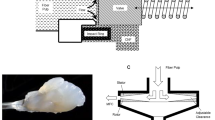
In nature, cellulose is a ubiquitous structural polymer that confers its mechanical properties to higher plant cells. Wood and plants are cellular hierarchical biocomposites designed by nature and they basically consist of semicrystalline cellulose microfibril-reinforced amorphous matrix made of hemicellulose, lignin, waxes, and extractive and trace elements. Lignocellulosic fibers consist therefore of a cemented microfibril aggregate. As a consequence, the structure of plants spans many length scales, in order to provide maximum strength with a minimum of material (Fig. 1). Wood, which is approximately 40–50 wt% cellulose with about half in nanocrystalline form and half in amorphous form, is an example.
Details of the cellulosic fiber structure with emphasis on the cellulose microfibrils (in color). Reproduced with permission from Ref. [5]. Copyright 2012 Elsevier
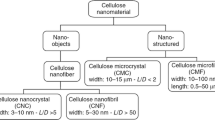
Purification of cellulose from plant fibers involves chemical treatments consisting of alkali extraction and bleaching. Owing to its hierarchical structure and semicrystalline nature, nanoparticles can be extracted from this naturally occurring polymer using a top-down mechanically or chemically induced deconstructing strategy. This review is limited to cellulose nanomaterials obtained through top-down strategies and therefore excludes bacterial cellulose (BC). The potential of cellulosic nanoparticles or nanocellulose has been proved for special functional nanomaterials [6] but it is as a biobased reinforcing nanofiller that they have attracted significant interest over the last 20 years [1••, 7, 8•, 9, 10•].
Preparation of Cellulose Nanomaterials
Purification of Cellulose
The first step consists in the purification of cellulose, i.e., removal of most of non-cellulosic components from the natural fiber. Bleached pulps can be used to skip this matrix removal process. The biomass is generally the first ground to increase the accessibility of the material to further treatments. Dewaxing in a Soxhlet apparatus with a toluene/ethanol or benzene/ethanol mixture is sometimes performed. An alkali extraction (2% sodium hydroxide (NaOH) solution at 80 °C) followed by filtration and washing with water is then carried out to remove the soluble polysaccharides. The washed product is then bleached with a sodium chlorite (NaClO2) solution in a buffer medium under mechanical stirring removing most of the residual phenolic molecules like lignin or polyphenols and proteins. This treatment results in different changes in the properties of the fibers [11] such as the disappearance of the peaks observed by Fourier transform infrared (FTIR) spectroscopy around 1730 and 1250 cm−1 attributed to C=O stretching of acetyl group and uranic ester groups of hemicellulose or ester linkage of carboxylic group ferulic and p-coumaric acids of lignin and/or hemicellulose, and C–O stretching of aryl group in lignin, respectively. It also induces an increase in cellulose content and concomitant decrease of lignin and hemicellulose content, a decrease in the diameter of the fiber due to removal of the cementing materials, an increase in the crystallinity index, and an increase in thermal stability due to the removal of poorly thermally stable hemicelluloses.
Mechanically Induced Deconstructing Strategy
This purified cellulosic material suspended in water can then be submitted to a mechanically induced deconstructing treatment involving strong multiple mechanical shearing actions in order to release more or less individually the nanofibrils. Different equipment such as high-pressure homogenizer, microfluidizer, ultra-fine friction grinder, high-intensity ultrasonifier, aqueous counter collision, ball milling, and twin-screw extruder and refiner can be used. A detailed description of these different equipment can be found elsewhere [1••]. However, these processes are highly energy-consuming and a pretreatment of the cellulosic fiber is generally necessary. Several strategies have been proposed to obtain fibers that are less stiff and cohesive, thus decreasing the energy needed for fibrillation, as detailed in [5]. There are basically three alternatives: (i) limiting the hydrogen bonding in the system, and/or (ii) adding a repulsive charge, and/or (iii) decreasing the degree of polymerization (DP) or the amorphous link between individual fibrils. The most common pretreatments are enzymatic hydrolysis, 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation and carboxymethylation/acetylation. Tightly associated hemicellulose/pectin to cellulose is important to facilitate cell wall disruption during mechanical treatment and for the stability of aqueous suspensions.
The ensuing material is generally called cellulose nanofibrils (CNF), but other terminologies are used such as microfibrillated cellulose (MFC) or nanofibrillated cellulose (NFC). Transmission electron microscopy (TEM) or atomic force microscopy (AFM) are commonly used to investigate the morphology of CNF. Regardless of the source, this material consists of long entangled filaments as shown in Fig. 2a. Both individual nanofibrils and microfibril bundles can be observed. Larger fragments and unfibrillated fibers are sometimes observed. The manufacturing process and source of cellulose influence the particle diameter distribution of CNF that is generally in the range 2–100 nm. The length is more difficult to determine because of entanglements and difficulties in identifying both ends of individual microfibrils. However, methods based on the gel point of the suspension (solid content at which transition from dilute to semi-dilute behavior occurs) [13] or on shear viscosity measurements [14] have been proposed.
Besides direct microscopic observations, other measurements can be carried out to access indirectly the extent of fibrillation. It includes the turbidity of the suspension that becomes more transparent as can be accessed by measuring the UV-visible transmittance through the suspension. During fibrillation, the water retention increases and the suspension changes from a low viscosity to a high viscosity medium and it becomes a gel at low solid content as shown in Fig. 2b. It is attributed to the strong increase in the specific surface area of the fibers upon fibrillation. Due to high shear rates involved during the mechanical isolation process, a decrease in the degree of polymerization of cellulose is generally observed as well as an increase in its crystallinity index because of the partial degradation and removal of amorphous cellulose during mechanical treatment. The films prepared from CNF suspensions also exhibit fibrillation-dependent properties. Their porosity decreases and then their density increases, resulting in increased stiffness but lower tear strength. This dense packing of cellulose nanofibers can also result in optically transparent films. However, any single characterization method cannot be used to describe the properties and behavior of CNF [15]. A multi-criteria method to characterize high-quality CNF suspensions and establish a quality ranking has been proposed [16]. From the raw values of different tests, a final and single grade called the “quality index” can be determined.
In 2013, FPInnovations in Canada launched a 3-year project on cellulose filaments (CFs) with Kruger Biomaterials Inc., a newly created company, to develop new materials from wood fiber and use them in different traditional and non-traditional applications. CF is produced from pulp “by peeling the filaments from wood fibers using a mechanical process that uses no chemical or enzymes” [17]. The kraft pulp fibers are “peeled” (top-down approach) into cellulose filaments that are similar to CNF, but with higher aspect ratio (up to 1000), 30–500 nm wide, and up to 2 mm long. CFs have high flexibility, high surface area, and high bonding potential. Because the “peeling” is done “in a gentle manner,” the original fiber length is preserved, and very thin filaments are obtained. Also, because no chemicals are used, no effluent is generated. Moreover, the process is expected to use only well-known commercial equipment. The leading producers of CF are Kruger, Canada, which can produce 6000 tons per year.
