Abstract
It is time to recognize the existence of plant memory, a phenomenon that allows plants to store and recall information from previous events and then change their responses to future stressful conditions. Although many recent publications have addressed this topic and concluded about plant memory using either classical or advanced approaches, several scientists are still armored against this important concept inherent to life and found in plants. Herein, our aims are to clarify the concept of memory and its use in plants and to overview the mechanisms underlying plant memory. How do plants store information? Where is such information stored? How long can information be stored? How does memory affect the organization of a given system with such ability? These are some questions that deserve attention from the scientific community rather than inadequate and incoherent ones challenging the existence of plant memory. Let us move on to the next page and deal with plants as living beings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Despite abundant studies addressing several aspects of memory in plants, many plant scientists are still repellent to this subject and show a kind of prejudice. In the last 20 years, a quick search using the input words “plant memory” returns more than 800 documents in the Google Scholar database, including articles in indexed journals, books and book chapters, of which most texts were published between 2016 and 2019. As examples of such repellency, some papers from our research groups on plant memory have been submitted to publication in high quality journals and overwhelmingly criticized by some of the reviewers, which claimed that plant memory does not exist and kindly asked us to withdraw any mention about memory throughout the manuscript. Frequently, the reviewers’ argument is that “plants do not have a brain”, which is a logical and correct argument IF we had indeed claimed such a thing. But we didn’t at all!
Although there is already a consolidated literature conceptualizing and characterizing memory in plants (e.g. Thellier and Lüttge 2013; Baluska et al. 2018; Michmizos and Hilioti 2019; Demongeot et al. 2019), we were urged to support the concept and applicability of memory as an actual biological phenomenon expressed by plants, instead of a simple and useful metaphor. Therefore, our aim with this piece of paper is to put at straight lines the scientific concept of memory, as well as its underlying mechanisms.
2 Understanding memory
According to the Merriam-Webster Dictionary, the very broad definitions of memory are (i) “the power or process of reproducing or recalling what has been learned and retained especially through associative mechanisms”, (ii) “the store of things learned and retained from an organism’s activity or experience as evidenced by modification of structure or behavior or by recall and recognition” or, simply, (iii) “capacity for storing information”. Thus, memory is NOT necessarily found in beings with brains; computers, for instance, are brainless and can be endowed with powerful memory capacities. Hence, the commonsense argument that “plants cannot have memory because they are brainless” do not resist to a simple check in a good dictionary.
However, the claims that plants do not have memory could be settled on a scientific definition of what memory really is. But the question is: what scientific field could be asked for a final and everywhere definition of memory? Many scientific fields are commonly based on concepts such as memory, from medical ones as neuroscience and psychology, to computational and informational sciences. Each of them confers memory to very different kinds of systems, from human brains to memory cards. Notwithstanding, the common ground is the capacity for storing information.
Memory capacity is a pivotal feature of all sort of living organisms in all aspects of their lives. Living beings need to face a plethora of environmental stimuli frightening their survival. In order to keep their stability, a variety of responses to those challenges are achieved by many different mechanisms from shifts on gene expression to whole body changes, allowing organism survival and fitness and, ultimately, adaptive evolution (Witzany 2018). Achieving such responses is particularly challenging for plants because of their sessile nature, preventing plants from simply escaping from an unfavorable situation. Thus, plants must stay and deal with all sort of environmental variation and resource limitations in their surroundings. Another remarkable feature is the modular structure of plant body. Overall, modules can be considered as the knots of networks that are connected via the edges (different modes of short and long-distance signaling). Modules are biological entities (individuals, structures, processes or pathways) characterized by more internal than external integration (Hütt 2019; Wegner 2019). As consequence of their modular nature, plants are decentralized organisms and then “the response of a plant to its environment is the sum of all modular responses to their local conditions plus all interaction effects that are due to integration” (de Kroon et al. 2009). Hence, whatever the mechanisms of memory are, memory in plants shall be a non-centralized process. This claim does not imply that all tissues and organs have the same ability or capacity to memorize stressful conditions. When considering plant modularity, some challenging questions also arise: how do plants enlist the several stimuli coming from different modules and trigger a unified response for the entire organism? Would the mitotic division of mother cell or organelles such as chloroplasts and mitochondria be responsible for such a modular organization?
