Abstract
Research on nanocellulose has significantly increased over the past few decades, owing to the various attractive characteristics of this material, such as renewability, widespread availability, low density, excellent mechanical properties, economic value, biocompatibility, and biodegradability. Nanocellulose categorized into two main types, namely cellulose nanofibrils (CNFs) and cellulose nanocrystals (CNCs). In this review, we present the recent advances made in the production of CNFs and CNCs. In addition to the conventional mechanical and chemical treatments used to prepare CNFs and CNCs, respectively, other promising techniques as well as pretreatment processes have been also proposed in recent times, in an effort to design an economically efficient and eco-friendly production route for nanocellulose. Further, while the hydrophilic nature of nanocellulose limits its use in polymeric matrices and in some industrial applications, the large number of hydroxyl groups on the surface of nanocellulose provides a suitable platform for various kinds of modification treatments. The various chemical and physical surface treatment procedures reported for nanocellulose have been reviewed in this paper. Finally, in this review, we summarize the life cycle assessment studies conducted so far on nanocellulose, which quantify the environmental impact of nanocellulose products. The current paper is a comprehensive review of the recent literature on nanostructured cellulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There has been an increasing demand for products made from renewable and sustainable resources that are biodegradable, non-petroleum based, and carry low environmental, animal/human health, and safety risks. Cellulose, which is the most abundant biopolymers on earth, as well as its derivatives have been widely studied as renewable materials. Cellulose is present in wood, cotton, hemp, and other plant-based materials and serves as the dominant reinforcement material in plant structures. Materials based on cellulose and its derivatives have been used for more than 150 years in a wide variety of applications such as food, paper production, biomaterials, and pharmaceuticals. In addition, natural cellulose-based materials such as wood, hemp, cotton, and linen have been used in our society as engineering materials for thousands of years. The enormous number of industries engaged in the manufacture of forest-based products, paper, textiles, and so on worldwide is testament to the continuing popularity of this material.
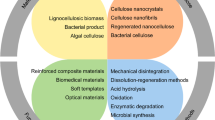
In recent years, researchers have focused on isolating, characterizing, and developing applications for a novel form of cellulose known as nanocellulose. Generally, one-dimensional isolated cellulosic materials with dimensions in the nanometer range are referred to as nanocelluloses. Nanocellulose combines the key properties of cellulose, such as high specific strength and modulus, hydrophilicity, and extensive ability for chemical modification, with specific properties characteristic of nanoscale materials, originating from the very large surface area of these materials. Based on the appearance and preparation methods, nanocellulose can be classified into two main subcategories, namely cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs). CNCs are short and needle-shaped, with diameter in the nanoscale and length generally in the range 100–500 nm. On the other hand, CNFs are flexible long nanofibers with diameter in the nanoscale and length in the micron scale. The final features, properties, and yield of the nanocellulose materials are dependent on the cellulose sources and preparation conditions used. Various terminologies have been used in the literature for CNCs and CNFs which unfortunately leads to ambiguities and misunderstanding, including cellulose nanowhiskers, nanocrystaline cellulose, nanofibrilated cellulose and cellulose microfibrils. Recently, the Technical Association of the Pulp and Paper Industry (TAPPI) proposed standard terms and their definition for cellulose nanomaterial (TAPPI WI 3021), based on the nanocellulose size (Trache et al. 2017; Kargarzadeh et al. 2017). The nomenclature, abbreviation, and dimensions applicable to each subgroup are shown in Fig. 1.

Adapted with permission from Mariano et al. (2014), Copyright 2014. Reproduced with permission of John Wiley & Sons
Standard terms for cellulose nanomaterials (TAPPI W13021).
The large number of hydroxyl groups on the nanocellulose surface is responsible for the inherent hydrophilic nature of the material, which limits its applications in polymeric matrices. However, the presence of a large number of hydroxyl groups also results in a unique substrate that is amenable to various types of surface modifications, extending its use to sophisticated applications. Most of the chemical modifications performed on nanocelluloses are a logical extension of those applied previously to cellulose fibers. However, it is very important to ensure that the surface treatment does not change or damage the cellulose structure and the original morphology of nanocellulose.
One of the most important issues concerning nanotechnology and nanomaterials is environmental health and safety (EHS) of the materials. Owing to the nanoscale features of nanocellulose and breakthroughs in the ability to produce and apply nanocellulose in a variety of sectors from food to medicine, it is necessary to assess its environmental and safety aspects before using it in applications in the wider society, in order to ensure that it can be safely used in commercial applications. For this purpose, life cycle assessment (LCA), which is a comprehensive modeling framework that attempts to measure the total environmental impact of a product “from cradle to grave”, is utilized. Despite outstanding developments in nanotechnology and more specifically, in the field of nanocellulose, environmental issues related to this product remain poorly understood and only a few studies have examined the LCA of nanocellulose and its products.
A number of review articles focusing on the production, characterization, and modification of nanocellulose have been published recently. Abdul Khalil et al. (2014) focused on the developments made in the pretreatment of nanocellulose, CNF production, and their applications in the production of nanopaper, coating, additives, and food packaging, as well as surface modification of CNFs. Wei et al. (2014) reviewed techniques available for the preparation of nanocellulose, nanocellulose papers, and nanocellulose films as well as their potential applications. Habibi (2014), Islam et al. (2013), and Missoum et al. (2013) reviewed the various approaches available for the surface chemical modification of nanocellulose, specifically CNFs. Jonoobi et al. (2015) described the various approaches reported in the literature for isolating nanocellulose from various natural sources. They also studied the key properties of cellulose nanomaterials, such as morphology, crystallinity, and thermal stability, for materials prepared from various natural sources. However, they described only the common preparation techniques for producing nanocellulose, whereas a number of novel techniques have been proposed in the literature. Nechyporchuk et al. (2016) reviewed the various production methods for CNFs including conventional and novel mechanical disintegration techniques, as well as biological and chemical pretreatment methods aimed at facilitating the isolation of CNFs. Additionally, the preparation of various forms of CNFs such as suspensions, water-redispersible powders, films or nanopapers, hydrogels, and aerogels was discussed.
However, there are some recent advances that have not been adequately addressed so far, including i) certain techniques for CNF production (e.g., extrusion and aqueous counter collision) and CNC production (e.g., solid and gashouse acid hydrolysis), ii) physical modification methods for nanocellulose such as plasma and irradiation treatment, and iii) the LCA of nanocellulose. Therefore, this review aims at providing a comprehensive review on nanocellulose, with particular focus on the recent advances achieved in the production, characterization, and modification of CNCs and CNFs, as well as the LCA of nanocellulose.
Cellulose nanostructure
Cellulose is a semi-crystalline polymeric material and is representative of nanostructures existing in natural fibers and other plant-based materials. It acts as the main reinforcing phase in plant structures and is also synthesized by algae, tunicates, and bacteria. Cellulose fibers consist of bundles of microfibrils, which are present in the cell walls and reinforce an amorphous matrix consisting of lignin, hemicellulose, proteins, extractive organic substances, and trace elements. Cellulose nanocrofibrils with diameter in the range of 5–30 nm are composed of cellulose macromolecules in an extended chain conformation. The building blocks of the cellulose polymer chain are composed of D-glucopyranose molecules linked together by the β-1,4-glucosidic bond. More details on the chemistry and properties of cellulose can be found in various references in the literature (Dufresne 2013; Moon et al. 2011; Nechyporchuk et al. 2016).
Nanocellulose refers to cellulose particles with at least one dimension in the range of 1–100 nm. The dimension, composition, and properties of nanocellulose depend on the production conditions. There are two main types of nanocellulose, namely CNCs and CNFs. Even if considered as cellulose nanomaterial, bacterial cellulose results from a different strategy since it involves a bottom-up procedure, and will consequently not be addressed in this review. The major difference between CNCs and CNFs lies in the proportion of the amorphous phase and dimensions of the material, which are directly affected by the production condition.
CNCs exhibit an elongated rod-like shape with diameter in the nanoscale and higher length. They have very limited flexibility compared to CNFs. The most important method used for the extraction of CNCs is acid hydrolysis. In this process, a strong acid such as H2SO4 dissolves the amorphous portions (disordered regions) of cellulose, resulting in the formation of a nanocrystal structure. The dimensions of CNCs can vary widely, with diameter ranging from 3 to 50 nm and length ranging from 100 to 500 nm. The dimensions and crystallinity of CNCs depend on the cellulose source and extraction conditions (Abdul Khalil et al. 2014; Habibi et al. 2010; Klemm et al. 2011; Nechyporchuk et al. 2016).
On the other hand, CNFs are generally produced by the mechanical delamination of cellulosic pulp aqueous suspensions in a high-pressure homogenizer (HPH). During this process, a highly entangled network of nanofibrils with both crystalline and amorphous domains is produced owing to the high shear force used. Depending on the processing conditions, CNFs can get disintegrated into flexible nanofibers that are 20–50 nm in diameter and 500–2000 nm in length (Abdul Khalil et al. 2012). An in-depth review of the methods for the production of CNFs and CNCs and their properties are presented in the following sections.
Production of CNFs
A number of studies have been conducted on the isolation and characterization of CNFs from various natural sources (Abe et al. 2007; Chirayil et al. 2014; Deepa et al. 2011; He et al. 2014; Li et al. 2014). CNFs can be extracted from plant cell walls by simple mechanical shearing or by a combination of chemical and mechanical routes. Examples of such methods include HPH, grinding, cryocrushing, and high intensity ultrasonic treatment, which lead to transverse cleavage of the cellulose fibers. A brief review of the most common CNF preparation routes and equipment is provided below.
High-pressure homogenization
HPH is a widely used method for the large-scale production of CNFs. Two types of equipment are typically employed for this process, namely homogenizers and microfluidizers. The homogenization process, as well as the equipment, has been extensively used in the dairy and food industries, primarily to stabilize food emulsions. During the homogenization process for the production of CNFs, cellulose slurry is pumped at a high pressure and fed through a spring-loaded valve assembly. As the valve opens and closes rapidly, the fibers are exposed to a large pressure drop and subjected to shearing and impact forces. This combination of forces leads to a high degree of microfibrillation of the cellulose fibers. The extent of cellulose fibrillation depends on the number of homogenization cycles as well as applied pressure. The higher the pressure, the higher would be the disruption efficiency per pass through the equipment.
Methods for the production of CNFs were first reported by Herrick et al. (1983) and Turbak et al. (1985). They fed dilute cellulosic wood pulp-water suspensions through a mechanical homogenizer and the large pressure drop in the homogenizer facilitated micro fibrillation. Li et al. (2014) extracted CNFs from de-pectinated sugar beet pulp by combining chemical treatment with HPH. The diameter of the resultant CNFs ranged from several nanometers to 70 nm and it was shown that the crystallinity of the nanofibers increased significantly after treatment. Chen et al. (2014) also reported a method to fibrillate raw dried cotton fibers into separate CNFs by chemical purification and pretreatment using a high speed blender combined with nanofibrillation by HPH. The resultant nanofibers were found to be approximately 10–30 nm in diameter and high aspect ratios. Further, Pelissari et al. (2014) isolated CNFs from banana peel using a combination of chemical treatment methods including alkaline treatment, bleaching, and acid hydrolysis. Suspensions of chemically treated fibers were passed through an HPH to produce CNFs (Pelissari et al. 2014).
Microfluidizers have been used as an alternative to homogenizers for CNF production. Unlike homogenizers, which operate at a constant pressure, the microfluidizer operates at a constant shear rate. In this technique, cellulose suspension is passed through a thin chamber of a specific geometry (e.g., Z or Y-shape) with an orifice width of 100–400 µm. By applying a high pressure, strong shear forces are produced. In addition, the suspension impacts the channel walls allowing cellulose fibrillation. It is necessary to repeat the process several times and use chambers of various sizes in order to improve the degree of fibrillation. Lee et al. (2009a, b) examined the effect of the number of passes of microcrystalline cellulose (MCC) slurry through a microfluidizer on the morphology of the resultant CNFs. They found that the aspect ratio of the nanofibrillar bundles increased after 10–15 passes, whereas an additional 20 passes led to agglomeration of the CNFs owing to increased surface area and increased amount of surface hydroxyl groups.
Grinding
In the grinding process, cellulose fibers are forced through a gap between two specially modified grooved discs, one of which is static and the other revolving at about 1500 rpm. During the process, the cell wall structure is broken down by the high shear forces, resulting in the production of individual nanosized fibers. The extent of fibrillation depends on the distance between the discs, morphology of the disc channels, and number of passes through the grinder. Abe et al. (2007) obtained CNFs with a uniform diameter of 15 nm from wood by grinding in the undried state. This study demonstrates a powerful, yet quite simple method for the production of CNFs from plant fibers. In another study by Iwamoto et al. (2005), when homogenized cellulosic pulp was subjected to grinding treatment, the fibril bundles were further fibrillated. Ten repetitions of the grinder treatment resulted in the production of uniform nanofibers that were 50–100 nm wide.
Cryocrushing
Cryocrushing is yet another method for producing nanofibers, in which the fibers are frozen using liquid nitrogen and then subjected to high shear forces. In this process, high impact forces act on the frozen fibers and the ice crystals exert pressure on the cell walls, causing them to rupture and leading to the formation of microfibrils. Cryocrushing combined with a high pressure fibrillation process was used by Wang and Sain (2007) for isolating nanofibers from soybean stock. The resultant fibers were 50–100 nm in diameter. In another study, Alemdar and Sain (2008) reported the extraction of CNFs from wheat straw and soy hull by mechanical treatment involving cryocrushing. They investigated the chemical composition, morphology, as well as physical and thermal properties of the nanofibers in an effort to study the feasibility of using the nanofibers in biocomposite applications. They showed that the diameter of the wheat straw nanofibers was in the range of 10–80 nm with length on the order of a few thousand nanometers. Chakraborty et al. (2005) also reported a novel technique for producing cellulose microfibrils through mechanical methods. Their method involved severe shearing in a refiner followed by high-impact crushing under liquid nitrogen.
High intensity ultrasonication
This process involves a combination of chemical pretreatment and high-intensity ultrasonication. Owing to the impact of ultrasonic waves, the micron-sized cellulose fibers are gradually disintegrated into nanofibers. Before ultrasonication, the plant fibers are purified to prepare cellulose fibers by mild acid hydrolysis followed by alkali and bleaching treatments. In this process, non-cellulosic materials such as lignin and hemicelluloses are removed. After chemical pretreatment, the purified cellulose fibers are soaked in distilled water, following which about 110 mL of the solution containing chemically purified cellulose fibers is placed in an ultrasonic generator operating at a frequency of 20–25 kHz. Ultrasonication is conducted for 30 min to isolate the nanofibers. Li et al. (2014) prepared nanocellulose fibers by pretreating cellulose in a NaOH/urea/thiourea solution and then defibrillating the fibers by ultrasonication. They achieved a high yield of 85.4% and the prepared nanocellulose fibers were about 30 nm in diameter with the cellulose II crystal structure. In addition, the nanofibers possessed high thermal stability with thermal degradation commencing at 270 °C and a maximum degradation temperature of 370 °C. Chen et al. (2014) reported the separation of CNFs from poplar wood by explosive chemical pretreatment and high-intensity ultrasonication. When the output power of the ultrasonic generator used for chemically purified cellulose fibers was greater than 1000 W, CNFs that were 5–20 nm in diameter and several microns in length were obtained.
Steam explosion process
This process involves vapor phase cooking for a short duration at a temperature in the range of 180–210 °C, followed by explosive decompression and sudden release of pressure. In this process, the cellulosic biomass is pressurized for a short period of time in an autoclave with steam, following which it is explosively discharged to the atmospheric pressure. This results in the sudden disintegration of the starting material into a fibrous dispersed solid. Substantial breakdown of the lignocellulosic structure, hydrolysis of the hemicellulose fraction, de-polymerization of the lignin components, and defibrillation occur. Deepa et al. (2011) reported the extraction of CNFs from the banana plant by the steam explosion process in an autoclave. In another study, Chirayil et al. (2014) employed the steam explosion process for extracting CNFs from isora fiber in an autoclave. Their technique involved alkaline treatment, bleaching, acidic steam treatment, and homogenization. The results of their study showed that the prepared CNFs had a nanofibrillar network-like structure with high crystallinity and good thermal stability. Cherian et al. (2010) also reported the application of the steam explosion process for the successful extraction of CNFs from pineapple leaf fibers. Steam coupled with acid treatment of the pineapple leaf fibers was found to be effective for depolymerization and defibrillation, resulting in the production of nanofibrils.
Electrospinning
Electrospinning is a rather simple and cost-effective method for the production of nanofibers, in which a solution is extruded and electrospun under the action of a high electric field. Once the voltage is sufficiently high, a charged stream of matter is ejected following a rather complicated loop. During this process, the solvent evaporates leaving behind randomly oriented nanofibers that accumulate on the collector. Direct dissolution of cellulose is a difficult process. Therefore, CNF production using electrospinning requires a suitable solvent or chemical derivatization of cellulose (Dufresne 2013).