Chemically Induced Deconstructing Strategy
Cellulose is a semicrystalline polymer. A controlled strong acid hydrolysis treatment can be applied to cellulosic fibers allowing dissolution of amorphous domains. During the acid hydrolysis process, the hydronium ions can penetrate the cellulose chains in the amorphous domains promoting the hydrolytic cleavage of the glycosidic bonds and releasing individual crystallites. The ensuing material is generally called cellulose nanocrystals (CNC), but other terminologies are used such as cellulose whiskers, cellulose nanowhiskers, or nanocrystalline cellulose. This material consists of rod-like or needle-like nanoparticles as shown in Fig. 3a, b. Each rod can be considered a cellulosic crystal with no apparent defect and the precise physical dimensions of the nanocrystal depend on several factors, including the source of the cellulose, the exact hydrolysis conditions, and the ionic strength. The length is generally of the order of few hundreds of nanometers and the width is of the order of few nanometers. They generally present a relatively high distribution in length because of the diffusion-controlled nature of the acid hydrolysis process. Their surface chemistry depends on the nature of the acid used for hydrolysis. The hydrolysis treatment of cellulose is generally performed with sulfuric acid resulting in the introduction of sulfate esters at the surface of CCN, leading to improved electrostatic stabilization of the suspension but decreased thermal stability. When observed in polarized light between cross-nicols, the CNC suspensions shows the formation of birefringent domains (Fig. 3c).
a, b TEM from a dilute suspension of CNC from soy hulls. Reproduced with permission from Ref. [18]. Copyright 2016 Elsevier. c Photograph of an aqueous suspension of capim dourado CNC (0.50 wt%) observed between cross-nicols showing the formation of birefringent domains. Reproduced with permission from Ref. [19]. Copyright 2010 Springer Nature
Other processes have been proposed for the preparation of CNC. It includes enzymatic hydrolysis [20], TEMPO oxidation [21], hydrolysis with gaseous acid [22], ionic liquid [23], ammonium persulfate oxidation [24], periodate oxidation [25], ultrasonication-assisted FeCl3-catalyzed hydrolysis [26], subcritical water [27], liquefaction [28], and acid deep eutectic solvents [29].
Main Properties of Cellulose Nanomaterials
When decreasing the size of a material from the microscale as for cellulosic fibers down to the nanoscale as for cellulose nanomaterials, several properties change and are expected to drive new potential applications. The main properties that are impacted by this change of scale are reported below.
Specific Surface Area
When decreasing the size of a material, the total surface area per unit of mass, i.e., the specific surface area, increases. The determination of the specific surface area from gas adsorption isotherm is often incorrect because of the irreversible aggregation of the nanoparticles upon drying. It can be estimated from the average geometrical dimensions of the nanoparticles, assuming a rod-like geometry and a density of 1.5 or 1.6 g·cm−3 for crystalline cellulose. Values of 51 and 533 m2·g−1 were reported for CNF and CNC, respectively, extracted from sisal fibers [30]. A sharp increase is observed when the diameter falls below a value of 20 nm as shown in Fig. 4a, which corresponds to the diameter range of CNC [1••]. This high specific surface area coupled with the low concentration of the suspensions allows the preparation of aerogels which can be used as porous templates, potentially useful in various nano-applications. The aerogel network can be chemically modified to tune its wetting properties towards non-polar liquids/oils. It can also be impregnated with different precursors, which can readily be transformed into functional nanoparticles along the cellulose nanofibers.
Some specific properties of cellulose at the nanoscale. a Evolution of the specific surface area of rod-like nanoparticles as a function of their diameter, assuming a density of 1.5 g.cm−3 for crystalline cellulose [1••]. b Evolution of Young’s modulus of CNC films determined from tensile tests as a function of the aspect ratio of the constituting CNC. Reproduced with permission from Ref. [31]. Copyright 2011 Elsevier. c Tensile modulus values for crystalline native cellulose (cellulose I) at room temperature: experimental (●) and calculated data (○). The x-axis data correspond to different references which detail can be found in [32]. The solid and dashed horizontal lines correspond to the average value for experimental and calculated data, respectively. Reproduced with permission from Ref. [32]. Copyright 2011 Elsevier. d Steady-state viscosity versus shear rate for cellulose nanomaterials prepared from cotton: CNF (■), NaOH-neutralized CNC (×) and TEMPO-oxidized CNC (●) suspensions (0.6 wt%) [33]. e Images of neat PLA film (1) compare with those of bionanocomposites reinforced with 2.5 wt% (2), 7.5 wt% (3) and 15 wt% CNC grafted by n-octadecyl-isocyanate. Reproduced with permission from Ref. [34]. Copyright 2013 Elsevier. f Oxygen transmission rate (OP) as a function of relative humidity for CNF films after varying pressing times (to emphasize the differences between the samples, a close-up of OP below 10 cm3 μm m−2 d−1 kPa−1 is shown in the inset). Reproduced with permission from Ref. [35]. Copyright 2013 American Chemical Society. g Common surface covalent chemical modifications of cellulose nanocrystals. PEG, poly (ethylene glycol); PEO, poly (ethylene oxide); PLA, poly (lactic acid); PAA, poly (acrylic acid); PNiPAAm, poly(N-isopropylacrylamide); PDMAEMA, poly(N,N-dimethylaminoethyl methacrylate). Reproduced with permission from Ref. [5]. Copyright 2012 The Royal Society of Chemistry
Aspect Ratio
An important parameter for cellulosic nanomaterials is the aspect ratio, which is defined as the ratio of the length to the width. It determines the anisotropic phase formation and reinforcing properties. It is indeed well-known that the mechanical percolation approach is highly relevant to describe the mechanical behavior of cellulose nanomaterials-based nanocomposites when prepared by casting/evaporation [1••, 17]. This mechanism suggests the formation of a stiff continuous network of nanoparticles linked through hydrogen bonding, which lead to an unusual and outstanding reinforcing effect. Furthermore, this phenomenon is expected to occur only above a critical volume fraction of filler phase, defined as the percolation threshold, which in turn depends on the aspect ratio of the nanoparticle. In addition, it was shown by tensile tests performed on films prepared by water evaporation of a series of CNC suspensions that their tensile modulus increases when increasing the aspect ratio of the nanoparticles as shown in Fig. 4b [31]. Films can also be prepared by dewatering process to analyze the tensile index and TEA index, to determine the quality of the MFC or CF. However, CNC and CNF cannot be utilized to make film by dewatering process due to the small size of the particles. Difficulties associated with the determination of the length make it challenging to assess its value for CNF. It is considered very high but strongly decreases when increasing the strength of oxidation for TEMPO-pretreated fibers [36]. For CNC, the aspect ratio is easier to determine and it is found to depend on both the source of cellulose and hydrolysis conditions. The aspect ratio varies between 10 for cotton and 67 for tunicin or capim dourado (golden grass) [1••] and can be higher than 100 for soy hulls [17].