So, the presence of memory in computers, brains or plants is not in question. In fact, the relevant questions are: how do plants store information? Where is information stored, if anywhere? How long can information be stored? Utterly, what are the mechanisms of memory and in what extension memory affects the organization of a given system with such ability?
3 Memory in plants: here’s how
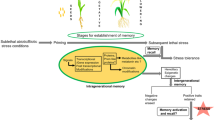
Most often, plants in the wild respond to simultaneous and/or subsequent stimuli (biotic and/or abiotic) through modifications in their metabolism, changing growth and/or morphogenesis. It is well known that previous responses can affect the subsequent ones, which is referred as priming when talking about plant-biotic interactions, or acclimation when plant performance is improved under recurrent or crossed non-lethal abiotic stimuli, such as drought, high or low temperature, and excessive or low light (Crisp et al. 2016; Demongeot et al. 2019). Over the last decades, the use of the term “priming” has been extended and is currently used to describe the enhanced resistance to both biotic and abiotic stresses (Conrath et al. 2006). Subsequent responses clearly must be based on some sort of stored information from past events, i.e. a memory-based process. For instance, plant memory has already been explored in agriculture. Rustification (sometimes called as hardening) is a common practice under nursery conditions that improves the performance of both crops and forest trees under field conditions (Fernández et al. 2013; Bompadre et al. 2018). Actually, recurrent responses and cross-tolerance are indeed “learning” processes (Thellier and Lüttge 2013; Souza et al. 2018; Michmizos and Hilioti 2019). Although there is a common claim of positive relationship between memory and performance, there is no reason to assign that memory will always improve plant metabolism or growth under constraining conditions. Instead, it is important to remark that, insofar memory is a basic capacity to store and eventually recall information, memory can also enable disruptive effects on plant organization.
Plants can follow at least three different pathways from the stimulus perception to the ultimate response: (1) a straightforward one, taking place almost immediately with non-dependency on previous stimulus; and two memory-dependent pathways, which are (2) learning and (3) storing/recalling. Those three pathways may be totally independent on each other or share some connections (Trewavas 2003; Demongeot et al. 2019). When learning, a recurrent stimulus changes the response intensity either negatively (lowering the response) that is known as “familiarization” or learning by habituation (Gagliano et al. 2014), or positively (reinforcing the response) that is called “sensitization” (Conrath et al. 2006).
Among the molecular mechanisms underpinning plant memory processes, sustained alterations in levels of many signaling metabolites and transcription factors were those first described and most elucidated to date. Such changes can explain how plant metabolism is altered and maintained even after the end of the stress period, and how plants deal with recurrent exposure to stresses (Bruce et al. 2007; Crisp et al. 2016). Conrath et al. (2006) suggested that the first stress event could trigger accumulation or post-translational modification of one or more signaling proteins that, after being synthetized or modified, remain inactive. This would allow an enhanced response when perceiving the second signaling event, due to a hyperactivation of the signaling protein. One of the most important reversible post-translational modifications that causes inactive proteins to become active and vice-versa is protein phosphorylation and dephosphorylation. Such processes modulate members of a diverse class of mitogen‐activated protein kinases (MAPK), with role in plant responses to biotic and abiotic stimuli (Gális et al. 2009).
Transcriptome studies have allowed the identification of a wide range of changes in gene expression, levels of proteins and other metabolites described as important in memory responses. Transcriptional responses resulting of a first exposure to a stimulus can last and induce either sustained changes in gene expression (activation or repression) or a modified response as hyperinduction after a secondary stimulus. In Arabidopsis, heat stress memory is regulated by transcription factors that hit a target promoter, which in turn initiate a rapid and sustained response (Lämke and Bäurle 2017). In many cases, such accumulation of transcription factors allows fast responses in gene expression (Conrath et al. 2006). Importantly, transcripts can be altered in different ways, depending on stimuli nature. As an example, recurrent dehydration in A. thaliana can either activate or repress the transcription of genes, as well as change the expression of genes previously modified by the first exposure to drought (Ding et al. 2013).