Various systems for the direct dissolution of cellulose without chemical derivatization have been studied. Examples of such systems include N,N-dimethylacetamide (DMAc)/LiCl (Frey 2008), dimethyl sulfoxide (DMSO)/triethylamine/SO2 (Quan et al. 2010), N-methylmorpholine-N-oxide (NMMO) (Kulpinski 2005), and NaOH/urea aqueous solution (Qi et al. 2010). In addition, CNFs have also been produced by the electrospinning technique by dissolving cellulose fibers in solvents such as ethylene diamine, with either potassium thiocyanate or potassium iodide utilized as the salt. However, only a few fundamental studies have been conducted on this method. Ma et al. (2005) prepared cellulose acetate (CA) nanofibers by the electrospinning technique with an acetone/dimethyl formamide (DMF)/trifluoroethylene (3:1:1) mixture as the solvent. The diameter of the resultant fiber ranged from 200 nm to 1 µm.
Extrusion
Twin-screw extrusion is considered a promising technology for biomass conversion as well as CNF production. It is a physico-chemical method, in which cellulose pulp is subjected to heat, compression, and shear force leading to the physical disruption and chemical treatment of cellulose as it passes through the extruder. The aspect ratio and porosity of the final product depends on the extrusion parameters such as barrel temperature, screw speed, and moisture. Compared to other production routes, extrusion is a highly productive process that can be completed in a short duration of time and uses water efficiently (Karunanithy and Muthukumarappan 2010, 2011; Olea et al. 2015). However, there also some disadvantages to the extrusion process such as low treatment rate, low pulp/liquid ratio, and the use of a relatively high temperature. These parameters need to be optimized in order to achieve sufficient shear forces for inducing delamination of the fibers as well as preventing cellulose degradation (Senturk-Ozer et al. 2011). CNFs with a high solid content of 25–40 wt% were produced in the powder form from never-dried refined needle-leaf bleached kraft pulp through the twin-screw extrusion process after 10–14 passes. Upon increasing the number of passes, while the moisture in the pulp evaporated and the degree of fibrillation of the pulp increased, some degradation also occurred (Ho et al. 2015).
Extrusion has also been used to produce composites with in situ CNF production. Pulp nanofibrillation and further melt compounding with polypropylene was reported by Suzuki et al. (2013). Never-dried refined needle-leaf bleached kraft pulp disintegrated into nano and micron-sized pieces, when it was processed in a twin-screw extruder with polypropylene, with maleic anhydride-grafted-polypropylene as a compatibilizer. Hietala et al. (2014) studied the twin-screw extrusion process for the production of composites from starch and CNFs, while Cobut et al. (2014) produced composites from thermoplastic starch and TEMPO (2,2,6,6-tetramethylpiperidine-1-oxyl)-CNFs.
Aqueous counter collision
Aqueous counter collision (ACC) is an eco-friendly extraction method for the preparation of CNFs. In this technique, two jets of cellulose aqueous suspensions collide against each other under high pressure, resulting in wet pulverization and the liberation of CNFs. One of the disadvantages of ACC is that the size of the processed cellulosic material needs to be lower than the nozzle diameter (150 µm) in order to avoid clogging (Kondo et al. 2014). Kose et al. (2011) produced CNFs using ACC from bacterial cellulose (BC) aqueous suspensions. The resultant CNFs had a diameter of 30 nm. They also found that the Iα crystalline phase in cellulose changed to the Iβ phase with over 70% crystallinity. Interestingly, the ACC-treated CNFs exhibit hydrophilic and hydrophobic behavior resulting in switching surface effects depending on the characteristics of the substrate. In another study by Kondo et al. (2014) NFC was produced by the ACC treatment of MCC. The resulting CNFs had a diameter of 15 nm and length of 700 nm.
Production of CNCs
Generally, the preparation of CNCs from natural fibers involves four steps: (1) mechanical size reduction; (2) purification by alkali and bleaching treatments, (3) controlled chemical treatment, predominantly by acid hydrolysis, which removes the inter-fibril regions and amorphous parts and releases CNCs, and (4) mechanical or ultrasound treatment.
In the first step, raw fibers are washed and dried. The cleaned raw fibers are then broken down by milling or grinding. The milled powder is of uniform size on the millimeter/micrometer scale, which allows it to be effectively treated by chemical methods. Alkali treatment is then conducted using aqueous KOH or NaOH solutions in order to remove alkali soluble materials like hemicellulose and other impurities, which cover the external surface of the fiber cell walls (Savadekar and Mhaske 2012; Zainuddin et al. 2013). It has been suggested that a minimum of 50% of hemicellulose should be removed to increase cellulose digestibility (Lee et al. 2014).
The primary mechanism behind alkali treatment is the interruption of OH bonding in the fiber network structure, which leads to the separation of inter-fibrillar regions from the cellulose fibers. The mechanism is as follows.
Generally, alkali treatment can be divided into two categories: (1) alkali solution heating and (2) alkali cooling by autoclaving. The former is carried out using a combination of high temperature (70–90 °C) and mechanical stirring, while the latter involves cooling the fibers and then subjecting to high temperature treatment (160 °C) (Shi et al. 2011; Shin et al. 2012).
Next, bleaching or delignification is an additional important step for the further removal of residual cementing materials, mainly lignin, in the alkali-treated fibers (Panaitescu et al. 2013; Shi et al. 2011). In fact, removal of lignin is necessary to enhance fibers digestibility up to the point where cellulose is exposed for solubilisation process and prepared for hydrolysis (Lee et al. 2014).
Typically, the alkali-treated fibers are bleached by boiling with sodium chlorite (NaClO2) solution under acidic conditions (Sundari and Ramesh 2012), which are created by means of an acetate buffer solution consisting of NaOH and glacial acetic acid diluted with distilled water (Acharya et al. 2011; Savadekar and Mhaske 2012). When the fibers are placed in the acidic buffer solution, sodium chlorite breaks down into chlorine dioxide (ClO2) in the presence of the buffer salts. These acids are capable of releasing hydronium ions (H+) for the hydrolytic cleavage of glycosidic bonds in cellular molecular chains within amorphous regions along the cellulose fibrils. Thus, the hierarchical structure of the nanofibril bundles are broken down into a nanocrystalline structure (Shi et al. 2011; Tee et al. 2013).
Acid hydrolysis
Acid hydrolysis of native cellulose with, for example, a high concentration solution of H2SO4 is a well-known and effective process for producing well-defined CNCs. In addition to H2SO4, other types of acids have also been used for the acid hydrolysis of native cellulose including HCl, oxalic acid, HBr, H3PO4, and HNO3 (Lee et al. 2009a, b; Maiti et al. 2013; Sonia et al. 2013).
As mentioned previously, during the acid hydrolysis process, the amorphous portions and local inter-filler contacts of cellulose are hydrolyzed, whereas stable crystallites remain intact and can be isolated in the form of rod-like nanocrystallite particles. CNCs suspended in a strong acid are diluted with water and washed. They are then neutralized or dialyzed with distilled water to remove the free acid from the suspension and finally, subjected to ultrasonication or other mechanical method to disintegrate them.
Anionically charged sulfate ester groups are introduced on the CNC surface during hydrolysis with H2SO4. While this negative charge causes sufficient dispersion at the individual CNC level in water, the introduction of acidic sulfate groups also compromises the thermostability of the CNCs. To increase the thermal stability of the CNCs, neutralization of the sulfate groups by increasing the pH to over 7 using NaOH has been proposed (Kargarzadeh et al. 2012; Ping and You-Lo 2010). It is worth noting that besides the source of cellulose, the acid hydrolysis conditions such as concentration of acid, temperature, and duration of reaction also play an important role in determining the final properties of CNCs. Typically, high acid concentrations, long reaction times, and high temperatures lead to high surface charge and narrow size. However, these conditions also result in lower yield, decreased crystallinity, and lower thermal stability of CNC (Kargarzadeh et al. 2012; Martínez-Sanz et al. 2011).
One of the major drawbacks of H2SO4 hydrolysis is that the sulfate groups introduced on the CNCs through acid hydrolysis accelerate the degradation of cellulose, which results in limited thermal stability of cellulose. This negatively affects the role of CNCs in various applications such as reinforcement in nanocomposites. Therefore, in recent years, there is increasing focus on the use of mineral acids other than H2SO4 for the hydrolysis of cellulose. Espinosa et al. (2013) compared the properties of CNCs extracted via both H2SO4 and H3PO4 hydrolysis. They observed that the CNCs extracted by H3PO4 hydrolysis exhibit flame resistance and higher thermal stability compared to those obtained by H2SO4 hydrolysis. Tang et al. (2015) extracted CNCs from old corrugated container fiber by a process involving a combination of H3PO4 hydrolysis, enzymatic hydrolysis, and sonication. They showed that the enzymatic hydrolysis step following H3PO4 was effective in enhancing the CNC yield. Moreover, they showed that enzymatic hydrolysis imparted improved dispersion, increased crystallinity, and enhanced thermal stability to the resulting CNCs. In another study, facile extraction of thermally stable CNCs via HCl hydrolysis of cellulose raw material under hydrothermal conditions was reported by Yu et al. (2013). They obtained thermally stable CNCs with a high yield of 93.7% by combining hydrothermal hydrolysis and neutralization of the acid with ammonia. They achieved highly stable aqueous CNC suspensions, owing to the presence of ammonium groups. Table 1 presents some of the recent data obtained on the effect of cellulose source and acid hydrolysis conditions on the yield of CNCs.
Hydrolysis with solid and gaseous acids
In addition to the poor thermal stability of the final CNCs, acid hydrolysis has some other disadvantages as well such as acid corrosion of equipment, health, and environmental hazards as in addition to high energy and chemical consumption. Recently, a number of studies have focused on replacing liquid acids with solid acid. Liu et al. (2014) demonstrated a green and sustainable method for the preparation of CNCs from bleached hardwood pulp by using concentrated phosphotungstic acid (H3PW12O40) hydrolysis. They obtained rod-like CNCs that were 15–40 nm in width and hundreds of nanometers in length. They concluded that the resulting CNCs exhibited much better thermal stability than the partially sulfated CNCs prepared by H2SO4 hydrolysis. Moreover, the solid acid could be easily recovered and recycled by extraction with diethyl ether. However, the high cost of solid acid, prolonged hydrolysis time, and low yield are disadvantages of solid acid hydrolysis. In order to overcome these drawbacks, Hamid et al. (2015), reported that sonication at an optimum sonication power of 225 W combined with hydrolysis using solid phosphotungstic acid dramatically reduced the operating time from 30 h to 10 min. The CNCs obtained were 15–35 nm in diameter and 150–300 nm in length with about 88% crystallinity and 85% yield.
Hydrolysis with gaseous acid is another technique for CNC production. In this technique, wet cellulose with high moisture content is hydrolyzed in the presence of an acidic gas, which is absorbed by the cellulose fibers. The acidic gas reacts with the moisture in the material, producing a high local acid concentration. This leads to a high rate of hydrolysis in the amorphous domains and local inter-fibril contacts. Subsequently, mechanical treatment such as grinding or ultrasonication is required for further defibrillation and for producing CNCs. Various types of gaseous acids such as HCl, HNO3, and trifluoroacetic acid can be used in this procedure (Kontturi et al. 2012).
Hydrolysis with metal salt catalysts
Transition metal salts have also been used as homogeneous acid catalysts for the cellulose hydrolysis process. They are generally categorized based on their valence state into monovalent (e.g., NaCl, KCl), divalent [e.g., CaCl2, FeCl2, FeSO4, Mn(NO3)2], and trivalent [e.g., FeCl3, Fe2(SO4)3, Al(NO3)3, Cr(NO3)3] catalysts. They are effective as catalysts for the degradation of the glycosidic linkages in cellulose during the acid hydrolysis process (Liu et al. 2009; Yi et al. 2013). It is worth noting that the valence state of the transition metal ions plays an important role in determining the hydrolysis efficiency. During hydrolysis, hydronium ions (H+) are generated due to polarization between the metal ions and water molecules and these ions effectively co-catalyze the acid hydrolysis reaction in the presence of metal ions (Kamireddy et al. 2013; Yahya et al. 2015). Li et al. (2013a, b) reported that the crystallinity of nanocellulose improved by 19% compared to native cellulose upon treatment with Fe(III) ions. On the other hand, Yahya et al. (2015) found that the addition of an Ni(II) inorganic salt resulted in an increase in the crystallinity of nanocellulose. In addition, the different valence states of the transition metals could affect the yield of the final product (Li et al. 2013a, b).
Recently, Chen et al. (2016) studied the efficiency of three different transition metal salts, namely Fe(NO3)3, Co(NO3)2, and Ni(NO3)2, which acted as co-catalysts along with H2SO4 for the preparation of CNCs. They found that these transition metal salts were able to selectively degrade the amorphous structure of cellulose and increase the crystallite size (8.12–27.8 nm) as well as improve the crystallinity index (65.5–70.3%), compared to native cellulose. In addition, the higher trivalent oxidation state of the Fe(III) cations allowed more effective hydrolysis during the preparation of the cellulose crystallites compared to systems with divalent cations (Co(II) and Ni(II)). The CNCs obtained were 18.5–31.5 nm in diameter. In another study conducted by Yahya et al. (2015), it was found that the CNCs produced by acid hydrolysis were shorter in length and had lower aspect ratio compared to nanocellulose produced by catalysis with nickel.
Pretreatment
There are two major difficulties associated with the mechanical fibrillation process for producing nanocellulose. Firstly, mechanical disintegration of the fibers into nanocellulose requires a high amount of energy and secondly, the fibers aggregate when the slurry is pumped through the disintegration device. Efficient pretreatment is known to help reduce energy consumption by 20–30 times (Siró and Plackett 2010). For example, by combining mechanical treatment with chemical or enzymatic treatments, it is possible to decrease the energy consumption from 20,000–30,000 kWh/ton to around 1000 kWh/ton (Pääkkö et al. 2007). The choice of the pretreatment method is dependent on the cellulose source and, to a lesser degree, on the desired morphology of initial cellulose for further treatment. It is worth noting that appropriate pretreatment of cellulose fibers promotes accessibility, increases the inner surface area, alters crystallinity, breaks hydrogen bonds, and boosts the reactivity of cellulose. Consequently, the energy demand is decreased and the nanocellulose production process is promoted with pretreatment (Abdul Khalil et al. 2014; Šturcová et al. 2005). For example, during the pretreatment of plant materials, non-cellulose components such as hemicellulose and lignin are partially or completely removed and the individual fibers are isolated (Hubbe et al. 2008). In the case of bacterial nanocellulose, bacteria and other impurities are removed from the slurry during pretreatment (Ashjaran et al. 2013). Pretreatment is a very important step, because it can alter the structural organization, crystallinity, and polymorphism of cellulose, as well as various properties of the pretreated feedstock (Mariano et al. 2014). Various pretreatment techniques have been reported in the literature including the pulping process, bleaching and alkali treatments (Chirayil et al. 2014), enzymatic treatment (Tibolla et al. 2014), and oxidation (Cao et al. 2012; Missoum et al. 2013). Carboxymethylation and acetylation of the fibers followed by homogenization is another effective technique for reducing energy consumption during CNF production (Aulin et al. 2009; Taipale et al. 2010; Tingaut et al. 2009). Similarly, mechanical refining, grinding, and cryocrushing have been also used to produced CNFs. Additionally, microwave-assisted pretreatment (Chowdhury and Abd Hamid 2016), e-beam irradiation (Kim et al. 2016), chemical swelling (Haafiz et al. 2014) and extrusion pretreatments (Olea et al. 2015) have been proposed recently for use in the CNC production process. More information on the energy consumption during the manufacturing of CNFs and CNCs as well as various effective pretreatment techniques for the energy efficient manufacturing of nanocellulose can be found in a report by Bharimalla et al. (2015).
Surface modification of nanocellulose
Nanocellulose particles exhibit some properties that can limit their applications. CNCs and CNFs are sensitive to moisture, are hydrophilic, and exhibit low thermal stability (Siqueira et al. 2010). During the last decade, many techniques have been suggested for overcoming these limitations. Methodologies for surface modification and fiber pretreatment are now well developed and can be used to improve specific properties of the nanoparticles, particularly to alleviate difficulties in dispersing them in apolar solvents or polymers.
The nanosized structure is responsible for the exponential increase in the hydrogen bonding-induced aggregation of these materials. Surface modification can be used, for example, to impart new steric or electrostatic effects to the particles (Araki 2013). Additionally, the generation of radical groups, covalent bonds, and coating of the particles have been suggested as methods for improving the surface properties of cellulose. These routes can decrease the surface energy of the particles, allowing the nanomaterial to be used in a broader number of systems.