Mechanical Properties
The mechanical modulus of cellulose nanomaterials is probably their main asset. Indeed, cellulose is a ubiquitous structural polymer that confers its mechanical properties to higher plant cells. The tensile modulus of single cellulose I (native cellulose) crystal has been estimated both experimentally and theoretically. A broad range of values ranging between 56 and 220 GPa was reported with an average value of 130 GPa as shown in Fig. 4c [32], with a tensile strength around 10 GPa. As expected, the lower crystallinity of CNF results in a lower modulus, which the average value is around 100 GPa [32]. Moreover, crystalline cellulose with a density around 1.5–1.6 g.cm−3 can be considered a lightweight material. The specific tensile modulus, which is the ratio between the tensile modulus and the density, was estimated around 85 and 65 J.g−1 for CNC and CNF, respectively, whereas it is around 25 J.g−1 for steel [37].
Thermal Properties
When cellulose nanomaterial suspensions are dried, a film forms, which thermal expansion coefficient (TEC) is very low because of both its high crystallinity and strength of the cellulose nanomaterial network interactions [38]. This low TEC of cellulose nanoparticles can be used to reduce the TEC of polymer nanocomposite sheets if the strength of the network is strong enough to restrict the thermal expansion of the matrix. However, the low thermal stability of cellulose nanomaterials may limit their use and manufacturing conditions of its nanocomposites at high temperatures. This issue is particularly important for sulfuric acid-hydrolyzed CNC that exhibits a significantly reduced thermal stability compared with the raw starting material or other forms of nanocellulose. It is attributed to the sulfate ester groups that are introduced on the surface of the nanoparticles during the hydrolysis reaction [39].
Rheological Properties
Cellulose nanomaterials exhibit both a high specific surface area and high density of surface hydroxyl groups. These properties influence their interactions with the surrounding medium when suspended in a liquid. After mechanical disintegration, the CNF suspension becomes a gel at low solid content as shown in Fig. 2b, and it exhibits a pseudoplastic, shear-thinning behavior, i.e., it becomes very fluid when stirred at high shear rate. The shear-thinning behavior of cellulose nanomaterial suspensions is illustrated in Fig. 4d [33]. Thixotropic viscosity (time-dependent rheological behavior) properties and high water holding capacity were also observed. The highly elongated nature of CNF in suspension favors irreversible mechanisms such as entanglement and non-repairable rupture of network structures.
Optical Properties
Small particles are considered “invisible” due to dimensions lower than the wavelength of visible light. Cellulose nanomaterials can therefore be considered for preparing transparent coatings/films. Films made only from CNF or CNC can be optically transparent if the cellulose nanofibers are densely packed, and the interstices between the fibers are small enough to avoid light scattering. This type of flexible, transparent, and renewable substrate is usually referred to as nanopaper. Surface light scattering can also be limited thanks to a surface polishing step [40]. Transparent composite films can also be obtained by impregnating the CNF/CNC film with a transparent resin or mixing if the difference in refractive index between both components is small and/or that the domain sizes in the different phases are smaller than the wavelength of visible light as shown in Fig. 4e [34].
Furthermore, at sufficiently high concentrations, CNCs self-align to form a chiral nematic liquid crystalline phase in equilibrium with isotropic phase in aqueous medium [41•]. Slow evaporation of the suspension produces semi-translucent films that retain the self-assembled chiral nematic liquid crystalline order formed in the suspension, and then CNCs can form chiral nematic, iridescent, colored films. The chiral nematic organization of CNCs is always left-handed and thus, chiral nematic CNC films selectively reflect left-handed light, and appear colorful when the helicoidal pitch (P) is on the order of magnitude of the wavelength of visible light. P can be controlled through ionic strength, temperature, concentration, exposure to magnetic field, and US treatment, and it is therefore relatively straightforward to modulate film color. This structure-color phenomenon is similar to iridescence observed in nature, for example, in butterfly wings, seed hull of the Margaritaria nobilis fruit or beetle shells. Mesoporous materials with long-range chiral nematic ordering imparted by CNC can be synthesized in the presence of the material of interest by evaporation-induced self-assembly and removal of cellulose by calcination [42].
Barrier Properties
Because of their small size resulting in high specific surface area, low permeability of cellulose enhanced by high crystallinity and ability to form dense percolating networks, cellulose nanomaterials exhibit increased tortuosity towards diffusing gazes. They can provide improved barrier properties against moisture and oxygen when used as self-standing films, coating for polymers and paper, or reinforcement in polymer composites. Cellulose nanomaterials are generally poor moisture barrier but good oxygen barrier below 70% relative humidity (RH) [35]. The barrier performance can be further improved by optimizing the morphology and surface chemistry of cellulose nanomaterials, or sandwiching them with high moisture-resistant polymers [43].
Functionalized Cellulose Nanomaterials
Cellulose nanomaterials can be used as such or their properties can be tuned using physical or chemical methods. The inherent high reactivity of cellulose and the pervasive surface hydroxyl groups associated with the nanoscale dimensions of cellulose nanomaterials provide the possibility of modification via a chemical reaction strategy and open up opportunities to develop new functional nanomaterials [6]. The objective of these modifications is to make available cellulose nanomaterials that can be used as reinforcing agents in composite materials or can contribute to the specific functions for the development of new nanomaterials, with the target of expanding their applications in the field of functional nanomaterials. A wide variety of chemical modification methods, including coupling hydrophobic small molecules, grafting polymers and oligomers, and adsorbing hydrophobic compounds to surface hydroxyl groups of cellulosic nanoparticles, can be employed (Fig. 4g). Experimental conditions should avoid swelling media and peeling effect of surface-grafted chains inducing their dissolution in the reaction medium. The chemical grafting process has to be mild in order to preserve the integrity of the nanoparticle.