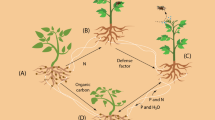
Phytohormones have also been identified as important molecules in plant memory responses. Various studies show that previous exposure to stresses affects the synthesis/degradation of gibberellin (GA), abscisic acid (ABA) and salicylic acid (SA) in plants. Under drought, GA levels decreased and remained low in double-stressed plants as compared with those exposed just once to drought. Oppositely, ABA levels were higher in double- than in single-stressed plants and were correlated to high H2O2 levels, a potential signaling molecule (Fleta-Soriano et al. 2015; Marcos et al. 2018). In A. thaliana, stomatal closure due to dehydration is ABA-dependent and both mesophyll and guard cells display transcriptional memory. Altered ABA production in guard cells maintains stomata partially closed during rehydration period (recovery), decreasing transpiration under recurrent drought (Virlouvet and Fromm 2015). Plant hormones in volatile forms, such as methyl salicylate (MeSA) and methyl jasmonate (MeJA), can activate plant immunity when applied repeatedly. Song and Ryu (2018) demonstrated that MeSA-MeSA treatment induces enhanced systemic acquired resistance (known as SAR) by upregulating the expression of SA-dependent defense genes. One would argue that changes in hormone levels are the end product of a transcriptional regulation and there is evidence that several epigenetic factors can modulate biosynthesis, transport and signal transduction of several plant hormones. On the other hand, some phytohormones may cause epigenetic modifications (Yamamuro et al. 2016). According to Turgut-Kara et al. (2020), some plant hormones have significant effects on chromatin packaging, which is conciliated by DNA methylation and histone modifications.
In general, epigenetic changes and hormone action are correlated to each other and one is not just a result of the other. Hormones and epigenetic memory mechanisms can operate altogether engendering responses to many external stimuli. For instance, high SA levels, which mediate SAR, induce certain modifications in the chromatin structure, such as H3 and H4 acetylation, and H3K4 methylation. Such modifications could prime pathogen-responsive genes and allow more effective responses to a subsequent attack (Iwasaki and Paszkowski 2014).
Epigenetic mechanisms are known as events that may provide mechanistic basis for the memory formation (Bruce et al. 2007) and changes in the epigenome play a fundamental role in memory responses to recurrent stimuli. Epigenetic modification may vary due to the nature of the stimulus and include changes in chromatin state, which may account for changes in the histone tail, control of gene expression through DNA methylation and demethylation, or inactivation of RNA polymerase II small RNAs and other non-coding RNAs (Crisp et al. 2016; Turgut-Kara et al. 2020). Biotic and abiotic stimuli lead to chromatin modifications that play a crucial role in initiation and maintenance of stress memories at various responsive loci in plants (Friedrich et al. 2018; He and Li 2018).
Responses to multiple exposure to drought include not only changes in gene expression patterns and transcripts accumulation, but also epigenetic modifications. Ding et al. (2012) pointed out that gene expression and transcripts accumulation in Arabidopsis may be the result of a progressive increase in H3K4 trimethylation and in RNA polymerase II phosphorylation. Such changes persist even after stress relief, representing a drought stress memory. In Arabidopsis, H3K4 trimethylation is also involved in heat stress memory, being associated with hyperinduction of gene expression upon a recurring heat shock and leading to acquired thermotolerance (Lämke et al. 2016). In relation to low temperatures, stress memory can stimulate springtime flowering, indicating that vernalization is regulated by epigenetic mechanisms (Song et al. 2013; Yang et al. 2017).
DNA methylation—directed by sRNAs—can act in a sequence-specific manner to regulate gene expression at both transcriptional and post‐transcriptional levels (Iwasaki and Paszkowski 2014). In Arabidopsis, DNA methylation regulates the responses to environmental factors. Tricker et al. (2012) found that the stomatal index is reduced under low relative air humidity, an environmental element that triggers RNA-directed de novo DNA methylation and suppression of genes controlling stomatal development. Such phenotypic responses and DNA methylation can be even inherited (Tricker et al. 2013). Espinas et al. (2016) emphasized the role of DNA methylation and demethylation under biotic stress, with hypomethylation of regions flanking both ends of defense-related genes enhancing their expression during the interaction with the pathogen.