Cellulose can be chemically modified owing to the presence of active sites that can be modified under certain conditions. There are three hydroxyl groups in each of the cellobiose member rings. The secondary (C2 and C3) and primary alcohol (C6) groups can be substituted by other functional groups and long chains. Additionally, they can be oxidized as well.
However, native cellulose is arranged in the form of fibers and most of the chains remain unexposed. In the solid state, a major portion of the hydroxyl groups resides within the structure and does not participate in surface chemical reactions. For example, in the microscopic cellulosic fibers, only 2% of the hydroxyl groups are accessible at the surface (Dufresne 2013). The degree of substitution of the hydroxyl groups (DS) can be increased by maximizing the available surface area and exposing the internal hydroxyl groups by using, for example, swelling agents (Crawshaw et al. 2002; Lazko et al. 2014).
In pristine fibers, the amorphous domains are considered to be regions with very different surface reactivity compared to crystalline domains. Since the amorphous phase has only a few hydrogen bonds among the chains and the bonds are weaker compared to those in the crystalline regions, these regions become more accessible. Experimental results show that the reaction rates for C2, C3, and C6 hydroxyl groups in the amorphous phase are similar, which is different from the behavior in the crystalline phase (Rowland and Howley 1988; Rowland and Roberts 1972). In the crystalline phase, the strong intra-molecular bonds make OH3 (OH bonded to C3) almost unavailable for reaction. However, the other hydroxyl groups exhibit intermediate or high reaction rates (Rowland and Howley 1988).
Covalent modifications
It is clear that the chemical reactions in nanocellulose exhibit some unique features. The higher crystallinity, accessibility to hydroxyl groups, and surface energy impart chemical properties to nanocellulose that are different from those of pristine fibers. First, it is important to keep the crystalline structure intact and avoid softening or liquid retention during reaction. This is crucial since the mechanical properties of the nanoparticles are important in most of the applications. The reactions occur on the particle surface and swelling or dissolution agents are not typically employed. Such agents are unnecessary because the higher surface area of the nanoparticles increases the total number of available –OH groups compared to the pristine fiber.
Hettegger et al. (2016) have visualized quite well, the influence of particle size on the surface reactivity. They managed to graft a fluorescent molecule onto micro and nanocellulose surfaces through a simple click-chemistry reaction. By measuring the fluorescence of the material, it was possible to observe a very high DS for the nanofibers. There are a number of other reports on the surface modifications of cellulose nanomaterials by the grafting of acrylamide (Yang and Ye 2012), tetra alkyl ammonium (Trifol et al. 2016), rosin (de Castro et al. 2016), ascorbic acid (Filpponen and Argyropoulos 2010), and others functional molecules. Grafting techniques such as esterification, etherification, oxidation, and radical reactions will be discussed in detail next.
Non-covalent modifications
Hydrophobicity can also be induced on the nanocellulose surface via adsorption of molecules or macromolecules such as surfactants, oligomers, or copolymers. These additives can cover the nanoparticles and interact with the surface by electrostatic and van der Waals forces or hydrogen bonds, thereby imparting hydrophobic characteristics to the particles (Habibi 2014).
Surfactants were used to stabilize CNC particles in nonpolar solvents for the first time towards the end of the 1990s (Heux et al. 2000). It is now clear that surfactants can modify the surface properties of nanoparticles and thereby improve their stability and dispersion. Recently, the effect of surfactant addition in CNC systems on the dispersion of the nanofiller in water (Hu et al. 2015), organic solvents (Abitbol, et al. 2014; Chi and Catchmark 2017), and even polymers during melt extrusion (Nagalakshmaiah et al. 2016a, b) was examined. The results of some small angle neutron scattering and UV spectroscopy experiments suggest the presence of very thin layers (15 Å) of surfactant around the CNCs (Bonini et al. 2002; Elazzouzi-Hafraoui et al. 2009). The use of anionic (Heux et al. 2000) or non-ionic molecules (Hu et al. 2015) as nanoparticle coatings has been reported in the literature. While the effect of anionic surfactants on the properties of iridescent solid films (Bardet et al. 2015) has been investigated recently, cationic surfactants are used more commonly in practice. The surface charges of CNCs (Lin and Dufresne 2014b; Shafeiei-Sabet et al. 2013) and CNFs (Li et al. 2015) are often reported to be negative in the literature. Therefore, cationic surfactants, which carry positive charges, would be attracted to the CNC or CNF surfaces.
Uncharged polymers also appear to adsorb or at least interact with CNCs. Ben Azouz et al. (2011) were able to decrease the viscosity of an aqueous solution of polyethylene oxide (PEO) by adding a certain amount of CNC to the system. This indicates that some of the PEO polymer chains interacted with the nanoparticle surface. With subsequent increase in the PEO concentration, a threshold level was overcome (surface saturation limit was overcome) and the system viscosity increased again.
Mercerization
While the mercerization process is not exactly a surface modification method used to achieve functionalization, it is related to the organization of the chains into different polymorphs. In this process developed by John Mercer, cellulose I is transformed into cellulose II using concentrated solutions of NaOH. Normally, this transformation occurs at an NaOH concentration (by weight) of around 17% (Dinand et al. 2002).
Since the 1980s, Sarko, Nishimura, and Okano have studied the mechanism involved in the mercerization process (Nishimura 1987a,b; Okano and Sarko 1984, 1985). The results of these studies suggest that the transformation of the crystalline phase from cellulose I (with parallel chains) to cellulose II (antiparallel chains) passes through an intermediary state known as Na-cellulose (with antiparallel chains), which consists of organized swollen cellulose induced by fiber hydration.
There are different theories on how cellulose conversion actually occurs. Some authors suggest that the network of hydrogen bonds is disrupted during mercerization owing to cellulose swelling. In that case, long chains can undergo conformational change through folding (Simon et al. 1988). Further, it has also been suggested that the crystalline conversion process, in fact, depends on the presence of the amorphous phase. At the interface between the crystalline and amorphous domains, it is possible to find parallel and antiparallel chains close to each other with random placement. This allows the deposition of antiparallel chains in the crystalline form during precipitation. This antiparallel cellulose II structure appears to possess a lower energy and is more stable than the pristine cellulose I arrangement. Na-cellulose transforms into cellulose II with NaOH removal. The conversion of cellulose I to cellulose II is irreversible and depends on the amorphous phase content in the fiber, since type II cellulose is less crystalline than type I. Kobayashi et al. (2011) found that during the removal of water from hydrated cellulose, the chains progressively contract, resulting in reduced distance between the cellulose sheets. Simultaneously, the internal structure of the chains is maintained.
Mercerization as well as ball milling (Nge et al. 2013) can be useful methods for preparing CNCs with the type II morphology (Borysiak and Grząbka-Zasadzińska 2016; Yue et al. 2012). CNC nanoparticles with the cellulose-II structure can exhibit different morphologies depending on the synthesis processes used such as regeneration, hydrolysis of the mercerized cellulose II pulp, or mercerization of CNC nanoparticles with the type I structure (Hao et al. 2015; Jin et al. 2016; Kim et al. 2006). Some papers report the preparation of spherical or irregular nanocrystals composed of cellulose II or mixtures of I and II polymorphs. The different morphologies are well illustrated in the study by Dhar et al. (2015), who prepared various types of CNCs from raw bamboo. The authors were able to obtain CNC I, CNC II, and CNC I → II (i.e., a type II polymorph converted from previously isolated CNC I). The morphologies of the particles obtained are presented in Fig. 2.

Adapted with permission from Dhar et al. (2015), Copyright 2015. Reproduced with permission from Royal Society of Chemistry
SEM images of three different CNC polymorphs: a CNC I, b CNC II, and c CNC I → II.
Esterification
Esterification of nanocellulose particles may be used as a method for attaching functional molecules to the material surface. New ester bonds are created during this process by the reaction of carboxylic acids and alcohols, as illustrated in Fig. 3. This reaction is widely employed for the production of commercial grades of cellulose derivatives such as CA.
The acylation reaction is one such specific esterification route. This reaction is based on the introduction of acetyl-COCH3 groups on the cellulose surface, resulting in the formation of new ester bonds. For example, pristine cellulose can be converted into CA by its reaction with acetic anhydride in an acidic medium. This specific type of reaction is known as acetylation (Jonoobi et al. 2010; Lin et al. 2011).
Different routes have been suggested for the acylation of cellulosic materials. This includes the use of catalysts such as trimethylamine (de Menezes et al. 2014), imidazole (Pires et al. 2015), and lipase (Božic et al. 2015) to introduce different functional molecules on the cellulose surface by the formation of new ester bonds (Ji et al. 2015). Some specific routes were also proposed by Stenstad et al. (2008) who studied the oxidation of C3. This reaction involved glucose ring opening and the formation of a reactive three-member ring.
The esterification reactions can be performed in aqueous or non-aqueous media. Huang et al. (2012) used a single step process to produce functionalized CNFs through ball milling in DMSO, creating a flow-birefringent suspension of modified nanoparticles in the organic solvent. Pasquini et al. (2008) used dodecanoyl chloride in pyridine to promote a nucleophilic reaction with the elimination of HCl. This process resulted in the insertion of long chains on the surface of sugar cane cellulose fibers. Espino-Pérez et al. (2014), developed a “green” SolReact method, wherein a series of nontoxic carboxylic acids were simultaneously used as both grafting agents and solvents, facilitating the insertion of phenolic groups on the CNC surface without employing hazardous solvents.
Several interesting applications have been developed based on this reaction in various areas. Saini et al. (2015) used the esterification reaction to attach benzyl penicillin to MFC, in order to synthesize antimicrobial materials. Nielsen et al. (2010) grafted fluorescent molecules on CNCs, converting the nanocrystals into pH-sensors and leading the suspensions to present fluorescent properties as illustrated in Fig. 4a.

Adapted with permission from Nielsen et al. (2010) and Cunha et al. (2014), Copyright 2010 and 2014. Reproduced with permission from Royal Society of Chemistry and American Chemical Society
a Suspensions of pH responsive CNC at increasing pH values and DFM micrographs of o/w/o emulsions stabilized by b NFC/CNCC12, and c CNC/CNCC12.
Cunha et al. (2014) grafted CNFs and CNCs with lauroyl chloride (C12) through ester bonds. A combination of various nanoparticles was used to stabilize the oil/water/oil interfaces. Owing to the C12 chains grafted on the particles, they were able to build a double layer of nanoparticles and control the composition of the inner (CNF/CNC) and outer (CNCC12/CNFC12) layers. Some images of the resulting emulsions are shown in Fig. 4b, c. Alternatively, the oxidation of hydroxyl to carboxylic groups on nanocellulose can follow many routes. The newly introduced C = O groups can act as a receptor of free electrons from the nucleophile. The TEMPO reagent is the most frequently employed oxidant and will be discussed further in this section.
The most popular esterification reaction involving nanocellulose materials occurs during the isolation of CNCs using H2SO4 and is known as sulfonation. Sulfonation takes place as side reaction and leads to the formation of organosulfates during the hydrolysis of the amorphous phase of cellulose. During the reaction, H2SO4 attacks the C6, with subsequent elimination of water. The reaction scheme is given in Fig. 5.
The extent of esterification strongly depends on the reaction conditions employed such as temperature, fiber/acid ratio, and hydrolysis time (Bondeson et al. 2006; Teodoro et al. 2011). Besides, factors such as cellulose source and pretreatment methodologies that can modify the accessibility of the chains are also relevant. Desulfonation has been studied over the last several years and many techniques such as basic (Roman and Winter 2004), solvolytic (Jiang et al. 2010), and even auto-catalyzed acidic desulfation (Beck and Bouchard 2014) have been proposed.
Silylation
Reaction with silyl (R3Si-) groups, i.e., silylation, is possible for nucleophilic groups such as alcohols, carboxylic acids, and amines. Similar to esterification, silylation is used to attach molecules to the surface of the nanoparticles via covalent bonding. One of the first reports discussing the use of silylation to graft molecules on the nanocellulose surface was published by Goussé et al. (2002). In this work, a series of alkyldimethyl chlorosilanes were used to stabilize CNCs in organic solvents. Owing to the newly acquired hydrophobic characteristics imparted by the long chains, the CNC particles were stable in hydrophobic organic solvents. However, the authors called attention to the possibility of “over silylation”, wherein the particle chains on the surface become soluble in the reaction medium and microfibrillar characteristics are lost (Goussé et al. 2004). The scheme of the reaction between cellulose and chlorosilane is shown in Fig. 6.
Over the last several years, similar reaction mechanisms have been employed in other studies to introduce silane groups on the cellulose surface, with the goal of improving the mechanical properties of polymers (Pei et al. 2010), achieving antimicrobial activity (Saini et al. 2016), and improving the wetting properties of aerogels by non-polar liquids (Aulin et al. 2010).
Kämäräinen et al. (2016) have fabricated a material with potential biomedical uses, specifically for diagnostic applications. Using the photolithography technique, the researchers were able to deposit a chemisorbed hydrophobic alkylsilane layer on a CNF film. The shape of the alkylsilane layer could be controlled via oxidation by exposure to a UV/ozone environment. Photolithography technique allowed spatial control of the adsorption of bovine serum albumin (BSA) on the support by altering the pH. Figure 7 schematically illustrates the construction of the material.

Adapted with permission from Kämäräinen et al. (2016), Copyright 2016. Reproduced with permission from Springer
Schematic illustration of a CNF film modified through the chemisorption of n-octyldimethylchlorosilanes and creation of a hydrophilic-hydrophobic pattern using photolithography.
Etherification
Etherification involves the creation of new ester bonds (R–O–R′) on the surface of cellulose materials containing hydroxyl groups. This reaction is also well-known for cellulose materials because of its industrial importance during the preparation of carboxymethyl cellulose. It is an easy method for conducting the defibrillation of fibers during the preparation of NFCs (Habibi 2014). Similar to the esterification reaction, etherification of cellulose has been studied over the last 30 years and progressive improvements have been made in the regioselective mechanisms by using appropriate solvents (Fox et al. 2011). In the case of nanocellulose, cellulose dissolution should be avoided and etherification occurs only on the particle surface. There does not appear to be any preference for specific hydroxyl groups in this reaction, especially if the modification is aimed at improving the compatibility of nanocellulose with a hydrophobic polymer, for example.
Bae and Kim (2015) conducted the reaction shown in Fig. 8, wherein long hydrophobic chains were grafted on the nanocellulose surface by reacting a cellulose alcoholic group with an alkyl group with subsequent elimination of a halide ion (Br−). In this reaction, R refers to octane and dodecane groups. A similar mechanism was used by Hassan et al. (2012) to graft modified terpyridine, resulting in the formation of a highly fluorescent nanocellulosic material with potential applications in LEDs and solar cells.
Furthermore, etherification can also be performed by the copolymerization of acrylic derivatives (Anirudhan and Rejeena 2014) or a mixture of nanoparticles with the epoxy matrix (Dufresne and Belgacem 2013). Hasani et al. (2008) prepared cationic CNCs by the reaction of the hydroxyl groups on cellulose with the epoxy group present in the (2,3-epoxypropyl)-trimethylammonium chloride (EPTMAC) molecule. This reaction resulted in a positively charged nanoparticle with modified gelling and rheological properties. Cationic nanofibrillated cellulose was also produced by Ho et al. (2011). In this study, trimethylammonium was grafted on the surface of cellulose pulp by etherification using a HPH to disintegrate the pulp, resulting in the formation of CNFs. Recently, Gorgieva et al. (2015) also grafted a hydrophobic anthraquinone derivative on the surface of nanocellulose particles by creating ether bonds. Several characterization techniques such as light scattering were used to quantify the agglomeration of the particles. Modification of the nanofibers in the suspension was studied using labeled fibers.
Polymer grafting
Long chains such as polymers or oligomers can be grafted on the surface of nanocellulose by creating new covalent bonds using the previously described methodologies such as esterification, acetylation, and silylation, among others (Roy et al. 2009). Surface modification occurs by the reaction of the active sites on the cellulose surface with the reactive end groups found in the macromolecules used. In general, all the reactions can be achieved by two methodologies, namely “grafting from” and “grafting onto”. This nomenclature is more closely related to the general reaction steps than to the formation of new covalent bonds between the particles and polymeric chains.
The “grafting from” methodology is based on the in situ growth of polymer chains grafted onto the surface. The new chains will grow attached to the surface by the hydroxyl groups, which act as initiators. Some examples are based on ring opening polymerization (Tehrani and Neysi 2013) and radical polymerization techniques including atom transfer radical polymerization (Wang et al. 2015; Yin et al. 2016; Yu et al. 2016). On the other hand, “grafting onto” polymerization involves the simple attachment of the desired polymer onto the particle surface. Some of these reactions have been performed by click chemistry (Benkaddour et al. 2013; Nielsen et al. 2010), using epoxy groups (Li et al. 2010), or nucleophilic addition (Lin and Dufresne 2013). Besides the “grafting from” and “grafting onto” methodologies, many other interesting examples of polymer grafting can be found in the literature. A few examples are provided in Table 2.