Safety and Ecotoxicology
A great environmental concern relates to nanotechnology. Time they exhibit novel properties and may expose humans and environment to new risks. Even if cellulose and powdered cellulose are generally recognized as safe and can be used as a raw material for food contact materials or even as food additives, the biological effects of nanosized cellulose cannot be predicted solely from its chemical nature. This is the case for all other nanomaterials. Different parameters may affect the interactions of cellulose nanomaterials with cells and living organisms. Therefore, the safety of cellulose nanomaterials is not necessarily obvious. Current studies of the oral and dermal toxicity of cellulose nanomaterials have shown a lack of adverse health effects and so far, no adverse acute effects have been reported when using realistic concentrations [44, 45]. However, additional studies are needed to support this general conclusion. Studies of pulmonary and cytotoxicity, on the other hand, have yielded discordant results. The toxicity of cellulose nanomaterials strongly depends on their preparation protocol, cellulose source, post-processing, exposure time, but also physico-chemical properties such as surface chemistry, purity, degree of aggregation, shape, and dimensions.
Applications of Cellulose Nanomaterials
A broad range of applications is envisioned for cellulose nanomaterials across many sectors. Several academics with different backgrounds and coming from different scientific fields have focused their research integrating the concepts of “bio” and “nano,” broadening at the same time the applications of cellulose nanomaterials. It can also improve the environmental footprint of many industries by replacing synthetic or petrochemical-based materials. Simultaneously, companies and conference organizers seized this opportunity. The industrial production of cellulose nanomaterials is increasing rapidly with several companies already producing on the tons-per-day scale, intensifying the quest for viable products across many sectors. An overview of the sound markets impacted by cellulose nanomaterials is proposed below but this list is of course not exhaustive.
Composites
The properties of cellulose at the nanometer scale make it an attractive material for the reinforcement of plastics. The addition of cellulose nanomaterials in a polymeric matrix to prepare nanocomposites is probably the major application due to the structural function of cellulose in nature. From the pioneering work on this topic [46], it has been clearly demonstrated that the intrinsic mechanical properties of cellulose nanomaterials are not the main source of possible reinforcement of a polymeric matrix. The strong interactions between cellulose nanoparticles through hydrogen bonding are beneficial to exploit their full potential and reach the highest mechanical reinforcement effect that can be obtained from these nanoparticles. At the same time, it limits their dispersion within a continuous medium. In addition, because of the size of the reinforcing phase in the nano range, very thin polymer films with good mechanical properties and functionality can be obtained. The main issue with nanoparticles is their homogeneous dispersion in a continuous medium resulting from the high surface energy of these particles at the nano scale, associated with the polar nature of cellulose that tends to lead to aggregation, mainly when considering an apolar dispersing medium. Cellulose nanomaterials form a stable dispersion in water or any polar liquid medium, and for this reason, never-dried nanoparticles have been broadly used to process nanocomposites to ensure a proper level of dispersion in the polymeric matrix. However, the dispersion of cellulose nanomaterials in polymer melts and the development of suitable processing technologies for large scale production have been addressed in the literature to broaden their applicability [47•].
Paper and Board
Potential applications are envisaged for cellulose nanomaterials, mainly CNF, in paper and cardboard manufacturing as a strengthening agent. They should improve the bond strength between fibers and thus induce a strong mechanical reinforcing effect on these materials. In addition of improving the tensile strength, burst strength, density, smoothness and limiting the air permeability of paper and cardboard products, the capacity of retaining fillers and adsorbing dyes are also improved by the nanoparticles. However, one of the drawbacks of all CNF nanopapers is their relative brittleness and low tear resistance, measured as the force needed for crack propagation after introducing a notch [48]. Another important issue is the relatively high viscosity of CNF dispersions at low concentrations that limits their use in high-speed coating processes. CF is also well known for improving the wet-web strength for paper making, which decreases the breaking-up rate, thus improving the operation efficiency of paper making process.
In the paper sector and because of its transparency and strength, cellulose nanomaterial-based films can also be used for conservation and restoration of cultural heritage serving for filling paper losses on cultural objects and as a stabilizing agent for paper. It can be used as such in the form of thin films to consolidate damaged historic papers without the application of additional adhesives or in association with adhesives [49, 50].
Coatings
The durability of water-based paints and varnishes can be improved by adding cellulose nanomaterials. The mechanical properties, scratch resistance, and UV protection can be enhanced. The stabilizing, thickening, and iridescent properties of cellulose nanomaterials can also promote applications in this field. The shear-thinning behavior is particularly useful in a range of different coating applications. CNC-reinforced UV-water-based coatings for wood applications were developed and their wear properties were studied [51]. CNC was modified by either alkyl quaternary ammonium bromides or acryloyl chloride. The mechanical properties (abrasion and scratch resistances, hardness and adhesion) were analyzed and compared with the reference varnish without nanoparticles. The modified CNC addition in UV-water-based coatings results in an approximately 30–40% increase in wear resistance (abrasion and scratch), without any loss of appearance. Low amounts of CNC to UV curable wood coating systems were also found to limit the water vapor transmission rate (WVTR) values [52]. Because of the viscosity recovery effect (thixotropy) induced by the presence of cellulose nanomaterials, sag resistance can be obtained. Iridescent effects can also be profitably exploited for some applications. Syneresis, drying time, rub-off, and volatile organic compound (VOC) emissions can also be significantly improved using cellulose nanomaterials in paints and coatings [53]. Cellulose nanomaterial coatings can also be used in medical, biomedical, cosmetics, and bioengineering products.
Packaging
Cellulose nanomaterials can be used in fibrous, plastic, complex, or foam packaging. The interest lies of course in the improvement of the mechanical properties and strong expectation of industry for biobased solutions, but also in the improvement of barrier properties and to act as a binder. They exhibit a great potential to act as barrier material against oxygen, water vapor, and grease/oil when used as coating, filler in nanocomposites or as self-standing thin films [54]. The edibility, flexibility, biodegradability, transparency, and possibility to develop active packaging as cellulose nanomaterials can serve as carriers of some active substances such as antioxidants and antimicrobials are additional motivations. However, hydrophilic films are poor barrier under wet conditions. Indeed, when a hydrophilic film is exposed to water vapor, the water molecules adsorb in the material and increase the fractional free volume of the film serving as a moving carrier for gases. Therefore, a key need is the inadequate resistance of cellulose nanomaterial-based packaging films to water vapor and moisture. It was highlighted that the packaging applications of cellulose-based nanomaterials in various industries are technology-dependent [55]. These technologies include layer-by-layer assembly, electrospinning, composite extrusion, casting evaporation, coating, and all-cellulose composites. A growing sector is food and beverage packaging where cellulose nanomaterials can be used to prevent spoilage of food contents, block the entry of oxygen into the food contents, replace the use of polystyrene-based foams, extend food shelf-life, and improve food quality as they can serve as carriers of some active substances, such as antioxidants and antimicrobials. However, when a material is intended for food contact, there are also safety issues involved, since there is the possibility of migration of components to the food [56]. In the case of edible materials (such as edible films and coatings), the issue is even more important, since the compounds present in the materials are intended to be directly ingested, so any components should be considered food components (instead of food contact components). Although the properties and safety of bulk cellulose are well known, the nanosized counterparts must be evaluated in terms of any potential toxic effects, since their small dimensions may allow them to penetrate into cells and eventually remain in the system. Moreover, the occupational and environmental risks related to cellulose nanomaterials should be widely assessed as well.