More recently, it has been discovered that epigenetic memory may include transcript splicing. The exposure of Arabidopsis plants to a non-lethal heat stress results in de-repression of splicing after a second heat stress, while plants that did not experience such heat stress showed significant repression of splicing. Ling et al. (2018) pointed out that splicing memory affects “the ability of plants to survive subsequent and otherwise lethal heat stress”. This finding further increases the diversity of epigenomic processes that support plant memory, including the way how RNA is processed before coding proteins. Although the potential impact of DNA methylation and chromatin organization on memory have been addressed, many open questions remain around the specificity of DNA and chromatin marks, their persistence and stability during mitosis, and thus the maintenance of memory (Hilker and Schmülling 2019).
A mechanism for supporting memory in plants, very similar to the neuronal process already described for animals, is the plant synapse proposed by Baluska et al. (2005). Plant synapsis is the overall result of actin-driven endocytosis, endosomal sorting, and vesicle recycling, which together allow polar transport of auxin. Baluska et al. (2008) suggested that auxin-enriched vesicle recycling at plant synapse could also be involved in gravi- and electric-memory phenomena. Besides, animal and human learning via synaptic plasticity is based on the endocytic recycling of glutamate and GABA receptor (Baluska and Mancuso 2018).
The molecular mechanisms underlying memory are not yet fully understood and it is very likely that many pathways and interactions are involved. But, even more importantly, there is an urgent need to integrate the mechanisms described for a clear understanding of the memory process as a whole. Fleta-Soriano and Munné-Bosch (2016) suggested that the integration of ‘omics’ approaches, which includes epigenomics, transcriptomics and metabolomics, in addition to structural changes at various levels of organization, may contribute to the understanding of plant memory. In fact, the description of the mechanisms and molecular elements involved in memory has been the subject of many studies. However, few studies have offered a model that allows the understanding of how molecular architecture operates to guarantee the storage and subsequent recall of information (Inoue 2008; Thellier and Lüttge 2013; Demongeot et al. 2019). Further studies are needed to reveal whether such models based on ‘omics’ approaches described so far can indeed be applied to the study of memory in plants. For instance, Georgii et al. (2019) mapped the transcriptomic networks in Poplar plants during and after water stress and revealed that a complex interplay of network components contributes to the coordination of post-recovery responses to stress. In addition, it cannot be ruled out that when evaluating more complex ‘omic’ networks, interactions are even more significant and important for the creation and maintenance of stress memory.
To account for complex networks, a new and general framework for molecular information storage in cells was proposed by De la Fuente (2015), which is composed by two functionally and molecularly interrelated systems: a dynamic, flexible and adaptive system (metabolic memory); and an essentially conservative system (genetic memory). The molecular information of both systems seems to coordinate the physiological development of the whole cell. Accordingly, metabolic memory can be modeled as Hopfield networks that are networks where each unit (node) takes only on two different values for their states, in which the shift between both is determined when a certain threshold is exceeded. According to this model, the metabolic dynamics follows a self-organized pattern toward a local minimum (attractor), enabling storage of functional catalytic patterns. Thus, the metabolic dynamics governed by Hopfield-type attractors and structural changes based on the enzymatic covalent modifications of specific molecules would determine cellular memory and learning (De la Fuente 2015). As suggested by Demongeot et al. (2019), Hopfield-like boolean metabolic networks could store memory through the reinforcement of specific metabolic pathways, which could be activated more promptly when plants are subjected to recurrent stimuli. In that model, it is claimed a central role for calcium wave signaling, modulating the pathways to be reinforced (Demongeot et al. 2019).
So, memory is confirmed as a decentralized process that takes place within each cell. However, plant responses may occur not only at the level of a single cell but also at organism level (entire multicellular organism). Then, individual cells are able to store information and at the same time the whole plant can have permanent memories, for example, from cold exposure known as vernalization (Gális et al. 2009). Given that the memory mechanisms in plants are proposed at the cellular level, one still intriguing question raises: in a plant as a whole, are memories integrated? Likely, the long-distance processes of signaling, such as calcium and ROS waves, other small molecules as peptides as well as electrical signs, could drive such systemic memory (Sukhov et al. 2019; Takahashi and Shinozaki 2019). But, if so, how?