As discussed earlier, the insertion of polymers can decrease the surface energy of the nanomaterials as a result of increase in the hydrophobic characteristics of the particles. In addition, physical entanglements can be created between the matrix chains and grafted chains when the latter exhibit the same characteristics as the bulk polymer. As a result, the grafted chains become an effective part of the matrix, while being covalently bonded to the nanoparticles at the same time. This further increases the compatibility between the two components and promotes other effects such as co-crystallization of the polymer chains.
However, the grafted polymers are normally long chains with a higher volume than the pristine hydroxyl groups around the nanoparticles. The presence of these long chains restricts the interactions between the nanoparticles through hydrogen bonding. Consequently, in the case of CNCs, the formation of a particle percolation network can be prevented, which forms the basis of the outstanding mechanical properties presented by nanocomposites.
TEMPO-oxidation
TEMPO-oxidation is one of the most popular surface modification methods for nanocellulose materials. The purpose of this reaction is to transform the surface hydroxyl groups into carboxylic acid groups and the reaction is performed by the oxidation of nanocellulose using 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) reagent. This reaction was introduced by Davis and Flitsch (1993) and adapted to glucans by De Nooy et al. (1995) as a technique to selectively oxidize primary alcohols such as the hydroxyl group present in cellulose C6. As a result of this reaction, the hydroxyl group is oxidized to carboxylic acid (–COOH), which is useful for subsequent substitution reactions.
Under certain conditions, the TEMPO reagent transforms into an N-oxoammonium salt (R1R2N+=O), which acts as a catalyst for further reaction. The catalyst is restored to its TEMPO form by sodium hypochlorite and sodium bromide. The general reaction mechanism under basic conditions is presented in Fig. 9. In the case of cellulose, it has been found that the TEMPO reaction can cause some depolymerization. However, the probable cause of the 2,3-scission of glucose units is the presence of secondary oxidants that can be aggressive towards cellobiose such as sodium hypochlorite (De Nooy et al. 1995). The reaction can be conducted under acidic or basic pH values. The reaction mechanism and reaction kinetics strongly depend on the pH and temperature conditions. In addition, parameters such as reaction time and TEMPO concentration should be controlled in order to minimize secondary reactions.
The TEMPO-oxidation reaction is normally quantified by the DS of the hydroxyl groups, which is normally measured by conductometric titration. Carboxyl group concentrations of around 1 mmol/g indicate that the oxidation process occurred successfully (Hoeng et al. 2015; Martoïa et al. 2015; Fraschini et al. 2017). In the case of CNC, TEMPO modification is usually used to obtain unoxidized intermediaries, which serve as functional groups for posterior grafting. In a remarkable study, Mangalam et al. (2009) were able to attach DNA molecules to the CNC surface subjected to TEMPO reaction. The polynucleotide-cellulose complex obtained exhibited reasonable chemical stability and was promising for potential applications in nucleic acid research. TEMPO-oxidized materials are popular as support for biomedical and packaging applications. TEMPO-nanocellulose has already been reported as a promising material for the adhesion and proliferation of cells, in addition to exhibiting antimicrobial activity (Jiang et al. 2016; Lin and Dufresne 2014a).
Besides the creation of new organic functionalities, TEMPO-oxidation is a popular method of cellulose pretreatment (such as enzymatic treatment) to obtain CNFs through mechanical shearing (Martoïa et al. 2015; Nechyporchuk et al. 2014). When TEMPO-oxidation is applied to pristine fiber, defibrillation becomes easier and the process consumes less energy. Carboxylic acid content of around 300 μmol/g appears to be sufficient for reducing the number of passes required to obtain MFC gel (Besbes et al. 2011). In general, the gel can be obtained using 5–60 passes, depending on the cellulose source and pretreatment method applied (Nechyporchuk et al. 2014; Nge et al. 2013).
Radiation
The use of energy sources other than heat is not new in organic or polymer chemistry. Low energy radiation is normally used for material characterization. Additionally, a number of radical reactions are initialized by UV light, which is common in polymerization reactions. Additionally, different energy levels can be accessed depending on the wavelength of the radiation source.
High energy radiation can be applied for modifying the surface of cellulosic materials. For example, gamma radiation is a type of high energy ionizing radiation, which can lead to the formation of reactive intermediates such as ions and free radicals. These species promote several reaction pathways including polymer and molecule grafting, oxidation, cross-linking, and scission degradation. Some examples of the application of radiation in cellulose processing are listed in Table 3.
The application of gamma radiation in the processing of cellulose nanomaterials is quite new and only a few studies exploring this technique are available in the literature. Khan et al. (2013) were able to decrease the oxygen and carbon dioxide permeabilities of PCL/CNC by treating the composite surface with gamma radiation. A few years earlier, the same group had reported mechanical and permeability modifications of a nanocellulose/methylcellulose composite film and had demonstrated that the modifications depended on the radiation intensity.
UV light, which is lower in energy than gamma radiation, can be used to create supramolecular healable materials from cellulose nanocomposites (Coulibaly et al. 2013). UV light has been used in the traditional manner as a photo initiator in some studies, for producing composites by in situ polymerization. Recently, Wang et al. (2016) functionalized CNC surfaces by attaching ethacryloyl groups to produce CNC-bis(acyl)phosphane oxide, which was used as a polymerization initiator. The monomers used in this study included methyl methacrylate (MMA), butyl acrylate, N-isopropylacrylamide (NIPAAm), and 2-hydroxyethyl acrylate (HEA). All the reactions were activated by the incidence of light.
Microwave energy has also been applied to chemical processes for a number of decades. The so-called “microwave chemistry” is utilized in many processes for promoting sample digestion, extraction, chemical reactions, pre-concentration, and desorption of analytical substrates. Some common applications of “microwave chemistry” include catalysis, environmental engineering, and nuclear chemistry and engineering (Zlotorzynski 1995). In the context of cellulosic materials, this technique is applied for the purpose of improving hydrolysis by controlling the experimental conditions. The method exploits the fact that the polar water molecules held within the cellulose structure absorbs microwave energy, whereas nonpolar materials do not. An advantage of microwave chemistry is that it can provide excellent heat transfer within the cellulose samples, since the heat originates from electromagnetic energy and not by direct contact (Hesas et al. 2013). Many papers have reported the use of microwave radiation in cellulose hydrolysis (Ni et al. 2015; Teng et al. 2016).
Kaith and Kalia (2008) grafted flax fibers with several binary vinyl monomers from mixtures consisting of MMA/vinyl acetate, MMA/acrylamide, and MMA/styrene under the influence of microwave radiation and found that under optimal conditions, a power of 210 W was required to achieve 24.64% grafting. As expected, the grafted fibers became moisture depleted and the inclusion of the grafted fibers into a phenolic matrix improved the mechanical properties of the matrix. More recently, some papers have reported the use of microwave energy for cellulose acetylation via an iodine-catalyzed reaction. This approach is interesting because it is a solvent-free method and different DS values can be achieved by controlling the temperature and duration of the reaction, with best results achieved for only 30 min of reaction (Eranna et al. 2016; Zepič et al. 2015).
Plasma
A relatively new and rapidly developing strategy for surface modification involves the use of ionized gases (i.e., plasma). The gaseous matter in a plasma contains a large amount of active particles, which promote chemical and physical reactions on the surface of organic materials, imparting hydrophilic or hydrophobic characteristics to the material.
Plasma is formed when a high voltage is applied between two electrodes. The electrons produced are accelerated and collide with the substrate breaking covalent bonds and producing radicals. Similarly, electrons in the atmospheric plasma can collide with the air particles, forming ozone species, which oxidize the surface of the substrate (Rouette 2001). Plasma modification can be used to functionalize cellulose substrates with styrene (Parida et al. 2012), titanium, and zinc oxide (Jazbec et al. 2015; Mihailović et al. 2011), improve protein immobilization (Zhao et al. 2016), and for various other applications.
Nanocellulose aerogels were plasma modified by Lin et al. (2015) and Shi et al. (2013). In both studies, it was possible to obtain hydrophobic aerogels with excellent adsorption properties. These aerogels could be used as oil adsorbent materials or heat insulators. The functional characteristics of the modified surface, in fact, depend on the type of plasma used. Different types of plasmas such as O2, N2, and CF4 can generate hydrophilic (O2 and N2) or hydrophobic (CF4) materials (Kurniawan et al. 2012). Flynn et al. (2013) also reported surface modification by means of a combined ammonia–nitrogen plasma. Moderate nitrogen group functionalization was observed.
Further, Nourbakhsh (2015) studied the interactions of polyester and nylon with nanocellulose by plasma and laser treatment. Both treatment processes were able to enhance the compatibility of the polymers with the nanocellulose layer deposited from a suspension. However, the final materials showed different morphologies when observed by SEM.
Life cycle assessment of nanocellulose
LCA, also known as life cycle analysis, is a particularly useful environmental management tool for assessing and evaluating the environmental burden induced by the production, use, and disposal of a product or the provision of a service (Duda and Shaw 1997; Walker et al. 2015). In other words, LCA attempts to measure the total environmental effect of a product “from cradle to grave”. LCA has been recommended by the National Nanotechnology Initiative and National Research Council (2011, 2012) and is widely used for a variety of purposes including hotspot identification, material selection and product design, and product and process comparisons (Walker et al. 2015). In fact, LCA can be employed to identify methods that result in the best environmental performance of nanomaterials. Methods that cause minimal impact with no loss in the desirable technical attributes of nanomaterials constitute the best choices for up-scaling, while the other methods must be reanalyzed to identify alternative inputs or production pathways. Additionally, LCA is useful for comparing alternatives, identifying hotspots, choosing production routes and improving processes themselves. It can challenge conventional wisdom and advances the knowledge base (Curran 2014; Kralisch et al. 2015; Finnveden et al. 2009).
LCA is governed by a series of standards issued by the International Organization for Standardization (ISO), in particular 14040 and 14044 (2006a, b). Although the ISO gives a consensus definition for LCA and provides a general framework for conducting an assessment, it has some limitation and it leaves much to interpretation by the person conducting the assessment.
As example, data availability is one of the limitations for the early research stage LCA. Collecting data for an existing process can be difficult and this task becomes even more problematic when data for a not yet established process is needed. Laboratory processes can be completely different than the same step in a production plant as the equipment and vessels used are not comparable at all to the machineries of industrial plant. Furthermore, Laboratory processes are often far from being optimized, especially in terms of resources efficiency and do not have the advantage of economies of scale. Therefore, a comparison with an existing competing product often disfavors such a new, sustainable product under development through underestimation of its potential (Piccinno et al. 2018). Consequently, LCA studies have been criticized for producing different results for seemingly the same product. Furthermore, an aspect that is simply a characteristic of LCA methodology may be perceived as a limitation if it does not fulfil the user’s immediate need. For example, the LCA frame work does not take social welfare into consideration and someone who is interested in understanding the social aspects of product is recommended to apply some other tool. Other limitations are inherent in the design of LCA methodology and how it was intended to be conducted. Another limitation occurs during application when the modeler has alternative approaches from which to choose, leading to widely varying results from case to case. However, these limitations can be improved through research, or using alternate modeling choice (Curran 2014). Despite these limitation, LCA offers a strong environmental toll in the way toward sustainability.
LCA consists of 4 stages. The first stage involves definition of the scope of analysis. In this stage, the unit of analysis and system boundaries of the study are established. The second stage involves analysis of the life cycle inventory (LCI) and includes data collection and balance calculations for all the unit processes in the life cycle. The results are presented in the form of inputs and outputs for the entire system. The third stage involves life cycle impact assessment (LCIA). The results from the LCIA analysis can be converted into a quantified measure of the potential environmental and/or human health impact using the so-called characterization factors (CFs), in this stage. The final stage of LCA is the interpretation of results, which is based on all the three previous stages of assessment (Gilbertson et al. 2015; Hohenthal et al. 2012; Klöpffer 1997).
The assessment can be made from cradle-to-gate, cradle-to-customer, or end-of-life. In the cradle-to-gate studies, only the raw material extraction and production stages of the life cycle are considered. On the other hand, in the cradle-to-customer studies, steps until the product has been transported to the customer are considered. Most of the studies are restricted to the cradle-to-gate scope and do not consider the use or end-of-life impacts of a product. Only a few studies have reported the inclusion of end-of-life stage in the LCA. This stage may involve analysis of nanoemission from products in the pure form. Therefore, none or almost none of the studies are fully ISO-compliant (Gavankar et al. 2012; Hohenthal et al. 2012; Kekäläinen 2013).
LCA has been used for nanomaterials, products containing nanomaterials, and manufacturing processes involving nanomaterials such as CNFs (Khanna et al. 2008b), polymer nanocomposites (Khanna et al. 2008a), socks with silver nanoparticles (Meyer et al. 2011), semiconductor manufacturing (Krishnan et al. 2008), plasma spraying (Moign et al. 2010), and titanium dioxide (Grubb and Bakshi 2011). However, only a few studies have addressed the LCA of nanocellulose so far.
The first study on the environmental impact of nanocellulose was reported by Hohenthal et al. (2012). In this report, a cradle-to-gate LCA for NFC was conducted using the impact assessment methodology, ReCiPe, to provide values for climate change (CC) potential, eutrophication, terrestrial acidification (TA), and fossil fuel depletion. This LCA used a combination of lab and pilot scale measurements, estimates, and expert opinions to assess three different processing routes, including enzymatic pretreatment with HPH, chemical (TEMPO-oxidation) pretreatment with HPH, and chemical pretreatment with mechanical refinement in a Cavitron disperser. From an environmental perspective, the main differences between the various NFC production options include electricity consumption, raw material efficiency, and water consumption. NFC production with enzymatic pretreatment is an energy-intensive process, with high yield and low water consumption. While chemical pretreatment of NFC consumes less energy, more water is required for the process and it has a lower yield. The embodied environmental burden of the chemicals used in the production of NFC is another important threat. It was found that the effluents from TEMPO and NaBr treatments can cause sufficient cleaning technology addition and treatment can lower this burden. Assessment of the biodegradability of both functionalized and natural NFCs in an aqueous environment indicated that the functionalized NFCs have a lower biodegradation rate than unmodified NFCs. However, the threshold biodegradation limit of 90% was reached within the test duration. The authors also assessed the economic and social sustainability of NFCs, in addition to conducting an LCA assessment of NFC-coated board, for which data can be found (Hohenthal et al. 2012).
Another study on the LCA of nanocellulose was reported by Li et al. (2013a, b). They conducted a LCA of four comparable chemical–mechanical routes for lab scale nanocellulose fabrication through a cradle-to-gate approach with the Eco-Indicator 99 impact assessment method. Figure 10 shows the cradle-to-gate LCA system boundary of the lab-scale production of MFCs.
The nanocellulose fabrication routes considered in this LCA are presented in Fig. 11. The starting material for producing nanocellulose was delignified kraft pulp. The various routes considered included TOSO (TEMPO-oxidation for chemical modification, sonication for mechanical disintegration), TOHO (TEMPO-oxidation for chemical modification, homogenization for mechanical disintegration), CESO (chloroacetic acid etherification for chemical modification, sonication for mechanical disintegration), and CEHO (chloroacetic acid etherification for chemical modification, homogenization for mechanical disintegration). More details on the production of nanocellulose can be found in the paper by Li et al. (2013a, b).
The results of LCA studies are critically dependent on the impact assessment method used. Several impact assessment methods were used in this study and energy and global warming potential (GWP) were the two important environmental metrics investigated. It was observed that the fibrillation of MFCs via sonication demands a larger amount of energy than the homogenization process. In the homogenization process, the chemical steps (including CE or TO) were more energy consuming than the mechanical process. Overall, TOHO requires the least energy across the entire process life cycle. This is an important consideration in choosing appropriate industrial scale-up options for these processes. Thus, sonication does not appear to be competitive with homogenization (Li et al. 2013a, b). The GWP values follow the same trend as the energy demand for these processes, since the greenhouse gas emissions mostly contain carbon dioxide and result directly from the use of fossil fuels for energy production. The GWP values were in the range of 190–1169 kg CO2 equivalent/kg nanocellulose, which is higher than the GWP values (0.7–3.0 kg CO2 equivalent/kg nanocellulose) reported by Hohenthal et al. (2012).
In addition to energy and GWP, Eco-Indicator 99 (EI99, Sima Prov v2.08) also provides an end point damage assessment using a variety of environmental impact metrics. EI99 has three weighting sets reflecting different perspectives for evaluating the environmental damage, namely the egalitarian (E), hierarchist (H), and individualist (I) perspectives. The order of environmental impacts evaluated by EI99 was found to be the same as the energy and CC models: TOHO (lowest in each perspective) to CEHO to TOSO to CESO (highest perspective). Table 4 shows the EI99 results for the nanocellulose production routes.