Food Industry
Food applications have quickly been identified as a very interesting field due to the rheological behavior of cellulose nanomaterial gels. Carboxymethyl cellulose (CMC) is already sold as food additive. The high viscosity at low nanoparticle concentrations makes cellulose nanomaterials very interesting as fat replacer and low-calorie substitute for carbohydrate additives used as gelling agent, flavor carrier, or suspension stabilizer in a wide variety of food products. As an example, the gelation property of CNF was used to develop soy protein isolate complexes as fat substitutes [57]. Beyond the properties that made cellulose nanomaterials excellent food additives, some lines of studies considered potential functional properties, where it can improve the health of consumers, acting like a dietary fiber [58]. Moreover, the shear-thinning and thixotropic behavior of cellulose nanomaterial suspensions, which are viscous under normal conditions, but flow over time when shaken, agitated, or otherwise stressed, can be interesting in food industry.
Adhesives
The use of cellulose nanomaterials in wood adhesives significantly improves their adhesive bond performance and stability in both wet and dry conditions. It is expected to improve the hardness and modulus of the adhesive, but also its creep behavior. It also reduces VOC emissions from traditional adhesives used in wood and laminate panels. They can be used for brittle thermosets such as urea-formaldehyde as well as comparatively soft thermoplastics such as polyvinyl acetate [59]. However, its content is limited by the strong increase in viscosity caused by its addition.
Inks and Printing
Polymer dispersions containing CNC have been used as birefringent inks [60]. When printing on dark paper, the letters are darker than the background when viewed without polarizers, while they are brighter than the background with crossed polarizers. This type of contrast inversion can have applications in security printing and optical authentication. Also, in inkjet papers, especially those which are targeted for high-quality printing, cellulose nanopaper could be used in the ink receiving layer. It can prevent the spreading and bleeding of ink. The application of a plasticized CNF coating on woven cotton fabrics reduces the pigment penetration into the fabric bulk and enhances the pigment density on the surface of the fabric improving the print quality or reducing the amount of needed ink [61]. 3D printing is also a relevant domain of investigation mainly for biomedical applications [62]. The most important characteristics for this application are thixotropy and shape fidelity which can be improved by adding cellulose nanomaterials to the printing paste. A route for 3D printing both pure CNC and CNC-reinforced composite architectures by direct writing of concentrated, viscoelastic aqueous, and monomer-based CNC inks has been proposed [63]. This method yields cellulose-based structures with a high degree of CNC alignment along the printing direction.
Construction
Since cellulose nanomaterial films are transparent, solid, and thin, they can replace glass or plastic for screens and windows, or may be used as an additional layer to promote UV or IR barrier properties. They could also be used as a component of structural materials for buildings or as a “biofoam” in insulation design. It is believed that the addition of cellulose nanomaterial to cement and concrete mixes can accelerate the hydration process of cement cure, alter the rheology, and enhance flexural strength [64]. The mechanical properties and fracture characteristics of concrete can be enhanced with cellulose nanomaterials by arresting micro-cracks formed during hydration and preventing their further growth.
Electronics
Because cellulose nanomaterial films are highly transparent, lightweight, resistant, flexible, biodegradable, and display a low thermal expansion coefficient, they can be used instead of plastic or glass for flexible display panels and electronic devices, e.g., mobile phones, computers, TVs, touch sensors, solar cells, paper-based generators. [65]. Conductive nanopapers can also be prepared by adding conductive nanoparticles. In addition, a common high-speed printing process used in electronic manufacturing called “roll to roll” can be adapted for cellulose nanomaterials. Transparent and highly bendable microelectrodes necessary to develop lightweight, small electronic devices that are highly portable have been prepared from conductive silver nanowires and TEMPO-oxidized CNF [66].
Energy
Flexible cellulose nanomaterial-based films have been tested for energy storage applications as a substitute for usually rigid and thick separators used in lithium-ion batteries or batteries, including electrodes (e.g., active materials, binders, and structural support), electrolytes, and separators, but also cellulose-based triboelectric nanogenerators [67]. In solar energy harvesting, the preparation of flexible solar panels and photoelectrochemical electrodes is also being studied. Using cellulose nanomaterial as a functional component in modern energy devices and systems is expected to lead to a new paradigm of materials innovation. Identified challenges for broader use cellulose nanomaterials as energy materials are (1) synthesis of cellulose nanomaterials with well-controlled degree of polymerization, (2) molecular-level understanding of how cellulose molecules arrange into different phases, i.e., how the molecular chains organize and interact with each other in short- and long-range ordered fashion, and (3) new approaches to functionalization, modification, and assembly of cellulose nanomaterials in different length scale.
Sensors
Many of the (bio) sensors that are currently based on plastic, glass, or conventional paper platforms could be soon transferred to cellulose nanomaterials and this generation of (bio) sensing platforms could revolutionize the conventional sensing technology [68]. Cellulose nanomaterial-based platforms could be considered an emerging technology to fabricate efficient, simple, cost-effective, and disposable optical/electrical analytical devices for several (bio) sensing applications including health care, clinical/medical diagnostics, environmental monitoring, food quality control, forensic analysis, physical/mechanical sensing, labeling, and bioimaging. They exhibit plasmonic or photoluminescent properties that can be modulated and tailored using different reagents. Nanopaper not only has all the advantageous features exhibited by conventional paper but also brings optical transparency, low thermal expansion, low surface roughness; and high chemical, mechanical, and thermal stability. Furthermore, comparing with conventional paper, nanopaper maintains its 3D structure in water.
Detergent/Emulsions
Self-assembly of nanometric particles at the interfaces is a well-known phenomenon that has been exploited in the stabilization of Pickering emulsions, i.e., emulsions stabilized by solid particles that generally provide a more stable system than surfactant-stabilized emulsions. Cellulose nanomaterials also show this ability to self-assemble at the liquid interfaces, notably with a particular influence of the surface properties and the shape of the nanoparticles, as well as the interactions between particles and cellulose nanomaterial-solvent interactions [69]. Essential factors in the application of cellulose nanomaterials in emulsion stabilization include renewability, chemical stability, low toxicity, and biocompatibility. The preparation processes are facile and easy to handle because they are conducted in aqueous solutions, where cellulose nanomaterials are well dispersed and do not require any time-consuming solvent exchanging process. Therefore, material fabrication based on the cellulose nanomaterial-stabilized Pickering emulsions paves way towards novel biobased materials with a facile and scalable process.