4 How long some information can be stored?
Duration of the memory may vary widely. In some cases, it may contribute to environmental acclimation, being considered plastic and reversible. In general, memory based on increased level of metabolites and transcription factors probably mediates more transient or short-term effects and its duration can last from days to weeks (Bruce et al. 2007; Lämke and Bäurle 2017; Avramova 2018). In adittion, memory may last for months, which usually requires mitotic stability of the information and involves chromatin-based processes (Hilker and Schmülling 2019). In other cases, memory can contribute to adaptation, being stable and even extended to the offspring—known as inter or transgenerational stress memory (Crisp et al. 2016). It is assumed that transgenerational memory—one transmitted to two or more generations—is based on epigenetic mechanisms, whereas this may be or not the case for intergenerational memory—understood as one transmitted only to the first generation. Intergenerational memory may be the product of cues introduced into the seed or embryo by the mother plant or by environmental conditions during seed development (Lämke and Bäurle 2017). In fact, the epigenetic mechanism underpins more longer-lasting effects than the other suggested mechanisms (Bruce et al. 2007) and changes in DNA methylation pattern as well as histone modifications could be transmitted to the next generations (Iwasaki and Paszkowski 2014; Turgut-Kara et al. 2020).
Mechanisms for the resetting of epigenetic memory in plant paternal chromatin (which could be considered inheritable) have also been described (Borg et al. 2020). As each generation faces a different combination of environmental challenges, the loss or erasure of most environmental memories could enable a fresh start for the next generation (He and Li 2018). It is reasonable to suggest that the duration of the memories is not a simple result of the mechanism underlying the memory. Perhaps even more important are the resetting mechanisms involved. For example, it is known that the acquisition of heat memory dependent on DNA methylation involves the activation of heat shock transcription factors (HSFs) that induce the expression of heat shock proteins (Lämke et al. 2016). One mechanism described for resetting such heat memory is autophagy. In Arabidopsis, Sedaghatmehr et al. (2019) demonstrated that autophagy mediates the specific degradation of heat shock proteins at later stages of the thermo-recovery phase, compromising heat tolerance after a second heat shock.
5 Just a few insights on plant memory
At this point, we do believe that there is enough scientific knowledge to support the existence of plant memory. However, much has to be done to reveal all facets of memory and the underlying processes related to this phenomenon in plants. Would we be able to count plant memory as in computational systems? How can one measure memory capacity in a given plant species? Are plants able to transfer such “knowledge” based on previous experiences to their neighbors, as suggested by Ribeiro and Torres (2018)? In this case, do plants favor the species itself during the communication of memories? Are they able to choose not to communicate a memory? If so, important consequences for the resilience of (eco)systems would be proposed and further tested. For instance, it is reasonable to suppose differential memory capacity among species and such plant ability in storing information is likely affected by the developmental (or phenological) stage in which plants face stressful conditions (Kron et al. 2008; Leuendorf et al. 2020). Then, biodiversity would affect the ecosystem memory whereas older plants would have more experiences to share with younger ones, with both diversity and aging being two components of ecosystem resilience in a changing environment.
From a practical perspective, we need to explore the relevance of plant memory in both natural and agricultural systems, which would be the next step after recognizing it as one more ordinary phenomenon in nature. As one can see, there is a long and exciting pathway on plant memory to be explored and we—while scientists—must keep our minds open to new concepts derived from discoveries in plant science.