Piccinno et al. (2015) conducted a cradle-to-gate LCA of NFCs extracted from carrot waste. The fabrication route considered in this study consisted of a combination of enzymatic depolymerization pretreatment followed by homogenization to produce liberated MFCs. Two different spinning methods, namely wet spinning (routes 1a and 1b in Fig. 12) and electrospinning (route 2), were examined as alternatives for improving the orientation of the fibers. Briefly, in route 1a, the MFC was coated with a copolymer, GripX, consisting of a primary amine functionalized chitosan backbone and xyloglucan side-chains. In route 1b, the wet spinning method was examined without a coating. In both routes 1a and 1b, an aqueous solution of sodium alginate was added to the mixture, which acted as a carrier polymer for the subsequent spinning process. In route 2, poly(ethylene oxide) was used as the carrier polymer and added to the aqueous solution. Figure 12 shows the MFC liberation and production processes as well as the system boundaries for electrospun nanofibers. More details on the LCI assessment can be found in their report (Piccinno et al. 2015).

Adapted with permission from Piccinno et al. (2015), Copyright 2015. Reproduced with permission from the American Chemical Society
Production process and system boundaries for yarn spinning (I) and MFC liberation (II).
The impact assessment methods used in this study were ReCiPe, EI99, and IPCC 2007 Global Warming Potential. To evaluate the production process and improve it, ReCiPe midpoint and endpoint indicators were used with the hierarchist perspective as cumulative energy demand (CED). The results showed that there was a significant difference in the cumulative energy demand between the two wet-spinning and electrospinning routes. The difference could be due to the smaller scale of the electrospinning process, lower yield (60%) of the spun fiber, or importantly, due to the high-energy consumption of the electrospinning apparatus. The wet-spinning process demanded significantly less energy and the defibrillation of MFCs was identified as the most energy demanding step along this route. The defibrillation energy accounted for 72 and 88% of the total endpoint energy in routes 1a (with the GripX coating) and 1b (without coating), respectively. It was found that the enzymatic treatment step (conducted at 40 °C for 24 h) had the highest environmental impact contribution in the defibrillation process. On the other hand, GripX had a higher relative contribution to the terrestrial ecotoxicity potential derived from the hazardous solvent waste produced during the process. This waste goes to a hazardous waste incineration plant (Piccinno et al. 2015).
A thorough comparison was done between this study and the study by Li et al. (2013a, b). It was observed that the total energy for the enzymatic extraction process was lower than that for the wood pulp-based production process, i.e., HO and SO. This difference was attributed to the different energy inputs for the wood pulp production process, in which a high portion of the CED is generated by chemical inputs without contribution from electricity. On the other hand, in the carrot waste process, electricity accounts for at least 95% of the CED as the entire reaction takes place in water and therefore, almost no chemicals are used. A similar pattern was observed for GWP. The impact of the TOHO process was 1.9 kg CO2 equivalent, which is very close to the GWP reported in this study (1.5 or 1.6 kg CO2 equivalent) (Piccinno et al. 2015). Based on this comparison, the authors concluded that their technology had the potential to outperform other nanocellulose technologies from an environmental perspective.
A cradle-to-gate assessment of three production routes for NFCs from wood pulp was conducted by Arvidsson et al. (2015). The novelty of this study lay in the fact that two different pretreatment processes were employed before the mechanical treatment of pulps, with the assumption that the pretreatment would reduce the energy demand for NFC production. Three production routes, namely enzymatic route, carboxymethylation, and a route without pretreatment, were examined. Figure 13 shows the steps in the three production routes considered.

Adapted with permission from Arvidsson et al. (2015), Copyright 2015. Reproduced with permission from American Chemical Society
Flowchart for the three production routes a enzymatic route, b carboxymethylation route, and c no pretreatment route.
The ReCiPe impact assessment method was also used in this study. Four impact categories including energy use (CED) and GWP were considered in addition (Li et al. 2013a, b; Piccinno et al. 2015) to water depletion (WD) and TA, which is an emission-based impact category and does not always correlate with CC for bio-based systems. Thus, it provides a different perspective on environmental impact. A hierarchist scenario was also selected in this study (Arvidsson et al. 2015).
The results indicate that although a pretreatment process was used, the carboxymethylation route had a higher CED than the no-pretreatment route. Reduction in chemical usage during carboxymethylation treatment is the most obvious way to reduce the CED of this route. For example, solvent recovery methods can be implemented, if technically feasible. The environmental impacts of the enzymatic and no-pretreatment routes were similar for the four impact categories. For the enzymatic route, pulp production was the main contributor to CED and GWP, whereas enzymatic treatment was the main contributor to TA (due to phosphate buffer production) and WD (due to water used for washing). Reducing direct water use for washing would, thus, be a method to reduce the WD of the enzymatic route. In the case of the no-pretreatment route, the treatment process was the main contributor to CED, GWP, and WD, whereas pulp production contributed significantly to TA. Reduced electricity use during treatment would be the most obvious method of reducing the environmental impacts of CNFs in this route (Arvidsson et al. 2015). The authors also claimed that the environmental impacts of the production of NFC by the enzymatic and no-pretreatment routes were lower than those of MFC/NFC produced by TEMPO-oxidation and homogenization reported by Li et al. (2013a, b).
The LCA of CNCs was first reported by de Figueirêdo et al. (2012). The environmental impacts of two CNC production processes, namely acid hydrolysis extraction of CNCs from unripe coconut fiber (EUC system) and from white cotton fiber (EC system) were evaluated. Figure 14 shows the boundaries for both the EUC and EC systems. Many environment aspects were studied including energy, water, and emissions present in the liquid effluents. Some of the metrics studied included chemical oxygen demand (COD), biological oxygen demand (BOD), total nitrogen, nitrate, total phosphorus, phenols, furfural, and hydroxymethylfurfural (HMF). The environmental impact categories assessed by the ReCiPe method included CC, WD, eutrophication, and human toxicity.

Adapted with permission from de Figueirêdo et al. (2012), Copyright 2012. Reproduced with permission from Elsevier
Boundaries for the a EUC and b EC systems.
LCI assessment revealed that water, energy, and other environmental footprints increased when unripe coconut fiber was used to produce CNCs. The energy demanded by the EUC system was significantly higher than that necessary for EC. The energy demand in both systems was mostly related to the extraction step in the production of CNCs (processes 1 and 2 in Fig. 14). The energy associated with these steps stems from the electricity required to chop fibers and warm up the chemical solution. In addition, most of the water required for EUC (99.57%) and EC (96.16%) is used in turbines at hydropower plants for producing the energy required for these processes. Thus, electricity production and distribution are the main processes responsible for water use in both the systems. Processes 1 and 2 in the EUC and EC systems required 131 and 138 L/g of water for producing 1 g of CNC (de Figueirêdo et al. 2012). This volume of water is significantly higher than the water required for producing carbon nanotubes, which is around 0.108–0.121 L/g (Singh et al. 2008). Thus, the CNC production process may be considered to be water-intensive.
Besides water and energy, the EUC system also generated effluents with significantly higher pollution loads as indicated by metrics such as COD, BOD, total nitrogen, phenol, furfural, and HMF, compared to the EC system. Among the extraction processes, process 1 contributed to high loads of COD, BOD, total nitrogen, furfural, and HMF. The phenol load came from the extraction of unripe coconut fibers. The washing of the fibers and pretreatment to remove extractives and lignin in process 1 was the main cause of the COD, BOD, phenol and total nitrogen loads. On the other hand, furfural and HMF were produced during fiber hydrolysis and were related to the dehydration of hemicellulose and cellulose at high temperatures, particularly during acid hydrolysis. On the other hand, the main cause for the high nitrate and total phosphorous load in the EC system was cotton production in the farms and application of fertilizers (de Figueirêdo et al. 2012).
LCA of the EC and EUC systems shows that EUC exerts a greater impact on CC, human toxicity, and eutrophication compared to EC, whereas it has a lower impact on WD compared to the EC system. It was found that the production and distribution of electricity is primarily responsible for the impact on CC, human toxicity, and eutrophication in both systems. The production of copper for use in the electricity distribution cables is mainly responsible for the emission of toxic substances and nutrients causing human toxicity as well as marine and freshwater eutrophication. While process 1 in the EUC system also generates toxic substances and nutrients, these substances have lower human toxicity impacts and eutrophication compared to the substances released during the production and distribution of electricity. In the EC system, besides electricity production and distribution, the production of cotton in the farms also significantly contribute to eutrophication (de Figueirêdo et al. 2012). Table 5 presents the results of the effects of the EUC and EC systems on various impact categories.
The results reported in this study indicate that the production of 1 g of CNFs with methane as the feedstock contributes 0.7–1.3 kg of CO2 equivalent to CC and 0.5–0.53 kg of 1.4-DB equivalent towards human toxicity. These values are at the same level as those calculated for the EUC system. However, the corresponding values for the EC system are lower (Table 5) (de Figueirêdo et al. 2012; Khanna et al. 2008c).
In a recent work presented by do Nascimento et al. (2016a, b) the environmental impact of CNC fabrication from coconut fiber using four different methods, was compared. Lignin was recovered from coconut fibers via four different methods and one of the following chemicals was used to hydrolyze cellulose: (1) dilute sulfuric acid (CNH1); (2) concentrated sulfuric acid (CNH2); (3) ammonium persulfate (CNO); and iv) high powered ultrasound (CNU). Figure 15 shows the extraction and production routes for CNC from coconut fiber. The hierarchical version of the ReCiPe method at the midpoint level was used for impact assessment and the following categories were considered: CC, TA, water body eutrophication (FE), marine eutrophication (ME), human toxicity (HT), and WD.
Inventory analysis revealed that the CNU method used the least amount of resources and generated the least emission loads for the production of 1 g of CNC. Interestingly, a positive correlation between yield and power consumption was observed in this study. On the other hand, the CNH2 and CNO methods exhibited the lowest yield and highest energy consumption, whereas the CNU method resulted in a higher yield and reduced energy use. The CNH1 process exhibited higher yields of CNCs than CNH2. This decrease in yield was attributed to the high concentration (64% w/w) of H2SO4 used, which speeds up the hydrolysis of the amorphous domains of cellulose chains, resulting in nanocrystals with smaller diameters. On the other hand, the low CNO yield was related to the high reaction time, high demand for equipment use, and low selectivity of ammonium persulfate oxidation. CNO had high nitrogen emission compared to the other methods because of the use of ammonium persulfate (APS), which contains NH4+ ions (do Nascimento et al. 2016a, b). The CNH2 method resulted in the highest BOD, COD, total phosphorus, furfural, and HMF emission loads as reported by de Figueirêdo et al. (2012). Moreover, CNU exhibited the least environmental impact in all categories considered in the impact evaluation process. Similar behavior was observed for mass and economic allocation procedures. Mass allocation reduced the environmental impact of CNCs, while economic allocation increased the impact. The higher impact of economic allocation was attributed to the higher price of CNC than lignin.
do Nascimento et al. (2016a, b) also compared the environmental effect of the extraction of CNC by CNU with other studies. A lower impact was observed in all the assessed categories (CC, terrestrial acidification, water body eutrophication, human toxicity, and water depletion), for the CNU method compared to the method proposed by Rosa et al. (2010). This was due to the higher yield, lignin recovery, better energetic efficiency, use of totally chlorine-free (TCF) reagent in the bleaching process, no use of sulfuric acid, and no requirements to dialyze the CNC solution.
The comparison also showed that CNCs produced from the CNU process had a lower impact on CC compared to the MFCs obtained from wood pulp using TEMPO or mechanical treatment methods, as reported by Li et al. (2013a, b). However, NFCs extracted by enzymatic pretreatment followed by microfluidization, as reported by Arvidsson et al. (2015) and Piccinno et al. (2015), presented a smaller environmental impact than the CNU CNCs.
Based on the results obtained in this study, the authors also concluded that to reduce the environmental impact of nanocellulose produced from plant fiber with high lignin contents such as coconut fiber, pressed oil palm mesocarp fiber, and sugarcane bagasse, both nanocellulose and lignin should be extracted. The extracted lignin has superior properties and can be used in a wide range of applications as antioxidants, antimicrobial agents, composite reinforcements, binder, and for hydrogel fabrication (do Nascimento et al. 2016a, b; Thakur and Thakur 2015).
Besides evaluating the environmental impact of nanocellulose, it is very important to also investigate the environmental effect of other products prepared from nanocellulose such as composite-reinforced nanocellulose. The environmental impacts of manufacturing BC and NFC-reinforced epoxy composites were evaluated by Hervy et al. (2015). This evaluation was done with a cradle-to grave LCA approach, by considering the manufacturing phase, use phase, and end-of-life. This report is the first to examine the cradle-to grave life cycle of a product prepared from nanocellulose.
A comparison of the environmental impacts of BC/NFC reinforced polymer composites and glass fiber-reinforced polymer composites has also been reported. Two commercially available benchmark materials were chosen for this comparison: (1) 30 wt. % randomly oriented glass fiber-reinforced polypropylene (GF/PP) composites and (2) polylactide. The system boundary for the nanocellulose reinforced epoxy composites and benchmark materials is shown in Fig. 16. The CML 2001 impact assessment method (April 2013 version) developed by the Centre for Environmental Science at Leiden University was used for this LCA analysis (Guinee 2001). In the LCA, the use phase of parts made from the polymer and composite in a car and their end-of-life were considered as a hypothetical scenario. To evaluate the environmental impact associated with the use phase of the polymer and composite parts in a car, fuel consumption was considered to be a function of the weight of the parts in the car. The end-of-life of plastic wastes were divided into three categories based on their final destiny: wastes that go into landfills; wastes that are incinerated to recover energy, or wastes that are recycled. Owing to the uncertainty associated with the recycling process, the LCA model assumed that 60% of the composite panels would go into the landfill, whereas 40% would be incinerated to recover energy (Hervy et al. 2015).

Adapted with permission from Hervy et al. (2015), Copyright 2015. Reproduced with permission from Elsevier. (Color figure online)
System boundaries for the model representing the life cycle of BC and NFC-reinforced polymer composites (left), and PLA and GF/PP composites (right). The red, blue, and green arrows represent consumables or raw materials required, energy input, and waste (materials and energy), respectively. For interpreting the colors in the figure, the reader is referred to the web version of this article.
The cradle-to-gate analysis revealed that the BC and NFC-reinforced epoxy composites had higher GWP and abiotic depletion potential for fossil fuels (ADF) compared to neat polylactic acid (PLA) and glass fiber-reinforced polypropylene (GF/PP). It was also observed that the major environment impact was related to the production of NFCs and the biosynthesis of BC. Moreover, the composite manufacturing process was also an important contributor to the environmental impact. Among the various composite production routes considered in this study, the composite produced via vacuum assisted resin infusion (VARI) exhibited very poor environmental performance. This may be attributed to the use of environmentally unfriendly consumables in the manufacturing process (Hervy et al. 2015).
The use phase and end-of-life LCA studies showed that PLA contributed significantly to the cradle-to-grave GWP and ADF, whereas GF/PP composites had lower contributions. This was due to the differences in the mass required between the two materials to achieve the same performance. Conversely, it was found that the use of nanocellulose-reinforced epoxy composites led to lower cradle-to-grave GWP and ADF compared to PLA and GF/PP. The LCA study showed that while the manufacturing of nanocellulose-reinforced epoxy composites might not be as environmental friendly as the manufacture of neat PLA and GF/PP, the “green credentials” of nanocellulose-reinforced epoxy composites were comparable to those of the neat PLA and GF/PP composites when the use phase and end-of-life of the composites were considered. This suggests that nanocellulose-reinforced epoxy composites with high nanocellulose loading are desirable for producing materials with “greener credentials” compared to the best performing commercially available bio-derived polymers (Hervy et al. 2015).
Conclusion
Research on nanocellulose and the production of nanocellulose-based materials have significantly increased over the last few decades owing to their nanoscale dimensions and unique optical, electrical, magnetic, and mechanical properties, as well as biodegradability and nontoxicity. Nanocellulose can be extracted from various lignocellulosic biomass sources. Based on the preparation technique used and the appearance of nanocellulose, they are divided into two general groups, namely CNFs and CNCs. CNFs are produced mainly via mechanical treatment such as grinding or homogenization and are flexible fibers that are 500–2000 nm in length and 20–50 nm in diameter. On the other hand, CNCs are produced via acid hydrolysis, which is a chemical treatment process. CNCs are whiskers or needle-shaped and are shorter with length in the range of 100–500 nm and diameter in the range of 3–50 nm. The size of the isolated CNFs and CNCs depends on the preparation conditions and source of cellulose. Besides the conventional mechanical and chemical treatment processes, which are traditionally used to prepare CNFs and CNCs, other promising production techniques as well as pretreatment processes have also been proposed in order to develop an economically efficient and eco-friendly production route for nanocellulose. The hydrophilic nature of nano-sized cellulose fibers prevents good dispersion of these materials in hydrophobic polymers, thereby leading to poor mechanical properties. Surface modification before or after mechanical defibrillation could be a solution for this problem. Various surface modification techniques including both chemical as well as physical treatment procedures have been used to modify nanocellulose and enhance its compatibility with polymeric matrices, as discussed in this review paper. So far, most of the reports on the LCA of nanocellulose have assessed the cradle-to-gate impact, while the gate-to-grave impact of nanocellulose and the LCA of other nanocellulose-based materials remain less understood. Additionally, it should be noted that the current LCA impact methods do not adequately characterize human and environmental toxicity effects from nanomaterials. There are several gaps in knowledge regarding CNC and CNF, affecting the ability to draw conclusions from the available data about the safety of cellulose nanomaterials. While few available data indicate health or environmental hazards, the available data do not allow a conclusion of safety or harm from exposure, at this time (Shatkin and Kim 2015, 2017). However, so far, it has been assumed that there are no nanocellulose emissions into the environment or nanocellulose interactions with humans (Li et al. 2014; Yanamala et al. 2014; Colombo et al. 2015).