Filtration
Cellulose nanomaterials are also believed to have great potential in filter and membrane applications used for water and air purification. Two applications of cellulose nanomaterials have generated attention and proved sound strategy, viz. as adsorbent and as membrane for the removal of contaminants [70]. This potential is attributed to its high aspect ratio, high specific surface area, high capacity retention, and environmental inertness. In addition to the aforementioned advantages, the presence of active sites allows the incorporation of chemical moieties that may enhance the binding efficiency of pollutants to the surface. Their nanostructure could be used to produce filters that can purify all kinds of liquids. Desalination of seawater, filtration of blood cells during transfusions, or trapping of hazardous chemicals in cigarettes may be considered.
Biomedical
A growing number of applications are envisaged for cellulose nanomaterials as biomedical material. This is due to their remarkable physical properties, extended surface area, surface functionality, and biological properties (biocompatibility, biodegradability, and low toxicity). The applications are seen at the molecule level (tissue skeleton for cell culture, excipient in pharmaceutical compositions, drug delivery, enzyme/protein immobilization and recognition), but also on the scale of macroscopic biomaterials (substitutes for blood vessels and soft tissues, repair and healing of skin, cartilage and bone tissue, antimicrobial materials) [71]. Potential biomedical application of cellulose nanomaterials can also be designed by their functional modification. Additional features such as possibility to form hydrogels, shear-thinning properties, and spontaneous self-gelation can be exploited. However, creating controlled properties, reliable and reproducible production techniques for biocompatible cellulose nanomaterials (not only for BC) will be essential and beneficial to pave the way for greater acceptance of cellulose nanomaterials as a commercially available material in biomedical applications.
Cosmetics
As an organic and sustainable material, cellulose nanomaterial is a valuable cosmetic ingredient which provides a texture that improves a product’s feel in facial and body applications. According to Asia Nano group [72], cellulose nanomaterials disperse other effective ingredients of the cosmetics because they have high dispersion stability in water and hold the effective contents longer thanks to their water holding power. Through the rheological properties of their dispersions, cellulose nanomaterials can find potential applications in cosmetic products for hair, eyelashes, eyebrows, or nails, and the iridescence properties could also be exploited.
Hygiene
Because they are lightweight, resistant, and exhibit high surface area, cellulose nanomaterials can be transformed into foams and aerogels that can withstand more than 10,000 times their own weight [73, 74]. These materials are extremely porous and capable to store large amounts of water. They can be used alone or in combination with superabsorbent polymers for dressings, diapers, and sanitary napkins or pads. The surface chemistry of these materials can be tuned to selectively absorb hydrophobic liquids.
Oil Recovery
The extraction of hydrocarbons from porous or fractured rock formations is a potentially interesting large scale application of cellulose nanomaterials [75]. They have been suggested for use in oil recovery applications as a drilling fluid and flooding agent. The injected fluids in secondary processes supplement the natural energy present in the reservoir to displace oil. The drilling fluids are used to lubricate, provide hydrostatic pressure, and to keep the drill cool and the hole as clean as possible of drill cuttings. The recovery efficiency mainly depends on the mechanism of pressure maintenance, but the injected fluids in tertiary or enhanced oil recovery (EOR) processes interact with the reservoir rock/oil system. It has been shown that by using CNCs as an additive, the proportion of drilling muds lost in the geological holes could be reduced to a minimum. CNC-containing drilling muds would also help to limit damage to formations, improve the characteristics of sludge cake formation, and ensure that other additives such as clays and polymers produce better yield at lower dosages.
Defense
Cellulose nanomaterials can form very dense and resistant mats. Its strength-to-weight ratio, eight times higher than stainless steel, makes it perfect for the manufacture of body armor and bullet-proof vests that are both solid and light.
Conclusions
Cellulose nanomaterials can be extracted from any cellulosic source in the form of cellulose nanofibrils (CNF) or cellulose nanocrystals (CNC) using a mechanical or acid hydrolysis treatment, respectively. Both nanomaterials exhibit the intrinsic properties of cellulose, i.e., renewability, non-toxicity, biodegradability, biocompatibility, and low density. Its transformation down to the nanoscale endows cellulose nanomaterials with new properties such as increased specific surface area and ensuing greater impact of surface functionalization, high aspect ratio, and enhanced mechanical, thermal, rheological, optical and barrier properties. These new and remarkable properties give cellulose many industrial applications in different sectors. However, isolation of cellulose nanomaterials remains costly and involves energy-consuming processes. Challenges are mainly related to affordable up-scaling production and cost-effective surface modification routes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Dufresne A. Nanocellulose: from nature to high performance tailored materials. 2nd ed. Berlin/Boston: Walter de Gruyter GmbH & Co KG; 2017. This specialist monograph provides an overview of the recent research on the fundamental and applied properties of nanoparticles extracted from cellulose. This revised and updated second edition expands the broad overview of recent research.
Nickerson RF, Habrle JA. Cellulose intercrystalline structure. Ind Eng Chem. 1947;39:1507–12.
Herrick FW, Casebier RL, Hamilton JK, Sandberg KR. Microfibrillated cellulose: morphology and accessibility. J Appl Polym Sci Polym Symp. 1983;37:797–813.
Turbak AF, Snyder FW, Sandberg KR. microfibrillated cellulose: a new cellulose product: properties, uses, and commercial potential. J Appl Polym Sci Polym Symp. 1983;37:815–27.
Lavoine N, Desloges I, Dufresne A, Bras J. Microfibrillated cellulose - its barrier properties and applications in cellulosic materials: a review. Carbohydr Polym. 2012;90:735–64.
Lin N, Huang J, Dufresne A. Preparations, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: a review. Nanoscale. 2012;4:3274–94.
Mariano M, El Kissi N, Dufresne A. Cellulose nanocrystals and related nanocomposites: review of some properties and challenges. J Polym Sci Polym Phys. 2014;52:791–806.
• Kargarzadeh H, Gopakumar D, Mariano M, Ahmad I, Thomas S, Dufresne A, et al. Recent developments on nanocellulose reinforced polymer nanocomposites: a review. Polymer. 2017;132:368–93 This review addresses critical factors in the manufacturing of cellulose nanomaterial composites. It also introduces and comprehensively discusses various processing techniques.
Ferreira FV, Dufresne A, Pinheiro IF, Souza DHS, Gouveia RF, Mei LHI, et al. How do cellulose nanocrystals affect the overall properties of biodegradable polymer nanocomposites: a comprehensive review. Eur Polym J. 2018;108:274–85.
• Kargarzadeh H, Huang J, Lin N, Ahmad I, Mariano M, Dufresne A, et al. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog Polym Sci. 2018;87:197–227 This paper presents an overview of recent developments in cellulose nanomaterials, including the production, characterization, properties, and range of applications of cellulose nanomaterial-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites.