References
Avramova Z (2018) Defence-related priming and responses to recurring drought: two manifestations of plant transcriptional memory mediated by the ABA and JA signaling pathways. Plant Cell Environ 42:983–997
Baluska F, Mancuso S (2018) Plant cognition and behavior: from environmental awareness to synaptic circuits navigating root apices. In: Baluska F, Gagliano M, Witzany G (eds) Memory and learning in plants. Springer Nature, Cham, pp 51–77
Baluska F, Volkmann D, Menzel D (2005) Plant synapses: actin-based domains for cell-to-cell communication. Trends Plant Sci 10:106–111
Baluska F, Gagliano M, Witzany G (2018) Memory and learning in plants. Springer Nature, Cham
Baluska F, Schlicht M, Volkmann V, Mancuso S (2008) Vesicular secretion of auxin. Plant Signal Behav 3:254–256
Bompadre MJ, Colombo RP, Silvani VA, Fernández BL, Pardo AG, Ocampo JA, Godeas AM (2018) Pomegranate transplant stress can be ameliorated by Rhizophagus intraradices under nursery management. J Soil Sci Plant Nutr 18:772–789
Borg M, Jacob Y, Susaki D, LeBlanc C, Buendía D, Axelsson E, Kawashima T, Voigt P, Boavida L, Becker J, Higashiyama T, Martienssen R, Berger F (2020) Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat Cell Biol 22:621–629
Bruce TJA, Matthes MC, Napier JA, Pickett JA (2007) Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci 173:603–608
Conrath U, Beckers GJ, Flors V, García-Agustín P, Jakab G, Mauch F, Newman MA, Pieterse CM, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B (2006) Priming: getting ready for battle. Mol Plant-Microbe Interact 19:1062–1071
Crisp PA, Ganguly D, Eichten SR, Borevitz JO, Pogson BJ (2016) Reconsidering plant memory: intersections between stress recovery, RNA turnover, and epigenetics. Sci Adv 2:e1501340
de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ (2009) A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant Cell Environ 32:704–712
De la Fuente IM (2015) Elements of the cellular metabolic structure. Front Mol Biosci 2:16
Demongeot J, Hasgui H, Thellier M (2019) Memory in plants: Boolean modeling of the learning and store/recall memory functions in response to environmental stimuli. J Theor Biol 467:123–133
Ding Y, Fromm M, Avramova Z (2012) Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat Commun 3:740
Ding Y, Liu N, Virlouvet L, Riethoven JJ, Fromm M, Avramova Z (2013) Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol 13:229
Espinas NA, Saze H, Saijo Y (2016) Epigenetic control of defense signaling and priming in plants. Front Plant Sci 7:1201
Fernández NV, Marchelli P, Fontenla SB (2013) Ectomycorrhizas naturally established in Nothofagus nervosa seedlings under different cultivation practices in a forest nursery. Microb Ecol 66:581–592
Fleta-Soriano E, Munné-Bosch S (2016) Stress memory and the inevitable effects of drought: a physiological perspective. Front Plant Sci 7:143
Fleta-Soriano E, Pintó-Marijuan M, Munné-Bosch S (2015) Evidence of drought stress memory in the facultative CAM, Aptenia cordifolia: possible role of phytohormones. PLoS ONE 10:e0135391
Friedrich T, Faivre L, Bäurle I, Schubert D (2018) Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ 42:762–770
Gagliano M, Renton M, Depczynski M, Mancuso S (2014) Experience teaches plants to learn faster and forget slower in environments where it matters. Oecologia 175:63–72
Gális I, Gaquerel E, Pandey SP, Baldwin IT (2009) Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ 32:617–627
Georgii E, Kugler K, Pfeifer M, Vanzo E, Block K, Domagalska MA, Jud W, Abdelgawad H, Asard H, Reinhardt R, Hansel A, Spannagl M, Schäffner AR, Palme K, Mayer K, Schnitzler JP (2019) The systems architecture of molecular memory in poplar after abiotic stress. Plant Cell 31:346–367
He Y, Li Z (2018) Epigenetic environmental memories in plants: establishment, maintenance, and reprogramming. Trends Genet 34:856–866
Hilker M, Schmülling T (2019) Stress priming, memory, and signalling in plants. Plant Cell Environ 42:753–761
Hütt M-T (2019) Modular organization and emergence in systems biology. In: Wegner LH, Lüttge U (eds) Emergence and modularity in life sciences. Springer Nature, Cham, pp 37–49
Inoue J (2008) A simple Hopfield-like cellular network model of plant intelligence. In: Banerjee R, Chakrabarti BK (eds) Models of brain and mind—physical, computational and psychological approaches. Elsevier, Amsterdam, pp 169–174
Iwasaki M, Paszkowski J (2014) Epigenetic memory in plants. EMBO J 33:1987–1998
Kron AP, Souza GM, Ribeiro RV (2008) Water deficiency at different developmental stages of Glycine max can improve drought tolerance. Bragantia 67:693–699