Up to date there has been significant progress in understanding the various nanocellulose particle forms, their production, commercialization and application. Although several commercial-scale applications have been started recently, there has not been as much progress on useful and cost-effective applications yet and sooner or later it will be reached. There is a huge market for nanocellulose and it can be an appropriate future sustainable material for wide range applications in our daily life. It is anticipated that the world market of nanocellulose will be 60 billion dollars in 2020 and of course a breakthrough is necessary in nanocellulose research. Additionally, regulation and standardization of nanocellulose for safety and properties will be necessary for future commercialization. We anticipate that nanocellulose provide scientist and technologists fascinating options in the coming years.
References
A Research Strategy for Environmental, Health, and Safety Aspects of Engineered Nanomaterials, National Research Council of the National Academies. Washington, DC (2012)
Abdul Khalil HPS, Bhat AH, Ireana Yusra AF (2012) Green composites from sustainable cellulose nanofibrils: a review. Carbohydr Polym 87:963–979
Abdul Khalil H, Davoudpour Y, Islam MN, Mustapha A, Sudesh K, Dungani R, Jawid M (2014) Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr Polym 99:649–665
Abe K, Iwamoto S, Yano H (2007) Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 8:3276–3278
Abitbol T, Marway H, Cranston ED (2014) Surface modification of cellulose nanocrystals with cetyltrimethylammonium bromide. Nord Pulp Pap Res J 29:46–57
Acharya SK, Mishra P, Mehar SK (2011) Effect of surface treatment on the mechanical properties of bagasse fiber reinforced polymer composite. BioResources 6:3155–3165
Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues—wheat straw and soy hulls. Bioresour Technol 99:1664–1671
Anirudhan TS, Rejeena SR (2014) Poly(acrylic acid-co-acrylamide-co-2-acrylamido-2-methyl-1-propanesulfonic acid)-grafted nanocellulose/poly(vinyl alcohol) composite for the in vitro gastrointestinal release of amoxicillin. J Appl Polym Sci 1–2:40699
Araki J (2013) Electrostatic or steric? Preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 9:4125–4141
Araki J, Wada M, Kuga S (2001) Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 17:21–27
Arvidsson R, Nguyen D, Svanstrom M (2015) Life cycle assessment of cellulose nanofibrils production by mechanical treatment and two different pretreatment processes. Environ Sci Technol 49:6881–6890
Ashjaran A, Yazdanshenas ME, Rashidi A, Khajavi R, Rezaee A (2013) Overview of bio nanofabric from bacterial cellulose. J Text Inst 104:121–131
Aulin C, Ahola S, Josefsson P, Nishino T, Hirose Y, Österberg M, Wågberg L (2009) Nanoscale cellulose films with different crystallinities and mesostructures: their surface properties and interaction with water. Langmuir 25:7675–7685
Aulin C, Netrval J, Wågberg L, Lindström T (2010) Aerogels from nanofibrillated cellulose with tunable oleophobicity. Soft Matter 6:3298–3305
Azzam F, Heux L, Putaux JL, Jean B (2010) Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 11:3652–3659
Bae JH, Kim SH (2015) Alkylation of mixed micro- and nanocellulose to improve dispersion in polylactide. Polym Int 64:821–827
Bardet R, Belgacem NM, Bras J (2015) Flexibility and color monitoring of cellulose nanocrystal iridescent solid films using anionic or neutral polymers. ACS Appl Mater Interfaces 7:4010–4018
Beck S, Bouchard J (2014) Auto-catalyzed acidic desulfation of cellulose nanocrystals. Nord Pulp Pap Res J 29:6–14
Ben Azouz K, Ramires EC, Van den Fonteyne W, El Kissi N, Dufresne A (2011) Simple method for the melt extrusion of a cellulose nanocrystal reinforced hydrophobic polymer. ACS Macro Lett 1:236–240
Benkaddour A, Jradi K, Robert S, Daneault C (2013) Grafting of polycaprolactone on oxidized nanocelluloses by click chemistry. Nanomaterials 3:141–157
Besbes I, Alila S, Boufi S (2011) Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: effect of the carboxyl content. Carbohydr Polym 84:975–983
Bettaieb F, Khiari R, Hassan ML, Belgacem MN, Bras J, Dufresne A, Mhenni MF (2015) Preparation and characterization of new cellulose nanocrystals from marine biomass Posidoniaoceanica. Ind Crop Prod 72:175–182
Bharimalla AK, Deshmukh SP, Patil PG, Vigneshwaran N (2015) Energy efficient manufacturing of nanocellulose by chemo- and bio-mechanical processes: a review. World J Nano Sci Eng 5:204–212
Bhatti IA, Zia KM, Ali Z, Zuber M (2012) Modification of cellulosic fibers to enhance their dyeability using UV-irradiation. Carbohydr Polym 89:783–787
Bondeson D, Mathew A, Oksman K (2006) Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 13:171–180
Bonini C, Heux L, Cavaillé JY, Lindner P, Dewhurst C, Terech P (2002) Rodlike cellulose whiskers coated with surfactant: a small-angle neutron scattering characterization. Langmuir 18:3311–3314
Borysiak S, Grząbka-Zasadzińska A (2016) Influence of the polymorphism of cellulose on the formation of nanocrystals and their application in chitosan/nanocellulose composites. J Appl Polym Sci 133. https://doi.org/10.1002/app.42864
Božič M, Vivod V, Kavčič S, Leitgeb M, Kokol V (2015) New findings about the lipase acetylation of nanofibrillated cellulose using acetic anhydride as acyl donor. Carbohydr Polym 125:340–351
Bras J, Sadocco P, Belgacem MN, Dufresne A, Thielemans W (2010) Surface functionalization of cellulose by grafting oligoether chains. Mater Chem Phys 120:438–445
Cao X, Ding B, Yu J, Al-Deyab SS (2012) Cellulose nanowhiskers extracted from TEMPO-oxidized jute fibers. Carbohydr Polym 90:1075–1080
Chakraborty A, Sain M, Kortschot M (2005) Cellulose microfibrils: a novel method of preparation using high shear refining and cryocrushing. Holzforschung 59:102–107
Chen W, Abe K, Uetani K, Yu H, Liu Y, Yano H (2014) Individual cotton cellulose nanofibers: pretreatment and fibrillation technique. Cellulose 21:1517–1528
Chen YW, Lee HV, Abd Hamid SB (2016) Preparation and characterization of cellulose crystallites via Fe(III)-, Co (II)-and Ni (II)-assisted dilute sulfuric acid catalyzed hydrolysis process. J Nano Res Trans Tech Publ 41:96–109
Cherian BM, Leão AL, de Souza SF, Thomas S, Pothan LA, Kottaisamy M (2010) Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr Polym 81:720–725
Chi K, Catchmark JM (2017) Enhanced dispersion and interface compatibilization of crystalline nanocellulose in polylactide by surfactant adsorption. Cellulose 24:4845–4860
Chirayil CJ, Joy J, Mathew L, Mozetic M, Koetz J, Thomas S (2014) Isolation and characterization of cellulose nanofibrils from Helicteres isora plant. Ind Crops Prod 59:27–34
Chowdhury ZZ, Abd Hamid SB (2016) Preparation and characterization of nanocrystalline cellulose using ultrasonication combined with a microwave-assisted pretreatment process. BioResources 11:3397–3415
Cobut A, Sehaqui H, Berglund LA (2014) Cellulose nanocomposites by melt compounding of TEMPO-treated wood fibers in thermoplastic starch matrix. BioResources 9:3276–3289
Colombo L, Zoia L, Violatto MB, Previdi S, Talamini L, Sitia L, Nicotra F, Orlandi M, Salmona M, Recordati C, Bigini P (2015) Organ distribution and bone tropism of cellulose nanocrystals in living mice. Biomacromolecules 16:2862–2871
Coulibaly S, Roulin A, Balog S, Biyani MV, Foster EJ, Rowan SJ, Fiore GL, Weder C (2013) Reinforcement of optically healable supramolecular polymers with cellulose nanocrystals. Macromolecules 47:152–160
Couret L, Irle M, Belloncle C, Cathala B (2017) Extraction and characterization of cellulose nanocrystals from post-consumer wood fiberboard waste. Cellulose 24:2125–2137
Crawshaw J, Bras W, Mant GR, Cameron RE (2002) Simultaneous SAXS and WAXS investigations of changes in native cellulose fiber microstructure on swelling in aqueous sodium hydroxide. J Appl Polym Sci 83:1209–1218
Cunha AG, Mougel JB, Cathala B, Berglund LA, Capron I (2014) Preparation of double pickering emulsions stabilized by chemically tailored nanocelluloses. Langmuir 30:9327–9335
Curran MA (2014) Strengths and limitations of life cycle assessment. In: Klopffer W (ed) Background and future prospects in life cycle assessment, LCA compendium—the complete world of life cycle assessment. Springer, Dordrecht, pp 189–206
Danial WH, Majid ZA, Muhid MNM, Triwahyono S, Bakar MB, Ramli Z (2015) The reuse of wastepaper for the extraction of cellulose nanocrystals. Carbohydr Polym 118:165–169
Davis NJ, Flitsch SL (1993) Selective oxidation of monosaccharide uronic acids derivatives to uronic acids. Tetrahedron Lett 34:1181–1184
de Castro DO, Bras J, Gandini A, Belgacem NM (2016) Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr Polym 137:1–8
de Figueirêdo MCB, de Freitas Rosa M, Ugaya CML, de Souza MSM, da Silva Braid ACC, de Melo LFL (2012) Life cycle assessment of cellulose nanowhiskers. J Clean Prod 35:130–139
de Menezes AJ, Siqueira G, Curvelo AAS, Dufresne A (2009) Extrusion and characterization of functionalized cellulose whiskers reinforced polyethylene nanocomposites. Polymer 50:4552–4563
de Menezes AJ, Longo E, Leite FL, Dufresne A (2014) Characterization of cellulose nanocrystals grafted with organic acid chloride of different sizes. J Renew Mater 2:306–313
De Nooy AEJ, Besemer AC, van Bekkum H (1995) Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr Res 269:89–98
Deepa B, Abraham E, Cherian BM, Bismarck A, Blaker JJ, Pothan LA, Leao AL, de Souza SF, Kottaisamy M (2011) Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour Technol 102:1988–1997
Dhar P, Tarafder D, Kumar A, Katiyar V (2015) Effect of cellulose nanocrystal polymorphs on mechanical, barrier and thermal properties of poly(lactic acid) based bionanocomposites. RSC Adv 5:60426–60440
Dinand E, Vignon M, Chanzy H, Heux L (2002) Mercerization of primary wall cellulose and its implication for the conversion of cellulose I → cellulose II. Cellulose 9:7–18
do Nascimento DM, Almeida JS, Vale MS, Leitão RC, Muniz CR, de Figueirêdo MCB, Morais JPS, de Freitas Rosa M (2016a) A comprehensive approach for obtaining cellulose nanocrystal from coconut fiber. Part I: proposition of technological pathways. Ind Crop Prod 93:66–75
do Nascimento DM, Dias AF, de Araújo Junior CP, de Freitas Rosa M, Morais JPS, de Figueirêdo MCB (2016b) A comprehensive approach for obtaining cellulose nanocrystal from coconut fiber. Part II: environmental assessment of technological pathways. Ind Crop Prod 93:58–65
Duda M, Shaw JS (1997) Life cycle assessment. Society 35:38–43
Dufresne A (2013) Nanocellulose: from nature to high performance tailored materials. Walter de Gruyter, Berlin
Dufresne A, Belgacem MN (2013) Cellulose-reinforced composites: from micro-to nanoscale. Polímeros 23:277–286
Elazzouzi-Hafraoui S, Putaux J-L, Heux L (2009) Self-assembling and chiral nematic properties of organophilic cellulose nanocrystals. J Phys Chem B 113:11069–11075
Eranna PB, Pandey KK, Nagarajappa GB (2016) A note on the effect of microwave heating on iodine-catalyzed acetylation of wood. J Wood Chem Technol 36:205–210
Espino-Pérez E, Domenek S, Belgacem N, Sillard C, Bras J (2014) Green process for chemical functionalization of nanocellulose with carboxylic acids. Biomacromolecules 15:4551–4560
Espinosa SC, Kuhnt T, Foster EJ, Weder C (2013) Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 14:1223–1230
Filpponen I, Argyropoulos DS (2010) Regular linking of cellulose nanocrystals via click chemistry: synthesis and formation of cellulose nanoplatelet gels. Biomacromolecules 11:1060–1066
Finnveden G, Hauschild MZ, Ekvall T, Guinee J, Heijungs R, Hellweg S, Koehler A, Pennington D, Suh S (2009) Recent developments in life cycle assessment. J Environ Manag 91:1–21
Flynn C, Byrne C, Meenan B (2013) Surface modification of cellulose via atmospheric pressure plasma processing in air and ammonia–nitrogen gas. Surf Coat Technol 233:108–118
Fortunati E, Luzi F, Puglia D, Petrucci R, Kenny JM, Torre L (2015) Processing of PLA nanocomposites with cellulose nanocrystals extracted from Posidonia oceanica waste: innovative reuse of coastal plant. Ind Crop Prod 67:439–447
Fox SC, Li B, Xu D, Edgar KJ (2011) Regioselective esterification and etherification of cellulose: a review. Biomacromolecules 12:1956–1972
Fraschini C, Chauve G, Bouchard J (2017) TEMPO-mediated surface oxidation of cellulose nanocrystals (CNCs). Cellulose 24:2775–2790
Frey MW (2008) Electrospinning cellulose and cellulose derivatives. Polym Rev 48:378–391
Gavankar S, Suh S, Keller AF (2012) Life cycle assessment at nanaoscale: review and recommendation. Int J Life Cycle Ass 17:259–303
Gilbertson LM, Wender BA, Zimmerman JB, Eckelman MJ (2015) Coordinating modeling and experimental research of engineered nanomaterials to improve life cycle assessment studies. Environ Sci Nano 2:669–682
Goffin A-L, Raquez J-M, Duquesne E, Siqueira G, Habibi Y, Dufresne A, Dubois Ph (2011) Poly(ɛ-caprolactone) based nanocomposites reinforced by surface-grafted cellulose nanowhiskers via extrusion processing: morphology, rheology, and thermo-mechanical properties. Polymer 52:1532–1538
Gorgieva S, Vogrinčič R, Kokol V (2015) Polydispersity and assembling phenomena of native and reactive dye-labelled nanocellulose. Cellulose 22:3541–3558
Goussé C, Chanzy H, Excoffier G, Soubeyrand L, Fleury E (2002) Stable suspensions of partially silylated cellulose whiskers dispersed in organic solvents. Polymer 43:2645–2651
Goussé C, Chanzy H, Cerrada M, Fleury E (2004) Surface silylation of cellulose microfibrils: preparation and rheological properties. Polymer 45:1569–1575
Grubb GF, Bakshi BR (2011) Life cycle of titanium dioxide nanoparticle production. J Ind Ecol 15:81–95
Guinee J (2001) Handbook on life cycle assessment: operational guide to the ISO standards. Int J Life Cycle Asses 6:311–313
Haafiz MKM, Hassan A, Zakaria Z, Inuwa IM (2014) Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr Polym 103:119–125
Habibi Y (2014) Key advances in the chemical modification of nanocelluloses. Chem Soc Rev 43:1519–1542
Habibi Y, Goffin A-L, Schiltz N, Duquesne E, Dubois P, Dufresne A (2008) Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J Mater Chem 18:5002–5010
Habibi Y, Lucia L, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Hamid SBA, Zain SK, Das R, Centi G (2015) Synergic effect of tungstophosphoric acid and sonication for rapid synthesis of crystalline nanocellulose. Carbohydr Polym 138:349–355
Hao X, Shen W, Chen Z, Zhu J, Feng L, Wu Z, Wang P, Zeng X, Wu T (2015) Self-assembled nanostructured cellulose prepared by a dissolution and regeneration process using phosphoric acid as a solvent. Carbohydr Polym 123:297–304
Hasani M, Cranston ED, Westman G, Gray DG (2008) Cationic surface functionalization of cellulose nanocrystals. Soft Matter 4:2238–2244