Kargarzadeh H, Ahmad I, Abdullah I, Dufresne A, Zainudin SY, Sheltami RM. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose. 2012;19:855–66.
Malainine ME, Dufresne A, Dupeyre D, Mahrouz M, Vuong R, Vignon MR. Structure and morphology of cladodes and spines of Opuntia Ficus-Indica. Cellulose extraction and characterization. Carbohydr Polym. 2003;51:77–83.
Varanasi S, He R, Batchelor W. Estimation of cellulose nanofibre aspect ratio from measurements of fibre suspension gel point. Cellulose. 2013;20:1885–96.
Tanaka R, Saito T, Ishii D, Isogai A. Determination of nanocellulose fibril length by shear viscosity measurement. Cellulose. 2014;21:1581–9.
Kangas H, Lahtinen P, Sneck A, Saariaho A-M, Laitinen O, Hellén R. Characterization of fibrillated celluloses. A short review and evaluation of characteristics with a combination of methods. Nord Pulp Pap Res J. 2014;29:129–43.
Desmaisons J, Boutonnet E, Rueff M, Dufresne A, Bras J. A new quality index for benchmarking of different cellulose nanofibrils. Carbohydr Polym. 2017;174:318–29.
Flauzino Neto WP, Mariano M, Vieira da Silva IS, Silvério HA, Putaux JL, Otaguro H, et al. Mechanical properties of natural rubber nanocomposites reinforced with high aspect ratio cellulose nanocrystals isolated from soy hulls. Carbohydr Polym. 2016;153:143–52.
Doris GM, Ben Y, Hu T-Q, Neault P. Dry cellulose filaments and the method of making the same. 2014; WO 2014/071523 Al.
Siqueira G, Abdillahi H, Bras J, Dufresne A. High reinforcing capability cellulose nanocrystals extracted from Syngonanthus nitens (Capim Dourado). Cellulose. 2010;17:289–98.
Filson PB, Dawson-Andoh BE, Schwegler-Berry D. Enzymatic-mediated production of cellulose nanocrystals from recycled pulp. Green Chem. 2009;11:1808–14.
Hirota M, Tamura N, Saito T, Isogai A. Water dispersion of cellulose II nanocrystals prepared by TEMPO-mediated oxidation of mercerized cellulose at pH 4.8. Cellulose. 2010;17:279–88.
Kontturi E. Process for preparing micro- and nanocrystalline cellulose. Patent WO2011/114005; 2011.
Man Z, Muhammad N, Sarwono A, Bustam MA, Kumar MV, Rafiq S. Preparation of cellulose nanocrystals using an ionic liquid. J Polym Environ. 2011;19:726–31.
Leung ACW, Hrapovic S, Lam E, Liu Y, Male KB, Mahmoud KA, et al. Characteristics and properties of carboxylated cellulose nanocrystals prepared from a novel one-step procedure. Small. 2011;7:302–5.
Yang H, Alam MDN, van de Ven TGM. Highly charged nanocrystalline cellulose and dicarboxylated cellulose from periodate and chlorite oxidized cellulose fibers. Cellulose. 2013;20:1865–75.
Lu Q, Tang L, Lin F, Wang S, Chen Y, Chen X, et al. Preparation and characterization of cellulose nanocrystals via ultrasonication-assisted FeCl3-catalyzed hydrolysis. Cellulose. 2014;21:3497–506.
Novo LP, Bras J, García A, Belgacem N, Curvelo AAS. Subcritical water: a method for green production of cellulose nanocrystals. ACS Sustain Chem Eng. 2015;3:2839–46.
Kunaver M, Anžlovar A, Žagar E. The fast and effective isolation of nanocellulose from selected cellulosic feedstocks. Carbohydr Polym. 2016;148:251–8.
Sirviö JA, Visanko M, Liimatainen H. Acidic deep eutectic solvents as hydrolytic media for cellulose nanocrystals production. Biomacromolecules. 2016;17:3025–32.
Siqueira G, Bras J, Dufresne A. New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir. 2010;26:402–11.
Bras J, Viet D, Bruzzese C, Dufresne A. Correlation between stiffness of sheets prepared from cellulose whiskers and nanoparticles dimensions. Carbohydr Polym. 2011;84:211–5.
Dufresne A. Cellulose nanomaterial reinforced polymer nanocomposites. Curr Opin Colloid Interface Sci. 2017;29:1–8.
Mariano M, El Kissi N, Dufresne A. Cellulose nanomaterials: size and surface influence on the thermal and rheological behavior. Polimeros. 2018;28:93–102.
Espino-Pérez E, Bras J, Ducruet V, Guinault A, Dufresne A, Domenek S. Influence of chemical surface modification of cellulose nanowhiskers on thermal, mechanical, and barrier properties of poly (lactide) based bionanocomposites. Eur Polym J. 2013;49:3144–54.
Österberg M, Vartiainen J, Lucenius J, Hippi U, Seppälä J, Serimaa R, et al. A fast method to produce strong NFC films as a platform for barrier and functional materials. ACS Appl Mater Interfaces. 2013;5:4640–7.
Benhamou K, Dufresne A, Magnin A, Mortha G, Kaddami H. Control of size and viscoelastic properties of nanofibrillated cellulosefrom palm tree by varying the TEMPO-mediated oxidation time. Carbohydr Polym. 2014;99:74–83.
Dufresne A. Nanocellulose: a new ageless bionanomaterial. Mater Today. 2013;16:220–7.
Sheltami RM, Kargarzadeh H, Abdullah I, Ahmad I. Thermal properties of cellulose nanocomposites. In Handbook of nanocellulose and cellulose nanocomposites, Vol. 1. Ed. Kargarzadeh H, Ahmad I, Thomas S, Dufresne A. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2017 :523–52.
Lin N, Dufresne A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale. 2014;6:5384–93.
Nogi M, Iwamoto S, Nakagaito AN, Yano H. Optically transparent nanofiber paper. Adv Mater. 2009;21:1595–8.
• Gray DG. Order and gelation of cellulose nanocrystal suspensions: an overview of some issues. Phil Trans R Soc A. 2018;376:20170041 Some insight into the processes that govern the formation of solid chiral nematic films from CNC suspensions is provided.
Wang P-X, MacLachlan MJ. Liquid crystalline tactoids: ordered structure, defective coalescence and evolution in confined geometries. Phil Trans R Soc A. 2018;376:20170042.
Wang J, Gardner DJ, Stark NM, Bousfield DW, Tajvidi M, Cai Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain Chem Eng. 2018;6:49–70.
Roman M. Toxicity of cellulose nanocrystals: a review. Ind Biotechnol. 2015;11:25–33.