Lämke J, Bäurle I (2017) Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol 18:124
Lämke J, Brzezinka K, Altmann S, Bäurle I (2016) A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J 35:162–175
Leuendorf JE, Frank M, Schmülling T (2020) Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci Rep 10:689
Ling Y, Serrano N, Gao G, Atia M, Mokhtar M, Woo YH, Bazin J, Veluchamy A, Benhamed M, Crespi M, Gehring C, Reddy A, Mahfouz MM (2018) Thermopriming triggers splicing memory in Arabidopsis. J Exp Bot 69:2659–2675
Marcos FCC, Silveira NM, Mokochinski JB, Sawaya ACHF, Marchiori PER, Machado EC, Souza GM, Landell MGA, Ribeiro RV (2018) Drought tolerance of sugarcane is improved by previous exposure to water deficit. J Plant Physiol 223:9–18
Michmizos D, Hilioti Z (2019) A roadmap towards a functional paradigm for learning and memory in plants. J Plant Physiol 232:209–215
Ribeiro RV, Torres RS (2018) Sentinel plants as programmable processing units: insights from a multidisciplinary perspective about stress memory and plant signaling and their relevance at community level. Plant Signal Behav 13:e1526001
Sedaghatmehr M, Thirumalaikumar VP, Kamranfar I, Marmagne A, Masclaux-Daubresse C, Balazadeh S (2019) A regulatory role of autophagy for resetting the memory of heat stress in plants. Plant Cell Environ 42:1054–1064
Song GC, Ryu C-M (2018) Evidence for volatile memory in plants: boosting defence priming through the recurrent application of plant volatiles. Mol Cells 41:724–732
Song J, Irwin J, Dean C (2013) Remembering the prolonged cold of winter. Curr Biol 23:R807–R811
Souza GM, Toledo GRA, Saraiva GFR (2018) Towards systemic view for plant learning: ecophysiological perspective. In: Baluska F, Gagliano M, Witzany G (eds) Memory and learning in plants. Springer Nature, Cham, pp 163–189
Sukhov V, Sukhova E, Vladimir Vodeneev V (2019) Long-distance electrical signals as a link between the local action of stressors and the systemic physiological responses in higher plants. Prog Biophys Mol Biol 146:63–84
Takahashi F, Shinozaki K (2019) Long-distance signaling in plant stress response. Curr Opin Plant Biol 47:106–111
Thellier M, Lüttge U (2013) Plant memory: a tentative model. Plant Biol 15:1–12
Trewavas A (2003) Aspects of plant intelligence. Ann Bot 92:1–20
Tricker PJ, Lopez CM, Gibbings G, Hadley P, Wilkinson MJ (2013) Transgenerational, dynamic methylation of stomata genes in response to low relative humidity. Int J Mol Sci 14:6674–6689
Tricker PJ, Gibbings JG, Rodríguez López CM, Hadley P, Wilkinson MJ (2012) Low relative humidity triggers RNA-directed de novo DNA methylation and suppression of genes controlling stomatal development. J Exp Bot 63:3799–3813
Turgut-Kara N, Arikan B, Celik H (2020) Epigenetic memory and priming in plants. Genetica 148:47–54
Virlouvet L, Fromm M (2015) Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol 205:596–607
Wegner LH (2019) Modularity versus emergence: how to cope with complexity in whole-plant physiology. In: Wegner LH, Lüttge U (eds) Emergence and modularity in life sciences. Springer Nature, Cham, pp 75–95
Witzany G (2018) Memory and learning as key competences of living organisms. In: Baluska F, Gagliano M, Witzany G (eds) Memory and learning in plants. Springer Nature, Cham, pp 1–16
Yamamuro C, Zhu JK, Yang Z (2016) Epigenetic modifications and plant hormone action. Mol Plant 9:57–70
Yang H, Berry S, Olsson TSG, Hartley M, Howard M, Dean C (2017) Distinct phases of polycomb silencing to hold epigenetic memory of cold in Arabidopsis. Science 357:1142–1145
Acknowledgements
The authors acknowledge the reviewers that challenged us during our attempts to publish about plant memory, and also the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) for the scholarship (Finance Code 001) granted to YFG. RVR and GMS are fellows of the National Council for Scientific and Technological Development (CNPq, Brazil; Grants #302460/2018-7 and #302715/2018-5). RVR is also grateful to the São Paulo Research Foundation (FAPESP, Brazil) for funding research on sugarcane memory (Grants #2019/06161-2; and #2019/08047-2). We appreciate the insights from an anonymous reviewer, who suggested some exciting and unsolved questions about plant memory.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galviz, Y.C.F., Ribeiro, R.V. & Souza, G.M. Yes, plants do have memory. Theor. Exp. Plant Physiol. 32, 195–202 (2020). https://doi.org/10.1007/s40626-020-00181-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-020-00181-y