Hassan ML, Moorefield CM, Elbatal HS, Newkome GR, Modarelli DA, Romano NC (2012) Fluorescent cellulose nanocrystals via supramolecular assembly of terpyridine-modified cellulose nanocrystals and terpyridine-modified perylene. Mater Sci Eng B 177:350–358
He W, Jiang X, Sun F, Xu X (2014) Extraction and characterization of cellulose nanofibers from Phyllostachys nidularia Munro via a combination of acid treatment and ultrasonication. BioResources 9:6876–6887
Herrick FW, Casebier RL, Hamilton JK, Sandberg KR (1983) Microfibrillated cellulose: morphology, and accessibility. In: Sarko A (ed) Proceedings of the ninth cellulose conference applied polymer symposia. Wiley, New York, pp 797–813
Hervy M, Evangelisti S, Lettieri P, Lee KY (2015) Life cycle assessment of nanocellulose-reinforced advanced fiber composites. Compos Sci Technol 118:154–162
Hesas RH, Daud WMAW, Sahu J, Arami-Niya A (2013) The effects of a microwave heating method on the production of activated carbon from agricultural waste: a review. J Anal Appl Pyrol 100:1–11
Hettegger H, Beaumont M, Potthast A, Rosenau T (2016) Aqueous modification of nano- and microfibrillar cellulose with a click synthon. Chemsuschem 9:75–79
Heux L, Chauve G, Bonini C (2000) Nonflocculating and chiral-nematic self-ordering of cellulose microcrystals suspensions in nonpolar solvents. Langmuir 16:8210–8212
Hietala M, Rollo P, Kekäläinen K, Oksman K (2014) Extrusion processing of green biocomposites: compounding, fibrillation efficiency, and fiber dispersion. J Appl Polym Sci 131:1–9
Ho TTT, Zimmermann T, Hauert R, Caseri W (2011) Preparation and characterization of cationic nanofibrillated cellulose from etherification and high-shear disintegration processes. Cellulose 18:1391–1406
Ho TTT, Abe K, Zimmermann T, Yano H (2015) Nanofibrillation of pulp fibers by twin-screw extrusion. Cellulose 22:421–433
Hoeng F, Denneulin A, Neuman C, Bras J (2015) Charge density modification of carboxylated cellulose nanocrystals for stable silver nanoparticles suspension preparation. J Nanopart Res 17:1–14
Hohenthal C, Ovaskainen M, Bussini D, Sadocco P, Pajula T, Lehtinen H, Kautto J, Salmenkivi K (2012) Final assessment of nanoenhanced new products. VTT Technical Research Center of Finland, Espoo
Hu Z, Ballinger S, Pelton R, Cranston ED (2015) Surfactant-enhanced cellulose nanocrystal Pickering emulsions. J Colloid Interfaces Sci 439:139–148
Huang P, Wu M, Kuga S, Wang D, Wu D, Huang Y (2012) One-step dispersion of cellulose nanofibers by mechanochemical esterification in an organic solvent. Chemsuschem 5:2319–2322
Hubbe MA, Rojas OJ, Lucia LA, Sain M (2008) Cellulosic nanocomposites: a review. Bioresources 3:929–980
Islam MT, Alam MM, Zoccola M (2013) Review on modification of nanocellulose for application in composites. Int J Innov Res Sci Eng Technol 2:5444–5451
ISO 14040 (2006) Environmental management—life cycle assessment—principles and framework, Switzerland
ISO 14044 (2006) Environmental management—life cycle assessment—requirements and guidelines, Switzerland
Iwamoto S, Nakagaito A, Yano H, Nogi M (2005) Optically transparent composites reinforced with plant fiber-based nanofibers. Appl Phys A 81:1109–1112
Jazbec K, Šala M, Mozetič M, Vesel A, Gorjanc M (2015) Functionalization of cellulose fibres with oxygen plasma and ZnO nanoparticles for achieving UV protective properties. J Nanomater 2015:25
Ji B, Tang P, Yan K, Sun G (2015) Catalytic actions of alkaline salts in reactions between 1,2,3,4-butanetetracarboxylic acid and cellulose: II. Esterification. Carbohydr Polym 132:228–236
Jiang F, Hsieh YL (2015) Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr Polym 122:60–68
Jiang F, Esker AR, Roman M (2010) Acid-catalyzed and solvolytic desulfation of H2SO4-hydrolyzed cellulose nanocrystals. Langmuir 26:17919–17925
Jiang C, Oporto GS, Zhong T, Jaczynski J (2016) TEMPO nanofibrillated cellulose as template for controlled release of antimicrobial copper from PVA films. Cellulose 23:713–722
Jin E, Guo J, Yang F, Zhu Y, Song J, Jin Y, Rojas OJ (2016) On the polymorphic and morphological changes of cellulose nanocrystals (CNC-I) upon mercerization and conversion to CNC-II. Carbohydr Polym 143:327–335
Jonoobi M, Harun J, Mathew AP, Hussein MZB, Oksman K (2010) Preparation of cellulose nanofibers with hydrophobic surface characteristics. Cellulose 17:299–307
Jonoobi M, Oladi R, Davoudpour Y, Oksman K, Dufresne A, Hamzeh Y, Davoodi R (2015) Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: a review. Cellulose 22:935–969
Kaith B, Kalia S (2008) Preparation of microwave radiation induced graft copolymers and their applications as reinforcing material in phenolic composites. Polym Compos 29:791–797
Kämäräinen T, Arcot LR, Johansson L-S, Campbell J, Tammelin T, Franssila S, Laine J, Rojas OJ (2016) UV-ozone patterning of micro-nano fibrillated cellulose (MNFC) with alkylsilane self-assembled monolayers. Cellulose 23:1847–1857
Kamireddy SR, Li J, Tucker M, Degenstein J, Ji Y (2013) Effects and mechanism of metal chloride salts on pretreatment and enzymatic digestibility of corn stover. Ind Eng Chem Res 52:1775–1782
Kargarzadeh H, Ahmad I, Abdullah I, Dufresne A, Zainudin SY, Sheltami RM (2012) Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 19:855–866
Kargarzadeh H, Ioelovich M, Ahmad I, Thomas S, Dufresne A (2017) Methods for extraction of nanocellulose from various sources. In: Kargarzadeh H, Ahmad I, Thomas S, Dufresne A (eds) Handbook of nanocellulose and cellulose nanocomposites. Wiley, Germany, pp 1–50
Karunanithy C, Muthukumarappan K (2010) Effect of extruder parameters and moisture content of switchgrass, prairie cord grass on sugar recovery from enzymatic hydrolysis. Appl Biochem Biotechnol 162:1785–1803
Karunanithy C, Muthukumarappan K (2011) Influence of extruder and feedstock variables on torque requirement during pretreatment of different types of biomass—a response surface analysis. Biosyst Eng 109:37–51
Kekäläinen J (2013) Assesment of environmental impact with life cycle methods in nanotechnology industry. Dissertation, Univerity of Jyväskylä
Khafaga MR, Ali HE, El-Naggar AWM (2016) Antimicrobial finishing of cotton fabrics based on gamma irradiated carboxymethyl cellulose/poly(vinyl alcohol)/TiO2 nanocomposites. J Text Inst 107:766–773
Khan RA, Salmieri S, Dussault D, Uribe-Calderon J, Kamal MR, Safrany A, Lacroix M (2010) Production and properties of nanocellulose-reinforced methylcellulose-based biodegradable films. J Agric Food Chem 58:7878–7885
Khan RA, Beck S, Dussault D, Salmieri S, Bouchard J, Lacroix M (2013) Mechanical and barrier properties of nanocrystalline cellulose reinforced poly(caprolactone) composites: effect of gamma radiation. J Appl Polym Sci 129:3038–3046
Khanna V, Bakshi BR, Lee LJ (2008) Assessing life cycle environmental implications of polymer nanocomposites. In: 2008 IEEE international symposium on electronics and the environment, 2008 ISEE. IEEE, pp 1–6
Khanna V, Bakshi BR, Lee LJ (2008b) Carbon nanofiber production. J Ind Ecol 12:394–410
Khanna V, Bakshi BR, Lee LJ (2008c) Carbon nanofiber production: life cycle energy consumption and environmental impact. J Ind Ecol 12:394–410
Kim N-H, Imai T, Wada M, Sugiyama J (2006) Molecular directionality in cellulose polymorphs. Biomacromolecules 7:274–280
Kim DY, Lee BM, Koo DH, Kang PH, Jeun JP (2016) Preparation of nanocellulose from a kenaf core using e-beam irradiation and acid hydrolysis. Cellulose 23:3039–3049
Kleiner Y, Gal’braikh L, Irklei V, Chernukhina A (2013) Properties of radiation-modified celluloses and their derivatives. Fibre Chem 45:17–20
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerforts M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 24:5438–5466
Klöpffer W (1997) Life cycle assessment. Environ Sci Pollut Res 4:223–228
Kloser E, Gray DG (2010) Surface grafting of cellulose nanocrystals with poly(ethylene oxide) in aqueous media. Langmuir 26:13450–13456
Kobayashi K, Kimura S, Togawa E, Wada M (2011) Crystal transition from cellulose II hydrate to cellulose II. Carbohydr Polym 86:975–981
Kondo T, Kose R, Naito H, Kasai W (2014) Aqueous counter collision using paired water jets as a novel means of preparing bio-nanofibers. Carbohydr Polym 112:284–290
Kontturi E, Meriluoto A, Nuopponen M (2012) Process for preparing micro- and nanocrystalline cellulose. Patent# WO2012127117A1
Kose R, Kasai W, Kondo T (2011) Switching surface properties of substrates by coating with a cellulose nanofiber having a high adsorbability. Fiber 67:163–167
Kralisch D, Ott D, Gericke D (2015) Rules and benefits of life cycle assessment in green chemical process and synthesis design: a tutorial review. Green Chem 17:123–145
Krishnan N, Boyd S, Somani A, Raoux S, Clark D, Dornfeld D (2008) A hybrid life cycle inventory of nano-scale semiconductor manufacturing. Environ Sci Technol 42:3069–3075
Kulpinski P (2005) Cellulose nanofibers prepared by the N-methylmorpholine-N-oxide method. J Appl Polym Sci 98:1855–1859
Kurniawan H, Lai J-T, Wang M-J (2012) Biofunctionalized bacterial cellulose membranes by cold plasmas. Cellulose 19:1975–1988
Lacroix M, Khan R, Senna M, Sharmin N, Salmieri S, Safrany A (2014) Radiation grafting on natural films. Radiat Phys Chem 94:88–92
Lamaming J, Hashim R, Sulaiman O, Leh CP, Sugimoto T, Nordin NA (2015) Cellulose nanocrystals isolated from oil palm trunk. Carbohydr Polym 127:202–208
Lazko J, Sénéchal T, Landercy N, Dangreau L, Raquez J-M, Dubois P (2014) Well defined thermostable cellulose nanocrystals via two-step ionic liquid swelling-hydrolysis extraction. Cellulose 21:4195–4207
Lee S-Y, Chun S-J, Kang I-A, Park J-Y (2009a) Preparation of cellulose nanofibrils by high-pressure homogenizer and cellulose-based composite films. J Ind Eng Chem 15:50–55
Lee S-Y, Mohan DJ, Kang I-A, Doh G-H, Lee S, Han SO (2009b) Nanocellulose reinforced PVA composite films: effects of acid treatment and filler loading. Fibers Polym 10:77–82
Lee HV, Hamid SBA, Zain SK (2014) Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci World J 2014:1–20
Li J, Zhang L-P, Peng F, Bian J, Yuan T-Q, Xu F, Sun RC (2009) Microwave-assisted solvent-free acetylation of cellulose with acetic anhydride in the presence of iodine as a catalyst. Molecules 14:3551–3566
Li ZQ, Zhou XD, Pei CH (2010) Synthesis of PLA-co-PGMA copolymer and its application in the surface modification of bacterial cellulose. Int J Polym Mater 59:725–737
Li J, Xiu H, Zhang M, Wang H, Ren Y, Ji Y (2013a) Enhancement of cellulose acid hydrolysis selectivity using metal ion catalysts. Curr Org Chem 17:1617–1623
Li Q, McGinnis S, Sydnor C, Wong A, Renneckar S (2013b) Nanocellulose life cycle assessment. ACS Sus Chem Eng 1:919–928
Li M, Wang L-J, Li D, Cheng Y-L, Adhikari B (2014) Preparation and characterization of cellulose nanofibers from de-pectinated sugar beet pulp. Carbohydr Polym 102:136–143
Li M-C, Wu Q, Song K, Qing Y, Wu Y (2015) Cellulose nanoparticles as modifiers for rheology and fluid loss in bentonite water-based fluids. ACS Appl Mater Interfaces 7:5006–5016
Lin N, Dufresne A (2013) Physical and/or chemical compatibilization of extruded cellulose nanocrystal reinforced polystyrene nanocomposites. Macromolecules 46:5570–5583
Lin N, Dufresne A (2014a) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325
Lin N, Dufresne A (2014b) Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 6:5384–5393
Lin N, Huang J, Chang PR, Feng J, Yu J (2011) Surface acetylation of cellulose nanocrystal and its reinforcing function in poly(lactic acid). Carbohydr Polym 83:1834–1842
Lin R, Li A, Zheng T, Lu L, Cao Y (2015) Hydrophobic and flexible cellulose aerogel as an efficient, green and reusable oil sorbent. RSC Adv 5:82027–82033
Liu L, Sun J, Cai C, Wang S, Pei H, Zhang J (2009) Corn stover pretreatment by inorganic salts and its effects on hemicellulose and cellulose degradation. Bioresour Technol 100:5865–5871
Liu Y, Wang H, Yu G, Yu Q, Li B, Mu X (2014) A novel approach for the preparation of nanocrystalline cellulose by using phosphotungstic acid. Carbohydr Polym 110:415–422
Ma Z, Kotaki M, Ramakrishna S (2005) Electrospun cellulose nanofiber as affinity membrane. J Membr Sci 265:115–123
Maiti S, Jayaramudu J, Das K, Reddy SM, Sadiku R, Ray SS, Liu D (2013) Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr Polym 98:562–567
Majoinen J, Walther A, McKee JR, Kontturi E, Aseyev V, Malho JM, Ruokolainen J, Ikkala O (2011) Polyelectrolyte brushes grafted from cellulose nanocrystals using Cu-mediated surface-initiated controlled radical polymerization. Biomacromolecules 12:2997–3006
Mangalam AP, Simonsen J, Benight AS (2009) Cellulose/DNA hybrid nanomaterials. Biomacromolecules 10:497–504
Marcovich N, Auad M, Bellesi N, Nutt S, Aranguren M (2006) Cellulose micro/nanocrystals reinforced polyurethane. J Mater Res 21:870–881
Mariano M, El Kissi N, Dufresne A (2014) Cellulose nanocrystals and related nanocomposites: review of some properties and challenges. J Polym Sci Part B Polym Phys 52:791–806
Martínez-Sanz M, Lopez-Rubio A, Lagaron JM (2011) Optimization of the nanofabrication by acid hydrolysis of bacterial cellulose nanowhiskers. Carbohydr Polym 85:228–236
Martínez-Sanz M, Abdelwahab MA, Lopez-Rubio A, Lagaron JM, Chiellini E, Williams TG, Wood DF, Orts WJ, Imam SH (2013) Incorporation of poly(glycidylmethacrylate) grafted bacterial cellulose nanowhiskers in poly(lactic acid) nanocomposites: improved barrier and mechanical properties. Eur Polym J 49:2062–2072
Martoïa F, Perge C, Dumont P, Orgéas L, Fardin M, Manneville S, Belgacem MN (2015) Heterogeneous flow kinematics of cellulose nanofibril suspensions under shear. Soft Matter 11:4742–4755
Meyer DE, Curran MA, Gonzalez MA (2011) An examination of silver nanoparticles in socks using screening-level life cycle assessment. J Nanopart Res 13:147–156
Mihailović D, Šaponjić Z, Radoičić M, Lazović S, Baily C, Jovančić P, Nedeljković J, Radetić M (2011) Functionalization of cotton fabrics with corona/air RF plasma and colloidal TiO2 nanoparticles. Cellulose 18:811–825
Missoum K, Belgacem MN, Bras J (2013) Nanofibrillated cellulose surface modification: a review. Materials 6:1745–1766
Moawia RM, Nasef MM, Mohamed NHF, Ripin A (2016) Modification of flax fibres by radiation induced emulsion graft copolymerization of glycidyl methacrylate. Radiat Phys Chem 122:35–42
Mohamed M, Salleh W, Jaafar J, Asri S, Ismail A (2015) Physicochemical properties of “green” nanocrystalline cellulose isolated from recycled newspaper. RSC Adv 5:29842–29849
Mohd NH, Hadina Ismail NF, Zahari JI, Wan Fathilah WF, Kargarzadeh H, Ramli S, Ahmad I, Yarmo MA, Othman R (2016) Effect of aminosilane modification on nanocrystaline cellulose properties. J. Nanomater 2016:1–8
Moign A, Vardelle A, Themelis N, Legoux J (2010) Life cycle assessment of using powder and liquid precursors in plasma spraying: the case of yttria-stabilized zirconia. Surf Coat Technol 205:668–673
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Nagalakshmaiah M, El Kissi N, Dufresne A (2016a) Ionic compatibilization of cellulose nanocrystals with quaternary ammonium salt and their melt extrusion with polypropylene. ACS Appl Mater Interfaces 8:8755–8764