Camarero-Espinosa S, Endes C, Mueller S, Petri-Fink A, Rothen-Rutishauser B, Weder C, et al. Elucidating the potential biological impact of cellulose nanocrystals. Fibers. 2016;4:21.
Favier V, Canova GR, Cavaillé JY, Chanzy H, Dufresne A, Gauthier C. Nanocomposites materials from latex and cellulose whiskers. Polym Adv Technol. 1995;6:351–5.
• Dufresne A. Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Phil Trans R Soc A. 2018;376:20170040 This paper focuses on the processing techniques that can be used to prepare cellulose reinforced polymer nanocomposites, with an emphasis on melt processing that remains a challenge.
Desmaisons J, Gustafsson E, Dufresne A, Bras J. Hybrid nanopaper of cellulose nanofibrils and PET microfibers with high tear and crumpling resistance. Cellulose. 2018;25:7127–42.
Dreyfuss-Deseigne R. La nanocellulose en conservation-restauration: première application de ce nouveau matériau prometteur pour la consolidation des œuvres graphiques à caractère translucide et transparent. Tracé. 2016;16:75–83.
Völkel L, Ahn K, Hähner U, Gindl-Altmutter W, Potthast A. Nano meets the sheet: adhesive-free application of nanocellulosic suspensions in paper conservation. Herit Sci. 2017;5:23.
Vardanyan V, Poaty B, Chauve G, Landry V, Galstian T, Riedl B. Mechanical properties of UV-waterborne varnishes reinforced by cellulose nanocrystals. J Coat Technol Res. 2014;11:841–52.
Kaboorani A, Auclair N, Riedl B, Landry V. Physical and morphological properties of UV-cured cellulose nanocrystal (CNC) based nanocomposite coatings for wood furniture. Prog Org Coat. 2016;93:17–22.
https://www.celluforce.com/en/applications/#anchor26. Accessed 17 Feb 2019.
Hubbe M, Ferrer A, Tyagi P, Yin Y, Salas C, Pal L, et al. Nanocellulose in thin films, coatings, and plies for packaging applications: a review. Bioresources. 2017;12:2143–233.
Li F, Mascheroni E, Piergiovanni L. The potential of nanocellulose in the packaging field: a review. Packag Technol Sci. 2015;28:475–508.
Azeredo HMC, Rosa MF, Mattoso LHC. Nanocellulose in bio-based food packaging applications. Ind Crop Prod. 2017;97:664–71.
Sun L, Chen W, Liu Y, Li J, Yu H. Soy protein isolate/cellulose nanofiber complex gels as fat substitutes: rheological and textural properties and extent of cream imitation. Cellulose. 2015;22:2619–27.
Andrade DR, Mendonça MH, Helm CV, Magalhães WL, de Muniz GI, Kestur SG. Assessment of nano cellulose from peach palm residue as potential food additive: part II: preliminary studies. J Food Sci Technol. 2015;52:5641–50.
Gindl-Altmutter W, Veigel S. Nanocellulose-modified wood adhesives. In: Oksman K, Mathew AP, Qvintus P, Bismarck A, Rojas O, Sain M, editors. Handbook of green materials: processing technologies, properties and applications, vol. 2. Singapore: World Scientific Publishing Co Pte Ltd; 2014. p. chap 17.
Chaindawong C, Johannsmann D. An anisotropic ink based on crystalline nanocellulose: potential applications in security printing. J Appl Polym Sci. 2014;131:41063.
Nechyporchuk O, Yu J, Nierstrasz VA, Bordes R. Cellulose nanofibril-based coatings of woven cotton fabrics for improved inkjet printing with a potential in e-textile manufacturing. ACS Sustain Chem Eng. 2017;5:4793–801.
Abouzeid RE, Khiari R, Beneventi D, Dufresne A. Biomimetic mineralization of three-dimensional printed alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering. Biomacromolecules. 2018;19:4442–52.
Siqueira G, Kokkinis D, Libanori R, Hausmann MK, Gladman AS, Neels A, et al. Cellulose nanocrystal inks for 3D printing of textured cellular architectures. Adv Funct Mater. 2017;27:1604619.
Fu T, Moon RJ, Zavattieri P, Youngblood J, Weiss WJ. Cellulose nanomaterials as additives for cementitious materials. In Cellulose-reinforced nanofibre composites: production, properties and applications. Jawaid M, Boufi S, Khalil AHPS Eds. 2017:455–482.
Jung YH, Chang T-H, Zhang H, Yao C, Zheng Q, Yang VW, et al. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat Commun. 2015;6:7170.
Kim D, Ko Y, Kwon G, Kim U-J, You J. Micropatterning silver nanowire networks on cellulose nanopaper for transparent paper electronics. ACS Appl Mater Interfaces. 2018;10:38517–25.
Wang X, Yao C, Wang F, Li Z. Cellulose-based nanomaterials for energy applications. Small. 2017;13:1702240.
Golmohammadi H, Morales-Narváez E, Naghdi T, Merkoçi A. Nanocellulose in sensing and biosensing. Chem Mater. 2017;29:5426–46.
Fujisawa S, Togawa E, Kuroda K. Nanocellulose-stabilized Pickering emulsions and their applications. Sci Technol Adv Mater. 2017;18:959–71.
Abouzeid RE, Khiari R, El-Wakil N, Dufresne A. Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromolecules. 2019;20:573–97.
Lin N, Dufresne A. Nanocellulose in biomedicine: current status and future prospect. Eur Polym J. 2014;59:302–25.
https://www.personalcare1.com/articles/new-whitening-cosmetics-applying-nanocellulose-technology. Accessed 12 Feb 2019.
Kevin J, De France KJ, Hoare T, Cranston ED. Review of hydrogels and aerogels containing nanocellulose. Chem Mater. 2017;29:4609–31.
Nascimento DM, Nunes YL, Figueirêdo MCB, de Azeredo HMC, Aouada FA, Feitosa JPA, et al. Nanocellulose nanocomposite hydrogels: technological and environmental issues. Green Chem. 2018;20:2428–48.
Sun X, Zhang Y, Chen G, Gai Z. Application of nanoparticles in enhanced oil recovery: a critical review of recent progress. Energies. 2017;10:345.
Funding
LGP2 is part of the LabEx Tec 21 (Investissements d’Avenir - grant agreement n°ANR-11-LABX-0030) and of PolyNat Carnot Institut (Investissements d’Avenir - grant agreement n°ANR-16-CARN-025-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alain Dufresne declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Wood Structure and Function
Rights and permissions
About this article
Cite this article
Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr Forestry Rep 5, 76–89 (2019). https://doi.org/10.1007/s40725-019-00088-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-019-00088-1