Nagalakshmaiah M, Mortha G, Dufresne A (2016b) Structural investigation of cellulose nanocrystals extracted from chili leftover and their reinforcement in cariflex-IR rubber latex. Carbohydr Polym 136:945–954
National Nanotechnology Initiative: Environmental, Health, and Safety Research Strategy, National Science and Technology Council Committee on Technology (2011)
Nechyporchuk O, Belgacem MN, Pignon F (2014) Rheological properties of micro-/nanofibrillated cellulose suspensions: wall-slip and shear banding phenomena. Carbohydr Polym 112:432–439
Nechyporchuk O, Belgacem MN, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crop Prod 93:2–25
Nepomuceno NC, Santos AS, Oliveira JE, Glenn GM, Medeiros ES (2017) Extraction and characterization of cellulose nanowhiskers from Mandacaru (Cereus jamacaru DC.) spines. Cellulose 24:119–129
Nge TT, Lee S-H, Endo T (2013) Preparation of nanoscale cellulose materials with different morphologies by mechanical treatments and their characterization. Cellulose 20:1841–1852
Ni J, Teng N, Chen H, Wang J, Zhu J, Na H (2015) Hydrolysis behavior of regenerated celluloses with different degree of polymerization under microwave radiation. Bioresour Technol 191:229–233
Nielsen LJ, Eyley S, Thielemans W, Aylott JW (2010) Dual fluorescent labelling of cellulose nanocrystals for pH sensing. Chem Commun 46:8929–8931
Nishimura H, Sarko A (1987a) Mercerization of cellulose. III. Changes in crystallite sizes. J Appl Polym Sci 33:855–866
Nishimura H, Sarko A (1987b) Mercerization of cellulose. IV. Mechanism of mercerization and crystallite sizes. J Appl Polym Sci 33:867–874
Nourbakhsh S (2015) Comparison between laser application and atmospheric air plasma treatment on nanocellulose coating of polyester and nylon 66 fabrics. J Laser Appl 27:012005
Okano T, Sarko A (1984) Mercerization of cellulose. I. X-ray diffraction evidence for intermediate structures. J Appl Polym Sci 29:4175–4182
Okano T, Sarko A (1985) Mercerization of cellulose. II. Alkali-cellulose intermediates and a possible mercerization mechanism. J Appl Polym Sci 30:325–332
Olea EH, Carrillo EP, Chiw MM, Saldívar SOS (2015) Effects of extrusion pretreatment parameters on sweet sorghum bagasse enzymatic hydrolysis and its subsequent conversion into bioethanol. Biomed Res Int 2015:1–10
Pääkkö M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindström T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 8:1934–1941
Panaitescu DM, Frone AN, Nicolae C (2013) Micro-and nano-mechanical characterization of polyamide 11 and its composites containing cellulose nanofibers. Eur Polym J 49:3857–3866
Parida D, Jassal M, Agarwal AK (2012) Functionalization of cotton by in situ reaction of styrene in atmospheric pressure plasma zone. Plasma Chem Plasma Process 32:1259–1274
Pasquini D, de Morais Teixeira E, da Silva Curvelo AA, Belgacem MN, Dufresne A (2008) Surface esterification of cellulose fibres: processing and characterisation of low-density polyethylene/cellulose fibres composites. Compos Sci Technol 68:193–201
Pei A, Zhou Q, Berglund LA (2010) Functionalized cellulose nanocrystals as biobased nucleation agents in poly (l-lactide)(PLLA)-crystallization and mechanical property effects. Compos Sci Technol 70:815–821
Pelissari FM, do Amaral Sobral PJ, Menegalli FC (2014) Isolation and characterization of cellulose nanofibers from banana peels. Cellulose 21:417–432
Piccinno F, Hischier R, Seeger S, Som C (2015) Life cycle assessment of a new technology to extract, functionalize and orient cellulose nanofibers from food waste. ACS Sustain Chem Eng 3:1047–1055
Piccinno F, Hischier R, Seeger S, Som C (2018) Predicting the environmental impact of a future nanocellulose production at industrial scale: application of the life cycle assessment scale-up framework. J Clean Prod 174:283–295
Ping L, You-Lo H (2010) Preparation and properties of cellulose nanocrystals: rods, spheres, and network. Carbohydr Polym 82:329–336
Pires PAR, Malek NI, Teixeira TC, Bioni TA, Nawaz H, El Seoud OA (2015) Imidazole-catalyzed esterification of cellulose in ionic liquid/molecular solvents: a multi-technique approach to probe effects of the medium. Ind Crop Prod 77:180–189
Qi H, Sui X, Yuan J, Wei Y, Zhang L (2010) Electrospinning of cellulose-based fibers from NaOH/urea aqueous system. Macromol Mater Eng 295:695–700
Quan SL, Kang SG, Chin IJ (2010) Characterization of cellulose fibers electrospun using ionic liquid. Cellulose 17:223–230
Roman M, Winter WT (2004) Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 5:1671–1677
Rosa MF, Medeiros ES, Malmonge JA, Gregorski KS, Wood DF, Mattoso LHC, Glenn G, Orts WJ, Imam SH (2010) Cellulose nanowhiskers from coconut husk fibres: effect of preparation conditions on their thermal and morphological behavior. Carbohydr Polym 81:83–92
Rouette H-K (2001) Encyclopedia of textile finishing. Springer, Berlin
Rowland SP, Howley PS (1988) Hydrogen bonding on accessible surfaces of cellulose from various sources and relationship to order within crystalline regions. J Polym Sci Polym Chem 26:1769–1778
Rowland SP, Roberts EJ (1972) The nature of accessible surfaces in the microstructure of cotton cellulose. J Polym Sci Polym Chem 10:2447–2461
Roy D, Semsarilar M, Guthrie JT, Perrier S (2009) Cellulose modification by polymer grafting: a review. Chem Soc Rev 38:2046–2064
Saini S, Belgacem N, Mendes J, Elegir G, Bras J (2015) Contact antimicrobial surface obtained by chemical grafting of microfibrillated cellulose in aqueous solution limiting antibiotic release. ACS Appl Mater Interfaces 7:18076–18085
Saini S, Belgacem MN, Salon M-CB, Bras J (2016) Non leaching biomimetic antimicrobial surfaces via surface functionalisation of cellulose nanofibers with aminosilane. Cellulose 23:795–810
Savadekar N, Mhaske S (2012) Synthesis of nano cellulose fibers and effect on thermoplastics starch based films. Carbohydr Polym 89:146–151
Senturk-Ozer S, Gevgilili H, Kalyon DM (2011) Biomass pretreatment strategies via control of rheological behavior of biomass suspensions and reactive twin screw extrusion processing. Bioresour Technol 102:9068–9075
Shafeiei-Sabet S, Hamad WY, Hatzikiriakos SG (2013) Influence of degree of sulfation on the rheology of cellulose nanocrystal suspensions. Rheol Acta 52:741–751
Shatkin JA, Kim B (2015) Cellulose nanomaterials: life cycle risk assessment, and environmental health and safety roadmap. Environ Sci Nano 2:477–499
Shatkin JA, Kim B (2017) Environmental Health and safety of cellulose nanomaterials and composites. In: Kargarzadeh H, Ahmad I, Thomas S, Dufresne A (eds) Handbook of nanocellulose and cellulose nanocomposites. Wiley, Germany, pp 683–729
Shi J, Shi SQ, Barnes HM, Pittman JCU (2011) A chemical process for preparing cellulosic fibers hierarchically from kenaf bast fibers. BioResources 6:879–890
Shi J, Lu L, Guo W, Sun Y, Cao Y (2013) An environment-friendly thermal insulation material from cellulose and plasma modification. J Appl Polym Sci 130:3652–3658
Shin HK, Jeun JP, Kim HB, Kang PH (2012) Isolation of cellulose fibers from kenaf using electron beam. Radiat Phys Chem 81:936–940
Simon I, Glasser L, Scheraga HA, Manley RJ (1988) Structure of cellulose. 2. Low-energy crystalline arrangements. Macromolecules 21:990–998
Singh A, Lou HH, Pike RW, Agboola A, Li X, Hopper JR, Yaws CL (2008) Environmental impact assessment for potential continuous processes for the production of carbon nanotubes. Am J Environ Sci 4:522–534
Siqueira G, Bras J, Dufresne A (2009) New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 26:402–411
Siqueira G, Bras J, Dufresne A (2010) Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers 2:728–765
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17:459–494
Sonia A, Dasan KP, Alex R (2013) Celluloses microfibres (CMF) reinforced poly(ethylene-co-vinyl acetate) (EVA) composites: dynamic mechanical, gamma and thermal ageing studies. Chem Eng J 228:1214–1222
Stenstad P, Andresen M, Tanem BS, Stenius P (2008) Chemical surface modifications of microfibrillated cellulose. Cellulose 15:35–45
Šturcová A, Davies GR, Eichhorn SJ (2005) Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 6:1055–1061
Sun B, Zhang M, Hou Q, Liu R, Wu T, Si C (2016) Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 23:439–450
Sundari MT, Ramesh A (2012) Isolation and characterization of cellulose nanofibers from the aquatic weed water hyacinth—Eichhornia crassipes. Carbohydr Polym 87:1701–1705
Suzuki K, Okumura H, Kitagawa K, Sato S, Nakagaito AN, Yano H (2013) Development of continuous process enabling nanofibrillation of pulp and melt compounding. Cellulose 20:201–210
Taipale T, Österberg M, Nykänen A, Ruokolainen J, Laine J (2010) Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength. Cellulose 17:1005–1020
Tang Y, Shen X, Zhang J, Guo D, Kong F, Zhang N (2015) Extraction of cellulose nano-crystals from old corrugated container fiber using phosphoric acid and enzymatic hydrolysis followed by sonication. Carbohydr Polym 125:360–366
Tee T-T, Sin LT, Gobinath R, Bee S-T, Hui D, Rahmat A, Fang Q (2013) Investigation of nano-size montmorillonite on enhancing polyvinyl alcohol–starch blends prepared via solution cast approach. Compos Part B Eng 47:238–247
Tehrani AD, Neysi E (2013) Surface modification of cellulose nanowhisker throughout graft polymerization of 2-ethyl-2-oxazoline. Carbohydr Polym 97:98–104
Teng N, Ni J, Chen H, Ren Q, Na H, Liu X, Zhang R, Zhu J (2016) Initiating highly effective hydrolysis of regenerated cellulose by controlling transition of crystal form with sulfolane under microwave radiation. ACS Sustain Chem Eng 4:1507–1511
Teodoro KB, Teixeira EdM, Corrêa AC, Campos AD, Marconcini JM, Mattoso LH (2011) Whiskers de fibra de sisal obtidos sob diferentes condições de hidrólise ácida: efeito do tempo e da temperatura de extração. Polímeros 21:280–285
Thakur VK, Thakur MK (2015) Recent advances in green hydrogels from lignin: a review. Int J Biol Macromol 72:834–847
Tibolla H, Pelissari FM, Menegalli FC (2014) Cellulose nanofibers produced from banana peel by chemical and enzymatic treatment. LWT Food Sci Technol 59:1311–1318
Tingaut P, Zimmermann T, Lopez-Suevos F (2009) Synthesis and characterization of bionanocomposites with tunable properties from poly(lactic acid) and acetylated microfibrillated cellulose. Biomacromolecules 11:454–464
Trache D, Hazwan Hussin M, Haafiz MK, Thakur VK (2017) Recent progress in cellulose nanocrystals: sources and production. Nanoscale 9:1763–1786
Trifol J, Plackett D, Sillard C, Hassager O, Daugaard AE, Bras J, Szabo P (2016) A comparison of partially acetylated nanocellulose, nanocrystalline cellulose, and nanoclay as fillers for high-performance polylactide nanocomposites. J Appl Polym Sci 133. https://doi.org/10.1002/app.43257
Turbak AF, Snyder FW, Sandberg KR (1985) Suspensions containing microfibrillated cellulose. U.S. Patent 4378381A
Walker WC, Bosso CJ, Eckelman M, Isaacs JA, Pourzahedi L (2015) Integrating life cycle assessment into managing potential EHS risks of engineered nanomaterials: reviewing progress to date. J Nanopart Res 17:344–360
Wang B, Sain M (2007) Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos Sci Technol 67:2521–2527
Wang HD, Roeder RD, Whitney RA, Champagne P, Cunningham MF (2015) Graft modification of crystalline nanocellulose by Cu(0)-mediated SET living radical polymerization. J Polym Sci Polym Chem 53:2800–2808
Wang J, Siqueira G, Müller G, Rentsch D, Huch A, Tingaut P, Levalois-Grützmacher J, Grützmacher H (2016) Synthesis of new bis(acyl) phosphane oxide photoinitiators for the surface functionalization of cellulose nanocrystals. Chem Commun 52:2823–2826
Wei H, Rodriguez K, Renneckar S, Vikesland PJ (2014) Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ Sci Nano 1:302–316
Yahya M, Lee HV, Abd Hamid SB (2015) Preparation of nanocellulose via transition metal salt catalyzed hydrolysis pathway. BioResources 10:7627–7639
Yanamala N, Farcas MT, Hatfield MK, Kisin ER, Kagan VE, Geraci CL, Shvedova AA (2014) In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: a renewable and sustainable nanomaterial of the future. ACS Sustain Chem Eng 2:1691–1698
Yang J, Ye DY (2012) Liquid crystal of nanocellulose whiskers’ grafted with acrylamide. Chin Chem Lett 23:367–370
Yi J, He T, Jiang Z, Li J, Hu C (2013) AlCl3 catalyzed conversion of hemicellulose in corn stover. Chin J Catal 34:2146–2152
Yin Y, Tian X, Jiang X, Wang H, Gao W (2016) Modification of cellulose nanocrystal via SI-ATRP of styrene and the mechanism of its reinforcement of polymethylmethacrylate. Carbohydr Polym 142:206–212
Yu H-Y, Qin Z-Y, Liu Y-N, Chen L, Liu N, Zhou Z (2012) Simultaneous improvement of mechanical properties and thermal stability of bacterial polyester by cellulose nanocrystals. Carbohydr Polym 89:971–978
Yu H, Qin Z, Liang B, Liu N, Zhou Z, Chen L (2013) Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J Mater Chem A 1:3938–3944
Yu J, Wang C, Wang J, Chu F (2016) In situ development of self-reinforced cellulose nanocrystals based thermoplastic elastomers by atom transfer radical polymerization. Carbohydr Polym 141:143–150
Yue Y, Zhou C, French AD, Xia G, Han G, Wang Q, Wu Q (2012) Comparative properties of cellulose nano-crystals from native and mercerized cotton fibers. Cellulose 19:1173–1189
Zainuddin SYZ, Ahmad I, Kargarzadeh H, Abdullah I, Dufresne A (2013) Potential of using multiscale kenaf fibers as reinforcing filler in cassava starch-kenaf biocomposites. Carbohydr Polym 92:2299–2305
Zepič V, Poljanšek I, Oven P, Škapin AS, Hančič A (2015) Effect of drying pretreatment on the acetylation of nanofibrillated cellulose. BioResources 10:8148–8167
Zhang C, Su X, Xiong X, Hu Q, Amartey S, Tan X, Qin W (2016) 60Co-γ radiation-induced changes in the physical and chemical properties of rapeseed straw. Biomass Bioenergy 85:207–214
Zhao M, Li H, Liu W, Guo Y, Chu W (2016) Plasma treatment of paper for protein immobilization on paper-based chemiluminescence immunodevice. Biosens Bioelectron 79:581–588
Zlotorzynski A (1995) The application of microwave radiation to analytical and environmental chemistry. Crit Rev Anal Chem 25:43–76
Acknowledgments
The authors, Ishak Ahmad and Hanieh Kargarzadeh, would like to thank the Universiti Kebangsaan Malaysia (UKM) and Ministry of Higher Education of Malaysia (MOHE) for providing Research Grants, GUP-2016-009 and DIP-2016-026 respectively, and also of the National Science Centre, Poland on the basis of the Decision Number 2016/23/B/ST8/03509 to make this research possible.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kargarzadeh, H., Mariano, M., Gopakumar, D. et al. Advances in cellulose nanomaterials. Cellulose 25, 2151–2189 (2018). https://doi.org/10.1007/s10570-018-1723-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1723-5